Abstract
Objective
Sleep disturbance is a common problem in the general population. Sleep deprivation or dysfunction can have profound health consequences. However, how sleep duration is associated with thyroid function remains unclear. This study was thus developed to examine the association between sleep duration and thyroid function in the US adult population.
Methods
A total of 8102 participants from the NHANES 2007–2012 dataset were included in this study. Weighted data analyses were conducted, and the link between sleep duration and thyroid function was probed using linear regression models with smoothed curve fitting. Stratified analyses were also performed.
Results
Weighted mean (standard deviation) values for study variables were as follows: sleep duration 6.85 (0.02) hours, thyroid-stimulating hormone (TSH) 1.86 (0.03) mIU/ml, serum free T3 3.20 (0. 01) pg/mL, serum free T4 0.80 (0.01) ng/dL, serum total T3 115.12 (0.64) ng/dL, serum total T4 7.81 (0.04) ug/dL, TPOAb 16.20 (1.53) IU/mL, TgAb 5.75 (0.73) IU/mL, and Tg 15.11 (0.46) ng/mL. In unadjusted analyses, increased sleep duration was associated with higher serum TSH levels and decreased FT3 levels. After adjustment for potential confounders, a significant negative relationship was detected between sleep duration and FT3 levels in participants with ≤7 hours of sleep. When sleep duration exceeded 7 hours, no significant changes in FT3 levels were observed after further increases in sleep duration.
Conclusion
Increased sleep duration was related to decreased FT3 levels, primarily at short sleep durations, and this correlation was no longer evident when participants reached the recommended healthy sleep duration.
Introduction
Sleep is essential for human health, affecting a wide range of key physiological functions [1, 2]. Sleep is complex and subject to tight physiological regulation [3]. The recommend daily duration of sleep for adults is 7–9 hours [4, 5]. However, millions of Americans suffer from sleep-related disorders each year, with only 48% of adults reporting a habitual sleep duration within this range [6]. Similar patterns of gradually decreasing sleep duration have been observed in several Western nations over the past few decades [7]. Many studies have demonstrated that insufficient or disturbed sleep has far-reaching implications for health. A close relationship between sleep duration and health conditions such as cardiovascular events [8–10], mental disorders [11, 12], and mortality [13] has been observed in several epidemiological studies.
Thyroid hormone (TH) production and signaling activity plays a key role in human growth and development, shaping physiological processes such as digestion, respiration, heart rate, and thermoregulation [14]. The hypothalamic-pituitary-thyroid (HPT) axis tightly controls TH production and secretion. This central axis initiates TH production based on signaling in the hypothalamic paraventricular nucleus prior to signal transmission through the pituitary and thyroid glands [15, 16]. In addition, almost all hormones are produced in a cyclical rhythm over 24-hour intervals, with sleep having a varying impact on the regulation of this rhythm [17–19]. Notably, sleep strongly impacts thyroid-stimulating hormone (TSH) with respect to both the quality and duration of sleep, and free triiodothyronine (FT3) also showed a 24-hour circadian rhythm parallel to TSH, but delayed [20, 21]. How sleep impacts the HPT axis has been suggested to be dependent on the period of sleep restriction [22], with short-term sleep restriction increasing secretion of TSH [23] and FT4 [24], but long-term sleep restriction suppressing TSH and FT4 secretion [25], but the sample size is relatively small.
Currently, the relationship between sleep duration and thyroid function profiles remains unclear, and few large-sample epidemiological analyses have examined the association between sleep duration and thyroid function in the general adult population. Therefore, the aim of this study was to clarify how weekday sleep duration relates to thyroid function in the US adult population.
Methods
Study population
The cross-sectional National Health and Nutrition Examination Survey (NHANES) compiles large volumes of data pertaining to the demographic characteristics, healthy behaviors, and nutrition of individuals in the US. Data from the NHANES are accessible for researcher use online, along with corresponding statistics (www.cdc.gov/nchs/nhanes/). The present study was performed as per the Declaration of Helsinki.
The present study utilized NHANES 2007–2012 data, which received approval from the NCHS Research Ethics Review Board (ERB). All surveyed NHANES participants had provided written informed consent. For these analyses, 8,102 subjects ≥ 18 years of age for whom complete sleep- and thyroid function-related data were available were selected for inclusion. For further details regarding participant screening, see Fig 1.
Fig 1. Study flowchart.
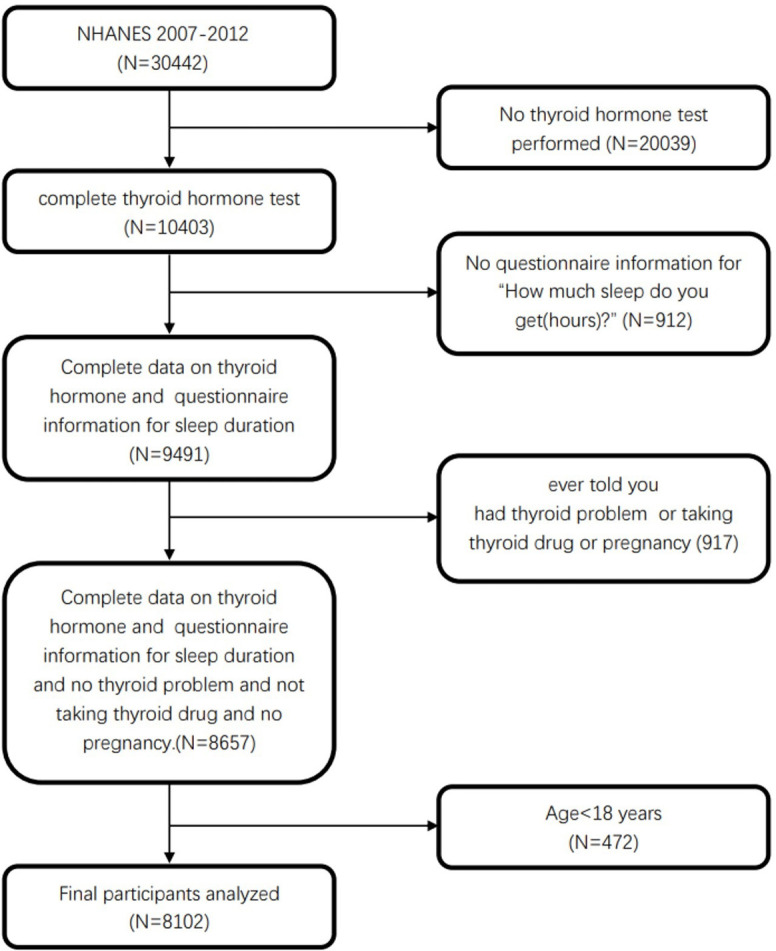
NHANES, National Health and Nutrition Examination Survey.
Measurements
Sleep duration
The assessment of sleep duration was based on the ’sleep disturbance’ data from the NHANES questionnaire. Question SLQ010H asked “How much sleep do you usually get at night on weekdays or workdays?”, with participants being requested to respond with a number of hours. Participants responded with their sleep duration from 2–11 hours. If they reported 12 or more hours, then a duration of 12 hours was recorded. Sleep duration was analyzed as a continuous and categorical variable. Based on the sleep duration recommendation of the National Sleep Foundation in the US and the consensus recommendations of the American Academy of Sleep Medicine and the Sleep Research Society regarding the duration of sleep necessary to promote optimal health in adults, sleep duration was stratified into three groups: < 7 h, 7–9 h, and >9 h [4, 26].
Thyroid outcomes
The thyroid parameters analyzed, including TSH, FT3, FT4, TT3, TT4, Tg, TgAb, and TPOAb levels, were determined using the Access 2 method (Beckman Coulter, from which the immunization reagents were purchased). Thyroid parameters were measured from 2007–2012. Participants were asked to fast for 9 hours and have their blood drawn within 4 hours of the morning, have their blood collected and processed for freezing and storage, and then have the collected blood samples sent together to a designated laboratory once a week for testing. TSH levels were analyzed using a third-generation two-site sandwich immunoassay with a reference range of 0.34–5.6 IU/mL. The reference range for the competitive binding immunoassay used to measure FT3 levels was 2.5–3.9 pg/mL, while the reference range for the two-step enzyme immunoassay used to measure FT4 levels was 0.6–1.6 ng/dL. Levels of TT3 and TT4 were also measured via competitive binding immunoenzymatic assays with respective reference ranges of 5.0–12.0 ug/dL and 80–220 ng/dL, respectively. A sequential two-step sandwich immunoassay was also used to measure TPOAb and TgAb titers, with respective 0–9.0 IU/mL and 0–4.0 IU/mL reference ranges. The Access Tg assay is a one-step simultaneous sandwich assay. The NHANES Laboratory/Medical Technologist Procedures Manual provides comprehensive instructions for specimen collection and processing.
Covariates
Participant age, race/ethnicity, poverty income ratio (PIR), smoking history, gender, and history of alcohol consumption were assessed using a standard questionnaire. Weight and height measurements, which are necessary for calculating participants’ body mass index (BMI), were collected by trained technicians in a mobile examination center (MEC). Additionally, considering the importance of iodine for the synthesis of thyroxine, urine samples were collected to determine participants’ urinary iodine concentration (UIC). The collected urine undergoes processing, storage, and is centrally shipped once a week to a designated laboratory for UIC testing (Inductively Coupled Plasma Dynamic Reaction Cell Mass Spectrometry, ICP-DRC-MS). Ethnicity was categorized into four groups (non-Hispanic black, non-Hispanic white, Mexican American, or other). Socioeconomic status was assessed using PIR values. BMI values were calculated by dividing participant weight (kg) by height squared (m2). Marital status was defined as a categorical variable with three groups: married or living with a partner; widowed, divorced, or separated; or never married. Alcohol consumption history was also categorized as never (< 12 lifetime drinks), former (≥ 12 drinks in 1 year but no drinks in the last year or ≥ 12 lifetime drinks but no drinks in the last year), light (≤ 2 and ≤ 1 daily drinks in males and females, respectively, in the last 12 months), moderate (3 or 2 daily drinks in males and females, respectively, in the last 12 months) or heavy (≥ 4 or ≥ 3 daily drinks in males and females, respectively, in the last 12 months). Smoking status was classified as never (< 100 lifetime cigarettes), former (> 100 lifetime cigarettes but not a current smoker), or current.
Statistical analysis
The analytical guidelines (NHANES:2007–2012) were used when calculating and analyzing weighted data. The NHANES data release file includes multiple sample weights, including interview weight (wtint2yr), or examination weight (wtmec2yr), and the most appropriate weighting is dependent on the selected variables of interest. Given that the MEC sample is an interview sample subset, this analysis was conducted with the combined MEC examination weight. However, the thyroid examination sample from NHANES 2009–2012 (two survey cycles) is a subset of the overall participants that were interviewed for the MEC examination. As a result, the subset weight (WTSA2YR) was used when analyzing data from these two survey cycles. The weight of the survey allows it to be extended to the civilian noninstitutionalized US population [27, 28].
The association between sleep duration and thyroid function was described with both unadjusted and adjusted linear regression models. Model 1 did not adjust for covariates, model 2 adjusted for gender, age, race/ethnicity, BMI, alcohol consumption, smoking status, marital status and UIC levels of participants. Results were further resolved with smooth curve fitting. Stratified analyses were also performed to interrogate the independent effect of sleep duration on thyroid function levels. Data are presented as means with standard deviations (SDs) or percentages, as appropriate. Effect values are reported as β values with corresponding 95% confidence intervals (CIs). P < 0.05 served as the cut-off for statistical significance. All analyses were performed with R v4.2.2 with the ’nhanesR’ package (v0.9.4.1) and the ’survey’ package (v 4.1–1).
Results
Baseline participant characteristics
Table 1 describes the characteristics of participants grouped according to sleep duration. Of these 8102 participants, 4649 (weighted percentage: 57.38%) slept 7–9 h, 3241 (weighted percentage: 40%) slept < 7 h, and 212 (weighted percentage: 2.62%) slept > 9 h. The weighted mean (SD) sleep duration of these participants was 6.85 (0.02) h. The mean TSH levels of these participants was 1.86 (0.03) mIU/mL, with serum free T3 levels of 3.20 (0.01) pg/mL, serum free T4 levels of 0.80 (0.01) ng/dL, serum total T3 levels of 115. 12 (0.64) ng/dL, serum total T4 levels of 7.81 (0.04) ug/dL, TPOAb levels of 16.20 (1.53) IU/mL, TgAb levels of 5.75 (0.73) IU/mL, and Tg levels of 15.11 (0.46) ng/mL. ANOVAs revealed significant differences in TSH and FT3 levels among sleep duration subgroups, with higher TSH and lower FT3 levels in the longer sleep duration group. The study did not include participants who were reported to have thyroid disease, who were taking thyroid medication, or who were pregnant. Our study did not exclude participants who were not clinically diagnosed with abnormal thyroid function.
Table 1. Thyroid function of NHANES (2007–2012) study population in sleep duration groups.
| Characteristics | Sleep duration | P-value | |||
|---|---|---|---|---|---|
| total | <7 h | 7–9 h | >9 h | ||
| N | 8102 | 3241 | 4649 | 212 | |
| Sleep duration(h) | 6.85(0.02)a | 5.48(0.02) | 7.60(0.02) | 10.49(0.09) | < 0.001 |
| Sex | 0.13 | ||||
| Male | 4318(53.3)b | 1761(54.78) | 2454(51.74) | 103(45.45) | |
| Female | 3784(46.7) | 1480(45.22) | 2195(48.26) | 109(54.55) | |
| Race/Ethnicity | < 0.001 | ||||
| White | 3554(43.87) | 1269(62.21) | 2189(69.97) | 96(60.05) | |
| Black | 1673(20.65) | 889(15.93) | 750 (8.25) | 34(10.30) | |
| Mexican | 1348(16.64) | 460 (7.52) | 856 (9.21) | 32(10.57) | |
| Other | 1527(18.85) | 623(14.34) | 854(12.57) | 50(19.08) | |
| Age (years) | 44.99(0.45) | 44.43(0.53) | 45.33(0.48) | 45.43(3.37) | 0.26 |
| Ever told doctor had trouble sleeping? | < 0.001 | ||||
| No | 6333(78.2) | 2224(66.49) | 3930(81.78) | 179(88.30) | |
| Yes | 1765(21.8) | 1014(33.51) | 718(18.22) | 33(11.70) | |
| Ever told by doctor have sleep disorder? | < 0.001 | ||||
| No | 7514(92.93) | 2914(89.51) | 4406(94.32) | 194(92.94) | |
| Yes | 572(7.07) | 319(10.49) | 236 (5.68) | 17 (7.06) | |
| BMI (kg/m2) | 28.37(0.14) | 29.04(0.18) | 27.99(0.18) | 27.32(0.93) | < 0.001 |
| TSH (mIU/L) | 1.86(0.03) | 1.79(0.04) | 1.90(0.04) | 2.11(0.14) | 0.02 |
| FT3 (pg/mL) | 3.20(0.01) | 3.24(0.02) | 3.18(0.01) | 3.14(0.04) | 0.002 |
| FT4 (ng/dL) | 0.80(0.00) | 0.80(0.01) | 0.80(0.01) | 0.82(0.01) | 0.12 |
| TT3 (ng/dL) | 115.12(0.64) | 116.11(0.85) | 114.51(0.70) | 114.57(2.81) | 0.18 |
| TT4 (ug/dL) | 7.81(0.04) | 7.81(0.05) | 7.80(0.04) | 8.09(0.12) | 0.06 |
| TPOAb (IU/mL) | 16.20(1.53) | 17.19(2.94) | 15.62(1.57) | 14.73(5.33) | 0.84 |
| TgAb (IU/mL) | 5.75(0.73) | 5.46(1.26) | 5.88(0.82) | 7.55(4.35) | 0.9 |
| Tg (ng/mL) | 15.11(0.46) | 15.68(0.70) | 14.69(0.66) | 16.73(1.46) | 0.28 |
| UIC (ug/dL) | 236.41(11.42) | 255.00(19.53) | 225.50(12.64) | 206.03(21.32) | 0.13 |
| Smoke | < 0.001 | ||||
| Never | 4085(53.21) | 1596(51.17) | 2399(56.44) | 90(52.48) | |
| Former | 1881(24.5) | 723(21.83) | 1116(25.33) | 42(19.61) | |
| Now | 1711(22.29) | 806(27.00) | 850(18.22) | 55(27.91) | |
| Alcohol.User | < 0.001 | ||||
| Never | 982(13.79) | 383 (9.54) | 555 (9.96) | 44(22.66) | |
| Former | 1353(19.01) | 608(16.84) | 698(13.91) | 47(24.31) | |
| Mild | 2182(30.65) | 838(31.84) | 1308(34.76) | 36(20.90) | |
| Moderate | 1041(14.62) | 414(15.73) | 616(18.49) | 11 (8.06) | |
| Heavy | 1561(21.93) | 635(26.05) | 890(22.87) | 36(24.06) | |
| Poverty-to-income ratio | 2.93(0.05) | 2.76(0.08) | 3.06(0.06) | 2.26(0.15) | < 0.001 |
| Marital | < 0.001 | ||||
| Never married | 1391(18.11) | 575(20.63) | 779(18.39) | 37(21.05) | |
| Widowed, divorced, or separated | 1668(21.72) | 772(19.45) | 836(15.35) | 60(22.81) | |
| Married, or living with partner | 4622(60.17) | 1778(59.91) | 2754(66.26) | 90(56.14) | |
a Continuous Variables: weighted mean (SD).
b Categorical variable: actual frequency (weighted percentage).
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TT3, total T3; TT4, total T4; Tg, thyroglobulin; TPOAb, thyroid peroxidase antibody; BMI, body mass index; UIC, urinary iodine concentration; SD, Standard deviation.
The association between thyroid function and sleep duration
Table 2 shows that in an unadjusted model, TSH was positively associated with sleep duration (β = 0.056, 95% CI: 0.023 to 0.089, P = 0.001). When sleep duration was categorized into 3 groups (<7h, 7-9h, and >9h), this positive association was still present (P for trend = 0.005), but this association did not remain after adjusting for confounders in model 2. In contrast, FT3 levels were negatively associated with sleep duration. This negative association remained after further adjustment for confounders in model 2 (β = -0.017, 95% CI: -0.028 to -0.005, P = 0.005) and after dividing sleep duration into 3 groups (P for trend = 0.013).
Table 2. The association between sleep duration and thyroid function.
| Model 1a | p-value | Model 2b | p-value | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | |||
| TSH(mIU/L) | ||||
| Sleep duration(h) | 0.056(0.023,0.089) | 0.001 | 0.029(-0.011,0.068) | 0.149 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | 0.110(0.022,0.198) | 0.016 | -0.002(-0.108,0.103) | 0.962 |
| >9 h | 0.319(0.022,0.616) | 0.036 | 0.328(-0.068,0.725) | 0.101 |
| p for trend | 0.005 | 0.624 | ||
| FT3(pg/mL) | ||||
| Sleep duration(h) | -0.021(-0.032,-0.009) | <0.001 | -0.017(-0.028,-0.006) | 0.005 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | -0.060(-0.098,-0.023) | 0.002 | -0.046(-0.083,-0.009) | 0.016 |
| >9 h | -0.102(-0.176,-0.027) | 0.009 | -0.072(-0.141,-0.002) | 0.044 |
| p for trend | <0.001 | 0.013 | ||
| FT4(ng/dL) | ||||
| Sleep duration(h) | 0.002(-0.001,0.006) | 0.169 | 0.001(-0.004,0.005) | 0.745 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | 0.003(-0.006,0.013) | 0.447 | -0.002(-0.012,0.009) | 0.750 |
| >9 h | 0.027(0.001,0.054) | 0.045 | 0.012(-0.021,0.045) | 0.461 |
| p for trend | 0.192 | 0.935 | ||
| TT3(ng/dL) | ||||
| Sleep duration(h) | -0.433(-1.052,0.185) | 0.166 | -0.244(-0.866,0.378) | 0.430 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | -1.594(-3.373,0.184) | 0.078 | -0.684(-2.385,1.017) | 0.418 |
| >9 h | -1.540(-6.769,3.688) | 0.556 | -2.628(-6.544,1.289) | 0.181 |
| p for trend | 0.065 | 0.320 | ||
| TT4(ug/dL) | ||||
| Sleep duration(h) | 0.015(-0.025,0.055) | 0.449 | 0.020(-0.023,0.064) | 0.352 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | -0.007(-0.117,0.102) | 0.893 | 0.032(-0.093,0.158) | 0.601 |
| >9 h | 0.280(0.049,0.510) | 0.018 | 0.140(-0.109,0.389) | 0.259 |
| p for trend | 0.658 | 0.472 | ||
| TPOAb(IU/mL) | ||||
| Sleep duration(h) | 0.360(-2.042,2.762) | 0.764 | -0.554(-3.771,2.664) | 0.728 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | -1.565(-8.009,4.880) | 0.628 | -4.777(-12.091,2.536) | 0.192 |
| >9 h | -2.458(-13.876,8.961) | 0.667 | -1.471(-19.320,16.379) | 0.867 |
| p for trend | 0.591 | 0.214 | ||
| TgAb (IU/mL) | ||||
| Sleep duration(h) | 0.371(-0.415,1.157) | 0.348 | -0.056(-1.057,0.946) | 0.910 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | 0.417(-2.459,3.294) | 0.772 | -0.512(-3.899,2.875) | 0.759 |
| >9 h | 2.082(-7.351,11.514) | 0.659 | 1.865(-12.566,16.296) | 0.793 |
| p for trend | 0.705 | 0.875 | ||
| Tg (ng/mL) | ||||
| Sleep duration(h) | -0.292(-1.271,0.687) | 0.552 | -0.020(-1.192,1.152) | 0.973 |
| categories | ||||
| <7 h | Reference | Reference | ||
| 7–9 h | -0.997(-3.006,1.012) | 0.323 | 0.076(-1.992,2.144) | 0.941 |
| >9 h | 1.044(-2.203,4.291) | 0.521 | 0.514(-3.986,5.013) | 0.817 |
| p for trend | 0.435 | 0.908 |
a Model 1: no covariates were adjusted.
b Model 2: age, gender, race/ethnicity, poverty-to-income ratio, marital, body mass index, alcohol use, smoke, urinary iodine concentration, sleep disorder(ever told doctor had trouble sleeping or ever told by doctor have sleep disorder) were adjusted.
Abbreviations: TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; TT3, total T3; TT4, total T4; TPOAb, anti-thyroid peroxidase antibody; TgAb, anti-thyroglobulin antibody; Tg, thyroglobulin.
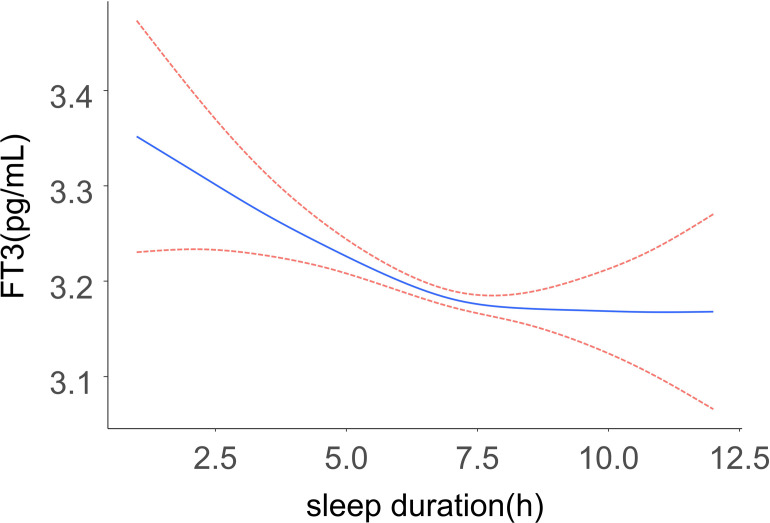
As shown in Fig 2, a generalized additive model revealed a flat L-shaped relationship between sleep duration and FT3 levels. FT3 levels were found to decrease with increasing sleep duration in participants with short sleep duration, but as sleep duration continued to increase after reaching a healthy sleep duration, FT3 levels leveled off and no longer decreased significantly. The solid line represents the smoothed curve fit between the variables while 95% CI for this fit is represented with a dashed line. Results were adjusted for all covariates (sex, age, race/ethnicity, marital status, BMI, sleep disturbance, poverty income ratio, smoking status, alcohol consumption status, UIC). A log-likelihood ratio test was also conducted, and comparisons of two-segment and one-line regression models were performed. A two-step recursive method revealed an inflection point of K = 7 h. For further details regarding these results, see Table 3.
Fig 2. A flat L-shaped association between sleep duration and FT3 was found in the generalized additive model (GAM).
The solid line represents the smoothed curve fit between the variables and the dashed line represents the 95% confidence interval of the fit. The model was adjusted for sex, age, race/ethnicity, marital status, BMI, sleep disturbance, poverty income ratio, smoking status, alcohol consumption status, UIC.
Table 3. Analysis of sleep duration and FT3 using segmented linear regression.
| Models | β (95%CI) | P value |
|---|---|---|
| Model I | ||
| One line effect | -0.017(-0.028,-0.006) | 0.005 |
| Model II | ||
| Inflection point (K) | 7 h | |
| sleep duration ≤ 7 h | -0.032 (-0.053, -0.011) | 0.004 |
| sleep duration > 7 h | 0.006 (-0.022, 0.033) | 0.664 |
| P for log-likelihood ratio test | 0.026 |
Model I, one-line linear regression; Model II, two-segment regression
β was the effect size and the 95% CI indicated the confidence interval
adjust for: sex, age, race/ethnicity, marital status, BMI, sleep disorder, poverty income ratio, smoking status, alcohol consumption status, UIC.
Subgroup analyses
Our study showed that sleep duration was negatively correlated with FT3 in thyroid function profiles, so we further evaluated the effect of sleep duration on FT3 in predefined and exploratory subgroups. Stratified analyses were performed as shown in Table 4. When sleep duration was used as a continuous variable, BMI and PIR had a significant interaction (p for interaction<0.05). With respect to BMI, overweight individuals (25 ≤ BMI < 30 kg/m2) had a greater decrease in FT3 levels compared to normal weight and obese individuals who slept longer. In terms of PIR, sleep duration was not associated with FT3 levels in low-income groups (PIR < 1), but there was a significant negative association in middle-income (1 ≤ PIR < 4) and high-income groups (PIR ≥ 4). When sleep duration was used as a categorical variable, PIR still had a significant interaction, and again sleep duration was significantly negatively associated with FT3 levels in middle-income and high-income populations (p for trend < 0.05), but not in low-income populations.
Table 4. Effect size of sleep duration on FT3 in prespecified and exploratory subgroups.
| Character | Total sleep duration | Categories sleep duration | ||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | p | p for interaction | <7 | 7–9 | >9 | p for trend | p for interaction | |
| Sex | 0.323 | 0.89 | ||||||
| Male | -0.014(-0.028,0.001) | 0.073 | reference | -0.053(-0.095,-0.011) | -0.067(-0.159, 0.026) | 0.01 | ||
| Female | -0.024(-0.038,-0.009) | 0.002 | reference | -0.058(-0.102,-0.013) | -0.102(-0.210, 0.006) | 0.008 | ||
| AGE (years) | 0.739 | 0.174 | ||||||
| >50 | -0.019(-0.030,-0.008) | 0.001 | reference | -0.038(-0.067,-0.008) | -0.146(-0.234,-0.059) | <0.001 | ||
| <50 | -0.016(-0.030,-0.002) | 0.029 | reference | -0.059(-0.104,-0.014) | -0.057(-0.153, 0.039) | 0.013 | ||
| Race/Ethnicity | 0.087 | 0.243 | ||||||
| White | -0.031(-0.048,-0.014) | <0.001 | reference | -0.081(-0.128,-0.034) | -0.13(-0.238,-0.023) | <0.001 | ||
| Black | -0.012(-0.041,0.018) | 0.435 | reference | -0.015(-0.079, 0.050) | -0.239(-0.376,-0.103) | 0.257 | ||
| Mexican | 0.01(-0.027,0.048) | 0.573 | reference | -0.008(-0.175,0.159) | 0.091(-0.196,0.379) | 0.956 | ||
| Other | -0.012(-0.029,0.005) | 0.164 | reference | -0.04(-0.103,0.022) | -0.084(-0.183,0.015) | 0.126 | ||
| Ever told doctor had trouble sleeping? | 0.536 | 0.889 | ||||||
| No | -0.024(-0.039,-0.008) | 0.003 | reference | -0.074(-0.119,-0.030) | -0.117(-0.204,-0.030) | <0.001 | ||
| Yes | -0.033(-0.057,-0.009) | 0.008 | reference | -0.067(-0.125,-0.010) | -0.14(-0.246,-0.033) | 0.016 | ||
| Ever told by doctor have sleep disorder? | 0.194 | 0.918 | ||||||
| No | -0.019(-0.031,-0.006) | 0.004 | reference | -0.062(-0.099,-0.024) | -0.104(-0.180,-0.028) | <0.001 | ||
| Yes | -0.045(-0.083,-0.007) | 0.020 | reference | -0.078(-0.182,0.026) | -0.105(-0.340,0.129) | 0.116 | ||
| Smoke | 0.408 | 0.178 | ||||||
| Never | -0.027(-0.043,-0.012) | <0.001 | reference | -0.078(-0.134,-0.021) | -0.157(-0.248,-0.065) | 0.003 | ||
| Former | -0.021(-0.039,-0.003) | 0.026 | reference | -0.026(-0.070, 0.018) | -0.187(-0.308,-0.065) | 0.077 | ||
| Now | -0.012(-0.036,0.012) | 0.318 | reference | -0.054(-0.125,0.017) | 0.013(-0.100,0.126) | 0.187 | ||
| Alcohol. User | 0.096 | 0.26 | ||||||
| Never | -0.009(-0.027,0.009) | 0.324 | reference | -0.031(-0.106,0.044) | -0.071(-0.220,0.078) | 0.3 | ||
| Former | -0.035(-0.062,-0.009) | 0.010 | reference | -0.088(-0.190, 0.013) | -0.143(-0.283,-0.004) | 0.055 | ||
| Mild | -0.037(-0.052,-0.022) | <0.0001 | reference | -0.093(-0.129,-0.057) | -0.157(-0.306,-0.008) | <0.0001 | ||
| Moderate | -0.012(-0.033,0.009) | 0.252 | reference | -0.024(-0.073,0.026) | -0.205(-0.469,0.058) | 0.212 | ||
| Heavy | -0.003(-0.033,0.027) | 0.842 | reference | -0.001(-0.094,0.093) | -0.083(-0.280,0.113) | 0.857 | ||
| BMI (kg/m2) | 0.013 | 0.069 | ||||||
| <25 | -0.01(-0.030,0.010) | 0.336 | reference | -0.063(-0.126,0.001) | -0.067(-0.179,0.046) | 0.042 | ||
| 25–29.9 | -0.041(-0.059,-0.023) | <0.0001 | reference | -0.104(-0.164,-0.045) | -0.136(-0.266,-0.006) | <0.001 | ||
| ≥30 | -0.013(-0.029,0.003) | 0.119 | reference | -0.013(-0.068, 0.041) | -0.135(-0.260,-0.011) | 0.369 | ||
| UIC (ug/dL) | 0.285 | 0.189 | ||||||
| <100 | -0.019(-0.041,0.002) | 0.078 | reference | -0.046(-0.097,0.005) | -0.078(-0.203,0.048) | 0.071 | ||
| 100–299 | -0.014(-0.028,-0.001) | 0.032 | reference | -0.05(-0.098,-0.003) | -0.073(-0.191, 0.045) | 0.028 | ||
| ≥300 | -0.036(-0.060,-0.012) | 0.004 | reference | -0.122(-0.195,-0.048) | -0.141(-0.259,-0.022) | 0.001 | ||
| PIR | 0.016 | 0.018 | ||||||
| <1 | 0.002(-0.015,0.020) | 0.809 | reference | 0.014(-0.043,0.070) | 0.025(-0.132,0.181) | 0.598 | ||
| 1–3.9 | -0.025(-0.041,-0.009) | 0.003 | reference | -0.06(-0.109,-0.011) | -0.175(-0.265,-0.084) | 0.003 | ||
| ≥4 | -0.035(-0.056,-0.014) | 0.002 | reference | -0.092(-0.146,-0.037) | -0.086(-0.234, 0.061) | <0.001 | ||
| Marital | 0.068 | 0.171 | ||||||
| Married, or living with partner | -0.026(-0.039,-0.013) | <0.001 | reference | -0.074(-0.118,-0.031) | -0.055(-0.126, 0.016) | 0.001 | ||
| Widowed, divorced, or separated | -0.039(-0.063,-0.015) | 0.002 | reference | -0.08(-0.142,-0.018) | -0.265(-0.373,-0.158) | 0.001 | ||
| Never married | -0.004(-0.025,0.018) | 0.725 | reference | -0.032(-0.103,0.040) | -0.092(-0.233,0.050) | 0.267 | ||
Each stratification adjusted for all factors (sex, age, race/ethnicity, marital status, BMI, sleep disturbance, poverty income ratio, smoking status, alcohol consumption status, UIC) except the stratification factor itself.
Discussion
This study was conducted with the goal of examining the relationship between sleep duration and thyroid function by analyzing data from a nationally representative cohort of adults in the US. These analyses revealed that sleep duration and FT3 levels were negatively correlated, with FT3 levels decreasing as sleep duration increased. When sleep duration was included as a categorical variable, FT3 levels were lower in the group with longer sleep duration. After adjusting for relevant confounders, this negative correlation remained, being primarily evident for participants who slept less than or equal to 7 hours, whereas for participants who slept more than 7 hours, FT3 levels did not change significantly with longer sleep duration. The present results also suggested an increase in TSH levels with increasing sleep duration, although this positive correlation was not significant after adjusting for confounders. No significant correlation was observed between other analyze thyroid parameters and sleep duration.
Multiple studies have investigated how sleep impacts thyroid function. A circadian variation in TSH secretion has been observed [20, 29]. Russell et al. also found similarly pronounced circadian rhythmicity for FT3, reporting that FT3 peaked 90 minutes following TSH in 86–100% of participants. In contrast, FT4 did not show a clear circadian rhythm [21], which may be because free T4 has a longer half-life (∼6.7 days). This also leads to a greater susceptibility of TSH and FT3 levels to sleep, which is also broadly similar to our study, where only TSH and FT3 levels were statistically different among sleep duration subgroups of analyzed thyroid function-related parameters, while FT4 was not. Recent studies have investigated the effects of sleep on TSH secretion based on relatively prolonged intervals of moderate sleep deprivation [25, 30]. These analyses repeatedly revealed that sleep deprivation contributed to the suppression of TSH secretion, although these analyses included relatively few subjects [23, 25]. Further studies confirming the link between sleep and thyroid function in a larger nationally representative cohort would thus be interesting.
Sleep and the endocrine system bidirectionally influence one another [31], and hormones and sleep are highly interdependent in the context of normal physiological function [32]. Indeed, sleep strongly influences thyroid hormone production, in turn impacting sleep duration and quality [16, 33]. The interaction between the HPT axis and the sleep cycle shapes metabolic processes and energy levels, and the disruption of one can lead to dysregulation of the other. Hyperthyroidism is a common cause of sleep disorders, and Stern et al. [34] conducted an analysis of 137 patients with Graves’ disease, which is the leading cause of hyperthyroidism, ultimately finding that difficulties falling asleep were reported by 66.4% of these individuals. Prolonged sleep latency was associated with TH-mediated changes in appetite, mood (i.e. increased anxiety), and bowel movements, ultimately contributing to reduced sleep duration. Xia et al. [35] found that TSH, T3, and T4 levels were directly correlated with insomnia symptom severity. Hyperthyroidism can also contribute to or aggravate anxiety, depression, and other conditions, thereby further impacting insomnia and sleep quality [36]. Hypothyroidism can similarly affect overall sleep quality, with multiple reports having documented a relationship between untreated subclinical hypothyroidism and poorer sleep. Song et al. [37] found that individuals exhibiting lower TH levels generally exhibited prolonged sleep latency, reduced sleep direction, and lower sleep quality as compared to people with normal thyroid function.
The study has several limitations. Firstly, as this was a cross-sectional analysis causal relationship could not be established with respect to the association between sleep and thyroid function. Future longitudinal research will be necessary to expand on these findings. Secondly, blood sampling for thyroid function was performed at individual time points during the morning period, and the blood was completed and processed and then cryopreserved before being sent together once a week to a designated laboratory for testing; therefore, differences in sampling time and storage time may affect thyroid function levels [38], and it may limit the interpretation of these results. In addition, participants’ sleep duration was based on self-reported durations rather than derived from a specific sleep quality scale, so the numbers provided may not accurately reflect their actual sleep duration, and these results may be affected by seasonal variation and recall bias [39]. Lastly, there may be additional confounding factors that were not appropriately accounted for. Even when taking these limitations, this is a population-based study of the association between sleep duration and thyroid function in US adults.
Conclusion
This analysis revealed that when analyzing a nationally representative group of adults in the US, FT3 levels were significantly and negatively correlated with sleep duration when sleep duration was ≤7 hours. In contrast, when sleep duration exceeded 7 hours, FT3 levels did not change significantly with further increases in sleep duration.
Acknowledgments
We would like to thank everyone who participated in NHANES and the NHANES team for their time and work. Also, we thanks to Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, the nhanesR package and webpage, makes it easier for us to explore the NHANES database.
Data Availability
All data files are available from the NHANES https://www.cdc.gov/nchs/nhanes/index.htm.
Funding Statement
This project was supported by the Public Interest Jinhua Science and Technology Research Program [2021-04-008 and 2021-04-219]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Steiger A. Sleep and endocrinology. Journal of Internal Medicine. 2003;254(1):13–22. doi: 10.1046/j.1365-2796.2003.01175.x [DOI] [PubMed] [Google Scholar]
- 2.Zielinski MR, McKenna JT, McCarley RW. Functions and Mechanisms of Sleep. AIMS neuroscience. 2016;3(1):67–104. Epub 2016/01/01. doi: 10.3934/Neuroscience.2016.1.67 ; PubMed Central PMCID: PMC5390528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjot T, Ray DW, Williams FR, Tomlinson JW, Armstrong MJ. Sleep and liver disease: a bidirectional relationship. The Lancet Gastroenterology & Hepatology. 2021;6(10):850–63. doi: 10.1016/S2468-1253(21)00169-2 [DOI] [PubMed] [Google Scholar]
- 4.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep health. 2015;1(4):233–43. Epub 2015/12/01. doi: 10.1016/j.sleh.2015.10.004 . [DOI] [PubMed] [Google Scholar]
- 5.Dong L, Xie Y, Zou X. Association between sleep duration and depression in US adults: A cross-sectional study. Journal of Affective Disorders. 2022;296:183–8. doi: 10.1016/j.jad.2021.09.075 [DOI] [PubMed] [Google Scholar]
- 6.Covassin N, Singh P. Sleep Duration and Cardiovascular Disease Risk: Epidemiologic and Experimental Evidence. Sleep medicine clinics. 2016;11(1):81–9. Epub 2016/03/15. doi: 10.1016/j.jsmc.2015.10.007 ; PubMed Central PMCID: PMC4791534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: A systematic review. Sleep medicine reviews. 2012;16(3):223–30. doi: 10.1016/j.smrv.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R, et al. Sleep Duration and Myocardial Infarction. Journal of the American College of Cardiology. 2019;74(10):1304–14. doi: 10.1016/j.jacc.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang T, Mariani S, Redline S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. Journal of the American College of Cardiology. 2020;75(9):991–9. doi: 10.1016/j.jacc.2019.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nature Reviews Cardiology. 2019;16(4):213–24. doi: 10.1038/s41569-018-0109-6 [DOI] [PubMed] [Google Scholar]
- 11.Paksarian D, Rudolph KE, Stapp EK, Dunster GP, He J, Mennitt D, et al. Association of Outdoor Artificial Light at Night With Mental Disorders and Sleep Patterns Among US Adolescents. JAMA Psychiatry. 2020;77(12):1266–75. doi: 10.1001/jamapsychiatry.2020.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faulkner SM, Bee PE, Meyer N, Dijk D-J, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep medicine reviews. 2019;46:108–23. doi: 10.1016/j.smrv.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. European Heart Journal. 2018;40(20):1620–9. doi: 10.1093/eurheartj/ehy695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiological reviews. 2001;81(3):1097–142. Epub 2001/06/28. doi: 10.1152/physrev.2001.81.3.1097 . [DOI] [PubMed] [Google Scholar]
- 15.Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacology & Therapeutics. 2017;173:135–45. doi: 10.1016/j.pharmthera.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shekhar S, Hall JE, Klubo-Gwiezdzinska J. The hypothalamic–pituitary–thyroid axis and sleep. Current Opinion in Endocrine and Metabolic Research. 2021;17:8–14. doi: 10.1016/j.coemr.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronfier C, Brandenberger G. Ultradian rhythms in pituitary and adrenal hormones: their relations to sleep. Sleep medicine reviews. 1998;2(1):17–29. Epub 2004/08/18. doi: 10.1016/s1087-0792(98)90051-x . [DOI] [PubMed] [Google Scholar]
- 18.Yuen KCJ, Llahana S, Miller BS. Adult growth hormone deficiency: clinical advances and approaches to improve adherence. Expert review of endocrinology & metabolism. 2019;14(6):419–36. Epub 2019/11/14. doi: 10.1080/17446651.2019.1689119 . [DOI] [PubMed] [Google Scholar]
- 19.Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26(9):3602–13. Epub 2012/06/05. doi: 10.1096/fj.12-203554 ; PubMed Central PMCID: PMC3425819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrenkranz J, Bach PR, Snow GL, Schneider A, Lee JL, Ilstrup S, et al. Circadian and Circannual Rhythms in Thyroid Hormones: Determining the TSH and Free T4 Reference Intervals Based Upon Time of Day, Age, and Sex. Thyroid: official journal of the American Thyroid Association. 2015;25(8):954–61. Epub 2015/06/11. doi: 10.1089/thy.2014.0589 . [DOI] [PubMed] [Google Scholar]
- 21.Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. The Journal of clinical endocrinology and metabolism. 2008;93(6):2300–6. Epub 2008/03/28. doi: 10.1210/jc.2007-2674 . [DOI] [PubMed] [Google Scholar]
- 22.Kim W, Lee J, Ha J, Jo K, Lim D-J, Lee J-M, et al. Association between Sleep Duration and Subclinical Thyroid Dysfunction Based on Nationally Representative Data. Journal of Clinical Medicine. 2019;8(11):2010. doi: 10.3390/jcm8112010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brabant G, Prank K, Ranft U, Schuermeyer T, Wagner TO, Hauser H, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. The Journal of clinical endocrinology and metabolism. 1990;70(2):403–9. Epub 1990/02/01. doi: 10.1210/jcem-70-2-403 . [DOI] [PubMed] [Google Scholar]
- 24.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet (London, England). 1999;354(9188):1435–9. Epub 1999/10/30. doi: 10.1016/S0140-6736(99)01376-8 . [DOI] [PubMed] [Google Scholar]
- 25.Kessler L, Nedeltcheva A, Imperial J, Penev PD. Changes in serum TSH and free T4 during human sleep restriction. Sleep. 2010;33(8):1115–8. Epub 2010/09/08. doi: 10.1093/sleep/33.8.1115 ; PubMed Central PMCID: PMC2910542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015;38(8):1161–83. Epub 2015/07/22. doi: 10.5665/sleep.4886 ; PubMed Central PMCID: PMC4507722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, et al. National Health and Nutrition Examination Survey: estimation procedures, 2007–2010. Vital and health statistics Series 2, Data evaluation and methods research. 2013;(159):1–17. Epub 2014/08/06. . [PubMed] [Google Scholar]
- 28.Chen TC, Parker JD, Clark J, Shin HC, Rammon JR, Burt VL. National Health and Nutrition Examination Survey: Estimation Procedures, 2011–2014. Vital and health statistics Series 2, Data evaluation and methods research. 2018;(177):1–26. Epub 2018/05/19. . [PubMed] [Google Scholar]
- 29.Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocrine reviews. 2013;34(5):619–57. Epub 2013/04/12. doi: 10.1210/er.2012-1076 . [DOI] [PubMed] [Google Scholar]
- 30.Schmid SM, Hallschmid M, Jauch-Chara K, Kück MC, Lehnert H, Schultes B. Partial sleep restriction modulates secretory activity of thyrotropic axis in healthy men. Journal of sleep research. 2013;22(2):166–9. Epub 2013/03/19. doi: 10.1111/jsr.12004 . [DOI] [PubMed] [Google Scholar]
- 31.Steiger A, Antonijevic IA, Bohlhalter S, Frieboes RM, Friess E, Murck H. Effects of hormones on sleep. Hormone research. 1998;49(3–4):125–30. Epub 1998/04/29. doi: 10.1159/000023158 . [DOI] [PubMed] [Google Scholar]
- 32.Dijk DJ, Landolt HP. Sleep Physiology, Circadian Rhythms, Waking Performance and the Development of Sleep-Wake Therapeutics. Handbook of experimental pharmacology. 2019;253:441–81. Epub 2019/06/30. doi: 10.1007/164_2019_243 . [DOI] [PubMed] [Google Scholar]
- 33.Green ME, Bernet V, Cheung J. Thyroid Dysfunction and Sleep Disorders. Frontiers in endocrinology. 2021;12. doi: 10.3389/fendo.2021.725829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern RA, Robinson B, Thorner AR, Arruda JE, Prohaska ML, Prange AJ Jr., A survey study of neuropsychiatric complaints in patients with Graves’ disease. The Journal of neuropsychiatry and clinical neurosciences. 1996;8(2):181–5. Epub 1996/01/01. doi: 10.1176/jnp.8.2.181 . [DOI] [PubMed] [Google Scholar]
- 35.Xia L, Chen GH, Li ZH, Jiang S, Shen J. Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical research. PloS one. 2013;8(8):e71065. Epub 2013/08/21. doi: 10.1371/journal.pone.0071065 ; PubMed Central PMCID: PMC3739817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trzepacz PT, McCue M, Klein I, Levey GS, Greenhouse J. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. General hospital psychiatry. 1988;10(1):49–55. Epub 1988/01/01. doi: 10.1016/0163-8343(88)90084-9 . [DOI] [PubMed] [Google Scholar]
- 37.Song L, Lei J, Jiang K, Lei Y, Tang Y, Zhu J, et al. The Association Between Subclinical Hypothyroidism and Sleep Quality: A Population-Based Study. Risk management and healthcare policy. 2019;12:369–74. Epub 2020/01/08. doi: 10.2147/RMHP.S234552 ; PubMed Central PMCID: PMC6927586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen E, Blaabjerg O, Petersen PH, Hegedüs L. Sampling time is important but may be overlooked in establishment and use of thyroid-stimulating hormone reference intervals. Clinical chemistry. 2007;53(2):355–6. Epub 2007/01/30. doi: 10.1373/clinchem.2006.078964 . [DOI] [PubMed] [Google Scholar]
- 39.Garrido ALF, Duarte AS, Santana PT, Rodrigues GH, Pellegrino P, Nogueira LFR, et al. Eating habits, sleep, and a proxy for circadian disruption are correlated with dyslipidemia in overweight night workers. Nutrition (Burbank, Los Angeles County, Calif). 2021;83:111084. Epub 2021/01/03. doi: 10.1016/j.nut.2020.111084 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data files are available from the NHANES https://www.cdc.gov/nchs/nhanes/index.htm.