Abstract
The actual prevalence of visceral leishmaniasis among human immunodeficiency type 1 (HIV-1)-infected patients in the Mediterranean basin remains unknown. There is also controversy about the risk factors for Leishmania infantum and HIV-1 coinfection. To appraise the prevalence of visceral leishmaniasis in patients infected with HIV-1 in southern Spain and to identify factors associated with this disease, 291 HIV-1 carriers underwent a bone marrow aspiration, regardless of their symptoms. Giemsa-stained samples were searched for Leishmania amastigotes. Thirty-two (11%) patients showed visceral leishmaniasis. Thirteen (41%) patients had subclinical cases of infection. Centers for Disease Control and Prevention (CDC) clinical category C was the factor most strongly associated with this disease (adjusted odds ratio [OR], 1.88 [95% confidence interval, 1.22 to 2.88]), but patients with subclinical cases of infection were found in all CDC categories. Female sex was negatively associated with visceral leishmaniasis (adjusted OR, 0.42 [95% confidence interval, 0.18 to 0.97]). Intravenous drug users showed a higher prevalence than the remaining patients (13.3 versus 4.9%; P = 0.04), but such an association was not independent. These results show that visceral leishmaniasis is a very prevalent disease among HIV-1-infected patients in southern Spain, with a high proportion of cases being subclinical. Like other opportunistic infections, subclinical visceral leishmaniasis can be found at any stage of HIV-1 infection, but symptomatic cases of infection appear mainly when a deep immunosuppression is present. There is also an association of this disease with male sex and intravenous drug use.
Visceral leishmaniasis is a frequent disease among human immunodeficiency virus type 1 (HIV-1)-infected patients in countries of the Mediterranean basin (15, 19). It also seems to be an emerging problem among this population in certain areas where leishmaniasis is not endemic (2, 20). Leishmania amastigotes have been found in 17% of HIV-1-seropositive individuals in Spain who showed at least a symptom or laboratory data suggesting that they had leishmaniasis (3). The reported visceral leishmaniasis incidence in AIDS patients in Sicily was 4.9% during the period from 1985 to 1994 (15). However, these rates have been obtained from studies carried out with symptomatic patients. Because subclinical cases of this disease are frequent in HIV-1-seropositive individuals (22), the data presented above could underestimate the true extent of Leishmania infantum and HIV-1 coinfection in our area. On the other hand, preliminary data suggest that visceral leishmaniasis could be a cofactor in HIV-1 disease progression (8). For these reasons, surveys that assess the actual prevalence of visceral leishmaniasis in this population are necessary.
In most case series visceral leishmaniasis was associated with advanced HIV-1 disease (15, 19, 23). This parasitic disease was also more frequent in intravenous drug users in some studies (6, 15) but not in others (19, 23). Nevertheless, these observations have also been reported in retrospective surveys dealing with symptomatic patients. Analyses of risk factors in unselected HIV-1-infected populations are needed. These studies have not been performed to date, probably due to methodological problems. In fact, the tools commonly used for the diagnosis of leishmaniasis in large-scale epidemiological surveys, such as serology or the leishmanin skin test, have low sensitivities when they are used to test HIV-1-infected patients (14, 15, 19). Consequently, invasive methods such as parasite detection in tissue samples, must be used.
We report herein the prevalence of visceral leishmaniasis in a group of HIV-1 infected patients with a wide spectrum of clinical conditions determined by searching for amastigotes in bone marrow aspirates. We also analyzed the association of this disease with potential risk factors in this population.
MATERIALS AND METHODS
Patients.
All 335 HIV-1-infected patients without severe clotting disorders who attended the Viral Hepatitis and AIDS Study Group’s unit between January 1993 and February 1997 were invited to participate in this cross-sectional study. Nine further HIV-seropositive individuals with a serious clotting disorder were seen at our unit during this period. This unit provides care to both inpatients and outpatients coming from Andalusia (southern Spain). In this area all HIV-1-infected individuals who seek medical care are seen in tertiary-care units like this one. Patients were invited to participate in the study regardless of their symptoms, and 291 (87%) agreed to participate. All of them answered an epidemiological questionnaire, had clinical and immunological evaluations, and underwent a sternal bone marrow aspiration.
The study was designed and performed according to the Helsinki Declaration and was approved by the Ethics Committee of the Hospital Universitario Virgendel Rocío; all patients gave their written informed consent to participate.
Laboratory methods.
Bone marrow aspirate samples were partially smeared on slides and were stained with Giemsa stain. Both thin and thick films were made. Examinations of the smears were carried out by an expert parasitologist at ×1,000 magnification for at least 20 min. Mononuclear cells from a part of the bone marrow aspirate taken from the last 166 patients included in the study were isolated by density gradient centrifugation on Ficoll and were cryopreserved in liquid nitrogen. Due to limitations in laboratory facilities, Leishmania cultures could be done only for a restricted number of patients; thus, cultures were performed retrospectively for the first eight individuals with a positive Giemsa staining result and for whom a cryopreserved sample was available. Myelocultures were also done with fresh bone marrow aspirate taken from 32 patients consecutively included in the study between February 1996 and April 1996. Samples were seeded into Evans modified Tobie’s medium supplemented with RPMI, and the cultures were followed for at least 1 month. The parasites cultured were characterized by electrophoretic analysis on starch gels of 15 enzymatic loci by using the techniques and nomenclature of the World Health Organization Collaborating Centre in Montpellier, France (24). The full enzyme names, codes, and reference strains that were used were reported by Gramiccia et al. (16). Smear examinations and cultures were performed in two independent laboratories. The parasitologists who carried out microbiological studies were blinded to the clinical condition of the patients.
CD4+ cell counts were determined by standard flow cytometry.
Diagnostic criteria.
Visceral leishmaniasis was diagnosed when Leishmania amastigotes were seen by direct visualization. Subclinical visceral leishmaniasis was defined as described elsewhere (22); a case was considered subclinical if the following criteria were met: the patient had no fever, no splenomegaly, or a hemoglobin level of <9 g/dl. If these symptoms were present, they had to be attributable to another concomitant disease and to disappear after specific treatment for that disease, without antileishmanial therapy. All cases that did not fulfill these criteria were considered symptomatic visceral leishmaniasis. HIV-1 infection was categorized according to the 1993 classification of the Centers for Disease Control and Prevention (CDC) (9).
Statistical analysis.
The continuous variables are expressed as median (range), and the prevalence rates are expressed as a percentage. The associations between visceral leishmaniasis and the following parameters were assessed: age, sex, living area (urban, suburban, or rural), risk factors for HIV-1 infection, CDC clinical category, previous leishmaniasis diagnosis, and CD4+ cell count. Both univariate and multivariate analyses were performed. In the univariate analysis, continuous variables were compared by the Mann-Whitney U test. Frequencies were compared by the χ2 test or the Fisher test. All variables having a univariate association with visceral leishmaniasis with a significance level of <0.1 were entered into a stepwise logistic regression model. Since cultures were not done for the entire population, all the prevalence rates and statistical analyses are based on the results of direct visualization of the bone marrow aspirate smears. Statistical analyses were performed by using the software package SPSS.
RESULTS
Characteristics of the population studied.
All patients were white. The median age of the group was 32 years (range, 17 to 75 years). The median CD4+ cell count was 172 × 106 cells/liter (range, 1 × 106 to 1,520 × 106 cells per liter). Two hundred ten individuals were intravenous drug users, 38 were homosexual men, 36 were heterosexuals, and 7 reported no risk factors for HIV infection. Other data for the population are summarized in Table 1.
TABLE 1.
Frequency of visceral leishmaniasis in relation to sex, living area, risk factors for HIV-1 infection, CDC clinical category, and history of previous leishmaniasis
| Parameter | No. (%) of patients | No. (%) of patients with VLa | P value (univariate) |
|---|---|---|---|
| Sex | |||
| Male | 239 (82) | 31 (13) | 0.02 |
| Female | 52 (18) | 1 (1.9) | |
| Living area | |||
| Rural | 87 (30) | 10 (11.5) | |
| Suburban | 159 (55) | 18 (11.3) | 0.88 |
| Urban | 45 (15) | 4 (8.9) | |
| Risk factor for HIV-1 | |||
| IDU | 210 (72) | 28 (13.3) | 0.04 |
| Non-IDU | 81 (28) | 4 (4.9) | |
| Previous leishmaniasis | |||
| Yes | 11 (4) | 3 (27.3) | 0.1 |
| No | 280 (96) | 29 (10.4) | |
| CDC clinical category | |||
| C | 148 (51) | 25 (16.9) | 0.001 |
| A or B | 143 (49) | 7 (4.9) |
VL, visceral leishmaniasis.
IDU, intravenous drug use.
Visceral leishmaniasis prevalence.
Leishmania amastigotes were found in the bone marrow aspirates of 32 (11%) patients. Leishmania promastigotes were isolated from five of the eight cryopreserved, Giemsa stain-positive samples analyzed. On the other hand, 1 of 32 fresh aspirates which were cultured showed amastigotes by Giemsa staining; only from this fresh sample were promastigotes isolated. All isolates obtained by culture were identified as L. infantum MON-1.
Thirteen (4.5%) patients met the criteria for subclinical visceral leishmaniasis; this represents 41% of the total visceral leishmaniasis patients. On the other hand, 179 patients did not have fever when they were included in the study; remarkably, 11 (6.1%) of them were found to have amastigotes in their bone marrow aspirates.
Associations between visceral leishmaniasis and other parameters.
The ages were similar among patients with and without visceral leishmaniasis (32 years [range, 24 to 50 years] versus 32 years [range, 17 to 75 years]; P = 0.78). CD4+ cell counts were lower in individuals with this disease (55 × 106 cells/liter [range, 1 × 106 to 1,358 × 106 cells/liter] versus 200 × 106 cells/liter [range, 1 × 106 to 1,520 × 106 cells/liter]; P = 0.005). On univariate analysis, male sex, injection drug use, and CDC clinical category C were also associated with visceral leishmaniasis (Table 1). The stepwise logistic regression procedure sequentially selected CDC clinical category C (odds ratio [OR], 1.88 [95% confidence interval {CI}, 1.22 to 2.88]; P = 0.002] and female sex (OR, 0.42 [95% CI, 0.18 to 0.97]; P = 0.03) as the variables independently associated with this parasitic disease. The association between intravenous drug use and visceral leishmaniasis was close to statistical significance level (OR, 1.56 [95% CI, 0.90 to 2.66]; P = 0.08).
The proportions of males (75%), intravenous drug users (70%), and patients in CDC clinical category C (39%) among patients who did not agree to be included in the study were not statistically different from those found among individuals who participated in the study (P = 0.35, P = 0.95, and P = 0.17, respectively).
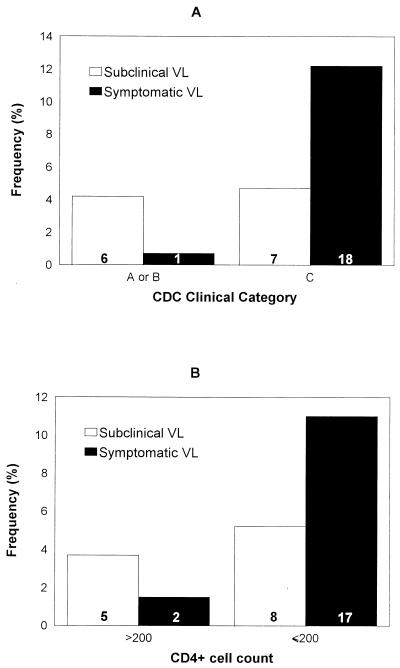
There was no association between subclinical visceral leishmaniasis and the CDC clinical category. In fact, the frequency of subclinical cases among patients included in category C was 4.7%, whereas it was 4.2% among those included in category A or B (P = 0.82) (Fig. 1). In the same way, after categorizing CD4+ cell counts, the frequency of subclinical visceral leishmaniasis was similar in patients with CD4+ cell counts of ≤200 × 106 cells/liter and those with more than 200 × 106 CD4+ cells/liter. Thus, 8 of 155 (5.2%) patients in the first subgroup and 5 of 136 (3.7%) patients in the second subgroup had subclinical disease (P = 0.54). In contrast, symptomatic cases were more frequent among patients in CDC clinical category C (P = 0.0008) and in those with CD4+ cell counts of ≤200 × 106/liter (P = 0.001) (Fig. 1).
FIG. 1.
Frequency of subclinical and symptomatic visceral leishmaniasis in relation to CDC clinical category (A) and CD4+ cell counts (cells per microliter) (B). The numbers inside the bars indicate the number of patients in each subgroup. VL, visceral leishmaniasis.
DISCUSSION
Our results indicate that visceral leishmaniasis is highly prevalent among HIV-1-infected patients in southern Spain. In fact, roughly 1/10 of this population had this disease, with nearly half of the patients having subclinical infection; even as many as 6% of afebrile individuals harbored L. infantum amastigotes in their bone marrow. The disease in most asymptomatic patients probably would have gone undetected in a retrospective case series. This fact explains why fever was present in 87 to 95% of the patients in a large case series (11, 19, 25) of visceral leishmaniasis in patients with HIV-1 infection and also why subclinical infections were described as exceptional in the first studies dealing with this issue (10, 11, 23). Therefore, these findings prove that the frequencies of L. infantum and HIV-1 coinfection based on studies performed with symptomatic patients were inaccurate.
This study could be biased because patients with severe immunodeficiency probably seek medical care more frequently than those with less advanced disease. This would lead to a higher proportion of strongly immunosuppressed individuals in the group analyzed here than in the whole HIV-1-infected population. In such a case, the prevalence of visceral leishmaniasis that we obtained would be an overestimation. However, this inherent bias should not be relevant, since patients with a wide spectrum of immune system impairments were included in this study. Anyway, our population represents the cluster of HIV-1-seropositive patients who attend hospitals in our area. Another limitation of this study may be that the diagnosis of leishmaniasis was based on direct visualization of amastigotes. Theoretically, this method can yield false-negative results if parasites are scanty or if the examiner is unskilled. Indeed, L. infantum has been detected by myeloculture (7, 13) or PCR (21) in individuals for whom direct examination of bone marrow aspirates was negative. However, culture is time-consuming and is therefore difficult to use in large-scale epidemiological surveys like the one described here. We performed cultures with fresh samples from 32 consecutive patients, and no further visceral leishmaniasis cases were detected. This suggests that the frequencies obtained in this study would have been very similar if myelocultures had been carried out for all patients. On the contrary, the culture of cryopreserved samples turned out to be negative for some patients for whom amastigotes were observed by direct examination. This fact has also been reported in other studies (21, 23) and could be at least in part a consequence of having used Ficoll-purified material. Despite the diversity of zymodemes isolated from HIV-Leishmania-coinfected patients (4, 5) and from sandflies (17) in our area, we found only L. infantum MON-1. The low number of cultures that we performed probably accounts for this fact.
We have found visceral leishmaniasis to be independently associated with the CDC clinical category. This finding confirms the relationship observed in most retrospective case series between this parasitic disease and advanced stages of HIV-1 infection (11, 15, 19, 23, 25). However, the frequency of subclinical cases was similar among patients with severe immunodeficiency and those with less advanced HIV-1 disease. These data indicate that visceral leishmaniasis may take place at any stage of HIV-1 infection, but it essentially becomes symptomatic in patients who are highly immunosuppressed. In this sense visceral leishmaniasis is similar to other AIDS-related opportunistic illnesses, such as tuberculosis, cytomegalovirus disease, or cryptococosis, in which silent infection may be present in any HIV-1-seropositive patient, whereas overt disease appears only in highly immunosuppressed patients.
The relationship between L. infantum and HIV-1 coinfection and injection drug use is a matter of controversy. Declared cases of visceral leishmaniasis are more frequent among AIDS patients who are or were intravenous drug addicts than among those who acquired HIV-1 infection in other ways (6). Also, L. infantum zymodemes from coinfected intravenous drug users show a higher variability than zymodemes from HIV-1-seronegative patients or dogs (4, 5, 16, 17). On the basis of these findings, some researchers have hypothesized that this parasite could be transmitted through the sharing of needles (4). However, the heterogeneity of zymodemes isolated from sandflies in our area is also high (17), so insect vector transmission cannot be completely ruled out. Moreover, no direct evidence of the spread of L. infantum through the sharing of needles has yet been obtained. In this study visceral leishmaniasis was more frequent in intravenous drug users, but such an association was not independent of clinical category and sex on multivariate analysis, although statistical significance was almost reached. Consequently, no definite conclusions on that subject can be drawn from this study, but in our opinion, transmission through the sharing of needles is very probable. The fact that L. infantum can be found in peripheral blood smears from some asymptomatic HIV-1-infected patients (12) supports this hypothesis.
Visceral leishmaniasis was independently associated with male sex. Other epidemiological studies performed in Mediterranean countries have found leishmaniasis to occur more frequently in men (15, 18), but other surveys failed to confirm such a difference (1). This finding is intriguing, and a satisfactory explanation is not easy to give. It could simply be a reflection of what happens in the south of Europe, where most drug addicts are males. Anyhow, the association of this parasitic disease with sex and drug abuse in people coinfected with HIV and L. infantum will require further attention.
The proportions of Leishmania-infected patients in the present study living in rural, suburban, and urban areas were similar. In other studies urban foci of L. infantum and HIV-1 coinfection have also been detected, such as in Campania, Italy (15). These findings suggest that modes of transmission other than phlebotomine bites may be involved in the spread of L. infantum among HIV-1-infected patients. Sharing of needles among intravenous drug users again comes to mind as a possible explanation.
ACKNOWLEDGMENTS
We are grateful to technicians Mercedes Olivera and Antonio Gayoso, to nurses Jose M. García, Manuela Mora, María L. Amo, Concepción Martínez, Concepción Almoguera, Maite Nuño, Rosario Martínez, Jacinto Flores, María O. Román, and Manuel J. García, and to the patients, without whose collaboration this study could not have been accomplished.
This study was partly supported by grants from the Plan Andaluz de Investigación (group code 3105) and the Servicio Andaluz de Salud (expediente 276/97).
REFERENCES
- 1.Acedo Sánchez C, Martín Sánchez J, Vélez Bernal I D, Sanchís Marín M C, Louassini M, Maldonado J A, Morillas Márquez F. Leishmaniasis eco-epidemiology in the Alpujarra region (Granada province, southern Spain) Int J Parasitol. 1996;25:303–310. doi: 10.1016/0020-7519(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht H, Sobottka I, Emminger C, Jablonowski H, Just G, Stoehr A, Kubin T, Salzberger B, Lutz T, van Lunzen J. Visceral leishmaniasis emerging as an important opportunistic infection in HIV-infected persons living in areas nonendemic for Leishmania donovani. Arch Pathol Lab Med. 1996;120:189–198. [PubMed] [Google Scholar]
- 3.Alvar J, Gutiérrez-Solar B, Molina R, López-Vélez R, García-Camacho A, Martínez P, Laguna F, Cercenado E, Galmes A. Prevalence of Leishmania infection among AIDS patients. Lancet. 1992;339:1427. doi: 10.1016/0140-6736(92)91255-7. [DOI] [PubMed] [Google Scholar]
- 4.Alvar J, Gutiérrez-Solar B, Pachón I, Calbacho E, Ramírez M, Vallés R, Guillén J L, Cañavete C, Amela C. AIDS and Leishmania infantum. New approaches for a new epidemiological problem. Clin Dermatol. 1996;14:541–546. doi: 10.1016/0738-081x(96)00046-6. [DOI] [PubMed] [Google Scholar]
- 5.Alvar J, Jiménez M. Could infected drug users be potential Leishmania infantum reservoirs? AIDS. 1994;8:6. doi: 10.1097/00002030-199406000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Amela C, López-Gay D, Alberdi J C, Castilla J. Injecting drug use as risk factor for visceral leishmaniasis in AIDS patients. Eur J Epidemiol. 1996;12:91–92. doi: 10.1007/BF00144435. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer J, Moreno S, Cercenado E, Bernaldo de Quirós J C L, García de la Fuente A, Bouza E. Visceral leishmaniasis in patients infected with human immunodeficiency virus (HIV) Ann Intern Med. 1989;111:129–132. doi: 10.7326/0003-4819-111-2-129. [DOI] [PubMed] [Google Scholar]
- 8.Cacopardo B, Nigro L, Preiser W, Fama A, Satariano M I, Braner J, Celesia B M, Weber B, Russo R, Doerr H W. Prolonged Th2 cell activation and increased viral replication in HIV-Leishmania coinfected patients despite treatment. Trans R Soc Trop Med Hyg. 1996;90:434–435. doi: 10.1016/s0035-9203(96)90538-6. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. 1993 revised classification system for HIV and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 10.Condom M A, Clotet B, Sirera G, Milla F, Foz M. Asymptomatic leishmaniasis in the acquired immunodeficiency syndrome. Ann Intern Med. 1989;111:767–768. doi: 10.7326/0003-4819-111-9-767_2. [DOI] [PubMed] [Google Scholar]
- 11.Dedet J P, Lambert M, Pratlong F. Leishmanioses et infection par le virus de l’inmunodeficiency humaine. Presse Med. 1995;24:1036–1040. [PubMed] [Google Scholar]
- 12.Delgado J, Pineda J A, Macías J, Regordán C, Gallardo J A, Leal M, Sánchez-Quijano A, Lissen E. Low sensitivity of peripheral blood smear for the diagnosis of subclinical visceral leishmaniasis in HIV-1 infected patients. J Clin Microbiol. 1998;36:315–316. doi: 10.1128/jcm.36.1.315-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dereure J, Reynes J, Pratlong F, Lamaury I, Rioux J A, Janbon F, Dedet J P. Visceral leishmaniasis in HIV-1 infected patients in the south of France. Bull W H O. 1995;73:245–246. [PMC free article] [PubMed] [Google Scholar]
- 14.Gallardo J A, Pineda J A, Macías J, Torronteras R, Lissen E. Specificity of a commercial indirect immunofluorescence technique in the diagnosis of visceral leishmaniasis in HIV-1 infected patients. Trans R Soc Trop Med Hyg. 1996;90:383. doi: 10.1016/s0035-9203(96)90514-3. [DOI] [PubMed] [Google Scholar]
- 15.Gradoni L, Scalone A, Gramiccia M, Troiani M. Epidemiological surveillance of leishmaniasis in HIV-1-infected individuals in Italy. AIDS. 1996;10:785–791. doi: 10.1097/00002030-199606001-00014. [DOI] [PubMed] [Google Scholar]
- 16.Gramiccia M, Gradoni L, Troiani M. Heterogeneity among zymodemes of Leishmania infantum from HIV-positive patients with visceral leishmaniasis in south Italy. FEMS Microbiol Lett. 1995;128:33–88. doi: 10.1111/j.1574-6968.1995.tb07496.x. [DOI] [PubMed] [Google Scholar]
- 17.Martín Sánchez J, Morillas Márquez F, Acedo Sánchez A, Sanchiz Marín M C. The variability of the etiological agent of leishmaniasis in the north east of the Almeria Region, south-east Spain. Syst Parasitol. 1995;30:233–238. [Google Scholar]
- 18.Marty P, Le Fichoux Y, Pratlong F, Gari-Toussaint M. Human visceral leishmaniasis in Alpes-Maritimes, France: epidemiological characteristics for the period 1985–1992. Trans R Soc Trop Med Hyg. 1994;88:33–34. doi: 10.1016/0035-9203(94)90485-5. [DOI] [PubMed] [Google Scholar]
- 19.Medrano F J, Hernández-Quero J, Jiménez E, Pineda J A, Rivero A, Sánchez-Quijano A, Vélez I D, Viciana P, Castillo R, Reyes M J, Carvajal F, Lissen E. Visceral leishmaniasis in HIV-1-infected individuals: a common opportunistic infection in Spain? AIDS. 1992;6:1499–1503. doi: 10.1097/00002030-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Parkas V, Godwin J, Murray H W. Kala-azar comes to New York. Arch Intern Med. 1997;157:921–923. [PubMed] [Google Scholar]
- 21.Piarroux R, Gambarelli F, Dumon H, Fontes M, Dunan S, Mary C, Toga B, Quilici M. Comparison of PCR with direct examination of bone marrow aspiration, myeloculture, and serology for diagnosis of visceral leishmaniasis in immunocompromised patients. J Clin Microbiol. 1994;32:746–749. doi: 10.1128/jcm.32.3.746-749.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pineda J A, Hernández-Quero J, Gallardo J A, López-Ruz M A, Martínez-Pérez M A, Macías J, Lissen E. Frequency of subclinical visceral leishmaniasis in HIV-1 infected patients in Spain. Eur J Clin Microbiol Infect Dis. 1996;15:263–264. doi: 10.1007/BF01591371. [DOI] [PubMed] [Google Scholar]
- 23.Ribera E, Cucurull E, Ocaña I, Vallespi T, Gasser I, Juste C. Leishmaniasis visceral en pacientes con infección por VIH. Enf Infecc Microbiol Clin. 1995;13:73–79. [PubMed] [Google Scholar]
- 24.Rioux J A, Lanotte G, Serres E, Pratlong F, Bastien P, Perières J. Taxonomy of leishmania. Use of isoenzymes. Suggestion for a new classification. Ann Parasitol Hum Comp. 1990;65:111–125. doi: 10.1051/parasite/1990653111. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal E, Marty M, Poizot-Martin I, Reynes J, Pratlong F, Lafeuillade A, Jaubert D, Boulat O, Dereure J, Gambarelli F, Gastaut J A, Dujardin P, Dellamonica P, Cassuto J. Visceral leishmaniasis and HIV-1 co-infection in southern France. Trans R Soc Trop Med Hyg. 1995;89:159–162. doi: 10.1016/0035-9203(95)90476-x. [DOI] [PubMed] [Google Scholar]