Perioperative Neurocognitive Disorders (PND) are increasingly recognized as an area of concern in our aging surgical population1,2. For decades, postoperative disturbances in cognition and behavior were studied in the context of postoperative delirium (POD) or postoperative cognitive dysfunction (POCD). More recently, PND has emerged as a more inclusive overarching term, based on Diagnostic and Statistical Manual 5th edition (DSM-V) terminology for neurocognitive disorder, which includes preexisting cognitive disorders, POD, and POCD1. Over the past three decades significant progress has been made to improve the quality of PND studies by standardizing nomenclature and how PND are defined, measured, and assessed to better compare results across studies1,2. However major gaps still exist in patient cohort selection, which often focuses on convenience samples that in turn contribute to poor external validity and generalizability of results to a more diverse population1,3.
Ethnoracial factors and social determinants of health, encompassing race, ethnicity, primary language, socioeconomic status, education and other social and racial factors, are known to play a role in neurocognitive disorders such as Alzheimer’s Disease and Related Dementias (ADRD) and PND4. Populations underrepresented in PND research tend to be from developing countries as well as marginalized groups in high-income countries5. Ethnoracial factors and social determinants of health have been shown to impact disease progression, phenotype expression, and treatment response, which inform risk stratification and management strategies4. The reasons for this are likely multifactorial and vary from biomarker expression and genetic predisposition to environmental exposures4. Another reason may be a lack of preventative care or adequate education on early signs or symptoms allowing diseases to progress unchecked for years before a diagnosis is made and treatment initiated, such as in the setting of mild cognitive impairment (MCI).
The world’s population of people aged 65 and older is rapidly growing, accounting for an increasing percentage of healthcare resource utilization and expenditure1–3. A meaningful proportion of those believed to be cognitively intact before surgery and anesthesia will develop PND1,2. Furthermore, ethnoracial disparities exist in dementia screening, resulting in older adults from disadvantaged backgrounds to be diagnosed with cognitive impairment or dementia further along in their disease process4. This delay in diagnosis may have implications on postoperative cognitive outcomes. Therefore, it is likely that a large proportion of older adults presenting for surgery have baseline cognitive impairment, which makes them susceptible to PND6.
The population of the US continues to become more ethnically and racially diverse. Racial and ethnic minority groups and those with limited-English proficiency are the largest growing demographic in the US7,8. Nevertheless there is a paucity of research examining PND in aging minoritized populations. The perioperative community has the opportunity to be better informed about our understanding of how PND is assessed, managed, treated, and potentially prevented in diverse populations. In this manuscript we will 1) discuss our perspective on the current state of PND research and suggest benefits PND research may attain by increasing diversity in patient selection; and 2) discuss practical approaches for increasing diversity in PND research to understand PND pathophysiology in diverse communities. In this review, the racial and ethnic categories used by the original studies will be used.
ETHNORACIAL FACTORS AND SOCIAL DETERMINANTS OF HEALTH
Hundreds of publications on PND have focused on identifying and understanding risk factors and pathophysiologic mechanisms underlying the disease. Like ADRD, PND research has studied biological differences (biomarkers) and physiologic effects (frailty or resiliency) that predispose to or protect from cognitive decline. Recently, ADRD researchers have realized that psychosocial differences (an interplay of social, cultural and environmental influences9) also have an integral role in the development and expression of the disease4,9 (Figure 1). For example, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) network created a repository to understand the biological differences affecting ADRD10. ADNI was instrumental in identifying imaging biomarkers that track preclinical buildup of amyloid plaques in the brain leading to significant advances in tracking disease progression. In its nearly 20 years of existence, the majority of ADNI participants, like those in most ADRD studies, were white and highly educated10. In the latest iteration, ADNI4, which has yet to initiate recruitment, the focus is to increase diversity. This need for increased diversity is due to differences in dementia risk factors and findings indicating biomarkers from white populations may not translate to geographically, ethnically and language diverse populations10.
Figure 1. Psychosocial Framework for Brain Health and Cognition.
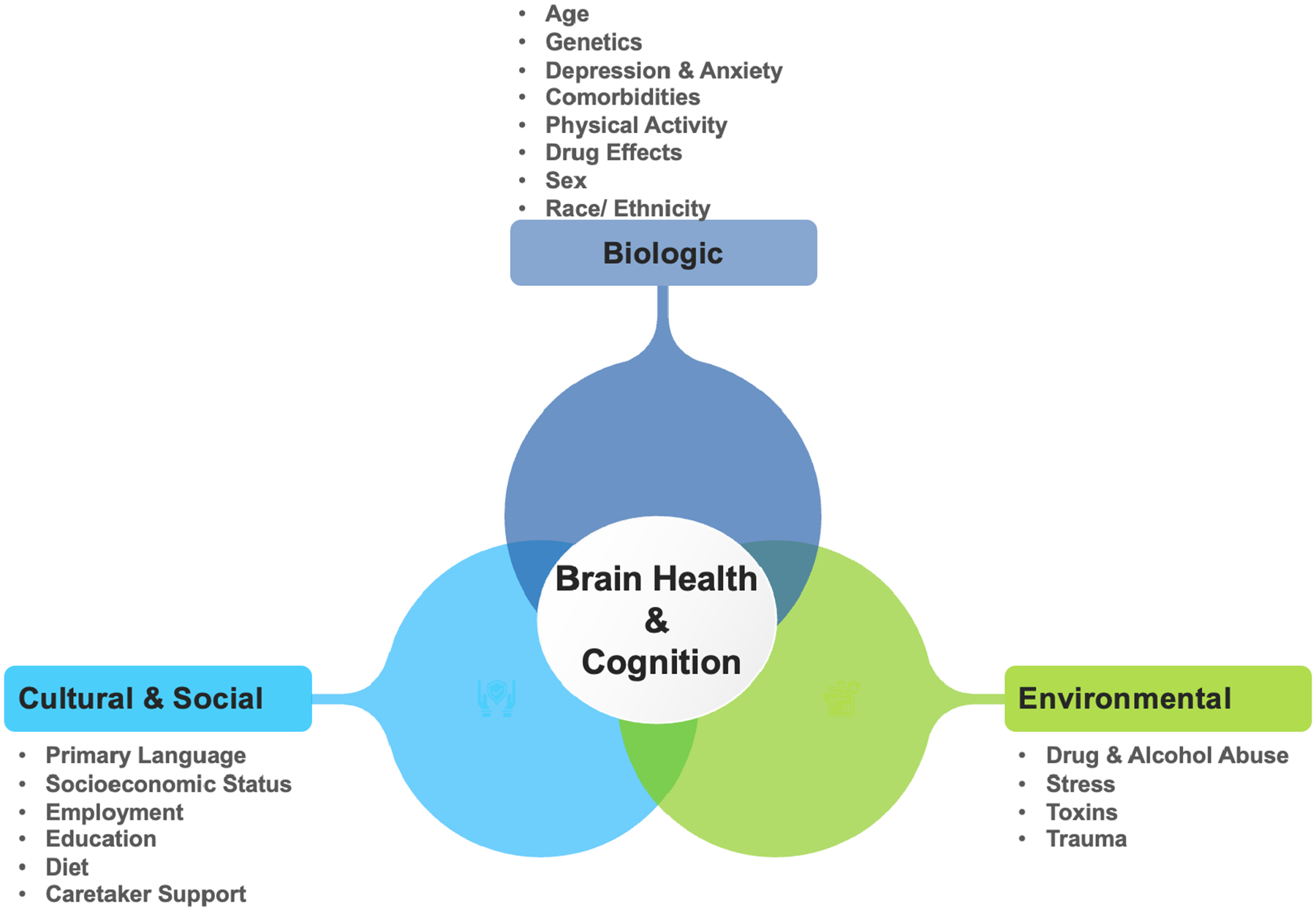
The psychosocial framework has interconnections between biological, cultural, environmental and social factors that contribute to overall brain health and cognition. Ethnoracial factors and social determinants of health contribute to phenotype expression. For instance, environmental factors including stress due to socioeconomic status or social drivers of health may act as exposures that in turn affect cognitive trajectories based on genetic predisposition.
Acculturation, education, socioeconomic factors, and language spoken affect the results of neurocognitive assessments11. Instead of validating or referencing published norms for cultural and language appropriate neurocognitive assessments, the vast majority of PND studies exclude participants who do not speak the predominant language, which is often English. Exluding based on language, can lead to neglect of other ethnoracial factors and social determinants of health that may be important for PND and may identify subgroup phenotypes in biomarker analysis12. PND researchers have an opportunity to significantly improve the quality of the science by assessing the impact of ethnoracial factors and social determinants of health on biomarker analysis, disease progression, treatment modalities, risk factors and overall outcomes. In Table 1 and discussed below, we outline the implications of diversity on various tools used to study PND in three main themes: 1) psychosocial factors and social drivers of health; 2) biological differences; and 3) physiologic effects.
Table 1.
Implications of Diversity on Research Tools Involved in Studying PND
| Screening/functional domain assessment | MOCA1, RAVLT2, Trailmaking3 | Language can affect performance on screening and cognitive assessment tools impacting timing of diagnosis, treatment or management | Increasing recruitment of ethnoracial diverse patients can provide language and cultural normative values for assessment of PND |
| Imaging | MRI4, fMRI5, PET6 | Ethnoracial differences have been found in neurodegeneration, treatment effect, brain volumes | By studying ethnoracial factors in PND, improved discrimination for biomarker analysis may be possible |
| CSF, Blood, Plasma, Saliva | APOE ε45, amyloid- beta7, tau8 | Ethnoracial differences have been found in gene mutation, expression | Diverse samples present opportunities to better understand the pathogenesis, diagnosis, and treatment of PND |
| Inflammatory Markers | CRP9, Cytokines5, TNF5 | Differences exist in inflammatory expression and treatment response between diverse groups | PND research would benefit by exploring how ethnoracial factors affect the perioperative inflammatory response |
| EEG | Raw or processed brain wave monitoring5 | EEG studies suggest ethnoracial differences in sleep disturbances which is closely associated to cognitive decline | As EEG analysis grows in PND research, exploring ethnoracial differences may better identify EEG patterns associated with increased risk |
| Frailty, resilience | Prehabilitation5, cognitive training10 | Cultural differences have been found in frailty and resilience factors as well as adherence to prehabilitation or cognitive training | Identifying culturally different protective factors and barriers to prehabilitation can identify ways to improve outcomes |
PND= Perioperative Neurocognitive Disorders; ADRD= Alzheimer’s Disease and Related Dementias; MOCA= Montreal Cognitive Assessment; RAVLT= Rey Auditory Verbal Learning Test; MRI= Magnetic Resonance Imaging; f= functional; PET= Positron Emission Tomography; CSF= Cerebral Spinal Fluid; APOE= Apolipoprotein E; EEG= Electroencephalographic; CRP= C-reactive Protein; TNF= Tumor Necrosis Factor.
Khan G, Mirza N, Waheed W: Developing guidelines for the translation and cultural adaptation of the Montreal Cognitive Assessment: scoping review and qualitative synthesis. BJPsych Open 2022; 8: e21
Ferreira Correia A, Campagna Osorio I: The Rey Auditory Verbal Learning Test: normative data developed for the Venezuelan population. Arch Clin Neuropsychol 2014; 29: 206–15
Lee TM, Cheung CC, Chan JK, Chan CC: Trail making across languages. J Clin Exp Neuropsychol 2000; 22: 772–8
Gavett BE, Fletcher E, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D: Ethnoracial differences in brain structure change and cognitive change. Neuropsychology 2018; 32: 529–540
Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, Bahar-Fuchs A, Bell J, Bowman GL, Brickman AM, Chetelat G, Ciro C, Cohen AD, Dilworth-Anderson P, Dodge HH, Dreux S, Edland S, Esbensen A, Evered L, Ewers M, Fargo KN, Fortea J, Gonzalez H, Gustafson DR, Head E, Hendrix JA, Hofer SM, Johnson LA, Jutten R, Kilborn K, Lanctot KL, Manly JJ, Martins RN, Mielke MM, Morris MC, Murray ME, Oh ES, Parra MA, Rissman RA, Roe CM, Santos OA, Scarmeas N, Schneider LS, Schupf N, Sikkes S, Snyder HM, Sohrabi HR, Stern Y, Strydom A, Tang Y, Terrera GM, Teunissen C, Melo van Lent D, Weinborn M, Wesselman L, Wilcock DM, Zetterberg H, O’Bryant SE, International Society to Advance Alzheimer’s R, Treatment AsA: Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement 2019; 15: 292–312
Wilkins CH, Windon CC, Dilworth-Anderson P, Romanoff J, Gatsonis C, Hanna L, Apgar C, Gareen IF, Hill CV, Hillner BE, March A, Siegel BA, Whitmer RA, Carrillo MC, Rabinovici GD: Racial and Ethnic Differences in Amyloid PET Positivity in Individuals With Mild Cognitive Impairment or Dementia: A Secondary Analysis of the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) Cohort Study. JAMA Neurol 2022; 79: 1139–47
McDonough IM: Beta-amyloid and Cortical Thickness Reveal Racial Disparities in Preclinical Alzheimer’s Disease. Neuroimage Clin 2017; 16: 659–667
Garrett SL, McDaniel D, Obideen M, Trammell AR, Shaw LM, Goldstein FC, Hajjar I: Racial Disparity in Cerebrospinal Fluid Amyloid and Tau Biomarkers and Associated Cutoffs for Mild Cognitive Impairment. JAMA Netw Open 2019; 2: e1917363
Farmer HR, Wray LA, Xian Y, Xu H, Pagidipati N, Peterson ED, Dupre ME: Racial Differences in Elevated C-Reactive Protein Among US Older Adults. J Am Geriatr Soc 2020; 68: 362–369
McDonough IM, Byrd DR, Choi SL: Resilience resources may buffer some middle-aged and older Black Americans from memory decline despite experiencing discrimination. Soc Sci Med 2022: 114998
Psychosocial Factors and Social Drivers of Health
In the context of neurocognition, the term psychosocial refers to the interplay between biological, cultural, social and environmental factors that influence brain health and cognition9. These non-clinical factors including occupation, stable transportation for health care maintenance, access to nutrient-rich food, sleep, exercise, and mobility influence cognition8,9.
Linguistic Diversity
Language is a multidimensional factor in diagnosing PND. Ignoring the major influence of primary language can lead to diagnostic errors and treatment failure. Spoken and written language can be early indicators of cognitive decline, as language centers are often affected during disease progression4. Moreover, primary language affects performance on neurocognitive assessment tests, as they are sensitive to language, cultural norms, and education4. Psychosocial factors including primary language can also affect biomarker expression in ways discussed below. Despite the growing population of persons with limited English proficiency in English speaking countries, there are few studies examining ethnoracial factors and social determinants of health in PND research, with studies predominantly restricted to those who speak English4,13. Nonetheless, the Mini-Cog for screening for dementia and the Confusion Assessment Method tool for assessing delirium have been translated into over 20 languages. Studies on delirium have shown that patients may require re-orientation with language-concordant approaches, as patients often revert to their primary language during the acute phase of delirium13. Furthermore, it is critically important to highlight that simple translations are often inadequate as language often requires cultural humility to fully account for cross-cultural communication differences amongst different groups regardless of the language they speak. For example, the literal translation of “heartburn” in Spanish would be “ardor de corazón,” which would not convey the intended meaning.
Biological Differences
Neuroimaging Biomarkers
The use of neuroimaging biomarkers has steadily increased in PND studies. PND neuroimaging biomarker studies have been used to identify those at risk, to determine the severity of cognitive impairment, to identify those who may be responsive to specific treatment, and to monitor the efficiency of the treatment10. Researchers have yet to examine how ethnoracial factors and social determinants of health contribute to specific phenotypes of imaging biomarkers in PND. For example, older Black patients have shown disproportionately greater brain atrophy compared with other racial and ethnic groups with the differences in accelerated aging in underrepresented minority communities secondary to ethnoracial factors and social determinants of health4. It is plausible that variations in neuroimaging biomarkers exist between those who are bilingual or monolingual and may vary depending on primary language. Indeed, structural brain differences have been observed in monolingual versus bilingual individuals4. In China, researchers created the Taizhou Imaging Study to gain a better understanding of dementia and cerebrovascular diseases in Chinese populations, realizing that there are key differences in genetic, sociocultural, and lifestyles amongst Chinese patients when compared to other ethnic or racial groups14.
Biospecimen / Fluid Biomarkers
It is well documented that ethnoracial factors and social determinants of health influence blood, plasma, or cerebral spinal fluid (CSF) biomarkers expression4. Fluid biomarker analyses show the levels of plasma amyloid-beta (Aβ), which are associated with increased levels of plaque deposition in ADRD and tau protein, which are associated with senile plaque deposition and neurofibrillary tangle formation, vary greately depending on race or ethnicity4,15–17. Investigators are studying blood, CSF, urine, and saliva biomarker analysis, including Aβ and tau levels, to establish their relevance to PND. The exploration of ethnoracial factors and social determinants of health in PND biomarker analysis may present an opportunity to better understand the pathogenesis, diagnosis, and treatment of PND.
Immunity and Neuroinflammation
A growing body of literature underscores the role of immunity and neuroinflammation as a possible pathophysiological mechanism in PND18. C-reactive protein (CRP) correlates with the degree of systemic inflammation. Studies show that CRP levels have been found to be significantly elevated among Japanese American and Mexican American populations diagnosed with MCI when compared to non-Hispanic white populations4. Inflammatory cytokines have also shown to be associated with ethnoracial factors and social determinants of health with circulating levels of interleukin (IL)-6 consistently elevated in Black patients, while IL-10 and tumor necrosis factor alpha were found to be unchanged when comparing Black and non-Hispanic white populations4. Thus, PND studies may yield a better understanding of the neuroinflammatory mechanisms involved if these ethnoracial factors and social determinants of health are taken into account.
Physiologic Effects
Frailty and Resilience
Physical and cognitive reserve and their relationship to frailty is an important topic of research in the perioperative community, as we know that frailty is closely associated to PND and increased morbidity and mortality19. Active areas of scientific inquiry are measurements of frailty, the state of increased vulnerability with physical and cognitive decline, and resilience, the ability to recover after a major stressor. It is important to understand the relative contributions of different genetic, ethnic and sociocial determinats of health to resilience and the observed differences among ethnoracial groups in overall cognition4. Differences in resilience with associated pathology among ethnoracial groups may reflect different relationships between brain health and cognitive performance such as higher levels of brain pathology for a given degree of cognitive impairment4. PND research exploring the same themes of frailty and resilience, should also consider the relative contributions of different genetic, socio-behavioral, and lifestyle factors among ethnoracial groups to better understand protective factors against cognitive decline.
APPROACHES FOR INCREASING DIVERSITY IN PND RESEARCH
The world’s population of older adults is ethnically diverse and represents a wide array of cultural norms, languages, formal education, exposure to environmental factors, stressors, and genetic mutations. This diversity translates to variations in disease phenotype, expression, and treatment response, which compromises the external validity of applying diagnostic and treatments norms based on Western, educated, industrialized, rich, and democratic samples to diverse populations4. By exploring ethnoracial differences in PND research, we have the opportunity to create brain health equity and to offer greater scientific insight, rather than solely focusing on populations already well-studied4.
One of the commonly cited limitations in PND research is the lack of diversity in the scientific workforce with the ability to implement language and culturally competent neurocognitive assessments4. Although efforts should be made so that the scientific community represents the patient population they serve by inclusion of a diverse scientific team with expertise in the subject matter, this goal will take time to achieve. Nonetheless, this effort is important because language translation does not equate to cultural norms, nor does it gain buy-in from often marginalized communities that may distrust the healthcare and scientific communities. This distrust can affect enrollment and retention4. One step forward would be for multi-national cross-cultural PND studies to compare ethnoracial differences and the mechanisms underlying such differences if they were to exist. By specifically looking at differences in ethnoracial factors and social determinants of health, one may be able to find a stronger signal when, for example, comparing neuroimaging or CSF biomarkers. A second approach would be for the perioperative workforce to create community partnerships and collaborate with investigators who may be involved in work with an emphasis on ethnoracial differences. For example, geriatricians, neuropsychologists, neurologists, and psychiatrist may already use culturally and language appropriate neurocognitive assessments with validated norms that are used for patient care or ongoing research efforts. The power lies in consortium studies combining efforts of heterogenous samples to improve the understanding of PND and its relation to overall brain health. A key point is that differences observed, as in the case of some neuroimaging biomarker studies, may not be directly rooted in race but may be a consequence of psychosocial and environmental factors that has produced a biodiverse response that should be further examined and taken into consideration in terms of PND research4. Further, if ethnoracial differences are found that pose a higher relative risk of PND, then investigating such differences can result in targeted areas of intervention.
CONCLUSION
The perioperative community has made great strides in PND research with promising results in studies in homogenous cohorts that are able to show internal validity whether exploring, risk factors, incidence, morbidity and mortality, or exploring the pathogenesis of PND through biomarker analysis. By focusing on ethnoracial factors and the mechanisms underlying such differences, we may be able to improve the overall science and improve the generalizabity and external validity of our studies so that the world’s heterogenous population of ethnoracially diverse people can achieve improved brain health. Neurocognitive assessments are sensitive to language, education, and other ethnoracial factors and social determinants of health. Genetic predispositions and biomarker analysis vary due to ethnoracial factors and social determinants of health, yet the mechanisms that underlie these differences have rarely been explored. Precisely because they are sensitive to these factors, the onus is on the scientific community to examine how ethnoracial factors and social determinants of health may impact PND.
Funding:
This work is supported by UCLA RCMAR/CHIME NIA Grant #P30-AG021684 and UCLA CTSI NCATS Grant #UL1TR001881 for Dr. Canales-RCMAR/CHIME Scientist. Dr. Ibarra is supported by the University of Pittsburgh Cluster Hire Initiative, Department of Anesthesiology and Perioperative Medicine and Bristol Myers Squibb Foundation. Dr Cannesson is supported by R01EB029751 and R01HL144692.
Conflict of Interest:
Dr. Canales was selected as a RCMAR/CHIME Scientist (2022–2023) to support her work on PND in Minority Elders. The award is funded by NIA Grant #P30-AG021684 and NCATS Grant #UL1TR001881. Dr. Cannesson is a consultant for Edwards Lifesciences and Masimo Corp, and has funded research from Edwards Lifesciences and Masimo Corp. He is also the founder of Sironis and Perceptive Medical and he owns patents and receives royalties for closed loop hemodynamic management technologies that have been licensed to Edwards Lifesciences.
Glossary of Terms:
- PND
Perioperative Neurocognitive Disorders
- POD
Postoperative Delirium
- POCD
Postoperative Cognitive Dysfunction
- DSM-V
Diagnostic and Statistical Manual 5th edition
- ADRD
Alzheimer’s Disease and Related Dementias
- MCI
Mild Cognitive Impairment
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- CSF
Cerebral Spinal Fluid
- Aβ
Amyloid-beta
- CRP
C-reactive Protein
- IL
Interleukin
REFERENCES
- 1.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG, Nomenclature Consensus Working G: Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 2018; 121: 1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, Browndyke JN, Wright CB, Culley DJ, Evered L, Scott DA, Wang NY, Brown CHt, Oh E, Purdon P, Inouye S, Berger M, Whittington RA, Price CC, Deiner S: State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth 2019; 123: 464–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, Whitson HE, Mathew JP: Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol Clin 2015; 33: 517–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, Bahar-Fuchs A, Bell J, Bowman GL, Brickman AM, Chetelat G, Ciro C, Cohen AD, Dilworth-Anderson P, Dodge HH, Dreux S, Edland S, Esbensen A, Evered L, Ewers M, Fargo KN, Fortea J, Gonzalez H, Gustafson DR, Head E, Hendrix JA, Hofer SM, Johnson LA, Jutten R, Kilborn K, Lanctot KL, Manly JJ, Martins RN, Mielke MM, Morris MC, Murray ME, Oh ES, Parra MA, Rissman RA, Roe CM, Santos OA, Scarmeas N, Schneider LS, Schupf N, Sikkes S, Snyder HM, Sohrabi HR, Stern Y, Strydom A, Tang Y, Terrera GM, Teunissen C, Melo van Lent D, Weinborn M, Wesselman L, Wilcock DM, Zetterberg H, O’Bryant SE, International Society to Advance Alzheimer’s R, Treatment AsA: Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement 2019; 15: 292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchers F, Spies CD, Feinkohl I, Brockhaus WR, Kraft A, Kozma P, Fislage M, Kuhn S, Ionescu C, Speidel S, Hadzidiakos D, Veldhuijzen DS, Yurek F, Evered LA, Ottens TH: Methodology of measuring postoperative cognitive dysfunction: a systematic review. Br J Anaesth 2021; 126: 1119–1127 [DOI] [PubMed] [Google Scholar]
- 6.Peden CJ, Miller TR, Deiner SG, Eckenhoff RG, Fleisher LA, Members of the Perioperative Brain Health Expert P: Improving perioperative brain health: an expert consensus review of key actions for the perioperative care team. Br J Anaesth 2021; 126: 423–432 [DOI] [PubMed] [Google Scholar]
- 7.Bureau UC: U.S. Census Bureau Releases 2019 Population Estimates by Demographic Characteristics, 2020
- 8.Daiello LA, Racine AM, Yun Gou R, Marcantonio ER, Xie Z, Kunze LJ, Vlassakov KV, Inouye SK, Jones RN, Alsop D, Travison T, Arnold S, Cooper Z, Dickerson B, Fong T, Metzger E, Pascual-Leone A, Schmitt EM, Shafi M, Cavallari M, Dai W, Dillon ST, McElhaney J, Guttmann C, Hshieh T, Kuchel G, Libermann T, Ngo L, Press D, Saczynski J, Vasunilashorn S, O’Connor M, Kimchi E, Strauss J, Wong B, Belkin M, Ayres D, Callery M, Pomposelli F, Wright J, Schermerhorn M, Abrantes T, Albuquerque A, Bertrand S, Brown A, Callahan A, D’Aquila M, Dowal S, Fox M, Gallagher J, Anna Gersten R, Hodara A, Helfand B, Inloes J, Kettell J, Kuczmarska A, Nee J, Nemeth E, Ochsner L, Palihnich K, Parisi K, Puelle M, Rastegar S, Vella M, Xu G, Bryan M, Guess J, Enghorn D, Gross A, Gou Y, Habtemariam D, Isaza I, Kosar C, Rockett C, Tommet D, Gruen T, Ross M, Tasker K, Gee J, Kolanowski A, Pisani M, de Rooij S, Rogers S, Studenski S, Stern Y, Whittemore A, Gottlieb G, Orav J, Sperling R, Group* SS: Postoperative Delirium and Postoperative Cognitive Dysfunction: Overlap and Divergence. Anesthesiology 2019; 131: 477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novak P, Chu J, Ali MM, Chen J: Racial and Ethnic Disparities in Serious Psychological Distress Among Those With Alzheimer’s Disease and Related Dementias. Am J Geriatr Psychiatry 2020; 28: 478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber CJ, Carrillo MC, Jagust W, Jack CR Jr., Shaw LM, Trojanowski JQ, Saykin AJ, Beckett LA, Sur C, Rao NP, Mendez PC, Black SE, Li K, Iwatsubo T, Chang CC, Sosa AL, Rowe CC, Perrin RJ, Morris JC, Healan AMB, Hall SE, Weiner MW: The Worldwide Alzheimer’s Disease Neuroimaging Initiative: ADNI-3 updates and global perspectives. Alzheimers Dement (N Y) 2021; 7: e12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enobi Y, Kemmotsu N, Robinson E, Murphy C: Effects of language and acculturation on neurocognitive performance of Japanese Americans. Neuropsychology 2022; 36: 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dilworth-Anderson P, Gibson BE: The cultural influence of values, norms, meanings, and perceptions in understanding dementia in ethnic minorities. Alzheimer Dis Assoc Disord 2002; 16 Suppl 2: S56–63 [DOI] [PubMed] [Google Scholar]
- 13.Reppas-Rindlisbacher C, Panov ED, Cuperfain AB, Rawal S: A Survey of Nurses’ Perspectives on Delirium Screening in Older Adult Medical Inpatients With Limited English Proficiency. J Gerontol Nurs 2021; 47: 29–34 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Cui M, Tian W, Zhu S, Chen J, Suo C, Liu Z, Lu M, Xu K, Fan M, Wang J, Dong Q, Ye W, Jin L, Chen X, Taizhou Imaging Study G: Lifestyle, multi-omics features, and preclinical dementia among Chinese: The Taizhou Imaging Study. Alzheimers Dement 2021; 17: 18–28 [DOI] [PubMed] [Google Scholar]
- 15.Miyashita A, Kikuchi M, Hara N, Ikeuchi T: Genetics of Alzheimer’s disease: an East Asian perspective. J Hum Genet 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchy-Dicey A, Howard B, Longstreth WT Jr., Reiman EM, Buchwald D: APOE genotype, hippocampus, and cognitive markers of Alzheimer’s disease in American Indians: Data from the Strong Heart Study. Alzheimers Dement 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleason CE, Zuelsdorff M, Gooding DC, Kind AJH, Johnson AL, James TT, Lambrou NH, Wyman MF, Ketchum FB, Gee A, Johnson SC, Bendlin BB, Zetterberg H: Alzheimer’s disease biomarkers in Black and non-Hispanic White cohorts: A contextualized review of the evidence. Alzheimers Dement 2022; 18: 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safavynia SA, Goldstein PA: The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front Psychiatry 2018; 9: 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, Annoni G, Bellelli G: Frailty and Delirium in Older Adults: A Systematic Review and Meta-Analysis of the Literature. J Am Geriatr Soc 2018; 66: 2022–2030 [DOI] [PubMed] [Google Scholar]


