Abstract
BACKGROUND
Findings from observational studies suggest that dietary patterns may offer protective benefits against cognitive decline, but data from clinical trials are limited. The Mediterranean–DASH Intervention for Neurodegenerative Delay, known as the MIND diet, is a hybrid of the Mediterranean diet and the DASH (Dietary Approaches to Stop Hypertension) diet, with modifications to include foods that have been putatively associated with a decreased risk of dementia.
METHODS
We performed a two-site, randomized, controlled trial involving older adults without cognitive impairment but with a family history of dementia, a body-mass index (the weight in kilograms divided by the square of the height in meters) greater than 25, and a suboptimal diet, as determined by means of a 14-item questionnaire, to test the cognitive effects of the MIND diet with mild caloric restriction as compared with a control diet with mild caloric restriction. We assigned the participants in a 1:1 ratio to follow the intervention or the control diet for 3 years. All the participants received counseling regarding adherence to their assigned diet plus support to promote weight loss. The primary end point was the change from baseline in a global cognition score and four cognitive domain scores, all of which were derived from a 12-test battery. The raw scores from each test were converted to z scores, which were averaged across all tests to create the global cognition score and across component tests to create the four domain scores; higher scores indicate better cognitive performance. The secondary outcome was the change from baseline in magnetic resonance imaging (MRI)–derived measures of brain characteristics in a nonrandom sample of participants.
RESULTS
A total of 1929 persons underwent screening, and 604 were enrolled; 301 were assigned to the MIND-diet group and 303 to the control-diet group. The trial was completed by 93.4% of the participants. From baseline to year 3, improvements in global cognition scores were observed in both groups, with increases of 0.205 standardized units in the MIND-diet group and 0.170 standardized units in the control-diet group (mean difference, 0.035 standardized units; 95% confidence interval, −0.022 to 0.092; P= 0.23). Changes in white-matter hyperintensities, hippocampal volumes, and total gray- and white-matter volumes on MRI were similar in the two groups.
CONCLUSIONS
Among cognitively unimpaired participants with a family history of dementia, changes in cognition and brain MRI outcomes from baseline to year 3 did not differ significantly between those who followed the MIND diet and those who followed the control diet with mild caloric restriction. (Funded by the National Institute on Aging; ClinicalTrials.gov number, NCT02817074.)
Although therapies have recently been approved for Alzheimer’s disease,1 there is a need for prevention strategies. Lifestyle interventions targeting diet are a possible approach that could have an effect on public health. Most clinical trials have investigated comprehensive diets, in contrast to dietary manipulation of single foods or nutrients, and most have been conducted predominantly with respect to cardiovascular health, which could influence the occurrence of dementia. Such diets include the Dietary Approaches to Stop Hypertension (DASH) or the Mediterranean diet to reduce cardiovascular risk factors. Data from trials of dietary interventions for brain health are limited.
We performed a randomized trial of the Mediterranean–DASH Intervention for Neurodegenerative Delay, known as the MIND diet, which is a hybrid of the DASH and Mediterranean diets that incorporates many components of the two but with modifications to include foods that have been putatively associated with a decreased risk of Alzheimer’s disease,2–4 slower cognitive decline,4 and fewer neuropathologic changes of Alzheimer’s disease.5,6 The MIND diet, like its constituent diets, emphasizes consumption of plant-based foods, including green leafy vegetables, nuts and berries, fish, and olive oil. The diet limits the intake of foods with high levels of saturated fat and sugar, such as red or processed meat, butter and margarine, whole-fat cheese, pastries and sweets, and fried foods. The MIND diet specifically incorporates foods that have been putatively associated with a decreased risk of dementia.7–11 Here, we compared the MIND diet with mild caloric restriction with a control diet with mild caloric restriction to evaluate the effects of a 3-year dietary intervention on cognitive decline and brain-imaging markers of dementia and Alzheimer’s disease in older, cognitively unimpaired adults at risk for dementia because of family history.
METHODS
TRIAL DESIGN AND OVERSIGHT
In this 3-year, two-site, randomized, controlled trial, the participants were randomly assigned to follow the MIND diet with mild caloric restriction for weight loss (goal of intake was to consume 250 kcal less per day) or their usual diet with the same mild caloric restriction for weight loss (control diet). The executive steering committee conceived the trial design and statistical analysis plan. The data coordinating center staff conducted regular safety checks and created data-monitoring reports and analyses for review by an independent National Institute of Health (NIH)–approved data and safety monitoring board of experts in Alzheimer’s disease, clinical trials, and statistics, who were unaware of the trial-group assignments. The trial followed the ethical standards of the 1964 Declaration of Helsinki and its later amendments and was approved by the institutional review boards at Rush University Medical Center, Harvard School of Public Health, and Brigham and Women’s Hospital. All the participants provided written informed consent. The authors vouch for the completeness and accuracy of the data and reporting of adverse events and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org.
ELIGIBILITY CRITERIA
Persons 65 years of age or older were eligible for inclusion if they had a score of at least 22 on the 30-item Montreal Cognitive Assessment12 (range, 0 to 30, with lower scores indicating greater impairment), were overweight (defined as a body-mass index [the weight in kilograms divided by the square of the height in meters] of ≥25), reported a family history of Alzheimer’s dementia (defined as having a first-degree blood relative with dementia), and had suboptimal diets (defined as a MIND-diet score of ≤8, as based on a 14-item diet questionnaire that was devised by the investigators and designed to detect inadequate diet with respect to brain health [scores range from 0 to 14, with lower scores indicating a less adequate diet with respect to putative brain health; see the Supplementary Appendix, available at NEJM.org]).
Participants were recruited from January 2017 through April 2018 at two sites in the United States (Chicago and Boston metropolitan areas) by means of mass mailings to commercially available lists, recruitment flyers distributed to local health care establishments, and advertisements in newspapers, buses, and subway cars and stations and on the radio. Interested participants contacted the team and underwent a two-stage prescreening protocol for the assessment of eligibility. The initial screening was conducted by telephone to collect information on age, family history of dementia, diet quality, food allergies, use of nutritional supplements, and use of medications for Alzheimer’s disease or Parkinson’s disease. Eligible persons had to agree to not take vitamins (e.g., folate, vitamin B12, or multivitamins) and complete a 3- to 4-week run-in period to assess their likelihood of complying with the trial intervention and activities. During the run-in period, participants had to answer all telephone calls pertaining to the trial and complete dietary records for at least 3 of the 4 weeks. Those who completed the 3- to 4-week run-in period were evaluated by dietitians and trial investigators for suitability for trial participation. The rationale for the trial has been published,13 and full inclusion and exclusion criteria are provided in the protocol.
RANDOMIZATION AND INTERVENTION
Participants were randomly assigned in a 1:1 ratio to the MIND-diet group or the control-diet group. Randomization was performed with the use of permuted blocks created with R software, version 3.3 (R Project for Statistical Computing). Assignments were stratified according to trial site, sex, and age categories (65 to 69 years, 70 to 74 years, 75 to 80 years, and 81 to 84 years). At baseline, measurements (e.g., height, weight, and blood pressure) were obtained, tests of cognition were performed, and questionnaires (e.g., 24-hour dietary recall and food-frequency questionnaire) were completed, and then participants were assigned to a diet group by research staff at the data coordinating center. Participants were instructed to follow their assigned diet for 3 years. Data for the key end points and covariates were collected at month 6 and then yearly until the end of the trial.
Dietary counseling for all participants, led by registered dietitians, was conducted by telephone in a planned schedule of weekly contact during the first 6 months and every other week during the second 6 months; during years 2 and 3, the participants were contacted by telephone at least twice monthly. Individualized guidance was available to participants in both groups. In the MIND-diet group, foods that were part of that diet but may have been lacking, as suspected on the basis of past dietary recall, were identified in order for their consumption to be increased. In the control-diet group, goals that were focused on portion control were suggested. These modifications were intended to alter the participants’ short-term plans to achieve expected targets but did not change the structure of the diet assignment. Dietary counseling for the MIND-diet group included instructions to incorporate foods from the MIND diet and to use MIND diet recipes for behavioral strategies to lose weight, to keep exercise levels the same as at baseline, and to monitor one’s own accountability for dietary intake through coaching and goal setting. Dietary counseling for the control-diet group consisted of an equivalent frequency of consultation but focused on calorie tracking, portion control, and behavioral strategies to lose weight (e.g., coaching, goal setting, and mindful eating techniques) without changing the types of foods consumed. Both groups received equivalent amounts of support with respect to mild caloric restriction with the aim of achieving a weight loss of 3 to 5% by year 3.
Participants were incentivized regularly to follow their assigned diets. The MIND-diet group received a monthly supply of blueberries (2.5 cups, or 15 oz [425 g], per week), mixed nuts (5 oz [142 g] per week), and extra virgin olive oil (14 tablespoons, or 7 fl oz [207 ml] per week) that was donated by nonprofit food-grower organizations. The participants in the control-diet group received $30 gift cards at the same frequency that the MIND-diet group received food. All the participants had at least five opportunities to connect with other members in their assigned trial group in group sessions that took place at months 3, 9, 18, and 30 to cultivate social support. Group sessions included education about their assigned diet, tips to promote mild weight loss, and other motivational activities such as cooking sessions, trivia games, competitions, and holiday celebrations.
Information was collected on several short-term measures of dietary adherence, such as weight assessments, participant-reported dietary assessments with the MIND-diet questionnaire (a 14-item questionnaire designed to capture adherence to the MIND diet) and food-frequency questionnaire, and individualized goal setting. Serum biomarkers of adherence related to key dietary components, including carotenoids (lutein and zeaxanthin, which reflect intake of green leafy vegetables and other cruciferous vegetables) and alpha and beta carotene (e.g., carrots, squash, and peppers), were measured in a subgroup of participants. These measures and biomarkers are summarized in the Supplementary Appendix.
During the coronavirus disease 2019 (Covid-19) pandemic, we were obliged to temporarily halt trial operations for approximately 3 months (March 19, 2020, to July 15, 2020; Fig. S1 in the Supplementary Appendix). During this time, participants in both trial groups continued to receive dietary counseling by telephone, but no in-person testing (e.g., cognition, weight measurements, and blood draws) was conducted. After stay-at-home orders were lifted, 44 participants (24 in the MIND-diet group and 20 in the control-diet group) declined to return to the office for the in-person cognitive testing at the year 3 assessment. We revised the protocol, with guidance from the data and safety monitoring board, to assess cognition (episodic and semantic memory only) by telephone and included these assessments in the primary analysis.
END POINTS
The primary end point was the change from baseline in global cognition and in specific cognitive domains through year 3. Cognition was assessed with an established battery of 12 publicly available cognitive function tests, details of which have been published.14 There were 5 tests of episodic memory (Word List Memory, Word List Recall, Word List Recognition,15 East Boston Story Immediate Recall, and East Boston Story Delayed Recall16); 2 tests of semantic memory (Category Fluency [animals and fruits and vegetables]15 and the Multilingual Naming Test17); 2 tests of executive function (Trail Making Test B18 and the Flanker Inhibitory Control and Attention Test from the NIH toolbox19); and 3 tests of perceptual speed (Oral Symbol Digit Modality Test,20 Pattern Comparison Test,19 and Trail Making Test A18). Raw scores from each test were converted to z scores with the use of the mean and standard deviation values at baseline, and the resulting z scores were averaged across all tests to create a global composite score and across component tests to create the four domain scores. Higher scores on the composite measures reflect better cognitive performance.14 Cognition was assessed at baseline and at months 6, 12, 24, and 36. Cognitive testing was conducted by research assistants who were certified in cognitive testing and unaware of the trial-group assignments.
The secondary end points were changes from baseline in magnetic resonance imaging (MRI)– derived measures of total brain volume, hippocampal volume, and volume of white-matter hyperintense lesions. At trial inception, all the participants were offered the opportunity of undergoing MRI (a 3-Tesla Philips Achieva MRI scanner was used at the Chicago site and a Siemens Skyra MRI scanner at the Boston site). After enrolling the first 100 participants, we estimated the proportion of persons in each diet group who would participate in undergoing MRI, and a nonrandom subgroup of participants were offered MRI to reach the target sample size of 300 (approximately half the enrolled participants). MRI scans were obtained only at baseline and at month 36. Details on data collection and postprocessing are provided in the Supplementary Appendix. The scans were evaluated by reviewers who were unaware of the trial-group assignments.
STATISTICAL ANALYSIS
The trial was powered to test the effect of the MIND diet over the control diet on the annual rate of change in the global cognition score over 3 years. Using a simulation approach in PASS (Power Analysis and Sample Size) 2008 software (NCSS), designed for mixed-model analyses, we analyzed data from two observational cohort studies (Rush Memory and Aging Project7 and Chicago Health and Aging Project21) and estimated the effect size of the MIND-diet score on cognitive decline in persons without cognitive impairment at baseline. We determined that a sample of 300 participants per trial group would provide the trial with more than 90% power to detect a between-group difference of 0.02 standardized units per year in the annual rate of cognitive decline, assuming a dropout rate of 20%. The determination of power for the secondary MRI outcomes was not part of the sample-size estimations. We conducted an intention-to-treat analysis that included all the participants who had undergone randomization.
Because the trajectories of global cognition across time were nonlinear, we estimated the mean change from baseline at each follow-up visit and then compared the changes between the trial groups over time. We used linear mixed-effect models with random intercept, as specified in the protocol, but instead of modeling time linearly, we added time as an indicator variable for each visit. The effect of the MIND diet, as compared with the control diet, on global cognition at each visit was estimated with the use of the product of the indicator variables for trial-group assignment and trial visit. Within-person residuals were assumed to follow a first-order autoregressive model that was conditional on the random intercepts; the autocorrelation coefficient for a lag of 1 year was estimated to be 0.28. A similar approach was used for the cognitive domain scores and other outcomes (e.g., weight change) when nonlinearity was observed. For the analysis of brain morphologic characteristics, we also adjusted models for the clinical site (i.e., Boston and Chicago) to account for differences in the scanners. The widths of confidence intervals for multiple outcomes, such as cognitive domains (four outcomes) and MRI-derived measures (three outcomes), were not corrected for multiple testing, so these confidence intervals should not be used to infer treatment effects for these outcomes.
To account for missing data, we performed multiple imputation using the R package Multiple Imputation by Chained Equations (MICE) algorithm,22 incorporating a wide range of cognition-related predictors such as age, sex, education, cognitive activities, clinical site, and baseline cognitive scores. We considered the analysis that included imputed data as supplementary to the primary analysis. To test whether the inclusion of assessments made by telephone after the Covid-19 pandemic affected primary results, we conducted additional analyses that excluded these telephone assessments.
We standardized brain MRI-derived volumes to intracranial volume and followed standard procedures to normalize the data. We then computed the mean and 95% confidence interval using estiated marginal means, with brain imaging outcomes and dietary assignment and clinical site as independent variables. We computed the difference from baseline to year 3 and then performed pairwise comparisons to evaluate differences between the MIND-diet group and the control-diet group. All analyses were performed with the use of R statistical computing, version 4.0 (R Foundation for Statistical Computing).
RESULTS
PARTICIPANTS
Participants were enrolled from January 2017 through April 2018; data collection was completed in June 2021. Of 1929 persons who had undergone screening, 1325 (68.7%) were excluded, among whom 545 had a MIND-diet score higher than 8, 305 had declined to participate in the trial, and 160 were ineligible on the basis of medical history or medication use (Fig. 1). Criteria for continuation during the run-in process were not met by 109 persons, among whom 93 withdrew, 5 did not complete the food diaries, and 11 moved from the area or were ineligible to continue on the basis of medical history or medication use. A total of 301 participants were assigned to the MIND-diet group and 303 to the control-diet group. A total of 26 participants (8.6%) in the MIND-diet group and 14 participants (4.6%) in the control-diet group were lost to follow-up by year 3.
Figure 1. Screening, Randomization, and Follow-up.
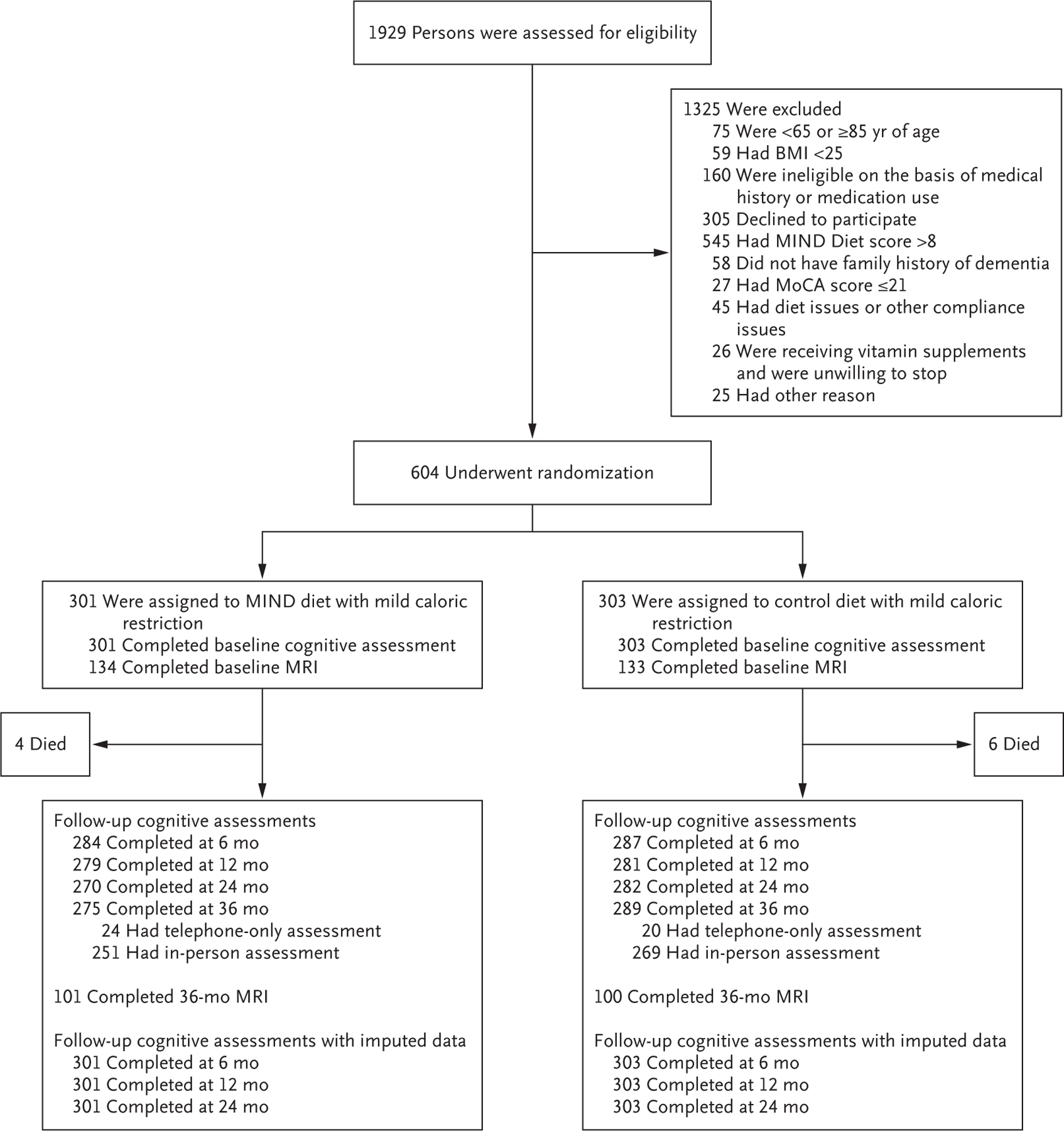
Scores on the Montreal Cognitive Assessment (MoCA) range from 0 to 30, with lower scores indicating greater impairment. BMI denotes body-mass index (the weight in kilograms divided by the square of the height in meters), MIND Mediterranean–DASH Intervention for Neurodegenerative Delay, and MRI magnetic resonance imaging.
The characteristics of the participants at baseline were similar across the trial groups, except the control-diet group had a slightly higher percentage of participants with an apolipoprotein E ε4 allele. The most common coexisting medical conditions were hypertension and type 2 diabetes, the prevalences of which were similar in the trial groups (Table 1). Over 3 years of the trial, 169 of 3020 planned cognitive assessments (5.6%) were not administered and required imputation, which was considered to be a sensitivity analysis.
Table 1.
Demographic and Clinical Characteristics of the Participants at Baseline.*
| Characteristic | MIND Diet (N = 301) | Control Diet (N = 303) |
|---|---|---|
| Demographic | ||
| Age — yr | 70.4±4.2 | 70.4±4.2 |
| Male sex — no. (%) | 105 (34.9) | 106 (35.0) |
| Race — no. (%)† | ||
| White | 263 (87.4) | 267 (88.1) |
| Black | 35 (11.6) | 31 (10.2) |
| Other | 3 (1.0) | 5 (1.7) |
| Education — yr | 16.9±2.7 | 17.0±2.6 |
| Clinical | ||
| Global cognition score‡ | 0.0±0.6 | 0.0±0.5 |
| Apolipoprotein E ε4 allele carrier — no. (%) | 76 (25.2) | 98 (32.3) |
| Body-mass index§ | 33.8±5.4 | 34.0±6.5 |
| CES-D scale score¶ | 1.2±1.6 | 1.2±1.4 |
| MIND-diet score‖ | 7.7±1.9 | 7.8±1.8 |
| Medical history — no. (%)** | ||
| Heart disease | 12 (4.0) | 15 (5.0) |
| Stroke | 9 (3.0) | 5 (1.7) |
| Diabetes | 45 (15.0) | 45 (14.9) |
| Hypertension | 154 (51.2) | 170 (56.1) |
Plus–minus values are means ±SD. MIND denotes Mediterranean–DASH Intervention for Neurodegenerative Delay.
Race was reported by the participants.
The global cognition score was derived from a 12-test battery. The raw scores from each test were converted to z scores, which were averaged across all tests to create the global cognition score; higher scores indicate better cognitive performance.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Scores on the Center for Epidemiological Studies Depression (CES-D) scale range from 0 to 10, with higher scores indicating a greater number of depressive symptoms.
MIND-diet scores were based on a 14-item food-frequency questionnaire that was devised by the investigators and designed to detect inadequate diet with respect to brain health. Scores range from 0 to 14, with lower scores indicating a less adequate diet with respect to putative brain health.
Medical history was reported by the participant.
NUTRITIONAL BIOMARKERS AND BODY WEIGHT
At baseline, the mean MIND-diet score was 7.7 (95% confidence interval [CI], 7.5 to 7.9) in the MIND-diet group and 7.8 (95% CI, 7.6 to 8.0) in the control-diet group. At 6 months, participants in the MIND-diet group increased their MIND-diet score by 3.3 points, for a mean score of 11.0, which was maintained at approximately that level throughout the trial. In the control-diet group, the MIND-diet score also increased, but only by 0.7 points, for a mean score of 8.5 (Fig. S2). The results of biochemical analyses of antioxidant nutrient levels in blood (lutein and zeaxanthin and alpha and beta carotene) supported adherence to the MIND diet (Fig. S3). Participants in both groups lost weight during follow-up (Fig. S4); the change in body weight from baseline was −5.0 kg (95% CI, −5.8 to −4.1) in the MIND-diet group and −4.8 kg (95% CI, −5.7 to −4.0) in the control-diet group.
PRIMARY AND SECONDARY END POINTS
From baseline through year 3, the estimated mean change in global cognition score (a primary end point) was 0.205 standardized units (95% CI, 0.164 to 0.246) in the MIND-diet group and 0.170 standardized units (95% CI, 0.130 to 0.210) in the control-diet group. In an intention-to-treat analysis, the mean change in score did not differ significantly between the trial groups, with an estimated mean difference at the end of the trial of 0.035 standardized units (95% CI, −0.022 to 0.092; P=0.23) (Table 2 and Fig. 2). Changes in the global cognition score from baseline followed a similar nonlinear pattern in both trial groups. The findings from analyses performed with imputation for missing data (Table S1), when cognitive assessments obtained by telephone during the Covid-19 pandemic were excluded (44 participants [1.5%]) (Table S2), were similar to those of the primary analysis. The findings with respect to the additional primary end points (i.e., changes from baseline in the four cognitive domain scores) were similar to those for the global cognition score (Table S3).
Table 2.
Global Cognition Score during 3 Years of Follow-up in the MIND-Diet Group and the Control-Diet Group.
| Years since Randomization | MIND Diet | Control Diet | Difference (95% CI) | ||
|---|---|---|---|---|---|
| No. of Participants | Estimated Mean Change from Baseline (95% CI) | No. of Participants | Estimated Mean Change from Baseline (95% CI) | ||
| Baseline | 301 | 0 (Reference) | 303 | 0 (Reference) | |
| 0.5 | 284 | 0.125 (0.093 to 0.158) | 287 | 0.117 (0.085 to 0.149) | 0.008 (−0.037 to 0.054) |
| 1.0 | 279 | 0.226 (0.188 to 0.264) | 281 | 0.211 (0.173 to 0.248) | 0.015 (−0.038 to 0.069) |
| 2.0 | 270 | 0.246 (0.205 to 0.287) | 282 | 0.200 (0.159 to 0.240) | 0.046 (−0.011 to 0.104) |
| 3.0 | 275 | 0.205 (0.164 to 0.246) | 289 | 0.170 (0.130 to 0.210) | 0.035 (−0.022 to 0.092)* |
P = 0.23 for the primary end point.
Figure 2. Changes in the Global Cognition Score during the Trial Period.
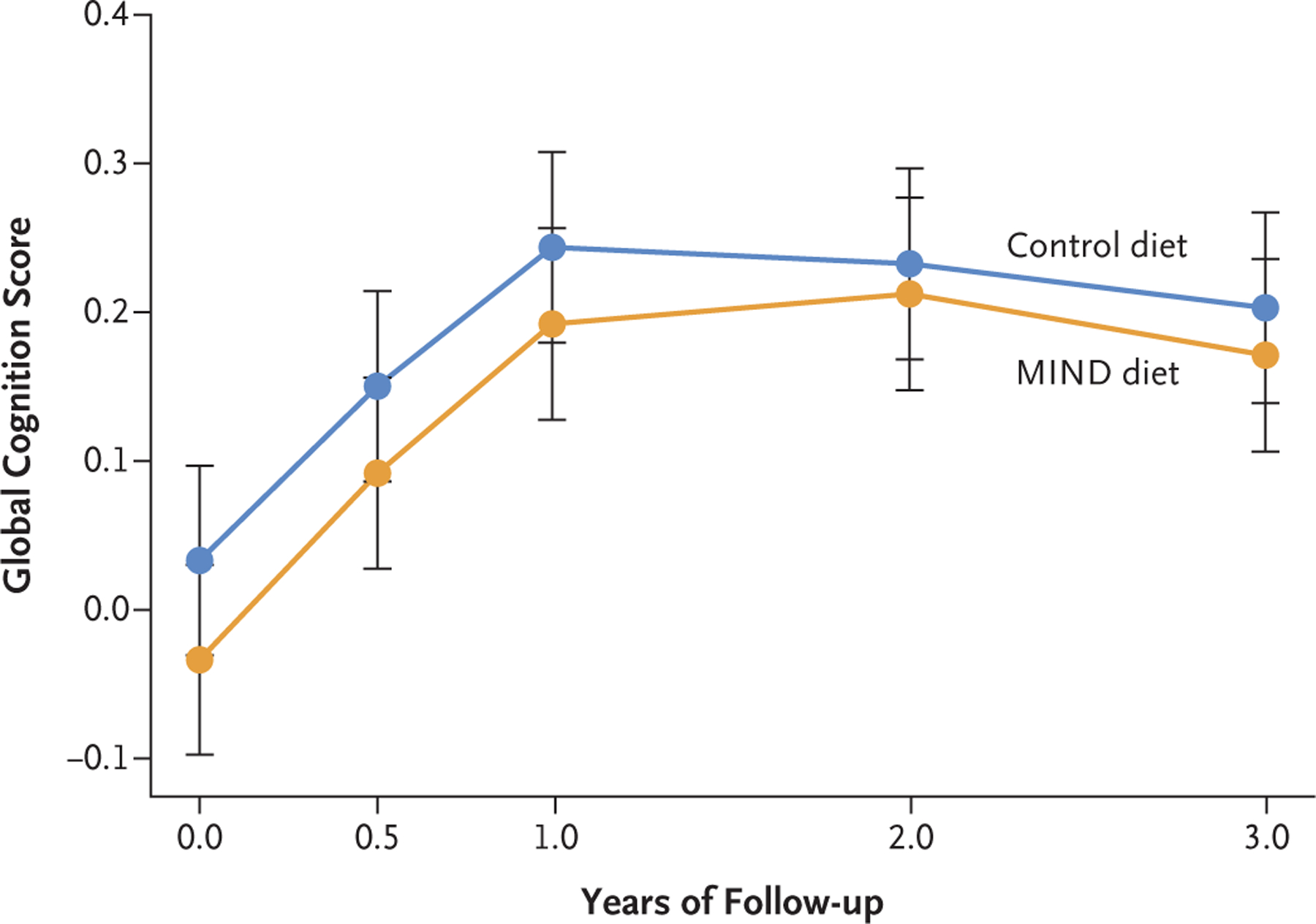
Shown are the mean global cognition scores over 3 years from a linear mixed-effect model; I bars indicate 95% confidence intervals. The global cognition score was derived from a 12-test battery. The raw scores from each test were converted to z scores, which were averaged across all tests to create the global cognition score; higher scores indicate better cognitive performance.
At year 1, the mean global cognition score increased from baseline by 0.226 standardized units (95% CI, 0.188 to 0.264) in the MIND-diet group and by 0.211 standardized units (95% CI, 0.173 to 0.248) in the control-diet group (Table 2); at year 2, the corresponding changes from baseline were 0.246 (95% CI, 0.205 to 0.287) and 0.200 (95% CI, 0.159 to 0.240). The results of additional analyses of the change in MIND-diet score, change in antioxidant levels, and change in body weight over time are provided in the Supplementary Appendix.
BRAIN IMAGING
A total of 267 of 604 participants agreed to undergo brain imaging at baseline, 201 of whom underwent follow-up imaging at year 3. We excluded 1 participant from the analysis because the baseline MRI was of inadequate quality, which left 200 participants for the analysis. At the end of the trial, white-matter hyperintense volumes increased in both the MIND-diet group and the control-diet group, whereas hippocampal and total brain volumes decreased in both groups (Table 3). No appreciable effect of the MIND diet, as compared with the control diet, was observed with respect to changes in white-matter hyperintense volume (mean difference, −0.019; 95% CI, −0.046 to 0.008), hippocampal volume (mean difference, 0.005; 95% CI, −0.016 to 0.026), and total gray- and white-matter volume (mean difference, 0.001; 95% CI, −0.003 to 0.005).
Table 3.
Results of Brain Imaging in the MIND-Diet Group and the Control-Diet Group.*
| Imaging Result | MIND Diet | Control Diet | Difference (95% CI)† | ||
|---|---|---|---|---|---|
| No. of Participants | Estimated Mean Change from Baseline (95% CI) | No. of Participants | Estimated Mean Change from Baseline (95% CI) | ||
| White-matter hyperintensities | |||||
| Baseline | 97 | 0 (Reference) | 96 | 0 (Reference) | |
| Year 3 | 97 | 0.086 (0.067 to 0.105) | 96 | 0.105 (0.086 to 0.124) | −0.019 (−0.046 to 0.008) |
| Hippocampal volume | |||||
| Baseline | 101 | 0 (Reference) | 99 | 0 (Reference) | |
| Year 3 | 101 | −0.075 (−0.09 to −0.06) | 99 | −0.080 (−0.095 to −0.065) | 0.005 (−0.016 to 0.026) |
| Gray and white matter | |||||
| Baseline | 101 | 0 (Reference) | 99 | 0 (Reference) | |
| Year 3 | 101 | −0.016 (−0.019 to −0.013) | 99 | −0.017 (−0.02 to −0.014) | 0.001 (−0.003 to 0.005) |
White-matter hyperintensities were standardized to intracranial volume, multiplied by 1000, and then log10-transformed to normalize the data. Hippocampal volume is the average of the right and left hippocampus, which was then standardized to intracranial volume and multiplied by 1000. Gray and white matter is the sum of gray matter and white matter volumes of the brain, which were standardized to intracranial volume.
The widths of confidence intervals have not been adjusted for multiplicity and cannot be used to infer treatment effects.
ADVERSE EVENTS
The incidence of adverse events over the 3 years was similar in the trial groups, with 127 events occurring in 89 participants in the MIND-diet group and 153 events occurring in 91 participants in the control-diet group (Table S4). The most common events were cardiovascular (17 events in the MIND-diet group and 32 in the control-diet group) and musculoskeletal (68 events in 60 participants, with 39 events in the MIND-diet group and 29 in the control-diet group). Ten deaths occurred during the trial (4 in the MIND-diet group and 6 in the control diet group). Neither deaths nor adverse events were categorized by the data and safety monitoring board as being related to the assigned diet.
DISCUSSION
The MIND diet, which is based on the established cardiovascular Mediterranean and DASH diets, includes foods and nutrients that have been putatively associated with preserving brain health.7,23 In this two-site randomized trial involving older adults with a family history of dementia, we found that the participants who followed the MIND diet had small improvements in a global measure of cognition that were similar to those who followed a control diet with mild caloric restriction.
The effects of a healthy diet on brain health are supported by findings from observational studies,5–11,24 and some evidence links high consumption of green leafy vegetables, nuts and berries, and olive oil with a reduction in the hallmark neuropathologic features of Alzheimer’s disease.25–28 The proposed underlying mechanisms point to antioxidant and antiinflammatory capacities of certain foods as being protective of brain functioning and against cognitive decline.29 Animal models have also shown the potential importance of vitamin E and docosahexaenoic acid for ostensible healthy brain functioning, including protection against lipid peroxidation,30 neuron loss,31 β-amyloid deposition,32 and decline in memory and learning.33 However, mechanistic evidence of these effects in humans is not well established. We included measurements of total brain volume, hippocampal volume, and white-matter hyperintense volume to investigate biologic mechanisms, but the planned 50% participation for these MRI scans was not attained. Nevertheless, although these studies were perhaps underpowered, the brain imaging outcomes were similar in the trial groups.
Previous meta-analyses of diet trials have shown mixed results and generally do not affirm the beneficial effects of diet on cognition that were observed in epidemiologic studies.34,35 Several reasons could contribute to this discrepancy. The presence of bias and confounding in observational studies is one possibility. It is also possible that differences in duration of follow-up and presence of preexisting coexisting medical conditions play a role. In our trial, we compared the MIND-diet group with a control-diet group that was instructed to restrict calories, and the participants in the control-diet group probably improved their diet, given evidence of weight loss that was similar in the two groups. It is also plausible that practice effects of repeated cognitive testing could account for improvement in both of our trial groups, as has been observed in previous randomized trials.36 Finally, it is possible that these interventions do not improve cognitive functioning or that it would take a longer period of adherence for an effect to be observed.
Our trial has limitations. A feature of the trial was that participants were required to have family history of dementia, have a suboptimal diet before randomization, and be overweight, all of which have been shown to be important risk factors for Alzheimer’s and cognitive decline.37 The participants were mostly well-educated, older adults of mostly European descent, and approximately 10% of the participants were Black. Our findings may not be generalizable to persons from diverse backgrounds and less education than that represented in the trial population (Table S5).
In a trial of the MIND diet that was designed to improve brain health, cognitive function and brain imaging outcomes at 3 years did not differ significantly between participants who followed the MIND diet and those who followed a control diet with a mild caloric restriction.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institute on Aging, National Institutes of Health (R01AG52583).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the participants from the MIND trial and Jeremy Furtado and his former laboratory at Harvard School of Public Health for work conducted on the adherence markers. This article is dedicated to the memory of Martha Clare Morris, Sc.D., a pioneer in nutrition–dementia science and co-creator of the MIND diet.
Contributor Information
Lisa L. Barnes, Rush Alzheimer’s Disease Center, Department of Neurology, Chicago
Klodian Dhana, Rush Institute for Healthy Aging, Departments of Internal Medicine, Chicago
Xiaoran Liu, Rush Institute for Healthy Aging, Departments of Internal Medicine, Chicago
Vincent J. Carey, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston
Jennifer Ventrelle, Clinical Nutrition, Preventive Medicine, Chicago
Kathleen Johnson, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston
Chiquia S. Hollings, Rush Institute for Healthy Aging, Departments of Internal Medicine, Chicago
Louise Bishop, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston
Nancy Laranjo, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston
Benjamin J. Stubbs, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston
Xavier Reilly, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston
Puja Agarwal, Rush Alzheimer’s Disease Center, Departments of Internal Medicine, Clinical Nutrition, Chicago
Shengwei Zhang, Rush Alzheimer’s Disease Center, Chicago
Francine Grodstein, Rush Alzheimer’s Disease Center, Departments of Internal Medicine, Chicago
Christy C. Tangney, Clinical Nutrition, Preventive Medicine, Chicago
Thomas M. Holland, Rush Institute for Healthy Aging, Departments of Internal Medicine, Chicago
Neelum T. Aggarwal, Rush Alzheimer’s Disease Center, Department of Neurology, Chicago
Konstantinos Arfanakis, Rush Alzheimer’s Disease Center, Diagnostic Radiology and Nuclear Medicine, Rush University Medical Center, and the Department of Biomedical Engineering, Illinois Institute of Technology, Chicago
Martha Clare Morris, Departments of Internal Medicine, Chicago.
Frank M. Sacks, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston
REFERENCES
- 1.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med 2023;388:9–21. [DOI] [PubMed] [Google Scholar]
- 2.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002;287:3223–9. [DOI] [PubMed] [Google Scholar]
- 3.Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol 2000;16: 357–63. [DOI] [PubMed] [Google Scholar]
- 4.Loef M, Walach H. Fruit, vegetables and prevention of cognitive decline or dementia: a systematic review of cohort studies. J Nutr Health Aging 2012;16:626–30. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C, Chen H, Wang Y, Schneider JA, Willett WC, Morris MC. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr 2021;113:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth SL, Shea MK, Barger K, et al. Association of vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement (N Y) 2022;8(1):e12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11: 1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol 2005;57:713–20. [DOI] [PubMed] [Google Scholar]
- 9.Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology 2018;90(3):e214–e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 2015;6:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devore EE, Kang JH, Breteler MMB, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol 2012;72:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Morris MC, Dhana K, et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp Clin Trials 2021;102:106270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krueger KR, Dhana K, Aggarwal NT, et al. Properties of the cognitive function battery for the MIND diet intervention to prevent Alzheimer’s disease. J Int Neuropsychol Soc 2022;28:790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39:1159–65. [DOI] [PubMed] [Google Scholar]
- 16.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 1991;57:167–78. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova I, Salmon DP, Gollan TH. The multilingual naming test in Alzheimer’s disease: clues to the origin of naming impairments. J Int Neuropsychol Soc 2013; 19:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitan RM, Wolfson D. The Halstead Reitan neuropsychological test battery Tucson, AZ: Neuropsychology Press, 1985. [Google Scholar]
- 19.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80:Suppl 3: S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A Symbol digit modalities test manual. Rev ed. Los Angeles: Western Psychological Services, 1982. [Google Scholar]
- 21.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr 2011;93:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 23.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015;11:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006; 67:1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner M, Agarwal P, Leurgans SE, et al. The association of MIND diet with cognitive resilience to neuropathologies Alzheimers Dement 2023 February 28 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 26.Agarwal P, Holland TM, James BD, et al. Pelargonidin and berry intake association with alzheimer’s disease neuropathology: a community-based study. J Alzheimers Dis 2022;88:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal P, Leurgans SE, Agrawal S, et al. Association of Mediterranean-DASH intervention for neurodegenerative delay and Mediterranean diets with Alzheimer disease pathology. Neurology 2023;100(22): e2259–e2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhana K, James BD, Agarwal P, et al. MIND diet, common brain pathologies, and cognition in community-dwelling older adults. J Alzheimers Dis 2021;83:683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fotuhi M, Zandi PP, Hayden KM, et al. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti-inflammatory drugs: the Cache County Study. Alzheimers Dement 2008; 4:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero AL, Dorado-Martínez C, Rodriguez A, Pedroza-Ríos K, Borgonio-Pérez G, Rivas-Arancibia S. Effects of vitamin E on ozone-induced memory deficits and lipid peroxidation in rats. Neuroreport 1999;10:1689–92. [DOI] [PubMed] [Google Scholar]
- 31.Bostanci MO, Bas O, Bagirici F. Alpha-tocopherol decreases iron-induced hippocampal and nigral neuron loss. Cell Mol Neurobiol 2010;30:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida Y, Ito S, Ohtsuki S, et al. Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem 2009;284:33400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada K, Tanaka T, Han D, Senzaki K, Kameyama T, Nabeshima T. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1–42)-induced learning and memory deficits in rats: implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. Eur J Neurosci 1999; 11:83–90. [DOI] [PubMed] [Google Scholar]
- 34.Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr 2018;107: 389–404. [DOI] [PubMed] [Google Scholar]
- 35.McBean L, O’Reilly S. Diet quality interventions to prevent neurocognitive decline: a systematic review and meta-analysis. Eur J Clin Nutr 2022;76:1060–72. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs DM, Ard MC, Salmon DP, Galasko DR, Bondi MW, Edland SD. Potential implications of practice effects in Alzheimer’s disease prevention trials. Alzheimers Dement (N Y) 2017;3:531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci 2016;1367:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


