Abstract
Elevated exposure to multiple trace metals can be neurotoxic even at relatively low levels. These findings are primarily evident from adult occupational studies as well as in children exposed prenatally or in early childhood. Less research has focused on the neurodevelopmental impacts of exposure to metals among school-aged children.
We examined associations between exposure to a mixture of four metals (arsenic, cadmium, manganese, lead) measured in hair and markers of cognition, attention, and behavior among 222 6–12 year old children who participated in a 2009–2010 neurodevelopmental follow-up to the C8 Health Project. Using quantile-based g-computation we estimated the adjusted overall metal mixture effect ψ (95% CI) as the change in outcome per decile increase in all metals in the mixture.
Hair metal levels varied by metal, with cadmium being lowest (median 0.007, interquartile range (IQR) 0.013 μg/g) and lead the highest concentration (median 0.152, IQR 0.252 μg/g). Children’s cognitive skills and development, attention/impulsivity, and behavior were all close to standardized population means. Each decile increase in all metals was associated with a Full Scale IQ reduction of 1.01 points (95% confidence interval (CI) −1.88, −0.15) and Verbal IQ reduction of 1.11 points (95% CI −1.97, −0.25), adjusted for child age, sex, secondhand smoke exposure, HOME score, maternal education, maternal IQ, and examiner. Maternal report of ADHD-like behaviors and executive functioning also showed adverse associations with the metal mixture.
Our findings suggest that similar to exposure during prenatal and early childhood periods, recent exposure to metals during middle childhood is associated with adverse neurodevelopmental consequences. Middle childhood may also be a developmental window of susceptibility to the negative consequences of exposure to environmental neurotoxicants.
Keywords: Metal mixtures, School Age Population, Neuropsychological Tests, Child Behavior
1. INTRODUCTION
Exposure to multiple trace metals can be neurotoxic even at low levels (Rodríguez-Barranco et al., 2013). Lead is harmful at any exposure level (2016; Lin-Fu, 1973; Prevention, 2012; Rabin, 1989; RUDDOCK, 1924). Manganese is an essential trace element, but toxic as exposure increases ((ATSDR), 2012). The neurotoxicity of arsenic (Vahidnia et al., 2007) and cadmium (Gonçalves et al., 2021) are also of well-established concern. These findings are primarily evident from adult occupational studies (Bowler et al., 2006; Lucchini et al., 1999; Roels et al., 1987), as well as in children exposed prenatally or in early childhood (Claus Henn et al., 2017; Freire et al., 2018; Leonhard et al., 2019; Muñoz-Rocha et al., 2018; Sanders et al., 2015).
Less is known about the neurodevelopmental impacts of exposure to metals among school-aged children, despite recognition of this age range as an important life stage to consider when assessing childhood exposures to environmental contaminants and adverse health outcomes (United States. Environmental Protection Agency. Risk Assessment Forum., 2005). This gap in knowledge is particularly concerning because studies have noted that childhood metal exposures are associated with adverse neurodevelopmental outcomes, sometimes even more strongly than prenatal or early life exposures (Lanphear et al., 2005; Mazumdar et al., 2011; Tellez-Rojo et al., 2006).
From a life stage exposure perspective, children 6–10 years start spending time in school environments and playing sports; beginning at ~11 years there is an increased rate of food consumption, increased risk of smoking, and more time away from home (United States. Environmental Protection Agency. Risk Assessment Forum., 2005). From a developmental perspective, middle childhood, colloquially referred to as 6–12 years of age, is a period of rapid neuro-adaptation (Mah and Ford-Jones, 2012). Synaptic pruning likely increases efficiency of processing in some brain regions (Giedd et al., 1999). Neurons responsible for cognition, language and social skills are consolidated (Szaflarski et al., 2006), and children gain cognitive control with an increasing ability to respond selectively to stimuli and can begin to process and understand material effectively (Carey, 2009). Emerging evidence indicates that for some types of adverse health outcomes the strongest impacts may be tied to specific windows of heightened plasticity that occur later, rather than earlier, in childhood development (Andersen et al., 2008; Pechtel et al., 2014; Teicher et al., 2018). Middle childhood, considered a sensitive period because of the active role that environment and experiences play in brain development (Knudsen, 2004), may be a window of susceptibility for developmental neurotoxicants like metals.
Humans are exposed to metals through diet/ingestion, air and soil pollution, and from industrial sites (Landrigan et al., 2018), resulting in concomitant exposure to numerous metals. Interrogating the health effects of one metal at a time may miss interactions among metals (von Stackelberg et al., 2015), and importantly does not reflect real world experience of multiple exposures and exposure mixtures.
We examined cross-sectional associations between exposure to a mixture of four metals (arsenic, cadmium, manganese, lead) measured in hair and markers of cognition, attention, and behavior among 222 6–12 year old children who participated in a neurodevelopmental follow-up to the C8 Health Project. The C8 Health Project was a study in the Mid-Ohio Valley to examine the health effects of exposure to perfluorooctanoate (PFOA) through contaminated drinking water (Frisbee et al., 2009). This industrial region of the Mid-Ohio Valley also experienced elevated exposure to metals. The U.S. Agency for Toxic Substances and Disease Registry (ATSDR) Health Consultation Marietta Area Air Investigation (Services and Registry, 2009) reported that levels of arsenic, cadmium, and manganese in this region exceeded ATSDR and U.S. Environmental Protection Agency (EPA) health-based comparison values at all monitoring locations during their 2007 – 2008 investigation.
2. METHODS
2.1. Study Population
The C8 Health Project enrolled 69,030 people from 2005–2006 and has been described in detail previously (Frisbee et al., 2009). From 2009–2010 we conducted a follow-up to the C8 Health Project to assess neuropsychological functioning among 6–12 year old children (Stein et al., 2013, 2014); 322 children and their biological mothers (73% of the known eligible) participated in the follow-up study. During the mother’s interview we requested contact information for the child’s teacher and 188 (60% of requested) teachers completed surveys. There was no difference in C8 Health Project participants between those who enrolled and did not enroll in the neuropsychological follow-up study by age (p=0.79), gender (p=0.32), or maternal smoking (p=0.99).
As part of the follow-up study, we collected hair samples for measurement of metals (Jursa et al., 2018). We collected two cm of hair closest to the scalp, which represents exposures from approximately two months prior to sampling (Smolders et al., 2009). Hair samples were collected concurrent to outcome assessments. From all children participating in the follow-up, hair metals data were available for 222 subjects (69% of follow-up study; Supplemental Figure 1). Subjects missing metals data occurred because the child or mother declined to allow the child’s hair be sampled (n=3), the child had insufficient hair to be sampled (n=55), or the sampled hair was insufficient for analysis (n=41). Compared to all children enrolled in the neurodevelopment follow-up study, the sub-sample with metals data differed by gender (p<0.001; Supplemental Table 1) because girls were more likely to have sufficient hair for sampling and analysis. Other characteristics differing significantly between children with and without hair metals data were maternal education (lower proportion of college graduates among those without metals data; p=0.03), NEPSY Design Copying Total scaled score (worse scores among those without metals data; p=0.02), and the Clinical Confidence Index from Conners’ Continuous Performance Test (less resemblance to ADHD canonical profile among those without metals data; p<0.001).
Mothers provided informed consent and children provided verbal assent. The Mount Sinai Program for the Protection of Human Subjects and the Battelle Centers for Public Health Research & Evaluation Institutional Review Board approved all study procedures; statistical analyses of de-identified data were categorized as exempt by the NYU Langone Institutional Review Board.
2.2. Exposure Assessment
Hair samples were collected proximal to the occipital lobe scalp and stored in zip top plastic bags at room temperature until analysis of a two cm segment of hair proximal to the scalp (Jursa et al., 2018). All cleaning and processing of hair samples was conducted in a HEPA filtered-air trace metal clean room using acid-cleaned labware and ultrapure trace metal grade reagents. Individual hair segments/samples weighing 5–30 mg each were cleaned of exogenous metal contamination as described previously (Eastman et al., 2013). Briefly, samples were placed in acid-cleaned 5 mL polypropylene syringe tubes and sonicated (20 minutes) in 0.5% Triton, rinsed five-times with ultrapure Milli-Q water, sonicated (10 minutes) in 1 N trace metal grade nitric acid (Fisher Scientific, Santa Clara, CA, USA), rinsed with 1 N nitric acid, rinsed five-times with Milli-Q water, and then dried at 65 C for 48 hours. Subsequently, samples were digested in 0.5 mL 15.7 N quartz-distilled nitric acid (Fisher Scientific, optima grade) at 80 C for 6 hours in a Class-100 HEPA filtered-air fume hood, and then diluted with 5 mL Milli-Q water. Typical analytical limits of detection (LOD) over five analytical runs were 0.0018, 0.0004, 0.0038, and 0.0077 ng/mL for arsenic, cadmium, lead, and manganese, respectively. For metal levels below the analytical LOD, the LOD was multiplied by 0.5 and adjusted using the sample dilution factor and sample weight of processed hair to derive a value for half the procedural detection limit; this value was used for statistical analyses. Standard reference material NIES 13 (human hair) was used to assess analytical accuracy; recoveries are means and standard deviations based on 17 replicates over five analytical runs. Arsenic: reference 0.10 μg/g, recovered 0.098 ± 0.008 μg/g (98%). Cadmium: certified 0.23 ± 0.03 μg/g, recovered 0.225 ± 0.015 μg/g (98%). Lead: certified 4.6 ± 0.4 μg/g, recovered 4.65 ± 0.47 μg/g (101%). Manganese: reference 3.9 μg/g, recovered 2.92 ± 0.42 μg/g (75%). Arsenic measurements were not speciated because of insufficient volume of hair and insufficient evidence demonstrating the utility of arsenic speciation in hair, in contrast to speciation in urine and blood.
2.3. Outcome Assessment
To collect information pertinent to clinically relevant behaviors, we selected a battery of tests that assess skills predictive of a child’s ability to make adequate progress in school. Outcome measures included direct assessment of the child and reports from mothers and teachers. Examined domains included cognitive skills and development; attention/impulsivity; and behavior. Eight examiners blinded to participants’ exposure histories were trained and certified (by D.C.B.) to administer the child assessments.
2.3.1. Cognitive Skills and Development
The Wechsler Abbreviated Scale of Intelligence (WASI) consists of four subtests similar to subtests of the Wechsler Intelligence Scale for Children—Fourth Edition (Corporation, 1999). These two verbal (Vocabulary, Similarities) and two nonverbal (Block Design, Matrix Reasoning) subtests have the highest loadings on general intellectual function. The tests are normed and provide age-standardized scores for Full Scale Intelligence Quotient (IQ), Verbal IQ, and Performance IQ. IQ scores range from 50 to 160 with mean = 100 and standard deviation (SD) = 15; higher scores reflect better performance.
We used three subtests from the Wechsler Individual Achievement Test—II (WIAT) to assess academic skills (Corporation, 2002). Two subtests measure word attack and decoding skills (Word Attack, Pseudoword Decoding); we averaged the standardized scores from these two subtests to create a composite measure of reading fluency. The third subtest measures arithmetic capabilities (Numerical Operations). Age-standardized scores range from 40 to 160 with mean = 100 and SD = 15; higher scores reflect stronger academic skills.
The NEPSY-II is a neuropsychologically-based instrument designed to test specific brain-behavior relationships and identify markers of atypical cognitive development (Korkman et al., 2007). We focused on three domains: Language (Comprehension of Instructions, Word Generation), Memory and Learning (Narrative Memory), and Visual-spatial Processing (Design Copying). Visual-spatial processing involves the combination of fine motor skills and spatial abilities, plus requires executive functioning; impairment in this domain may impact ability to complete school work. We averaged the Semantic and Initial Letter scaled scores from the Word Generation subtest. Age-standardized scaled scores range from 1 to 19 with mean = 10 and SD = 3; higher scores indicate better performance.
2.3.2. Attention/Impulsivity
The Conners’ Continuous Performance Test-II (CPT) is a computer-administered test that measures sustained attention and impulsivity (Conners, 2004). The child is instructed to push the spacebar every time a letter appears on the screen except when the letter is “X.” The Clinical Confidence Index measures the extent to which the child’s overall profile resembles the canonical profile of children with ADHD. The Clinical Confidence Index is a raw score ranging from 0 to 100; a higher score indicates greater resemblance to the canonical profile of children with ADHD. We also analyzed age-standardized T-scores for Omission Errors (no response after non-X), Commission Errors (response after X), and Hit Reaction Time (mean response time in milliseconds). T-scores have mean = 50 and SD = 10; lower scores indicate fewer ADHD-like behaviors.
Mother and teachers completed the Conners’ ADHD DSM-IV Scales-Revised (CADS) to report on ADHD-like behaviors (Conners, 2001). The ADHD Index score is based on responses to 12 items that best discriminate children who have been diagnosed with ADHD from those who have not. There are also three DSM-IV Symptoms subscales: Inattentive, Hyperactive-Impulsive, and Combined. Age-standardized T-scores have mean = 50 and SD = 10; lower scores indicate fewer ADHD-like behaviors.
2.3.3. Behavior
The Behavior Rating Inventory of Executive Function (BRIEF), completed by mothers and teachers, assesses executive function (Gioia, 2000). Executive function helps guide, direct, and manage cognition, emotion, and behavior. The BRIEF has eight clinical scales; we focused on three measures. The Global Executive Composite is a summary score that incorporates all eight clinical scales. The Behavioral Regulation Index focuses on inhibitory control – the ability to manage transitions and regulate emotions. The Metacognition Index reflects the ability to plan, organize, and monitor performance. To ensure validity, as recommended in the manual, we excluded mother (n=1) and teacher (n=1) reports with negativity scores greater than 6 or inconsistency scores greater than 8. The Negativity scale measures the extent to which the respondent answered selected BRIEF items in an unusually negative manner relative to the normed sample. Similarly, the inconsistency scale indicates the extent to which the respondent answered similar BRIEF items in an inconsistent manner. Age-standardized T-scores have mean = 50 and standard deviation (SD) = 10; higher scores represent greater difficulties with regard to executive functioning.
The Behavior Assessment System for Children-2 (BASC-2), completed by mothers and teachers, evaluates child personality, behavioral problems, and emotional disturbances (Reynolds and Kamphaus, 2004). We focus on the Behavioral Symptom Index and three composite scales: Adaptive Skills, Internalizing Problems, and Externalizing Problems. To ensure validity, as recommended in the manual, we excluded mother reports (n=6) with F-index (negativity scale) greater than 6, Pattern Response Index greater than 12, or Consistency Index greater than 17, as well as teacher reports (n=4) with F-index greater than 3, Pattern Response Index greater than 11, or Consistency Index greater than 15. The Pattern Response Index detects repeated and cyclical response patterns. Age-standardized T-scores have mean = 50 and SD = 10. For Adaptive Skills higher scores indicate better adaptive skills; for the Behavioral Symptom Index, Internalizing, and Externalizing Problems, lower scores indicate fewer problematic behaviors.
2.4. Covariate Assessment
We interviewed mothers to collect information to address confounding. The Home Observation for Measurement of the Environment—Short Form Mother Supplement (HOME) measures the quality and extent of stimulation in the home based on maternal report (Surveys). Maternal IQ was measured using the Wechsler Abbreviated Scale of Intelligence (Corporation, 1999). Mothers also completed an extensive interview to solicit data on factors that may have affected their child’s neurobehavioral development, such as pregnancy and delivery complications and presence of co-morbid health conditions in the child.
2.5. Statistical Analysis
We used quantile-based g-computation (QG-comp) (Keil et al., 2020) to estimate the overall effect of childhood hair metal mixture levels with neurodevelopmental and behavioral outcomes. QG-comp estimates the change in outcome per quantile increase in all metals in the mixture and the relative contribution of each component to that direction (positive or negative). The estimated overall mixture effect (ψ) can be interpreted as the change in outcome per quantile increase in all exposures together. We present results from deciles for ease of interpretation; specification of other quantiles produced very similar findings. The component weights are essentially the metal-specific beta divided by the total mixture effect across all metals acting in the same direction. Comparing the component weights for metals acting in the same direction can indicate which metals are driving the association between mixture and outcome. Additionally, we used Bayesian Kernel Machine Regression (BKMR) models (Bobb et al., 2018; Bobb et al., 2015) to assess potential non-linearity and interactions between metals as well as to corroborate the overall mixture results observed from QG-comp. For BKMR models, we scaled exposures and outcomes and specified Gaussian distributions and performed 50,000 iterations.
Although there was minimal missing data for any single variable (Supplemental Table 2), the cumulative missing-ness would have reduced our sample size and potentially introduced bias. To address this, we created 10 imputed datasets using the “mice” package in R (van Buuren and Groothuis-Oudshoorn, 2011) and pooled the QG-comp and linear regression estimates from each model using Rubin’s Rule (Rubin, 2004). To calculate the weights from QG-comp, we averaged across the 10 models from the imputed datasets. BKMR models were fitted using only complete case data. Missing data for teacher ratings were not imputed.
All multivariable models were adjusted for the same set of covariates based on a priori decisions or a covariate’s association with an exposure and outcome. PFOA did not meet confounding criteria (Rothman and Greenland, 1998) for this study of metal exposure (i.e., null associations between PFOA and metals (Stein et al., 2013, 2014)) so was not included as a covariate. Additionally, we had no hypothesis for why PFOA and these metals would have a synergistic impact on neurodevelopment. Analyses adjusted for child age at assessment (continuous); sex (female, male); secondhand smoke exposure from birth to 2 years of age (yes, no); cognitive and emotional HOME scores (continuous); maternal education (less than high school graduate, high school graduate, some college, college graduate); maternal IQ (continuous); and examiner (for WASI, WIAT, NEPSY, CPT outcomes only). Additionally, we ran QG-comp models stratified by sex to explore the possibility of sex-specific effects of metals on cognition and development. Lastly, to complement our mixture analysis and for comparison with previous publications, we also used linear regression models to estimate the relationships between each individual metal and subtest scores. In linear regression models, all metal biomarkers were log2-transformed and the resulting estimates are interpreted as the change in outcome per doubling of metal concentration.
3. RESULTS
Most of the 222 children included in the hair metal analyses were non-Hispanic white (95.9%) and female (76.1%), with an average age of 9.8 (SD 1.7) years (Table 1). Mothers reported that 9.5% of children had ever been diagnosed with ADHD. The plurality of mothers had some college. Mean measures of children’s cognitive skills and development, attention/impulsivity, and behavior were all close to the standardized population means. Hair metal concentration varied by metal (Table 2); cadmium was measured at the lowest concentration (median 0.007, interquartile range (IQR) 0.013 μg/g) and lead at the highest (median 0.152, IQR 0.252 μg/g). The four metals were moderately correlated (Supplemental Figure 2).
Table 1.
Characteristics of children aged 6–12 years with hair metal measurements, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009–2010 (n = 222)
| Characteristic | N | Percent or Mean | Standard Deviation |
|---|---|---|---|
| Child age, years | 222 | 9.8 | 1.7 |
| Child sex | |||
| Male | 53 | 23.9 | |
| Female | 169 | 76.1 | |
| Secondhand smoke for age 0–2 years | |||
| Yes | 41 | 18.0 | |
| No | 181 | 82.0 | |
| HOME score, maternal section only | |||
| Cognitive scale | 222 | 6.9 | 1.7 |
| Emotional scale | 222 | 9.8 | 1.5 |
| Maternal age, years | 221 | 38.6 | 6.0 |
| Maternal education | |||
| Less than high school | 10 | 4.5 | |
| High school graduate | 64 | 28.8 | |
| Some college | 87 | 39.2 | |
| College graduate | 61 | 27.5 | |
| Maternal Full Scale IQ (M 100, SD 15) ▲ | 221 | 99.4 | 12.6 |
| Child Neuropsychological Measures | |||
| WASI (M 100, SD 15) ▲ | |||
| Full Scale IQ | 220 | 101.9 | 12.9 |
| Verbal IQ | 221 | 103.2 | 13.1 |
| Performance IQ | 220 | 99.5 | 13.0 |
| WIAT (M 100, SD 15) ▲ | |||
| Word Reading/Pseudoword Decoding standard score mean | 212 | 102.4 | 11.1 |
| Numerical Operations standard score | 221 | 97.8 | 14.3 |
| NEPSY (M 10, SD 3) ▲ | |||
| Comprehension of Instructions scaled score | 221 | 10.5 | 2.9 |
| Design Copying Total scaled score | 209 | 8.6 | 3.4 |
| Narrative Memory Free and Cued Recall scaled score | 221 | 9.3 | 2.9 |
| Semantic/Initial Letter scaled score mean | 209 | 9.7 | 2.5 |
| CPT (M 50, SD 10) ▼ | |||
| Clinical Confidence Index | 215 | 47.8 | 23.5 |
| Omissions T-score | 215 | 53.6 | 12.3 |
| Commissions T-score | 215 | 56.3 | 8.2 |
| Hit Reaction Time T-score | 215 | 47.5 | 11.0 |
| CADS, mother report (M 50, SD 10) ▼ | |||
| ADHD Index | 221 | 54.1 | 11.6 |
| Combined Type | 221 | 53.9 | 10.6 |
| Inattentive Type | 221 | 52.3 | 10.7 |
| Hyperactive-Impulsive Type | 221 | 55.3 | 10.7 |
| CADS, teacher report (M 50, SD 10) ▼ | |||
| ADHD Index | 134 | 53.5 | 12.1 |
| Combined Type | 134 | 51.9 | 11.5 |
| Inattentive Type | 134 | 48.2 | 9.2 |
| Hyperactive-Impulsive Type | 134 | 51.7 | 11.5 |
| BRIEF, mother report (M 50, SD 10) ▼ | |||
| Global Executive Composite | 219 | 50.5 | 11.3 |
| Behavioral Regulation Index | 220 | 49.6 | 10.9 |
| Metacognition Index | 219 | 50.9 | 11.3 |
| BRIEF, teacher report (M 50, SD 10) ▼ | |||
| Global Executive Composite | 134 | 56.2 | 14.2 |
| Behavioral Regulation Index | 134 | 54.0 | 13.3 |
| Metacognition Index | 134 | 56.7 | 14.7 |
| BASC-2, mother report (M 50, SD 10) ▼ | |||
| Behavioral Symptom Index | 214 | 50.2 | 10.2 |
| Adaptive Skills (▲) | 214 | 50.5 | 10.0 |
| Internalizing Problems | 214 | 52.3 | 11.8 |
| Externalizing Problems | 214 | 50.3 | 9.4 |
| BASC-2, teacher report (M 50, SD 10) ▼ | |||
| Behavioral Symptom Index | 104 | 48.9 | 8.8 |
| Adaptive Skills (▲) | 104 | 51.9 | 9.9 |
| Internalizing Problems | 104 | 51.3 | 11.2 |
| Externalizing Problems | 104 | 47.8 | 7.2 |
M, mean; SD, standard deviation
higher score reflects better/more favorable performance
lower score reflects better/more favorable performance
Table 2.
Hair metal concentrations (μg/g) among children ages 6–12 years, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009–2010 (n = 222)
| N | LOD | Minimum | Mean | SD | Median | Interquartile Range | 95th-tile | Maximum | |
|---|---|---|---|---|---|---|---|---|---|
| Arsenic | 215 | 0.0018 | 0.0041 | 0.045 | 0.206 | 0.018 | 0.020 | 0.098 | 2.964 |
| Cadmium | 221 | 0.0004 | 0.0003 | 0.026 | 0.084 | 0.007 | 0.013 | 0.113 | 1.040 |
| Manganese | 222 | 0.0038 | 0.0025 | 0.237 | 0.441 | 0.109 | 0.215 | 0.696 | 4.100 |
| Lead | 221 | 0.0077 | 0.0080 | 0.380 | 0.829 | 0.152 | 0.252 | 1.330 | 7.726 |
LOD, limit of detection
3.1. Cognitive Skills and Development
Using QG-comp, a simultaneous increase in all four metal mixture components was associated with worse IQ scores on the WASI. Each decile increase in all metals was associated with a Full Scale IQ reduction of 1.01 points (95% confidence interval (CI) −1.88, −0.15) and a Verbal IQ reduction of 1.11 points (95% CI −1.97, −0.25), adjusted for child age, sex, secondhand smoke exposure, HOME score, maternal education, maternal IQ, and examiner (Figure 1, Supplemental Table 3). Performance IQ (–0.48, 95% CI −1.35, 0.39) was not associated with the metals mixture. Based on mixture component weights, manganese and lead were driving the associations with IQ (Figure 2). In adjusted BKMR models, when all metals were at their respective 75th percentiles compared to their respective 25th percentiles, there was a reduction in Full Scale IQ of 0.15 SD, or 1.5 points (Supplemental Figure 3). There was no evidence of interactions among metals in relation to any scale on the WASI, although manganese exhibited non-monotonic and non-linear associations (data not shown), which reflects manganese’s dual role as an essential element that is toxic at higher concentrations.
Figure 1.
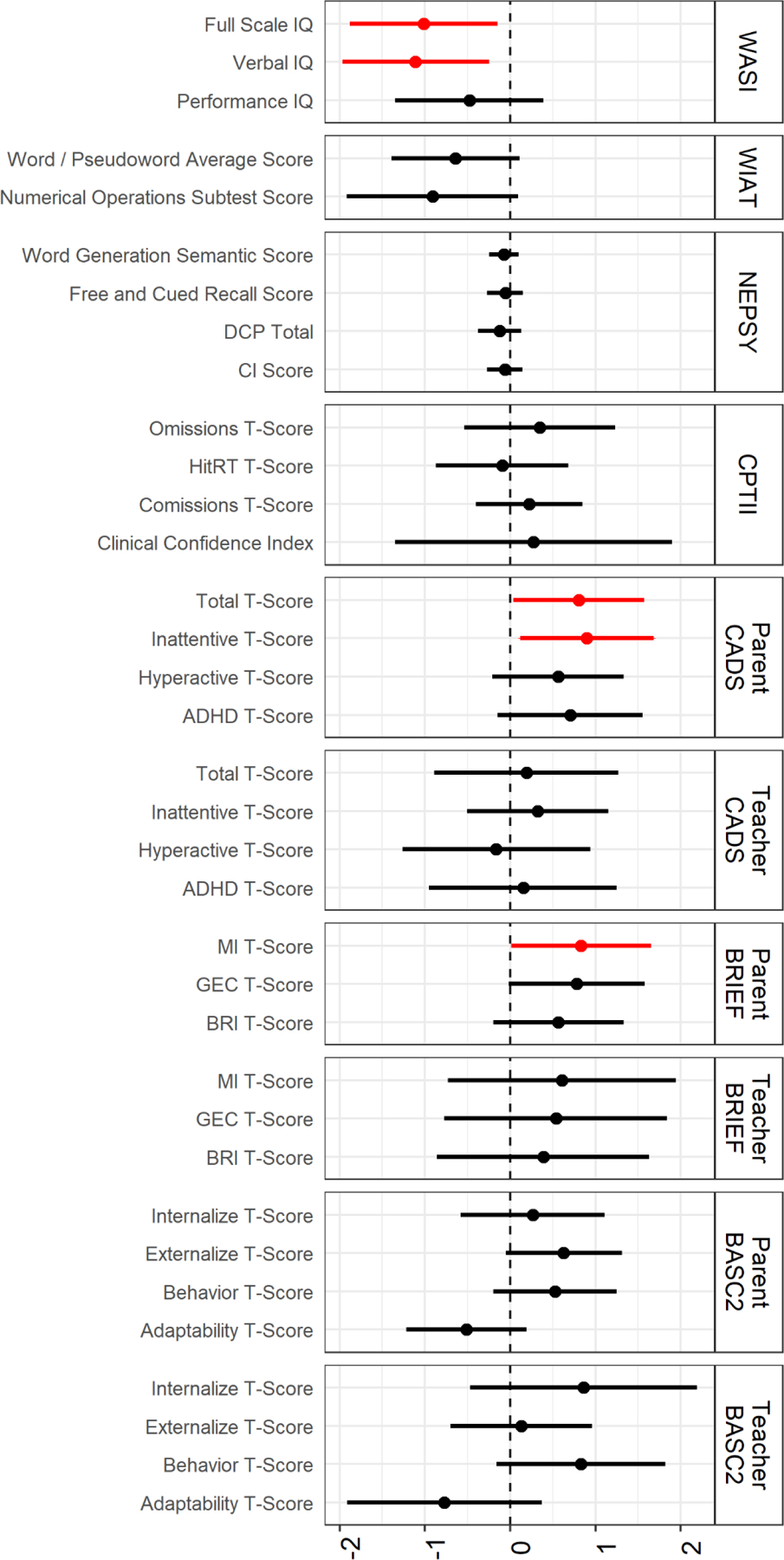
Estimated adjusted change (ψ; 95% confidence interval) in neuropsychological measure per decile increase in all metals. Children aged 6–12 years, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009–2010 (n = 222). Effect estimates from Quantile G-Computation are interpreted as change in outcome per decile increase in all metals (arsenic, cadmium, manganese, lead). Adjusted for child age, sex, secondhand smoke exposure from birth to 2 years of age, HOME score (cognitive and emotional), maternal education, maternal IQ, and child examiner (WASI, WIAT, NEPSY, CPT).
Figure 2.
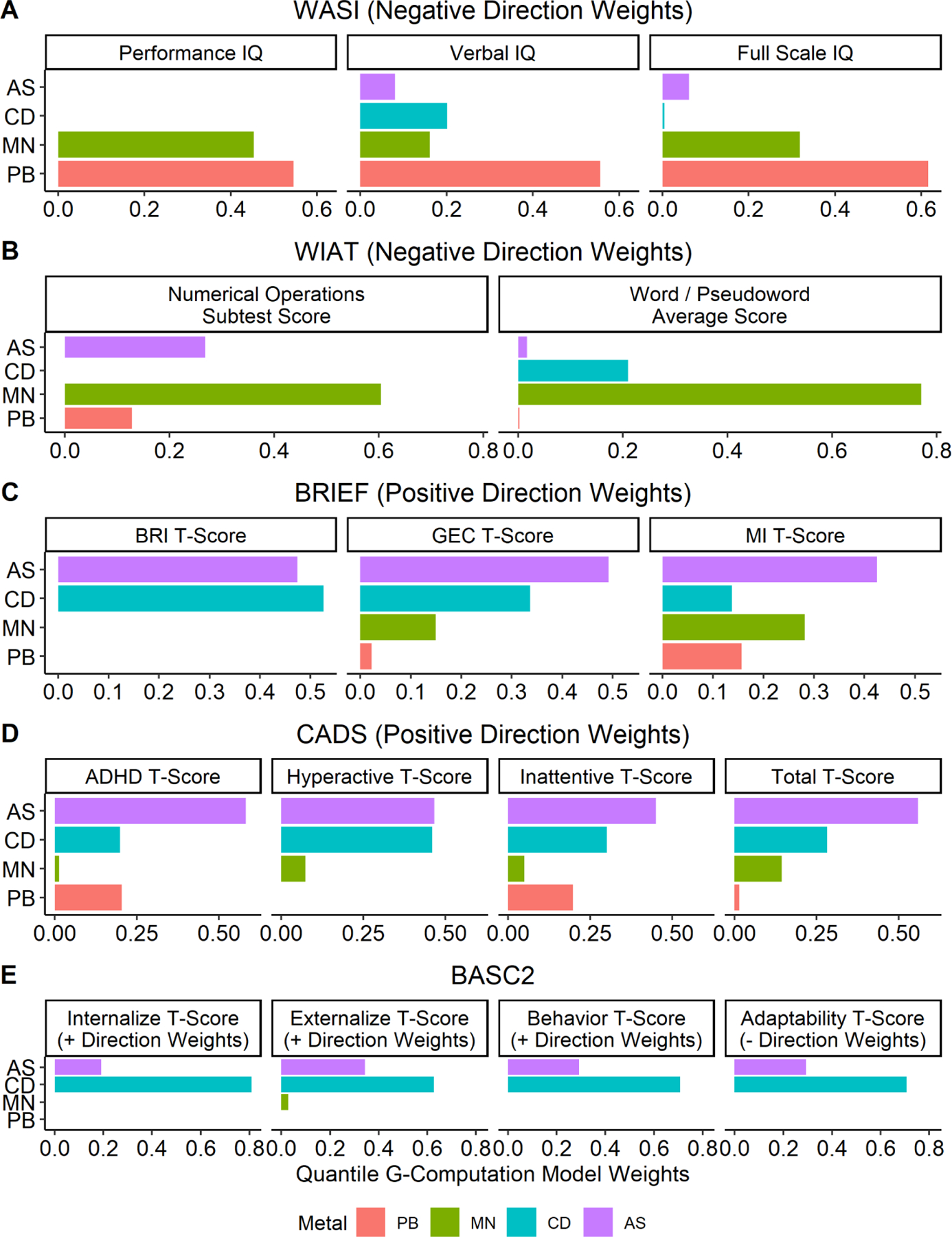
Estimated weights from Quantile G-Computation indicating contribution of each metal to adjusted association between hair metal mixture and neuropsychological measure averaged across 10 imputed datasets. Children aged 6–12 years, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009–2010 (n = 222). All models adjusted for child age, sex, secondhand smoke exposure from birth to 2 years of age, HOME score (cognitive and emotional), maternal education, maternal IQ, and child examiner (WASI, WIAT, NEPSY, CPT). Only weights for the direction of the mixture effect are shown. For example, the metal mixture is negatively associated with WASI scores so only weights from the negative direction are presented. Note: BASC-2 values are for parent report.
While there was a pattern of worse reading fluency and arithmetic skills on the WIAT in relation to a decile increase in all metals, the magnitude of the effect was small in relation to the SD (Figure 1, Supplemental Table 3). To the extent that there was an adverse association between metal mixture and academic skills manganese again appeared to be the key component (Figure 2). BKMR models exhibited similar patterns of small, negative, non-significant associations (Supplemental Figure 3), with no evidence of interactions or non-linearity among metals on the WIAT (data not shown).
Across QG-COMP and BKMR models there was no evidence of an association between the metal mixture and performance on the NEPSY (Figure 1, Supplemental Table 3, Supplemental Figure 3).
3.2. Attention/Impulsivity
In direct assessment of the children using Conners’ Continuous Performance Test-II, there were no associations between the metal mixture and measures of attention or impulsivity across QG-comp and BKMR models (Figure 1, Supplemental Table 3, Supplemental Figure 3). Using the CADS, however, mother report on child’s ADHD-like behavior showed worse scores on Inattentive (0.90, 95% CI 0.12, 1.68) and Combined (0.80, 95% CI 0.04, 1.57) scales per decile increase in all metals, adjusted for child age, sex, secondhand smoke exposure, HOME score, maternal education, and maternal IQ (Figure 1, Supplemental Table 3). These findings were primarily driven by arsenic and cadmium, with lead also a factor for the Inattentive scale (Figure 2). BKMR models exhibited comparable patterns (Supplemental Figure 3), with no indication of interactions or non-linearity (data not shown). Teacher report on the CADS showed null associations between the metal mixture and child ADHD-like behaviors across QG-comp and BKMR models (Figure 1, Supplemental Table 3, Supplemental Figure 3).
3.3. Behavior
Using the BRIEF, metal mixture exposure was associated with less favorable child executive functioning by maternal, but not teacher report. A decile increase in all metals was associated with an increase in the Global Executive Composite of 0.78 (95% CI −0.02, 1.58) points and an increase in the Metacognition Index of 0.83 (95% CI 0.01, 1.65) points, adjusted for child age, sex, secondhand smoke exposure, HOME score, maternal education, and maternal IQ (Figure 1, Supplemental Table 3). Arsenic and cadmium were the key components of the change in Global Executive Composite; arsenic and manganese were the key components of the change in Metacognition Index (Figure 2). BKMR models exhibited comparable patterns (Supplemental Figure 3), with no indication of interactions although there was a non-linear, non-monotonic association between cadmium and the BRIEF scales (data not shown), which indicates lack of a dose-response relationship.
On the BASC-2, the metal mixture was in the direction of worse Adaptive Skills for on both mother and teacher reports and more Externalizing Problems based on mother report (Figure 1, Supplemental Table 3). In QG-comp models, the magnitude of the decrement in Adaptive Skills, while not statistically significant, was comparable across mother and teacher report. BKMR models exhibited similar patterns (Supplemental Figure 3), with no indication of interactions or non-linearity (data not shown).
In sex-stratified analyses (Supplemental Figure 4), the adverse associations between metal mixture and IQ were more pronounced among females compared to males and the associations with executive function were observed exclusively among males. An examination of results from adjusted linear regression models estimating associations between individual metals and neurodevelopmental and behavioral outcomes (Supplemental Figure 5) did not alter the interpretation of results from the mixture models.
4. DISCUSSION
In this cross-sectional study of 6–12 year old children, greater exposure to a mixture of four metals measured in hair was associated with a decrease in Full Scale and Verbal IQ, increase in maternal report of ADHD-like behaviors, increase in maternal report of executive dysfunction, and reduced adaptive skills based on both mother and teacher report. However, exposure was not associated to any notable degree with Performance IQ, atypical cognitive development, or direct assessment of attention and impulsivity. We did observe some sex-specific associations with both IQ and behavior, although only 24% of the sample was comprised of males. The magnitudes of these adverse associations between childhood hair metal mixture exposure and neurobehavioral development may be small for a decile increase in all metals, but could impart meaningful decrements across exposure ranges at the population level. Also, estimates from metal mixture and individual metal models were similar. These results indicate that school-aged children may be susceptible to the neurotoxic effects of recent joint exposure to multiple metals. This finding is important because middle childhood is a period of rapid neuro-adaption when cognition, language, and social skills are consolidated and gains in cognitive control permit the effective processing and understanding of material. Disruptions to these processes may lead to long-term negative consequences in academic achievement and social-emotional development.
Despite numerous studies showing adverse associations between prenatal and/or early childhood exposure to select metals and neurobehavioral development, few studies provide a direct comparison when considering the specific metal mixture (arsenic, cadmium, manganese, lead) and age range (6–12 year olds) of this analysis. A 2013 systematic review and meta-analysis evaluated associations of pre or postnatal exposure to arsenic, cadmium and manganese with neurodevelopment and behavioral disorders in children through 16 years of age (Rodríguez-Barranco et al., 2013). In meta-analysis, a 50% increase in urinary arsenic concentration was associated with a −0.39 point decrease (95% CI −0.84, 0.06) in Full Scale IQ, but not behavior. There were too few studies to conduct meta-analyses for cadmium; separate study results were mixed with only some studies reporting associations between cadmium exposure and lower IQ or higher frequency of attention problems. Manganese, however, showed consistent negative associations with IQ. In meta-analysis, a 50% increase in hair manganese concentration was associated with a −0.70 point decrease (95% CI −1.07, −0.34) in Full Scale IQ, −1.26 point decrease (95% CI −2.20, −0.33) in Verbal IQ, and −0.42 point decrease (−0.82, −0.02) in Performance IQ. Manganese was also consistently associated with more behavioral problems. Our study showed IQ decrements of similar magnitude, but with a 10% increase in components in a mixture of four metals rather than a 50% increase in a single metal, underscoring the need to examine exposures in mixtures as they occur in real world situations.
A more recent review of the association between metals and neurodevelopment through a mean age of 8 years focused on susceptibility factors, including metal co-exposures (Bauer et al., 2020b). While there are mixed findings, adverse interactive associations with neurodevelopment were most frequently observed between lead and manganese (Al-Saleh et al., 2019; Frndak et al., 2019; Menezes-Filho et al., 2018), lead and arsenic (Freire et al., 2018), and lead and cadmium (Levin-Schwartz et al., 2019). Importantly, the review noted the limited availability of research on co-exposures, particularly research employing statistical mixture methods to investigate joint exposure, which is a strength of our study.
There are limited benchmark values for hair metal concentrations. ATSDR suggests arsenic concentrations in hair should not exceed 1 μg/g ((ATSDR), 2007), which is higher than our 95th percentile, but lower than our maximum value. Studies among children in neighboring regions in Ohio more directly impacted by manganese pollution reported geometric mean (SD) hair metal concentrations of 0.4165 μg/g (0.0024) (Haynes et al., 2015) and 0.3602 μg/g (0.0022) (Haynes et al., 2018), compared to 0.1194 μg/g (0.0089) in our population, although direct comparison of hair metal levels between studies is challenging due to differences in hair cleaning and analysis methods (Eastman et al., 2013; Jursa et al., 2018). In an Italian study of adolescents living in regions with current and historic ferroalloy activity, the median concentration of hair manganese was 0.08 μg/g (interquartile range 0.10) (Bauer et al., 2020a), which is close to the median in our study (both studies used identical hair cleaning and analysis methods). Overall, hair metal levels in our study population are generally lower than other studies in children, likely reflecting differences both in endogenous metal exposure and incorporation of metals from the circulation into hair as well as different hair cleaning methodologies (Jursa et al., 2018).
A key strength of this study is the inclusion of a direct assessment of the child as well as reports from mothers and teachers. We know, though, that different informants differentially report on child behavior, with parents typically reporting more problems than teachers (Briggs-Gowan, 1996; Touliatos, 1981). While parents observe their child’s behavior across a range of situations and teachers primarily observe behavior only in the classroom, parent report of child behavior is colored by their emotional connection to the child as well as their own mental health (Briggs-Gowan, 1996; Phares, 1989). Teachers, however, are able to gauge a child’s behavior in relation to the child’s peers, and comment on difficulties related to academic or social success that parents may never see. Reports on boys tend to have more agreement across raters than do reports on girls (Briggs-Gowan, 1996; Touliatos, 1981). Consequently, the concordance between mother and teacher reports on the BASC-2 Adaptive Skills scale is notable.
Associations between environmental exposures and worse adaptive skills have been observed previously. The New Hampshire Birth Cohort Study reported an association between prenatal exposure to manganese in a mixture of six metals and worse Adaptive Skills scores based on maternal report of preschool-aged children (Doherty et al., 2020). The HOME study found evidence of associations between blood lead concentration and worse Adaptive Skills among females, but not males, at age 8 years (Sears et al., 2021). PROGRESS found that higher concentrations of airborne PM2.5 during the first trimester of pregnancy were associated with worse Adaptive Skills by mother or caregiver report at ages 4–6 years (McGuinn et al., 2020). The New Bedford Cohort reported associations between increased concentrations of phthalates and worse teacher-reported Adaptive Skills among adolescents (Shoaff et al., 2019).
This study’s greatest limitation is its cross-sectional design, reliant on measures of recent metals exposure in hair and concurrent associations with outcomes. Without prenatal and early childhood measures of exposure we are unable to discern whether the observed pattern of results reflects adverse impacts of early childhood exposure combined with a strong correlation between early and middle childhood exposure levels. Exposure pathways, however, are known to change as children age (United States. Environmental Protection Agency. Risk Assessment Forum., 2005). Previous examinations in this population showed that hair metal levels varied considerably between subjects and less within subjects when comparing across proximal, medial, and distal hair segments representing approximately a six-month exposure period (Jursa et al., 2018). Still, the relevance of this exposure period during this age rage is uncertain. Additionally, this study population is overwhelming white and female, which restricts its generalizability. The predominantly white study sample does, however, reflect that of the original study as well as the original study’s recruitment pool. The skewed sex distribution, and consequent differences between children included and excluded from the analyses, results from more males having insufficient hair for sampling and analysis. Also, hair lead as a biomarker is not widely used because of the possibility of exogenous contamination. The use of hair lead measures may have reduced our ability to observe lead effects in this study. While we employed appropriate statistical methods to examine a mixture of four metals this still represents only a fraction of total metal exposures. Given the limited volume of hair we focused on the three metals (arsenic, cadmium manganese) that exceeded ATSDR and U.S. EPA health-based comparison values during their investigation, as well as lead, which we were able to quantify on the same panel. It is unclear how to compare the pattern of results we observed with the mixtures to what may occur for individual exposures. It is also possible the pattern of substantive associations arose because of the precision of measurement differs among neuropsychological instruments. Some of these associations were based on low concentrations of metals leading to imprecise measures and potentially unstable estimates.
4.1. Conclusion
Despite the above methodological constraints we demonstrate that middle childhood is a developmental window of susceptibility to the adverse neurodevelopmental consequences of environmental toxicant exposure. No segment of the childhood age spectrum appears to be spared from the negative consequences of exposure to environmental neurotoxicants.
Supplementary Material
HIGHLIGHTS.
For some individual metals, even exposure at low concentrations may be neurotoxic
Statistical analysis of metal mixtures is more representative of real world exposure
Metal mixtures were associated with neuropsychological outcomes among children aged 6–12
Higher metal mixture concentration was associated with lower IQ
Higher metal mixture concentration was associated with worse maternal-reported behaviors
Funding:
This research was supported by the National Institutes of Health [R21ES019643; P30ES023515] and by the C8 class action settlement agreement (Jack W. Leach, et al. v. E.I. du Pont de Nemours & Company (no. 01-C-608 W. Va., Wood County Circuit Court, WV) between DuPont and plaintiffs. Funds were administered by the Garden City Group (Melville, NY) that reports to the court.
Contributor Information
Cheryl R Stein, Hassenfeld Children’s Hospital at NYU Langone, Department of Child and Adolescent Psychiatry, Child Study Center, One Park Avenue, 7th Floor, New York, NY 10016.
Haotian Wu, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, 722 West 168th St, New York, NY, 10032.
David C Bellinger, Department of Neurology, Boston Children’s Hospital, Farley Basement Box 127, 300 Longwood Ave, Boston MA 02115.
Donald R Smith, Department of Microbiology and Environmental Toxicology, University of California, 442 Physical Sciences Building, Santa Cruz, CA 95064.
Mary S Wolff, Department of Environmental Medicine & Public Health, Icahn School of Medicine at Mount Sinai, 17 East 102 Street, New York, NY 10029.
David A Savitz, Department of Epidemiology, Brown University School of Public Health, 121 S. Main Street, Box G-S-121-2, Providence, RI 02912.
REFERENCES
- 2016. Prevention of Childhood Lead Toxicity. Pediatrics 138(1). [DOI] [PubMed] [Google Scholar]
- (ATSDR), A.f.T.S.a.D.R., 2007. Toxicological Profile for Arsenic, in: ToxFAQs, D.o.T.a.E.M (Ed.). U.S. Department of Public Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- (ATSDR), A.f.T.S.a.D.R., 2012. Toxicological Profile for Manganese, in: ToxFAQs, D.o.T.a.E.M (Ed.). U.S. Department of Public Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Al-Saleh I, Al-Mohawes S, Al-Rouqi R, Elkhatib R, 2019. Selenium status in lactating mothers-infants and its potential protective role against the neurotoxicity of methylmercury, lead, manganese, and DDT. Environ Res 176, 108562. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH, 2008. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 20(3), 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JA, Devick KL, Bobb JF, Coull BA, Bellinger D, Benedetti C, Cagna G, Fedrighi C, Guazzetti S, Oppini M, Placidi D, Webster TF, White RF, Yang Q, Zoni S, Wright RO, Smith DR, Lucchini RG, Claus Henn B, 2020a. Associations of a Metal Mixture Measured in Multiple Biomarkers with IQ: Evidence from Italian Adolescents Living near Ferroalloy Industry. Environ Health Perspect 128(9), 97002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JA, Fruh V, Howe CG, White RF, Henn BC, 2020b. Associations of metals and neurodevelopment: a review of recent evidence on susceptibility factors. Curr Epidemiol Rep 7(4), 237–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA, 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA, 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3), 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA, 2006. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 27(3), 315–326. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, 1996. Discrepancies among mother, child, and teacher reports: examining the contributions of maternal depression and anxiety. Journal of abnormal child psychology 24(6), 749–765. [DOI] [PubMed] [Google Scholar]
- Carey WB, 2009. Developmental-behavioral pediatrics, 4th ed. Saunders/Elsevier, Philadelphia, PA. [Google Scholar]
- Claus Henn B, Bellinger DC, Hopkins MR, Coull BA, Ettinger AS, Jim R, Hatley E, Christiani DC, Wright RO, 2017. Maternal and Cord Blood Manganese Concentrations and Early Childhood Neurodevelopment among Residents near a Mining-Impacted Superfund Site. Environ Health Perspect 125(6), 067020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, 2001. Conners’ Rating Scales-Revised. Multi-Health Systems, Inc., North Tonawanda, NY. [Google Scholar]
- Conners CK, 2004. Conners’ CPT II for Windows, in: Staff M (Ed.). Multi-Health Systems, Inc., North Tonawanda, NY. [Google Scholar]
- Corporation, T.P., 1999. Wechsler Abbreviated Scale of Intelligence. Harcourt Brace and Company, San Antonio, TX. [Google Scholar]
- Corporation, T.P., 2002. Wechsler Individual Achievement Test-Second Edition. Harcourt Assessment Company, San Antonio, TX. [Google Scholar]
- Doherty BT, Romano ME, Gui J, Punshon T, Jackson BP, Karagas MR, Korrick SA, 2020. Periconceptional and prenatal exposure to metal mixtures in relation to behavioral development at 3 years of age. Environmental epidemiology (Philadelphia, Pa.) 4(4), e0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR, 2013. Hair as a biomarker of environmental manganese exposure. Environ Sci Technol 47(3), 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Amaya E, Gil F, Fernández MF, Murcia M, Llop S, Andiarena A, Aurrekoetxea J, Bustamante M, Guxens M, Ezama E, Fernández-Tardón G, Olea N, 2018. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ 621, 340–351. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP Jr., Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM, 2009. The C8 health project: design, methods, and participants. Environ Health Perspect 117(12), 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frndak S, Barg G, Canfield RL, Quierolo EI, Manay N, Kordas K, 2019. Latent subgroups of cognitive performance in lead- and manganese-exposed Uruguayan children: Examining behavioral signatures. Neurotoxicology 73, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL, 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2(10), 861–863. [DOI] [PubMed] [Google Scholar]
- Gioia GA, 2000. BRIEF : behavior rating inventory of executive function : professional manual. Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Gonçalves JF, Dressler VL, Assmann CE, Morsch VMM, Schetinger MRC, 2021. Chapter Three - Cadmium neurotoxicity: From its analytical aspects to neuronal impairment, in: Aschner M, Costa LG (Eds.), Advances in Neurotoxicology. Academic Press, pp. 81–113. [Google Scholar]
- Haynes EN, Sucharew H, Hilbert TJ, Kuhnell P, Spencer A, Newman NC, Burns R, Wright R, Parsons PJ, Dietrich KN, 2018. Impact of air manganese on child neurodevelopment in East Liverpool, Ohio. Neurotoxicology 64, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, Parsons PJ, Aldous KM, Praamsma ML, Beidler C, Dietrich KN, 2015. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA). Environ Health Perspect 123(10), 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jursa T, Stein CR, Smith DR, 2018. Determinants of Hair Manganese, Lead, Cadmium and Arsenic Levels in Environmentally Exposed Children. Toxics 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ, 2020. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 128(4), 47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, 2004. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16(8), 1412–1425. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S, 2007. NEPSY II-Second Edition. NCS Pearson, Inc., San Antonio, TX. [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potocnik J, Preker AS, Ramesh J, Rockstrom J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K, Zhong M, 2018. The Lancet Commission on pollution and health. Lancet (London, England) 391(10119), 462–512. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R, 2005. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113(7), 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard MJ, Chang ET, Loccisano AE, Garry MR, 2019. A systematic literature review of epidemiologic studies of developmental manganese exposure and neurodevelopmental outcomes. Toxicology 420, 46–65. [DOI] [PubMed] [Google Scholar]
- Levin-Schwartz Y, Gennings C, Schnaas L, Del Carmen Hernández Chávez M, Bellinger DC, Téllez-Rojo MM, Baccarelli AA, Wright RO, 2019. Time-varying associations between prenatal metal mixtures and rapid visual processing in children. Environ Health 18(1), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Fu JS, 1973. Vulnerability of children to lead exposure and toxicity (first of two parts). N Engl J Med 289(23), 1229–1233. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L, 1999. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology 20(2–3), 287–297. [PubMed] [Google Scholar]
- Mah VK, Ford-Jones EL, 2012. Spotlight on middle childhood: Rejuvenating the ‘forgotten years’. Paediatr Child Health 17(2), 81–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL, 2011. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinn LA, Bellinger DC, Colicino E, Coull BA, Just AC, Kloog I, Osorio-Valencia E, Schnaas L, Wright RJ, Téllez-Rojo MM, Wright RO, Horton MK, 2020. Prenatal PM(2.5) exposure and behavioral development in children from Mexico City. Neurotoxicology 81, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Carvalho CF, Rodrigues JLG, Araujo CFS, Dos Santos NR, Lima CS, Bandeira MJ, Marques BLS, Anjos ALS, Bah HAF, Abreu N, Philibert A, Mergler D, 2018. Environmental Co-Exposure to Lead and Manganese and Intellectual Deficit in School-Aged Children. Int J Environ Res Public Health 15(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Rocha TV, Tamayo YOM, Romero M, Pantic I, Schnaas L, Bellinger D, Claus-Henn B, Wright R, Wright RO, Téllez-Rojo MM, 2018. Prenatal co-exposure to manganese and depression and 24-months neurodevelopment. Neurotoxicology 64, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH, 2014. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. NeuroImage 97, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares V, 1989. Perspectives on child behavior problems: Comparisons of children’s self-reports with parent and teacher reports. Psychological assessment 1(1), 68–71. [Google Scholar]
- Prevention, A.C.o.C.L.P., 2012. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention in: Prevention, U.S.C.f.D.C.a (Ed.). [Google Scholar]
- Rabin R, 1989. Warnings unheeded: a history of child lead poisoning. Am J Public Health 79(12), 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW, 2004. BASC-2 : Behavior Assessment System for Children : manual. Pearson, Minneapolis, MN. [Google Scholar]
- Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, Rojas-García A, 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454–455, 562–577. [DOI] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, de Fays M, Bernard A, Stanescu D, 1987. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med 11(3), 307–327. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, 1998. Modern epidemiology, 2nd ed. Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- Rubin DB, 2004. Multiple imputation for nonresponse in surveys. Wiley, New York;. [Google Scholar]
- RUDDOCK JC, 1924. LEAD POISONING IN CHILDREN: WITH SPECIAL REFERENCE TO PICA. Journal of the American Medical Association 82(21), 1682–1684. [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO, 2015. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Current environmental health reports 2(3), 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CG, Lanphear BP, Xu Y, Chen A, Yolton K, Braun JM, 2021. Identifying periods of heightened susceptibility to lead exposure in relation to behavioral problems. J Expo Sci Environ Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services, U.S.D.o.H.a.H., Registry, A.f.T.S.a.D., 2009. Health Consultation Marietta Area Air Investigation Marietta, Ohio. Atlanta, GA. [Google Scholar]
- Shoaff JR, Calafat AM, Schantz SL, Korrick SA, 2019. Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environ Res 172, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders R, Schramm KW, Nickmilder M, Schoeters G, 2009. Applicability of non-invasively collected matrices for human biomonitoring. Environ Health 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC, 2013. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology 24(4), 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC, 2014. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6–12-year-old children. Paediatr Perinat Epidemiol 28(2), 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveys, N.L., National Longitudinal Survey Program. http://www.bls.gov/nls/nlsy97.htm. (Accessed May 25 2012).
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK, 2006. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol 59(5), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, Rohan ML, Vitaliano GD, 2018. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage 169, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, Wright RO, Hernandez-Avila M, Hu H, 2006. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 118(2), e323–330. [DOI] [PubMed] [Google Scholar]
- Touliatos J, 1981. Congruence of parents’ and teachers’ ratings of children’s behavior problems. Journal of abnormal child psychology 9(3), 347–354. [DOI] [PubMed] [Google Scholar]
- United States. Environmental Protection Agency. Risk Assessment Forum., 2005. Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC. [Google Scholar]
- Vahidnia A, van der Voet GB, de Wolff FA, 2007. Arsenic neurotoxicity--a review. Hum Exp Toxicol 26(10), 823–832. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K, 2011. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 45(3), 1 – 67. [Google Scholar]
- von Stackelberg K, Guzy E, Chu T, Claus Henn B, 2015. Exposure to Mixtures of Metals and Neurodevelopmental Outcomes: A Multidisciplinary Review Using an Adverse Outcome Pathway Framework. Risk Anal 35(6), 971–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


