Abstract
BACKGROUND
Partial resistance of Plasmodium falciparum to the artemisinin component of artemisinin-based combination therapies, the most important malaria drugs, emerged in Southeast Asia and now threatens East Africa. Partial resistance, which manifests as delayed clearance after therapy, is mediated principally by mutations in the kelch protein K13 (PfK13). Limited longitudinal data are available on the emergence and spread of artemisinin resistance in Africa.
METHODS
We performed annual surveillance among patients who presented with uncomplicated malaria at 10 to 16 sites across Uganda from 2016 through 2022. We sequenced the gene encoding kelch 13 (pfk13) and analyzed relatedness using molecular methods. We assessed malaria metrics longitudinally in eight Ugandan districts from 2014 through 2021.
RESULTS
By 2021–2022, the prevalence of parasites with validated or candidate resistance markers reached more than 20% in 11 of the 16 districts where surveillance was conducted. The PfK13 469Y and 675V mutations were seen in far northern Uganda in 2016–2017 and increased and spread thereafter, reaching a combined prevalence of 10 to 54% across much of northern Uganda, with spread to other regions. The 469F mutation reached a prevalence of 38 to 40% in one district in southwestern Uganda in 2021–2022. The 561H mutation, previously described in Rwanda, was first seen in southwestern Uganda in 2021, reaching a prevalence of 23% by 2022. The 441L mutation reached a prevalence of 12 to 23% in three districts in western Uganda in 2022. Genetic analysis indicated local emergence of mutant parasites independent of those in Southeast Asia. The emergence of resistance was observed predominantly in areas where effective malaria control had been discontinued or transmission was unstable.
CONCLUSIONS
Data from Uganda showed the emergence of partial resistance to artemisinins in multiple geographic locations, with increasing prevalence and regional spread over time. (Funded by the National Institutes of Health.)
Malaria, which is caused primarily by Plasmodium falciparum, remains a substantial health challenge, particularly in Africa, where approximately 95% of malaria cases and deaths occur.1 Malaria control focuses on the use of long-lasting, insecticide-treated bed nets and indoor residual spraying of insecticides to limit mosquito vectors and on the use of efficacious drugs to treat and prevent malaria. Resistance to older drugs limited the efficacy of malaria treatment in Africa, where chloroquine resistance probably contributed to millions of excess malaria deaths.2 Early this century, treatment shifted to artemisinin-based combination therapy that was designed to limit drug resistance with a rapidly effective, but short-acting, artemisinin component combined with a long-acting partner drug.3 By 2005, artemisinin-based combinations, primarily artemether–lumefantrine and artesunate–amodiaquine, were standard therapy for uncomplicated malaria across Africa.4
Partial resistance to artemisinins, which manifests clinically as delayed parasite clearance after treatment with artemisinins5 and in vitro as enhanced parasite survival after exposure to an artemisinin pulse,6 was previously reported in Southeast Asia.5,6 The primary mediator was mutations in the kelch protein (PfK13) propeller domain,7 with secondary determinants mediating specific levels of resistance and fitness.8 Partial resistance to artemisinins is of particular importance when it is accompanied by resistance to partner drugs; this combined resistance led to decreased efficacies of artemisinin-based combination therapies in Southeast Asia.9,10 Among the many PfK13 mutations identified in P. falciparum, approximately 20 have been associated with partial resistance.11 These mutations have been reported at low prevalence in some regions outside Southeast Asia, including in Guyana, Papua New Guinea, and India, but to date these appear to have been sporadic outbreaks without stable PfK13 mutation prevalence or verified resistance.12
Of greatest concern has been the emergence and spread of partial resistance to artemisinins in Africa, where the effects are expected to be profound. In older studies, PfK13 mutations were seen in African parasites at low prevalence, but with few exceptions these were not validated resistance mediators.4,13 In East Africa, resistance-mediating PfK13 mutations14–16 and in vitro enhanced survival of parasites after artemisinin exposure17 were very uncommon,16 but this resistance status changed recently. In Rwanda, the PfK13 561H mutation, a validated resistance mediator, was first seen in 2014, had a prevalence of approximately 20% among isolates obtained from two sites in 2018, and was associated with clinical delayed parasite clearance.18–20 In Uganda, a single isolate with the 675V mutation and enhanced survival of the parasites after in vitro artemisinin exposure was identified in 2016.21 Subsequently, the 469Y and 675V mutations were seen at increasing prevalence in northern Uganda,22,23 where they were associated with partial resistance clinically and in vitro.24,25
Resistance to antimalarials has typically emerged in regions in which the intensity of malaria transmission has been relatively low, facilitated by low population immunity (allowing relatively unfit resistant parasites to spread) and low complexity of infection (limiting within-host competition between parasites).26 It is of interest to determine why partial resistance to artemisinins emerged in historically high-transmission regions of Uganda. To better characterize the extent of resistance and explore reasons for selection, we characterized parasite genotypes and assessed malaria metrics over the period in which resistance emerged and spread in Uganda.
METHODS
GENETIC SURVEILLANCE OF P. FALCIPARUM IN UGANDA
At multiple sites (10 sites from 2016 through 2017 and 16 sites from 2018 through 2022), we sequenced pfk13 in 50 isolates (2016–2019) or 100 isolates (2020–2022) annually from symptomatic persons who presented with uncomplicated malaria at malaria reference centers around Uganda (Fig. 1 and Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Symptomatic persons were selected according to convenience at each reference center at approximately the same time of year. Results from analyses of a subset of these isolates obtained from 2017 through 2019 were published previously.22,23
Figure 1. Map of Uganda and Study Districts.
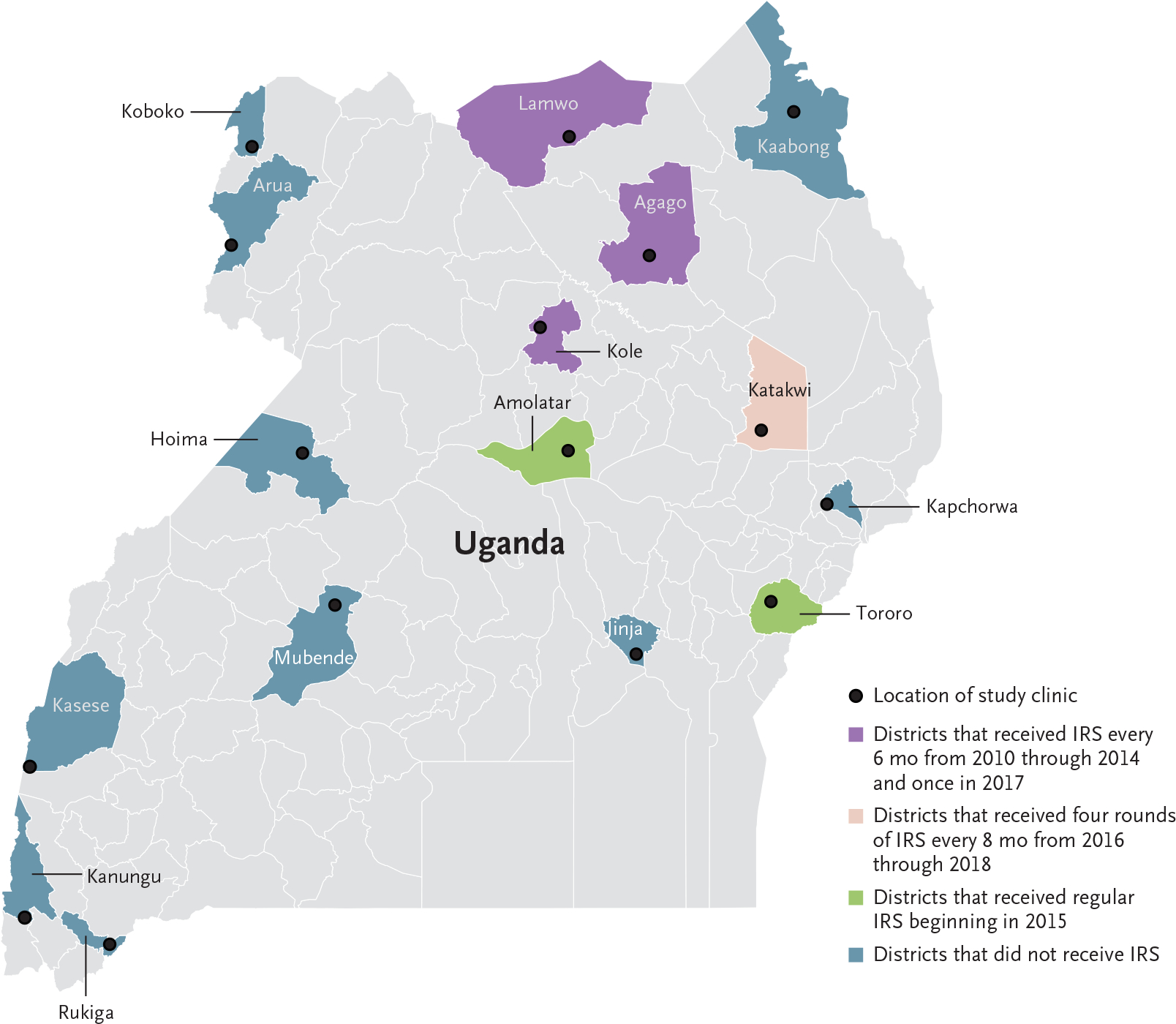
IRS denotes indoor residual spraying of insecticides.
For our analyses, we used dideoxy sequencing and molecular inversion probe technology, as previously described.23 For all analyses, mixed genotypes at any locus were categorized as mutant. These studies and assessments of malaria metrics were approved by the Makerere University Research and Ethics Committee, the Uganda National Council for Science and Technology, and the University of California, San Francisco, Human Research Protection Program.
PHYLOGENETIC ANALYSIS OF ISOLATES WITH PARTIAL RESISTANCE MARKERS
To assess the origins of mutant parasites, we genotyped seven microsatellites flanking pfk13 (Table S2) in parasites collected in Uganda from 2017 through 2021, removed samples missing more than one genotype or with multiple genotypes at any locus, and generated a neighborjoining tree with the use of R software, version 4.2.2.27 We also assessed phylogeny using genotypes of mutant parasites from Uganda (collected in 2020) and Southeast Asia (from the Malaria-GEN Pf6K repository, collected in 2008–2013).28 Parasites from Uganda were genotyped according to single-nucleotide polymorphisms that were distributed across the genome.29 Equivalent variant sites were extracted from Pf6K whole genomes after variant calling. After we filtered data for missingness and coverage, a cladogram was created with the use of R software. Details of our analytic methods are provided in Section S1 in the Supplementary Appendix.
EVALUATION OF MALARIA METRICS AT SITES ACROSS UGANDA
Enhanced malaria surveillance was established in 2006, and in 2014 it was extended to all sites described in this article.30 All outpatients who presented to malaria reference centers with suspected malaria underwent laboratory testing by means of rapid diagnostic test or microscopy. To evaluate the incidence of malaria, we considered monthly laboratory-confirmed cases and test positivity (the percentage of patients tested who had a positive malaria test) obtained from malaria reference centers. Because immunity wanes and the median age at presentation increases with effective control and decreased force of infection, we considered the median age of persons who presented with malaria in order to evaluate antimalarial immunity in the population.31
STATISTICAL ANALYSIS
Analysis of 50 samples per site provided the study with 92% power and 100 samples per site provided 99% power to detect a mutation at 5% prevalence and a 95% confidence level. Statistical analyses were performed with the use of StataSE software, version 14 (StataCorp). The prevalences of mutations were compared with the use of the two-sided Fisher’s exact test and assessed longitudinally with the use of the chi-square test of trend, implemented with the use of the ptrend command. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
STUDY SITES
We obtained isolates from patients presenting with uncomplicated malaria beginning in 2016; malaria metric data (monthly number of malaria cases, test positivity, and median age at time of presentation with malaria) were from a subset of sites with enhanced surveillance data available since 2014 (Fig. 1). Bed nets were distributed in 2013–2014, 2017–2018, and 2020–2021 at all study sites. Indoor residual spraying, a highly effective control measure that is limited in scope because of cost, was implemented selectively, with districts receiving one of the following: twice-yearly indoor residual spraying (as part of a national campaign) with the carbamate compound bendiocarb from 2010 through 2014 plus a single round of the organophosphate pirimiphos-methyl in 2017, four rounds of indoor residual spraying with pirimiphos-methyl in the context of a research study every 8 months from 2016 through 2018, once- or twice-yearly indoor residual spraying starting in 2015 (bendiocarb, primarily in 2015, then pirimiphos-methyl from 2016 through 2019 and clothianidin or clothianidin–deltamethrin in 2020), or no indoor residual spraying (Fig. 1).30,32
PREVALENCE OF RESISTANCE-ASSOCIATED MUTATIONS
Analysis of the oldest available samples identified modest prevalence of the PfK13 469Y and 675V mutations in 2016, particularly in Lamwo District in far north-central Uganda (Fig. 2 and Table S3). Since 2019, these two mutations were maintained at a combined prevalence greater than 10% in five northern districts (Agago, Kaabong, Katakwi, Koboko, and Lamwo), with the prevalence of each of the individual mutations increasing over time (test of trend, P<0.05) at four of the five sites. A different mutation at the 469 codon, 469F, was seen in Rukiga District in southwestern Uganda in 2016 and reached high prevalence in 2021 (40%) and 2022 (38%); a lower incidence of malaria at this site in 2017–2018 yielded few samples for study. The 561H mutation, which had reached a high prevalence in Rwanda, was first detected in 2021 in Rukiga District at a prevalence of 16%, increasing to 23% in 2022. The P441L mutation, at the upstream boundary of the propeller domain, reached a prevalence of 12 to 23% at three sites in western Uganda in 2021–2022. The five noted PfK13 mutations also occurred sporadically in other regions — for example, in Mubende (469F in 2018, 469Y in 2020, and 675V in 2020 and 2022) — but emergence was followed by stable high prevalence only in northern Uganda (469Y and 675V) and southwestern Uganda (469F, 561H, and 441L).
Figure 2. Prevalence of Indicated PfK13 Mutations in Studied Ugandan Districts.
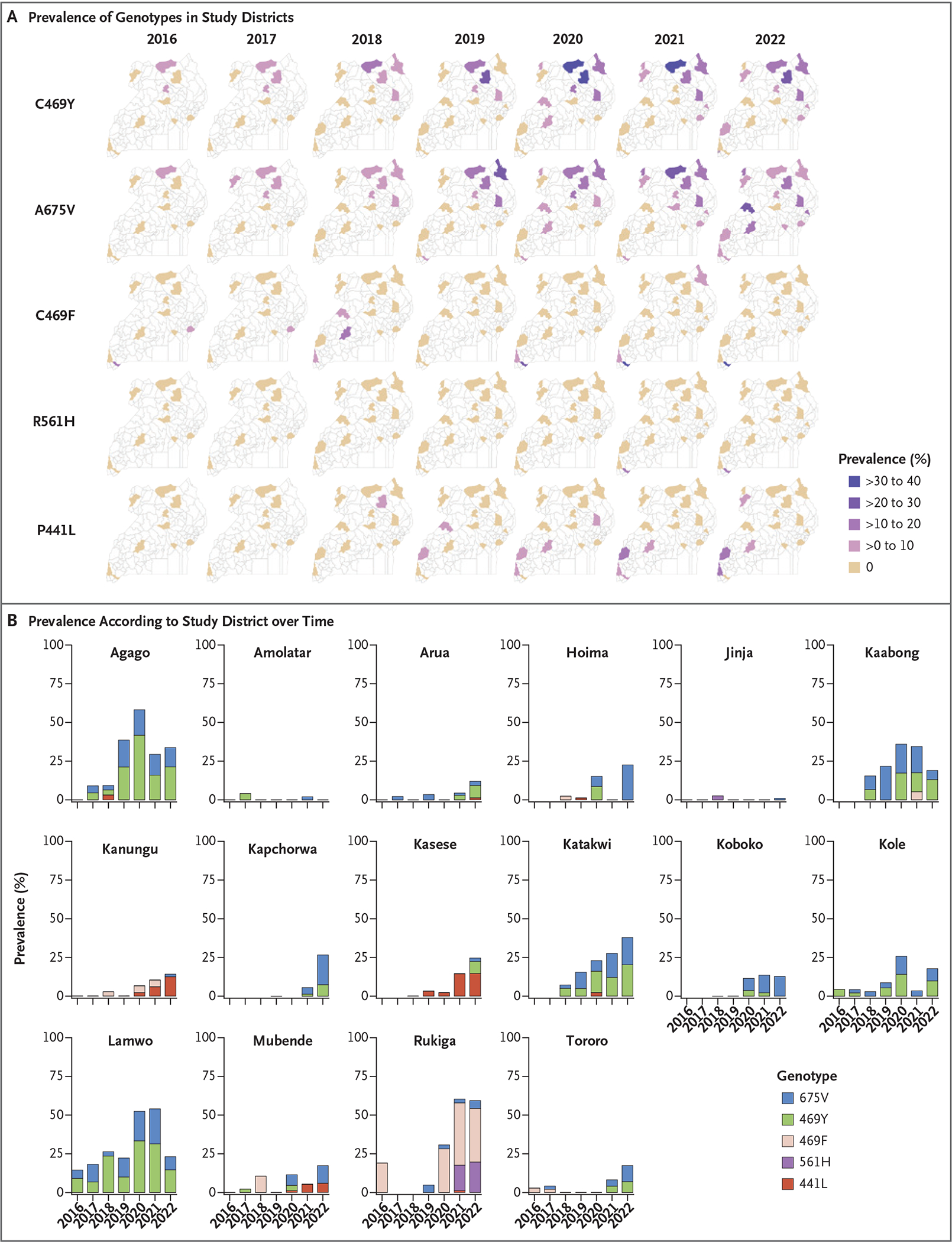
In Panel B, a horizontal dash along the x axis indicates no prevalence, and no dash means no samples were obtained in that year.
Additional PfK13 propeller domain mutations that are not candidate or validated resistance mediators were seen, mostly at a prevalence of less than 10%, with some clusters suggesting local emergence and spread (Table S4). Mutations outside the propeller domain were common (Table S5). Overall, multiple PfK13 mutations, including five candidate or validated resistance markers, have shown increased prevalence and spread over time.
RELATEDNESS OF ISOLATES WITH PARTIAL-RESISTANCE MARKERS
We explored the relatedness of mutant parasites using two methods. First, we characterized microsatellites flanking pfk13 in parasites from Uganda. Mutant parasites showed distinct haplotypes associated with each mutation, a finding consistent with singular origins (Fig. 3A and Table S6). The less-defined clustering of 675V parasites probably represents a more distant origin, but multiple origins cannot be ruled out.
Figure 3. Phylogenetic Relatedness of Parasites from Uganda.
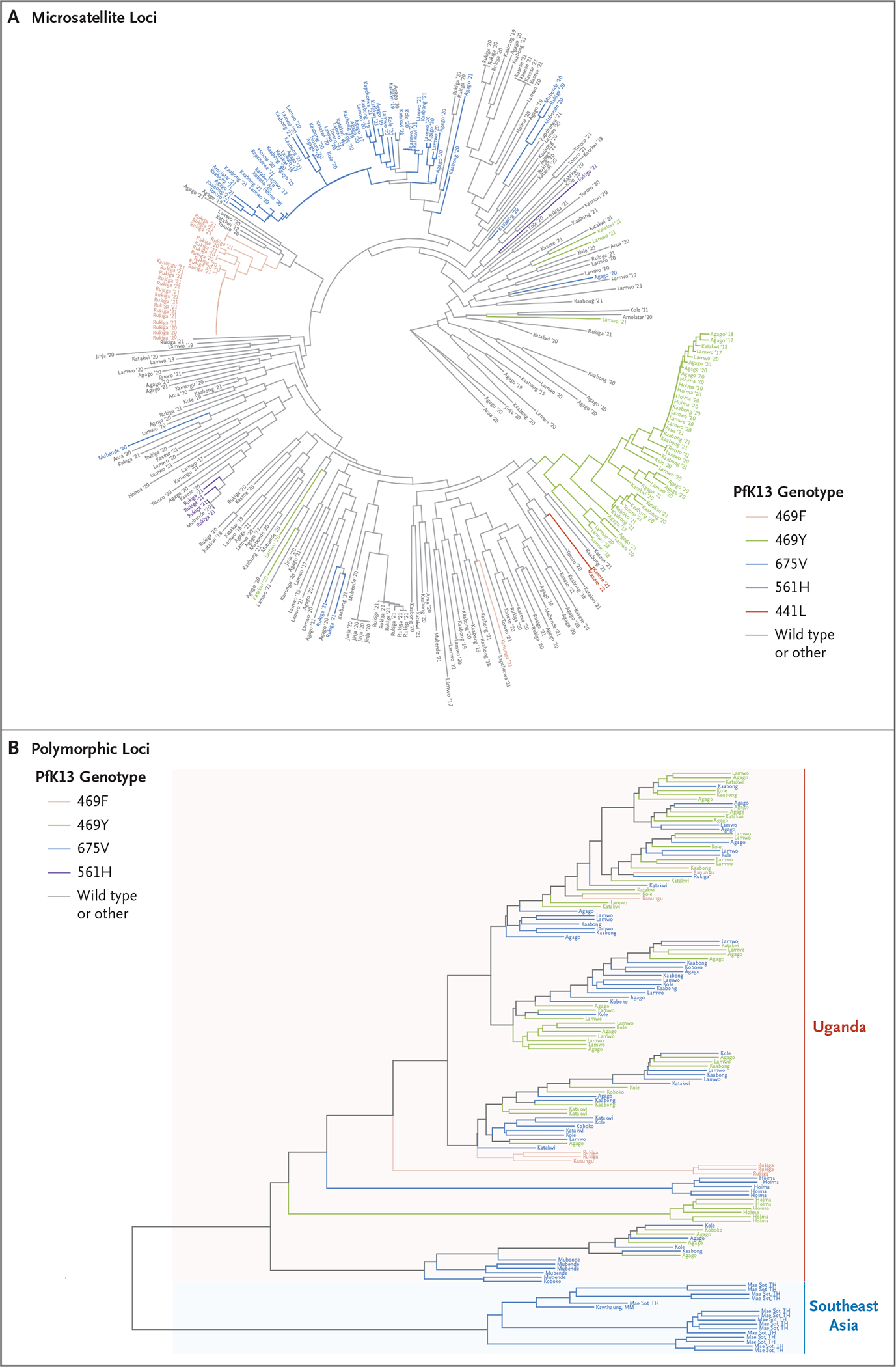
Dendrograms show the relatedness of parasites from Uganda with the indicated PfK13 mutations, wild-type parasites, and parasites containing other PfK13 mutations (other) according to the characterization of seven microsatellite loci flanking PfK13 (Panel A) and the relatedness of mutant isolates from Uganda and Southeast Asia according to polymorphic loci distributed across the genome (Panel B).
Second, we characterized polymorphisms across the P. falciparum genome in parasites from Uganda and Southeast Asia (Fig. 3B). The mutant parasites from Uganda were phylogenetically distant from parasites from Southeast Asia with the 675V mutation (parasites from Asia with other relevant mutations were not available), consistent with local emergence and spread within Uganda. The studied mutations in parasites from Uganda did not segregate in distinct genomic clusters, a finding that suggests that none were limited to a particular parasite background, and we found extensive recombination after emergence in these parasites as compared with mutations in parasites from Southeast Asia, where clonal transmission with fixed parasite backgrounds was generally observed.33
MALARIA METRICS AT SITES ACROSS UGANDA
To gain insight into factors that might facilitate resistance selection, we examined monthly malaria burden, measured as case counts and test positivity, at the eight malaria reference centers from which genomic data were also available from 2014 through 2021, capturing the interval during which partial resistance to artemisinins emerged in northern Uganda (Fig. 4 and Figs. S1, S2, and S3). Patterns of malaria incidence were strongly associated with the use of indoor residual spraying, a highly effective control measure when carbamate or organophosphate insecticides are used.30
Figure 4. Relationship between Malaria Metrics and Prevalence of PfK13 Mutations.
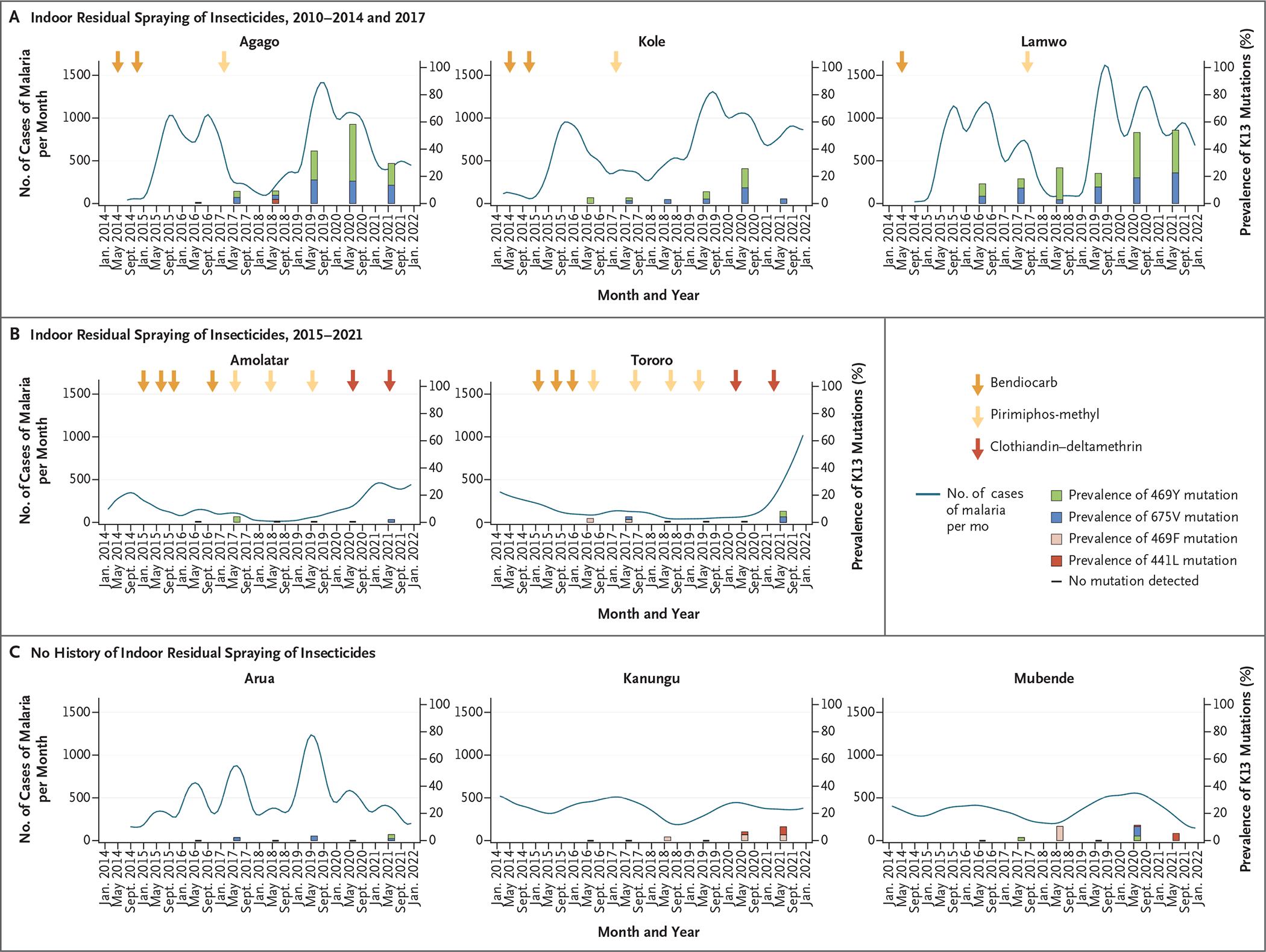
Malaria incidence and mutation prevalence are shown for sites that received indoor residual spraying of insecticides. The metrics shown were assessed monthly and are displayed with locally weighted scatterplot smoothing. Arrows indicate times of insecticide application; the darkest arrows indicate an apparently ineffective insecticide (clothiandin–deltamethrin). Histogram bars indicate the prevalence of PfK13 mutations. A horizontal dash along the x axis indicates sample collection occurred but no mutations were detected. Other metrics of malaria (test positivity and median age of presentation with malaria) are shown in Figures S1, S2, and S3.
The incidence was cyclic at many sites, probably affected by seasonal rainfall, but patterns that were associated with indoor residual spraying interventions were clearly discernible. Districts with regular indoor residual spraying from 2010 through 2014 and one additional round in 2017 had relatively low case counts and test positivity after a sustained period of spraying,30,34 with marked increases within approximately 4 to 8 months after indoor residual spraying ceased (Fig. 4A). Increased incidence was necessarily accompanied by more frequent treatment, primarily with artemether–lumefantrine, and thus increased selective pressure for drug resistance.35 Districts that had indoor residual spraying that began in 2015 had marked decreases in case counts and test positivity after implementation until a change to different insecticides for spraying and a marked increase in malaria incidence in 2020 (Fig. 4B). Districts with no history of indoor residual spraying, including sites with diverse malaria epidemiologic profiles, had varied case counts and test positivity over time, including transient seasonal increases in both (Fig. 4C).
We used the median age of patients at the time of presentation with malaria as a surrogate for antimalarial immunity, because as immunity decreases with effective control, the disease burden shifts to older persons.31 This analysis generally showed increased median age (indicating decreased immunity) during periods of malaria control, such that populations in northern Uganda had relatively low immunity at the time of cessation of indoor residual spraying and subsequent surges in malaria incidence (Fig. S1, S2, and S3). It is noteworthy that selection of PfK13 mutations occurred primarily in regions in which malaria-control measures were discontinued (i.e., northern Uganda) or where malaria transmission was unstable (i.e., southwestern Uganda).
DISCUSSION
The emergence of partial resistance to artemisinins in P. falciparum in East Africa is a serious challenge to the control of malaria. We examined the emergence and spread of five different resistance-mediating PfK13 mutations in Uganda since 2016. By 2021–2022, the prevalence of parasites with candidate or validated resistance markers exceeded 20% in 11 of the 16 districts where surveillance was conducted, with foci of more than 50% prevalence in both northern and southwestern Uganda. The full clinical consequences of these genetic changes are not yet known, but experience from Southeast Asia indicates that treatment of malaria may be compromised by the identified mutations. Improved characterization of the emergence and spread of artemisinin resistance and of factors that facilitate spread will assist policymakers in developing strategies to limit the spread and consequences of drug resistance.
Our findings with regard to the emergence of partial resistance to artemisinins in northern Uganda are notable in one important respect. Resistance to antimalarial drugs has typically first emerged in regions of relatively low malaria-transmission intensity. This scenario was seen with the emergence of resistance to chloroquine, antifolates, mefloquine, and artemisinins, which in each case was first observed in Southeast Asia (and for chloroquine, independently in South America). Emergence was followed by the spread of chloroquine resistance from Asia to Africa36 and selective sweeps of antifolate resistance in Africa.37 Our data, along with previous data from Rwanda18,19 and Uganda,24 indicate multiple independent emergences of PfK13 mutations in these regions of relatively high malaria burden, rather than the spread of resistant lineages from Asia.
Why has artemisinin resistance emerged and spread in northern Uganda, a region that historically has had a high incidence of malaria? We hypothesize that emergence and spread have been facilitated by events that have led to high malaria-transmission intensity in populations with relatively low malaria immunity. Key features of malaria control in Uganda have included the provision of effective antimalarial therapy with artemisinin-based combinations, national distributions of bed nets, and indoor residual spraying, which is the most effective vector-control intervention but one that has been limited in scope by its high cost. Indoor residual spraying with highly effective insecticides was used in 10 northern districts from 2010 through 2014 and was accompanied by sustained decreases in malaria-test positivity and parasite prevalence.34,38 The subsequent discontinuation of indoor residual spraying in 2014 was associated with the incidence of malaria increasing by a factor of five within 10 months, and a malaria epidemic was declared by the Uganda Ministry of Health in June 2015.30,39
In the four studied districts where cessation of indoor residual spraying was followed by resurgent malaria (Agago, Kole, and Lamwo, where indoor residual spraying was stopped in 2014, and Katakwi, a northern district that underwent four rounds of indoor residual spraying from 2016 through 2018 in the context of a research study),41 the prevalence of any of the five PfK13 mutations increased from 8.0% in 2016 to 32.0% in 2022 (Table S7). The prevalence of these mutations in these districts was significantly higher than in districts where there was sustained indoor residual spraying since 2015 (difference in prevalence, 36.0 percentage points for 2020 and 24.1 percentage points for 2021) or at sites without indoor residual spraying (difference in prevalence, 25.2, 14.8, and 11.3 percentage points for 2020, 2021, and 2022, respectively) for nearly all the years studied. More recently, the prevalence of the 469Y and 675V mutations has stabilized in northern Uganda but increased in other regions, which suggests a transition to stable prevalence across much of the country. Rukiga, in southwestern Uganda, has had emergences of 469F (an apparent independent emergence) and 561H (most likely spread from Rwanda), probably facilitated by unstable malaria transmission in that district. Finally, the 441L mutation, which is a candidate resistance mediator, has recently emerged in western Uganda.
Resistance selection in Uganda may also have been furthered by the use of artemisinin monotherapy, with the artemisinin component unprotected by a long-acting partner drug. The use of artemisinin monotherapy to treat uncomplicated malaria has been discouraged, but the practice persists. In Nigeria, oral artemisinin monotherapies made up 2.5% of the market share among studied drug outlets in 2015.40 The use of intravenous or rectal artesunate for severe malaria should be followed by a full course of an artemisinin-based combination,42 but follow-up therapy may be omitted. In addition, the use of parenteral drugs, including intravenous artesunate, to treat uncomplicated malaria, although not recommended, is a well-known practice that increases the potential for selection of resistant parasites.43
Our study had important limitations. The numbers of samples collected per site per year were small, which limited the precision of prevalence estimates. Assessments of associations between ecologic factors and drug-resistance markers relied on data collected for different purposes and did not cover all sites that were studied for drug resistance. Metrics for malaria incidence (monthly case counts and test positivity) and immunity (median age at presentation with malaria) were necessarily imprecise, but exact measures were challenging to obtain and were beyond the capacity of our surveillance network. Overall, our proposal that the establishment of resistance genotypes was facilitated by a high incidence of malaria after periods of low incidence in northern Uganda should be considered a hypothesis in need of additional testing.
Our results show worrisome, sustained prevalence in Uganda of P. falciparum with artemisinin-resistance–mediating PfK13 mutations. The 469Y and 675V mutations are extending their range, and three other mutations, 469F, 561H, and 441L have emerged in western Uganda. Resistance selection is possible under many scenarios, but our results suggest that in a population with a low level of immunity to malaria (owing to a sustained low burden of disease), a malaria epidemic increased the likelihood of resistance selection. In northern Uganda, this scenario occurred after the withdrawal of effective malaria control.
The epidemiology of malaria in Africa is highly varied, with many regions having major fluctuations in disease incidence, as seen in Uganda. Hence, there is concern about multiple additional emergences of partial resistance to artemisinins. Further study is needed to determine whether efforts to blunt malaria epidemics, including the maintenance of effective malaria control interventions and prompt attention to regions with increasing incidence, may decrease the emergence and spread of antimalarial drug resistance in Africa.
Supplementary Material
Acknowledgments
Supported by grants (AI075045, AI089674, and AI139520) from the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Adrienne Epstein, Bryan Greenhouse, and Isabel Rodriguez-Barraquer at the University of California, San Francisco, for helpful discussions; Deborah Chin at Brown University for assistance with sequencing; Venkatachalam Udhayakumar, Naomi Lucchi, and Samaly Svigel at the Centers for Disease Control and Prevention for providing advice and reagents for microsatellite analyses; the staff at Ugandan health centers for sample and data collection; and all the study participants for generously providing blood samples for genomic surveillance.
Contributor Information
Melissa D. Conrad, University of California, San Francisco, San Francisco
Victor Asua, Infectious Diseases Research Collaboration, Kampala, Uganda University of Tübingen, Tübingen, Germany.
Shreeya Garg, University of California, San Francisco, San Francisco
David Giesbrecht, Brown University, Providence, RI
Karamoko Niaré, Brown University, Providence, RI
Sawyer Smith, Brown University, Providence, RI
Jane F. Namuganga, Infectious Diseases Research Collaboration, Kampala, Uganda
Thomas Katairo, Infectious Diseases Research Collaboration, Kampala, Uganda
Jennifer Legac, University of California, San Francisco, San Francisco
Rebecca M. Crudale, Brown University, Providence, RI
Patrick K. Tumwebaze, Infectious Diseases Research Collaboration, Kampala, Uganda
Samuel L. Nsobya, Infectious Diseases Research Collaboration, Kampala, Uganda
Roland A. Cooper, Dominican University of California, San Rafael
Moses R. Kamya, Infectious Diseases Research Collaboration, Kampala, Uganda Makerere University, Kampala, Uganda.
Grant Dorsey, University of California, San Francisco, San Francisco
Jeffrey A. Bailey, Brown University, Providence, RI
Philip J. Rosenthal, University of California, San Francisco, San Francisco
References
- 1.World Health Organization. World malaria report 2021. December 6, 2021. (https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021).
- 2.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg 2001;64:Suppl:12–7. [DOI] [PubMed] [Google Scholar]
- 3.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 2007;77:Suppl:181–92. [PubMed] [Google Scholar]
- 4.Conrad MD, Rosenthal PJ. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect Dis 2019;19(10):e338–e351. [DOI] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009;361:455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkowski B, Amaratunga C, Khim N, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 2013;13:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014;505:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes BH, Dhingra SK, Rubiano K, et al. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. Elife 2021;10:e66277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaratunga C, Lim P, Suon S, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 2016;16:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phyo AP, Ashley EA, Anderson TJC, et al. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis 2016;63:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WWARN K13 Genotype-Phenotype Study Group. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments — a WWARN individual patient data metaanalysis. BMC Med 2019;17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhorda M, Amaratunga C, Dondorp AM. Artemisinin and multidrug-resistant Plasmodium falciparum — a threat for malaria control and elimination. Curr Opin Infect Dis 2021;34:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndwiga L, Kimenyi KM, Wamae K, et al. A review of the frequencies of Plasmodium falciparum Kelch 13 artemisinin resistance mutations in Africa. Int J Parasitol Drugs Drug Resist 2021;16:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad MD, Bigira V, Kapisi J, et al. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are Partial Resistance to Artemisinins in Malaria Parasites not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS One 2014;9(8):e105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad MD, Nsobya SL, Rosenthal PJ. The diversity of the Plasmodium falciparum K13 propeller domain did not increase after implementation of artemisinin-based combination therapy in Uganda. Antimicrob Agents Chemother 2019;63(10):e01234–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacoli C, Gai PP, Bayingana C, et al. Artemisinin resistance-associated K13 polymorphisms of Plasmodium falciparum in southern Rwanda, 2010–2015. Am J Trop Med Hyg 2016;95:1090–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper RA, Conrad MD, Watson QD, et al. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular assays. Antimicrob Agents Chemother 2015;59:5061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uwimana A, Legrand E, Stokes BH, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 2020;26:1602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uwimana A, Umulisa N, Venkatesan M, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 2021;21:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straimer J, Gandhi P, Renner KC, Schmitt EK. High prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis 2022;225:1411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda M, Kaneko M, Tachibana S-I, et al. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis 2018;24:71826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asua V, Vinden J, Conrad MD, et al. Changing molecular markers of antimalarial drug sensitivity across Uganda. Antimicrob Agents Chemother 2019;63:e01818–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asua V, Conrad MD, Aydemir O, et al. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis 2021;223:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balikagala B, Fukuda N, Ikeda M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 2021;385:1163–71. [DOI] [PubMed] [Google Scholar]
- 25.Tumwebaze PK, Conrad MD, Okitwi M, et al. Decreased susceptibility of Plasmodium falciparum to both dihydroartemisinin and lumefantrine in northern Uganda. Nat Commun 2022;13:6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masserey T, Lee T, Golumbeanu M, et al. The influence of biological, epidemiological, and treatment factors on the establishment and spread of drug-resistant Plasmodium falciparum. Elife 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talundzic E, Okoth SA, Congpuong K, et al. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog 2015;11(4):e1004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahouidi A, Ali M, Almagro-Garcia J, et al. An open dataset of Plasmodium falciparum genome variation in 7,000 worldwide samples. Wellcome Open Res 2021;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verity R, Aydemir O, Brazeau NF, et al. The impact of antimalarial resistance on the genetic structure of Plasmodium falciparum in the DRC. Nat Commun 2020;11:2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namuganga JF, Epstein A, Nank-abirwa JI, et al. The impact of stopping and starting indoor residual spraying on malaria burden in Uganda. Nat Commun 2021;12:2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kigozi SP, Kigozi RN, Epstein A, et al. Rapid shifts in the age-specific burden of malaria following successful control interventions in four regions of Uganda. Malar J 2020;19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein A, Maiteki-Sebuguzi C, Namuganga JF, et al. Resurgence of malaria in Uganda despite sustained indoor residual spraying and repeated long lasting insecticidal net distributions. PLOS Glob Public Health 2022;2(9):e0000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parobek CM, Parr JB, Brazeau NF, et al. Partner-drug resistance and population substructuring of artemisinin-resistant Plasmodium falciparum in Cambodia. Genome Biol Evol 2017;9:1673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raouf S, Mpimbaza A, Kigozi R, et al. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clin Infect Dis 2017;65:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mpimbaza A, Babikako H, Rutazanna D, et al. Adherence to malaria management guidelines by health care workers in the Busoga sub-region, eastern Uganda. Malar J 2022;21:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis 2001;184:770–6. [DOI] [PubMed] [Google Scholar]
- 37.Pearce RJ, Pota H, Evehe MS, et al. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med 2009;6(4):e1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kigozi R, Baxi SM, Gasasira A, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One 2012;7(8):e42857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okullo AE, Matovu JKB, Ario AR, et al. Malaria incidence among children less than 5 years during and after cessation of indoor residual spraying in Northern Uganda. Malar J 2017;16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Group ACTwatch, Ujuju C, Anyanti J, Newton PN, Ntadom G; ACTwatch Group. When it just won’t go away: oral artemisinin monotherapy in Nigeria, threatening lives, threatening progress. Malar J 2017;16:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulebeke R, Wanzira H, Bukenya F, et al. Implementing population-based mass drug administration for malaria: experience from a high transmission setting in North Eastern Uganda. Malar J 2019;18:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. WHO guidelines for malaria. March 14, 2023. (https://www.who.int/publications/i/item/guidelines-for-malaria).
- 43.Wang LT, Bwambale R, Keeler C, et al. Private sector drug shops frequently dispense parenteral anti-malarials in a rural region of Western Uganda. Malar J 2018;17:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


