Abstract
Background and Objectives
Due to current limitations in diagnosing chronic traumatic encephalopathy (CTE) clinically, traumatic encephalopathy syndrome (TES) has been proposed as the clinical presentation of suspected CTE. This study aimed to determine whether there was an association between a clinical diagnosis of TES and subsequent temporal decline in cognitive or MRI volumetric measures.
Methods
This was a secondary analysis of the Professional Athletes Brain Health Study (PABHS), inclusive of active and retired professional fighters older than 34 years. All athletes were adjudicated as TES positive (TES+) or TES negative (TES−) based on the 2021 clinical criteria. General linear mixed models were used to compare MRI regional brain volumes and cognitive performance between groups.
Results
A total of 130 fighters met inclusion criteria for consensus conference. Of them, 52 fighters (40%) were adjudicated as TES+. Athletes with a TES+ diagnosis were older and had significantly lower education. Statistically significant interactions and between-group total mean differences were found in all MRI volumetric measurements among the TES+ group compared with those among the TES− group. The rate of volumetric change indicated a significantly greater increase for lateral (estimate = 5,196.65; 95% CI = 2642.65, 7750.66) and inferior lateral ventricles (estimate = 354.28; 95% CI = 159.90, 548.66) and a decrease for the hippocampus (estimate = −385.04, 95% CI = −580.47, −189.62), subcortical gray matter (estimate = −4,641.08; 95% CI = −6783.98, −2498.18), total gray matter (estimate = −26492.00; 95% CI = −50402.00, −2582.32), and posterior corpus callosum (estimate = −147.98; 95% CI = −222.33, −73.62). Likewise, the rate of cognitive decline was significantly greater for reaction time (estimate = 56.31; 95% CI = 26.17, 86.45) and other standardized cognitive scores in the TES+ group.
Discussion
The 2021 TES criteria clearly distinguishes group differences in the longitudinal presentation of volumetric loss in select brain regions and cognitive decline among professional fighters 35 years and older. This study suggests that a TES diagnosis may be useful in professional sports beyond football, such as boxing and mixed martial arts. These findings further suggest that the application of TES criteria may be valuable clinically in predicting cognitive decline.
Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disorder related to prolonged exposure of repetitive head impacts (RHI).1 Due to current limitations in diagnosing CTE clinically, traumatic encephalopathy syndrome (TES) has been proposed as the clinical presentation of suspected CTE based on findings such as cognitive impairment, behavioral dysregulation, and other supportive features. Due to a weak specificity with wide CIs when assessing the predictive probability of the initial 2014 criteria,2,3 a consensus panel modified each of the diagnostic components and updated the clinical criteria in 2021 (NINDS consensus clinical criteria for traumatic encephalopathy syndrome [ccTES]).4 The panel determined the primary criteria required substantial exposure to RHI, the inclusion of core clinical features, signs and symptoms not fully accounted for by other diagnoses, and grading for the level of functional dependence or dementia. The first 3 of the 4 criteria are required for a definitive diagnosis of TES.4
To date, CTE remains a pathologic diagnosis. The ccTES criteria are intended for research use only and to be used to identify individuals most likely to be harboring CTE pathology. If validated, the ccTES could potentially classify individuals exposed to extensive RHI in epidemiologic studies or select groups for clinical trials. However, much of the available data used in developing the ccTES came from clinicopathologic studies of former American football players. How the ccTES perform in other cohorts exposed to RHI, and among living participants rather than a retrospective review of deceased athletes, are still unknown.
As a neurodegenerative disorder, CTE is considered a progressive condition. As such, having evidence of a progressive course is a core feature of ccTES. Consequently, one would expect that those who fulfill the ccTES would continue to display both clinical and biomarker worsening over time. One of the readily available potential biomarkers is the measurement of MRI regional volumes. Prior work from our group has shown that volumetric loss can be detected prospectively over a several-year period in a cohort of retired fighters in the hippocampus and amygdala.5 Other regions of interest that have been reported to show lower volumes in athletes exposed to RHI include the thalamus, caudate, and corpus callosum.6-9 Examples from other neurodegenerative disorders, such as Alzheimer disease and Parkinson disease, have demonstrated that MRI-based volumetric changes can precede clinical declines such as cognitive impairment and behavioral dysregulation and may be a useful tool to track change over time.10,11 Using a well-characterized cohort of professional fighters, this study aimed to determine whether the ccTES criteria can differentiate those who are more likely to show a temporal decline in cognitive or MRI volumetric imaging measures.
Methods
Data for this analysis were derived from the Professional Athletes Brain Health Study (PABHS), an observational longitudinal study of active and retired professional fighters (boxing, martial arts, and mixed martial arts [MMA]). Athletes enrolled in the PABHS primarily identified as “boxers” or “MMA” fighters, although the general term of “martial arts” was also included as a category for any professional fighter who competed in one of the many specific disciplines such as Jui-Jitsu, Karate, Muay Thai, or Taekwondo. A fourth category, “Mixed,” was included for any athlete who may have competed in more than 1 discipline of boxing and/or martial arts. The Nevada Athletic Commission, fight promoters, and local training facilities were provided with study information for dissemination to active and retired fighters. Active fighters were required to have at least 1 professional fight within the past 2 years during enrollment and be currently training with an intent to compete. Retired fighters required a minimum of 2 years since their most recent sanctioned fight, with no intention to return to competition. Both active and retired fighters required a minimum of 10 professional fights to meet criteria for substantial exposure to RHI and could not have any history of other neurologic or psychiatric disorders. Enrollment in the PABHS has been continuous since 2011. All participants attended annual follow-up visits. Active fighters were required to wait at least 45 days after a sanctioned event before attending their annual visit to minimize acute findings on examination. If an annual visit was missed, the subsequent study visit was conducted at the participant's soonest availability. More detailed methods of recruitment and study procedures have been previously described.12
Standard Protocol Approvals, Registrations, and Patient Consents
The original clinical PABHS was approved by the Cleveland Clinic Institutional Review Board (IRB) #10-944, and written informed consent was obtained from all participants. This study represents a secondary analysis of a deidentified database with no contact with participants; hence, it was submitted to and approved by the University of Nevada Las Vegas (UNLV) IRB for administrative review of exempt research. This study was conducted in full accordance with applicable UNLV Policies and Procedures and Federal and state laws and regulations, including 45 CFR 46 and the HIPAA Privacy Rule. The investigators performed the study in accordance with the study proposal.
Equity, Diversity, and Inclusion Statement
Our study included participants from a wide range of socioeconomic backgrounds. Discussing the limitations of our findings, we acknowledge the lack of representation among female participants and the population specificity due to the inclusion criteria of being a professional fighter. Our research and author team included both junior and senior researchers from a variety of disciplines and cultural backgrounds.
TES Diagnosis and Outcome Classification
A panel of 4 PABHS clinicians used the ccTES criteria to adjudicate a TES positive (TES+) or TES negative (TES−) diagnosis on the first visit in which the athlete was at least 35 years of age or had retired. A full description of the PABHS TES adjudication process has been recently published.13 To adhere to the ccTES, substantial exposure to RHI was measured with the cutoff threshold value of 10 professional fights.14 The core clinical feature of cognitive dysfunction was assessed through memory and executive function performance on the PABHS cognitive test battery, inclusive of the CNS Vital Signs and C3 Logix.15,16 Neurobehavioral dysregulation was not included for diagnosis among the PABHS athletes because it was not incorporated as a data point among the cohort during program initiation. Symptom progression was based on prior cognitive test performance, participant report, and PABHS clinician input. Self-reported medical history, clinician examination by either a neurologist or neuropsychiatrist, MRI findings, and scores on the patient health questionnaire–9 (PHQ-9) were used to rule out other potential contributing disorders. The consensus panel was not able to assign provisional levels of CTE pathology because the PABHS does not evaluate all supportive features in its data.
Cognitive outcomes were additionally derived from the CNS Vital Signs and C3 Logix testing procedures with the PABHS at each annual visit, including raw scores for simple reaction time and choice reaction time and standardized scores for processing speed, psychomotor speed, and reaction time. Processing speed was measured through the number of correct responses on the CNS Vital Signs symbol digit coding minus the number of errors. Psychomotor speed was measured through the total number of finger taps plus the number of correct responses on the symbol digit coding. Simple and choice reaction times were composed of scoring on the Stroop test. The scores were then standardized against age-matched norms.
A high-resolution T1-weighted anatomical MRI was performed at each visit. Between April 2011 and November 2015, a 3T MRI scanner (Siemens [Munich, Germany] Verio was used on all athletes, while the Siemens Skyra with a 32-channel head coil was used to acquire structural 3D T1-weighted magnetization-prepared rapid acquisition gradient echo images (repetition time ms/echo time ms = 2,300/2.98; resolution = 1 × 1 × 1.2 mm3) from December 2015 through the end of the study period. To maintain stringency of data, only high-quality cortical reconstruction from FreeSurfer and a signal-to-noise ratio of at least 16 were used in this study, ensured through a quality control procedure outlined by FreeSurfer's quality analysis tools (FreeSurfer 5.3 QATools, 2021). Based on prior studies evaluating RHI,5,14,17 10 regions of interest (ROI) were prespecified for analysis: hippocampus, corpus callosum, lateral and inferior lateral ventricles, white matter, total gray matter, and subcortical gray matter, including the thalamus, caudate, and putamen. Brain region volumes were calculated using FreeSurfer's automated full-brain segmentation process (version v.6; FreeSurfer, Boston, MA).
Data Analysis
Initial evaluation included descriptive statistics of the population and compared values using t tests for continuous variables (age, years of education, and the number of professional fights) and the Fisher-Freeman-Halton test for categorical variables (sex, race, ethnicity, fighting status, and type of fighting). Odds ratios (ORs) were computed for TES+ compared with TES− in the PABHS cohort to determine the odds of TES+ athletes being retired vs active fighters. The mean values of all hemispherical volumes were calculated to create an average of the left and right cerebral regions (hippocampus, lateral and inferior lateral ventricles, white matter, subcortical gray matter, thalamus, caudate, and putamen). A mean was not calculated for total volumes and corpus callosum volumes because neither distinguished between left and right hemispheres. All athletes completed an initial assessment (visit = 1) and at least 1 or more annual follow-up assessments (visits 2 through 7). The general linear mixed model was used to observe temporal differences in those with a TES+ diagnosis compared with those with a TES− diagnosis over repeated measurements of cognitive and MRI outcomes, while considering the within-subject correlations to control for artifactual variability between visits. All outcomes were predicted by TES diagnosis, age, sex, years of education, and race. Previous analyses of these data found no significant differences in the win/loss record among PABHS athletes12; thus, this predictor was not included in the model. Effect sizes were calculated for mean differences using Cohen's D. MRI outcomes, denoted by 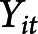 , were additionally predicted by scanner type because the MRI machine was upgraded within the time frame of data collection:
, were additionally predicted by scanner type because the MRI machine was upgraded within the time frame of data collection:
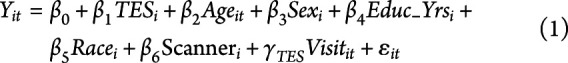 |
where 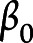 represents the fixed intercept,
represents the fixed intercept, 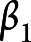 ,
, 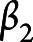 ,
, 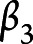 ,
, 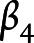 ,
, 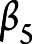 , and
, and 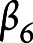 are the fixed-effect coefficients of each of the fixed-effect regressors (TES, age, sex, years of education, race, and scanner),
are the fixed-effect coefficients of each of the fixed-effect regressors (TES, age, sex, years of education, race, and scanner), 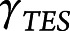 denotes the random slope of visit, and
denotes the random slope of visit, and 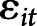 represents the error term.
represents the error term.
Cognitive outcomes were predicted by all independent variables except for scanner type (note the exclusion of the 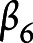 term):
term):
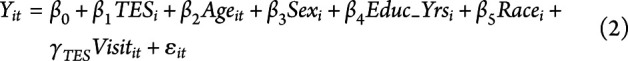 |
Neither model presented normality violations nor significant collinear variables. Model selections for various covariance structures were evaluated using the Akaike information criteria. The first-order autoregressive covariance structure provided the best approximation of the data for fixed effects, while the unstructured covariance structure was used for random effects. All analyses were 2-sided and evaluated with a significance level of 0.05. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
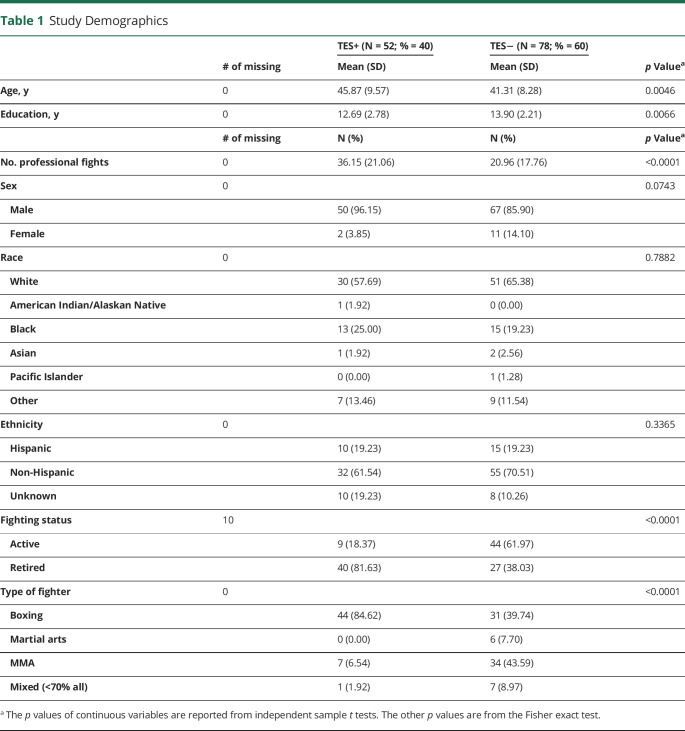
Of more than 700 enrolled fighters, 130 were identified as meeting inclusion in this analysis by having 1 or more follow-up visits, no missing data, and a) retired from professional fighting sports (or) b) an active fighter and being at least 35 years of age. The TES+ group had an average of 5.1 visits per participant (SD = 2.25), while the TES− group had an average of 4.7 visits (SD = 2.24). In this sample, individuals with TES had 7.2 times higher odds of being retired than an active professional boxer (OR = 7.24; 95% CI = 3.04, 17.24). More than 80% of athletes adjudicated as TES+ reported boxing as their sole form of fighting. Likewise, athletes with a TES+ diagnosis were older, had significantly lower education, and fought in a greater number of professional fights (Table 1).
Table 1.
Study Demographics
Regional Brain Volumes
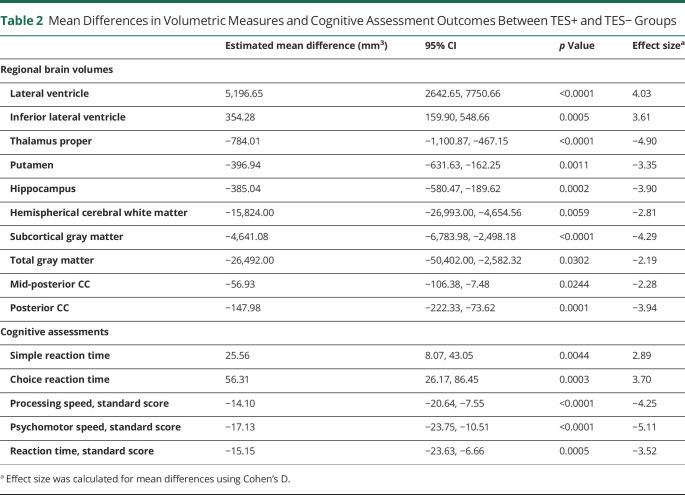
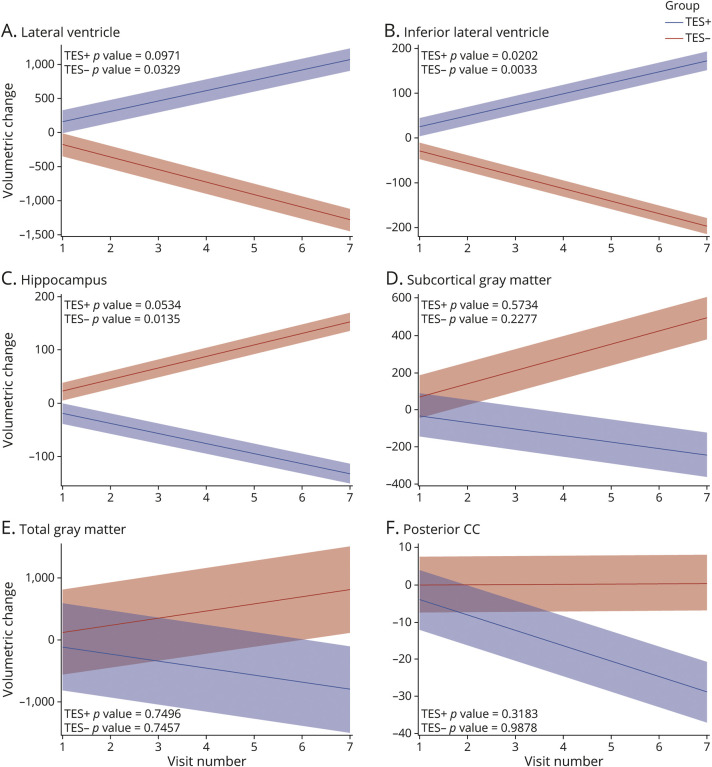
Across all 10 prespecified ROI and controlling for age, sex, education, race, and scanner, there were significant between-group total mean differences in MRI volumes among the TES+ compared with those among the TES− group. The lateral and inferior lateral ventricle volumes were significantly larger in those with a TES+ diagnosis, while the thalamus, putamen, hippocampus, white matter, gray matter, and corpus callosum were all significantly smaller in those with a TES+ diagnosis (Table 2). The average yearly rate of change was also calculated for all outcome measures. Figure provides the mean slope of each ROI among both the TES+ and TES− groups, with the shaded regions indicating the CIs for the slope of each group. The volumetric change increased at a significantly greater rate for both the lateral (estimate = 5,196.65; 95% CI = 2642.65, 7750.66) and inferior lateral ventricles (estimate = 354.28; 95% CI = 159.90, 548.66), with significantly different trends at all time points (Figure, A and B). TES+ volumetric trends for the hippocampus significantly decreased over time (estimate = −385.04, 95% CI = −580.47, −189.62), whereas TES− indicated an insignificant rate of change (Figure C). The rate of change for the subcortical gray matter and posterior corpus callosum volumes were significantly greater in TES+ for visits 3–7 and 4–7, respectively (Figure, D and F). The rate of volumetric change was not calculated for cerebral white matter, putamen, thalamus, and mid-posterior corpus callosum because of a lack of temporal variation within groups. The estimated annual rates of volumetric change are provided in eTable 1 (links.lww.com/WNL/C919).
Table 2.
Mean Differences in Volumetric Measures and Cognitive Assessment Outcomes Between TES+ and TES− Groups
Figure 1. Estimated Linear Trend in Regional Brain Volumes Among TES+ and TES− (in Cubic Millimeters).
X axis for all figures A-F = Visit number, Y axis for all figures = Volumetric change
Cognitive Measures
After controlling for age, sex, education, and race, there were significant between-group total mean differences in reaction times and all standardized cognitive measures among the TES+ compared with those among the TES− groups. Compared with the TES− group, simple reaction time (estimate = 25.56; p value = 0.0044) and choice reaction time (estimate = 56.31; p value = 0.0003) were significantly slower in the TES+ group. While a longitudinal decline in cognitive function was observed in the TES− group, it was significantly less than that of the TES+ group for all standardized cognitive assessments. Standardized processing speed was significantly lower across all visits for the TES+ group (estimate = −14.10; p value <0.0001). Standardized scores for psychomotor speed (estimate = −17.13; p value <0.0001) and reaction time (estimate = −15.15; p value = 0.0005) were also significantly lower (Table 2). The rate of cognitive decline was significantly greater in TES+ for visits 5–7 of standardized reaction time. The estimated annual rates of change for cognitive outcomes are provided in eTable 2 (links.lww.com/WNL/C919).
Discussion
Those with extensive exposure to RHI are at an increased risk of long-term neurologic impairment, including CTE. Although the phenomena of cognitive and motoric impairments were first observed among professional boxers in the early 1900s, termed “punch drunk syndrome,”18 an agreed-upon set of diagnostic clinical features for CTE had not been formulated until recently. In 2021, the first NINDS ccTES were published to reflect the clinical syndrome of CTE. One potential use of such criteria would be to identify individuals who may most likely develop a progressive neurodegenerative condition. However, the TES criteria have yet to be validated, and the subsequent course of those who fulfill the criteria is unknown. In this study, we examined the trajectory of MRI regional brain volumes and cognitive assessments among professional fighters, assessing the longitudinal differences between those who fulfill the criteria for TES based on the consensus criteria and those who do not.
Over a 6-year period of observation, there was a significant association between TES and regional MRI-based volumetric measurements and cognitive decline. A variety of cortical and subcortical structures showed a decline in the average yearly volume, whereas ventricular volumes increased in size. Individuals adjudicated as TES− presented with relatively minimal change among all volumetric outcomes. As with the volumetric outcomes, the cognitive outcomes also indicated significant differences between TES+ and TES− groups over time. Though by definition, the TES+ group required a history of progressive cognitive impairment for diagnosis, the domains involved were not just in executive or memory scores but also included declines in simple and choice reaction times. The TES− individuals did, as a group, show a longitudinal decline in cognitive function but to a lesser degree than the TES+ group.
The MRI findings in the TES+ group are not surprising based on prior work. Previous studies in this cohort have reported lower regional volumes and ventriculomegaly associated with exposure to RHI, though none have assessed changes using TES criteria.19-27 Longitudinal atrophy was observed in subcortical structures such as the thalamus and corpus callosum among active fighters, while atrophy of cortical structures such as the amygdala and hippocampus was observed in retired fighters compared with controls.5 Likewise, ventriculomegaly and atrophy of hippocampal and amygdala volumes have been found to be related to increased years of professional football participation.28 Prior work also described the correlation between decreased regional brain volumes and diminished cognitive functioning.14,29,30 However, in the professional fighter longitudinal analysis mentioned earlier, there was no relationship between volume decline and cognitive changes.5 This study provided a longer observational period than previously observed in the PABHS cohort analyses and may provide rationale for why cognitive changes were not previously observed, therefore leading to speculation that the cognitive decline might manifest in a delayed manner, with neuropathologic changes preceding clinical impairment. As such, because we required the presence of progressive cognitive impairment to fulfill the diagnosis of TES, the individuals in our study may be further along in the pathologic process. The TES+ classification does identify a group that is more likely to subsequently decline both clinically and with MRI as a biomarker.
Because the diagnostic criteria of TES were recently published, there are currently no additional analyses specifically on TES to contrast with our findings. Literature published before the NINDS consensus has a high level of heterogeneity, making it difficult to compare our results with previous cohorts accurately or with other types of athletes experiencing RHI. If validated in other groups exposed to RHI, the TES+ criteria could be applied to identify interventional study cohorts or classify groups in observational research. It is important to emphasize that the TES criteria are not yet intended for use in the clinical setting.
On reflection of our findings, there are certain limitations to be considered. The PABHS cohort is a convenience sample, potentially biasing the generalizability of the prevalence of TES in combat sports. However, previous studies of this cohort did compare those who entered the study as active fighters with a random sample of individuals who were licensed professional combatants in Nevada over the same time, with no significant differences observed in age, the number of fights, or win/loss record.12 In adjudicating whether cognitive impairment was present, a cutoff was used of 1.5 SDs below age and education norms on standardized cognitive tests. Given the varied socioeconomic backgrounds of the PABHS cohort, these norms may not be applicable to a subset of the cohort and may have added a bias to the classification. Future research developing a composite score for cognitive impairment may be of benefit. Similarly, the determination of executive impairment required only 1 abnormal score and clinician judgment; therefore, by only requiring 1 test to be abnormal, it is possible that some individuals were misclassified. Because this is a secondary analysis, all data were previously collected, thus limiting other potential analyses that may require larger sample sizes. Specifically, differences among certain demographic categories, such as sex and race, were unable to be assessed due to inadequate sample sizes. Likewise, neurobehavioral dysregulation was unable to be included for diagnosis among the PABHS athletes because it was not incorporated as a data point among the cohort during program initiation. Although analyses were adjusted for age, the TES+ group was older than the TES− group, which could affect volume measures. In addition, all data collected were self-reported by the athletes, and collateral information from an informant was not used. Because there are active fighters in both the TES+ and TES− groups, it is difficult to quantify the effect of ongoing exposure to RHI on regional brain volumes or cognitive measures. This study provides a strong foundation for hypothesis generation that may inform future studies and suggests the continued need to assess outcomes at longer temporal intervals.
In summary, this study reports the first longitudinal assessment of individuals who fulfilled the ccTES. Our results suggest that the ccTES can identify a group that is more likely to have worsening MRI volumetric change and cognitive performance over time, indicating a progressive course of those who are positive, whereas those who are negative remain relatively stable. Further clinicopathologic investigations are warranted to validate the accuracy of the ccTES criteria in detecting CTE or other pathologies that may have a progressive course.
Acknowledgment
The authors thank the PABHS research team at the Lou Ruvo Center for Brain Health in Las Vegas for their tremendous work on this cohort. Equally, the authors thank all the athletes who have volunteered their time to understand the long-term effects of repetitive head trauma.
Glossary
- ccTES
clinical criteria for traumatic encephalopathy syndrome
- CTE
chronic traumatic encephalopathy
- MMA
mixed martial arts
- OR
odds ratio
- PABHS
Professional Athletes Brain Health Study
- PHQ-9
patient health questionnaire–9
- RHI
repetitive head impacts
- ROI
regions of interest
- TES
traumatic encephalopathy syndrome
Appendix. Authors
Footnotes
Editorial, page 461
Study Funding
The authors report no targeted funding.
Disclosure
C. Bernick has received research funding from the UFC, Top Rank Promotions, Haymon Boxing, and Bellator Promotions. All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Alosco ML, Stern RA. The long-term consequences of repetitive head impacts: chronic traumatic encephalopathy. Handb Clin Neurol. 2019;167:337-355. [DOI] [PubMed] [Google Scholar]
- 2.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mez J, Alosco ML, Daneshvar DH, et al. Validity of the 2014 traumatic encephalopathy syndrome criteria for CTE pathology. Alzheimers Dement. 2021;17(10):1709-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz DI, Bernick C, Dodick DW, et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology. 2021;96(18):848-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernick C, Shan G, Zetterberg H, et al. Longitudinal change in regional brain volumes with exposure to repetitive head impacts. Neurology. 2020;94(3):e232-e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant BR, Narapareddy BR, Bray MJC, et al. The effect of age of first exposure to competitive fighting on cognitive and other neuropsychiatric symptoms and brain volume. Int Rev Psychiatry. 2020;32(1):89-95. [DOI] [PubMed] [Google Scholar]
- 7.Asken BM, Rabinovici GD. Identifying degenerative effects of repetitive head trauma with neuroimaging: a clinically-oriented review. Acta Neuropathol Commun. 2021;9(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner RC, Hess CP, Brus-Ramer M, et al. Cavum septum pellucidum in retired american pro-football players. J Neurotrauma. 2016;33(1):157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Wu J, Bullen J, et al. Association of cavum septum pellucidum and cavum vergae with cognition, mood, and brain volumes in professional fighters. JAMA Neurol. 2020;77(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer JB, Magda S, Airriess C, Smith ME. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR Am J Neuroradiol. 2009;30(3):578-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Fu Y, Zeng Y-F, et al. Six visual rating scales as a biomarker for monitoring atrophied brain volume in Parkinson's disease. Aging Dis. 2020;11(5):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernick C, Banks S, Phillips M, et al. Professional fighters brain health study: rationale and methods. Am J Epidemiol. 2013;178(2):280-286. [DOI] [PubMed] [Google Scholar]
- 13.Ritter A, Shan G, Montes A, Randall R, Bernick C. Traumatic encephalopathy syndrome: application of new criteria to a cohort exposed to repetitive head impacts. Br J Sports Med. 2023;57(7):389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernick C, Banks SJ, Shin W, et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the Professional Fighters' Brain Health Study. Br J Sports Med. 2015;49(15):1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623-643. [DOI] [PubMed] [Google Scholar]
- 16.Simon M, Maerlender A, Metzger K, Decoster L, Hollingworth A, Valovich McLeod T. Reliability and concurrent validity of select C3 logix test components. Developmental Neuropsychol. 2017;42(7-8):446-459. [DOI] [PubMed] [Google Scholar]
- 17.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25(3):350-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martland HS. Punch drunk. J Am Med Assoc. 1928;91(15):1103-1107. [Google Scholar]
- 19.Poca MA, Sahuquillo J, Mataro M, Benejam B, Arikan F, Baguena M. Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J Neurotrauma. 2005;22(11):1303-1310. [DOI] [PubMed] [Google Scholar]
- 20.Lepage C, Muehlmann M, Tripodis Y, et al. Limbic system structure volumes and associated neurocognitive functioning in former NFL players. Brain Imaging Behav. 2019;13(3):725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesman-Segev OH, La Joie R, Stephens ML, et al. Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. Neuroimage Clin. 2019;24:102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra V, Sreenivasan K, Banks SJ, et al. Investigating structural and perfusion deficits due to repeated head trauma in active professional fighters. Neuroimage Clin. 2018;17:616-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra VR, Sreenivasan KR, Zhuang X, et al. Understanding white matter structural connectivity differences between cognitively impaired and nonimpaired active professional fighters. Hum Brain Mapp. 2019;40(17):5108-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernick C, Banks S, Mahmoud S, Lowe M, Phillips M, Modic M. The Threshold Effect of Repeated Head Trauma on Brain. American Academy of Neurology; 2012. [Google Scholar]
- 25.Singh R, Meier TB, Kuplicki R, et al. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2014;311(18):1883-1888. [DOI] [PubMed] [Google Scholar]
- 26.Schultz V, Stern RA, Tripodis Y, et al. Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional American football players. J Neurotrauma. 2018;35(2):278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole JH, Jolly A, de Simoni S, et al. Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain. 2018;141(3):822-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misquitta K, Dadar M, Tarazi A, et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. Neuroimage Clin. 2018;19:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra VR, Zhuang X, Sreenivasan KR, et al. Multimodal MR imaging signatures of cognitive impairment in active professional fighters. Radiology. 2017;285(2):555-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs. 2014;28(2):147-156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.