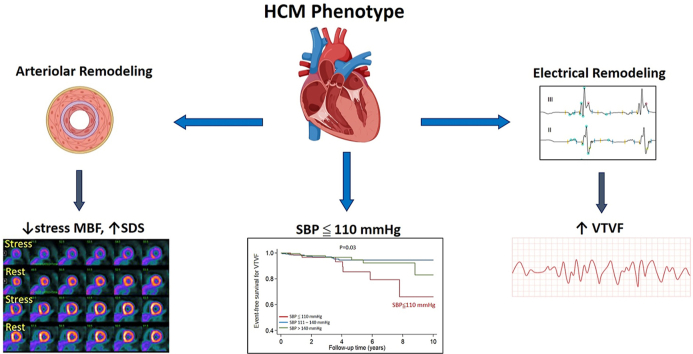
Abstract
Background
Coronary microvascular dysfunction (CMD) and hypertension (HTN) occur frequently in hypertrophic cardiomyopathy (HCM), but whether blood pressure (BP) influences CMD and outcomes is unknown.
Objective
The purpose of this study was to test the hypothesis that HTN is associated with worse CMD and outcomes.
Methods
This retrospective study included 690 HCM patients. All patients underwent cardiac magnetic resonance imaging, echocardiography, and rhythm monitoring; 127 patients also underwent rest/vasodilator stress 13NH3 positron emission tomography myocardial perfusion imaging. Patients were divided into 3 groups based on their rest systolic blood pressure (SBP) (group 1 ≤110 mm Hg; group 2 111–140; group 3 >140 mm Hg) and were followed for development of ventricular tachycardia (VT)/ventricular fibrillation (VF), heart failure (HF), death, and composite outcome.
Results
Group 1 patients had the lowest age and left ventricular (LV) mass but the highest prevalence of nonobstructive hemodynamics and restrictive diastolic filling. LV scar was similar in the 3 groups. Group 1 had the lowest rest and stress myocardial blood flow (MBF) and highest SDS (summed difference score). Rest SBP was positively correlated with stress MBF and negatively correlated with SDS. Group 1 had the highest incidence of VT/VF, whereas the incidences of HF, death, and composite outcome were similar among the 3 groups. In multivariate analysis, rest SBP ≤110 mm Hg was independently associated with VT/VF (hazard ratio 2.6; 95% confidence interval 1.0–6.7; P = .04).
Conclusion
SBP ≤110 mm Hg is associated with greater severity of CMD and coronary microvascular ischemia and higher incidence of ventricular arrhythmias in HCM.
Keywords: Hypertrophic cardiomyopathy, Myocardial blood flow, Rest systolic blood pressure, Summed difference score, Ventricular tachycardia, Ventricular fibrillation
Graphical abstract
Key Findings.
-
▪
Hypertrophic cardiomyopathy (HCM) patients with systolic blood pressure (SBP) ≤110 mm Hg are at high risk for lethal ventricular arrhythmias.
-
▪
SBP ≤110 mm Hg is associated with severe microvascular dysfunction and myocardial ischemia.
-
▪
SBP ≤110 mm Hg is a marker for severe cardiac HCM phenotype.
Introduction
Hypertrophic cardiomyopathy (HCM) is characterized by myocyte hypertrophy, fibrosis, electrical and microvascular remodeling.1, 2, 3 Angina resulting from myocardial ischemia due to coronary microvascular dysfunction (CMD) is common in HCM. However, the molecular mechanisms by which mutant sarcomeric proteins lead to medial hypertrophy of intramural coronary arterioles/arteries and CMD is unknown.
A significant proportion of HCM patients have hypertension (HTN).4 In the general population, HTN is associated with left ventricular (LV) hypertrophy, diastolic dysfunction, and microvascular remodeling consisting of medial hypertrophy,5 perivascular fibrosis, and CMD.6,7 However, the relationship between blood pressure, CMD, and cardiovascular outcomes has not been examined in HCM. Because of similarities in microvascular remodeling8 in HCM and HTN, we hypothesized that HCM patients with HTN would have greater severity of CMD and worse outcomes. In order to test this hypothesis, we performed a retrospective analysis of 690 HCM patients from the Johns Hopkins HCM Registry,9 stratified by systolic blood pressure (SBP), and examined the association of blood pressure with myocardial blood flow (MBF) and cardiovascular outcomes.
Methods
The HCM Registry is approved by the Institutional Review Boards of the Johns Hopkins Hospital and the University of California San Francisco (UCSF). All patients met the standard diagnostic criteria for HCM, namely left ventricular hypertrophy (LVH) ≥15 mm in the absence of other causes such as uncontrolled HTN, valvular heart disease and HCM phenocopies.10 Patients were enrolled in the Johns Hopkins HCM Registry from 2005–2016 during their first clinic visit. The research in this study was conducted according to the Helsinki Declaration guidelines on human research. Informed consent was obtained for use of medical records for research purposes. During their first clinic visit, all patients underwent rest and exercise echocardiography, 24-hour Holter monitoring, or implantable cardioverter-defibrillator (ICD) interrogation, and contrast-enhanced cardiac magnetic resonance (CMR) imaging as part of their clinical evaluation. Clinical data, which included symptoms, comorbidities, medications, and family history of HCM and sudden cardiac death, was ascertained by the examining physician (MRA, TPA) during the clinic visits. Self-reported functional capacity was graded according to the New York Heart Association classification.11 All patients were advised to meet with a genetic counselor and were offered clinical genotyping.
Patients who were asymptomatic or had stable symptoms were followed yearly; symptomatic patients were followed more frequently (every 1–3 months). During yearly follow-up visits, patients underwent exercise echocardiography and Holter monitoring or ICD interrogation. Patients with an ICD underwent device interrogation every 6 months or more frequently if they were symptomatic or experienced ICD discharges. Patients without an ICD, who had palpitations but no evidence of arrhythmias on Holter monitor or exercise electrocardiogram, were provided event monitors to document cardiac rhythm during symptoms.
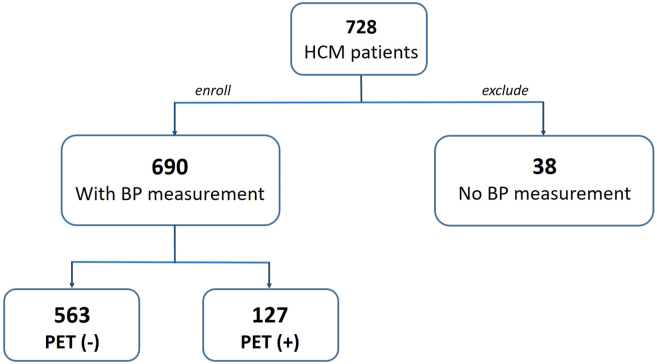
We retrospectively screened 728 HCM patients. Of these patients, 38 were excluded because of unavailable BP measurements (Figure 1). We used SBP to stratify patients because (1) SBP is reported to be superior to diastolic blood pressure (DBP) and mean arterial pressure (MAP) in terms of reflecting tissue perfusion12; and (2) SBP has a wider distribution and better separation compared to DBP in our patient cohort. HCM patients (n = 690) were divided into 3 groups using 2 cutoffs of rest SBP obtained before stress echocardiography: group 1 (≤110 mm Hg); group 2 (111–140 mm Hg)l and group 3 (>140 mm Hg). The lower cutoff point of 110 mm Hg was determined by locally weighted scatterplot smoothing analysis (Supplemental Figure 1), and the upper cutoff of 140 mm Hg was set at the threshold of stage 2 HTN.13
Figure 1.
Flowchart illustrating hypertrophic cardiomyopathy (HCM) patient selection. BP = blood pressure; PET = positron emission tomography.
Detailed cardiac imaging protocols and data analysis methods are given in the Supplemental Appendix. In brief, transthoracic echocardiography was performed at baseline and after maximum exercise on a treadmill. HCM subtypes were defined based on peak LV pressure gradients measured at the level of the left ventricular outflow tract (LVOT) and mid-LV, at rest, and after provocation (Valsalva, exercise, amyl nitrite). HCM was classified as nonobstructive (gradients <30 mm Hg at rest and provocation); labile obstructive (rest gradients <30 mm Hg, provoked gradients ≥30 mm Hg); or obstructive (rest and provoked gradients ≥30 mm Hg). CMR imaging was performed using a 1.5-T system, before/after contrast (gadopentetate dimeglumine 0.2 mmol/kg). LV mass and late gadolinium enhancement (LV-LGE) were quantified. Pixels with signal intensity >6 SD above the mean of normal myocardium were quantified as LV-LGE.14,15
Myocardial perfusion imaging was performed using a GE Discovery VCT positron emission tomography (PET)/computed tomographic system (GE Healthcare, Waukesha, WI) and a 1-day rest/stress protocol. Vasodilator was administered 60 minutes after injection of the rest tracer dose (370 MBq of 13N-ammonia). Rest SBP before PET imaging was used to stratify patients who underwent PET imaging into 3 groups. Myocardial flow reserve (MFR) was defined as the ratio of stress MBF to rest MBF. Coronary vascular resistance (CVR) was defined as MAP divided by mean MBF at rest and stress.16 Corrected rest MBF and Corrected MFR were computed as follows: Corrected rest MBF = (MBFrest × 10,000/RPPrest), where RPP = rate–pressure product17; and Corrected MFR = (Stress MBF/Corrected rest MBF).6,8,18 The summed stress score (SSS), summed rest score (SRS), and summed difference score (SDS) (SSS – SRS = SDS) were computed to assess regional differences in myocardial perfusion (inducible ischemia).
We defined 4 outcomes in this study: (1) sustained ventricular tachycardia (VT) (ventricular rate ≥130 bpm lasting for ≥30 seconds) or ventricular fibrillation (VF); (2) heart failure (HF), defined as new-onset or worsening HF to New York Heart Association functional class III or IV requiring hospitalization; (3) all-cause death; and (4) a composite outcome including the individual outcomes. If a patient had multiple adverse events, every event was included for the calculation of individual outcomes. However, for the calculation of the composite outcome, only the first event was counted. (For details of follow-up, see the Supplemental Appendix.)
All analyses were performed using STATA 14 (StataCorp LP, College Station, TX). Descriptive statistics were performed on patient demographics, hemodynamics, imaging parameters, and outcomes, stratified by SBP categories. Normality of distribution was determined via kernel density plots and the Shapiro-Wilk test. Continuous variables are given as mean ± SD and categorical variables as the total number and percentage. Differences in baseline characteristics among SBP subgroups were determined using analysis of variance for continuous variables and χ2 tests for categorical variables. The association between hemodynamic, echocardiographic, CMR, and PET parameters was analyzed by Pearson correlation. Kaplan-Meier analysis was used to examine the cumulative event-free survival for VT/VF, HF, all-cause death, and composite outcome, and significance was based on results of the log-rank test. The Cox proportional hazards model was developed to determine the association of SBP with individual endpoints with adjustment for age, sex, HCM type, and history of ICD implantation. HCM patients with SBP 111–140 mm Hg were considered the reference group. P <.05 was considered significant.
Results
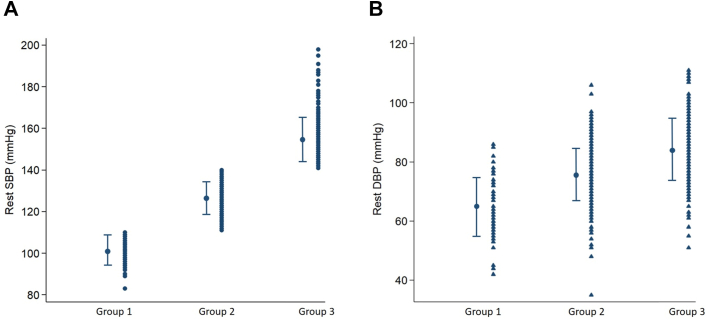
We retrospectively studied 690 HCM patients (mean age 53 ± 15 years; 64% men). Eighty patients had rest SBP ≤110 mm Hg (group 1; mean SBP 102; DBP 65; MAP 78), 395 patients had rest SBP 111–140 mm Hg (group 2; mean SBP 126; DBP 76; MAP 93), and 215 patients had rest SBP >140 mm Hg (group 3; mean SBP 154; DBP 84; MAP 107) (Table 1 and Figure 2). Group 1 had a higher proportion of younger patients, women, nonobstructive hemodynamics, history of VT/VF, and ICD implantation compared to groups 2 and 3. Analysis of exercise data revealed that group 3 (rest SBP >140 mm Hg) had the lowest exercise capacity and peak exercise heart rate, but resting heart rates were similar among the 3 groups. Group 1 had the highest prevalence of familial HCM (Table 1), but clinical genotyping performed in a small proportion of patients (n = 121) revealed a similar prevalence of pathogenic variants in the 3 groups (Supplemental Table 1).
Table 1.
Clinical characteristics of HCM population (N = 690) stratified by SBP
| Group 1 (≤110 mm Hg) (N = 80) | Group 2 (111–140 mm Hg) (N = 395) | Group 3 (>140 mm Hg) (N = 215) | P value | |
|---|---|---|---|---|
| Age (y) | 46 ± 15 | 50 ± 14 | 60 ± 13 | <.001 |
| Male | 44 (55) | 270 (68) | 127 (59) | .02 |
| Race | .6 | |||
| White | 7 (9) | 298 (75) | 158 (74) | |
| Black | 63 (79) | 52 (13) | 37 (17) | |
| Other | 10 (12) | 45 (12) | 20 (9) | |
| Body mass index (kg/m2) | 29 ± 6 | 29 ± 6 | 30 ± 7 | .1 |
| HCM type | .007 | |||
| Nonobstructive | 35 (44) | 127 (32) | 51 (24) | |
| Labile | 19 (24) | 144 (37) | 92 (43) | |
| Obstructive | 26 (32) | 124 (31) | 72 (33) | |
| NYHA functional class | .3 | |||
| I | 49 (61) | 213 (54) | 110 (51) | |
| II | 27 (34) | 134 (34) | 82 (38) | |
| III | 4 (5) | 48 (12) | 23 (11) | |
| Pathogenic mutation∗ | 11 (48) | 33 (47) | 6 (22) | .07 |
| Family history of HCM | 24 (30) | 84 (21) | 27 (13) | .002 |
| History of VT/VF | 7 (9) | 15 (4) | 3 (1) | .02 |
| History of ICD implantation | 13 (16) | 39 (10) | 12 (5) | .02 |
| Angina | 23 (29) | 168 (43) | 85 (40) | .07 |
| Dyspnea at exertion | 36 (45) | 222 (56) | 122 (57) | .2 |
| Syncope | 28 (35) | 75 (19) | 30 (14) | .001 |
| AHA SCD risk score† (%) | 4.12 ± 2.12 | 3.71 ± 2.72 | 3.41 ± 3.14 | .2 |
| Comorbidities | ||||
| History of hypertension | 18 (23) | 162 (41) | 160 (74) | <.001 |
| History of diabetes | 7 (9) | 29 (7) | 29 (14) | .05 |
| History of hyperlipidemia | 24 (30) | 178 (45) | 118 (55) | .001 |
| History of stroke | 3 (4) | 8 (2) | 8 (4) | .4 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | .3 |
| Medications | ||||
| Beta-blocker | 56 (70) | 277 (70) | 158 (74) | .7 |
| Calcium channel blocker | 11 (14) | 95 (24) | 82 (38) | <.001 |
| RAS blockade | 15 (19) | 78 (20) | 68 (32) | .002 |
| Echocardiography | ||||
| Septal wall thickness (mm) | 21 ± 5 | 21 ± 6 | 21 ± 5 | .8 |
| Posterior wall (mm) | 11 ± 3 | 12 ± 3 | 12 ± 4 | .005 |
| Left atrial diameter (mm) | 41 ± 7 | 42 ± 8 | 43 ± 7 | .2 |
| E/A | 1.5 ± 0.8 | 1.4 ± 0.7 | 1.2 ± 0.8 | .001 |
| E/A >2 | 17 (21) | 58 (16) | 18 (9) | .01 |
| E-wave DT (ms) | 231 ± 65 | 236 ± 70 | 257 ± 72 | .001 |
| E-wave DT <140 ms | 5 (6) | 17 (5) | 6 (3) | .4 |
| E/e′ | 18 ± 11 | 17 ± 10 | 20 ± 11 | .009 |
| Rest LVOT (peak) gradient (mm Hg) | 30 ± 40 | 28 ± 29 | 31 ± 33 | .4 |
| Stress LVOT (peak) gradient (mm Hg) | 58 ± 56 | 70 ± 54 | 75 ± 55 | .06 |
| LV GLS (%) | –15.9 ± 4.2 | –15.8 ± 3.7 | –16.2 ± 3.6 | .5 |
| CMR | ||||
| LVEDVI‡ (mL/m2) | 76 ± 20 | 72 ± 15 | 73 ± 15 | .5 |
| LVESVI‡ (mL/m2) | 27 ± 12 | 26 ± 9 | 24 ± 9 | .3 |
| LVEF‡ (%) | 68 ± 10 | 68 ± 9 | 70 ± 10 | .02 |
| Cardiac index‡ (L/m2) | 3.2 ± 0.9 | 3.1 ± 0.9 | 3.4 ± 1.0 | .007 |
| LV mass index‡ (g/m2) | 73 ± 28 | 77 ± 28 | 85 ± 32 | .02 |
| LGE presence† | 38 (66) | 216 (69) | 112 (68) | .8 |
| LGE percentage (% of LV mass)‡ | 16 ± 11 | 15 ± 13 | 13 ± 10 | .2 |
| Exercise stress hemodynamics | ||||
| METs | 10.2 ± 3.8 | 10.4 ± 4.3 | 8.6 ± 3.9 | <.001 |
| Rest (pre-exercise) HR (bpm) | 64 ± 13 | 66 ± 13 | 67 ± 14 | .4 |
| Rest (pre-exercise) SBP (mm Hg) | 102 ± 6 | 126 ± 8 | 154 ± 11 | <.001 |
| Rest (pre-exercise) DBP (mm Hg) | 65 ± 10 | 76 ± 9 | 84 ± 11 | <.001 |
| Rest (pre-exercise) PP (mm Hg) | 37 ± 9 | 50 ± 10 | 70 ± 14 | <.001 |
| Rest (pre-exercise) MAP (mm Hg) | 78 ± 7 | 93 ± 8 | 107 ± 9 | <.01 |
| Rest (pre-exercise) RPP (bpm∗mm Hg) | 6586 ± 1374 | 8252 ± 1751 | 10,249 ± 2295 | <.001 |
| Exercise stress HR (bpm) | 147 ± 29 | 143 ± 29 | 135 ± 27 | <.001 |
| Exercise stress SBP (mm Hg) | 135 ± 31 | 153 ± 33 | 178 ± 34 | <.001 |
| Exercise stress DBP (mm Hg) | 74 ± 15 | 78 ± 16 | 87 ± 19 | <.001 |
| Exercise stress PP (mm Hg) | 61 ± 27 | 75 ± 29 | 91 ± 32 | <.001 |
| Exercise stress MAP (mm Hg) | 94 ± 18 | 103 ± 19 | 117 ± 20 | <.001 |
| Exercise stress RPP (bpm∗mm Hg) | 20,082 ± 6741 | 22,198 ± 7420 | 24,075 ± 6878 | <.001 |
| ABPR | 21 (27) | 141 (37) | 69 (34) | .2 |
| Myectomy during follow-up | 18 (23) | 95 (24) | 47 (22) | .8 |
| Adverse outcomes | ||||
| VT/VF | 8 (10) | 12 (3) | 7 (3) | .04 |
| HF | 8 (10) | 18 (5) | 13 (6) | .2 |
| Death | 2 (3) | 5 (1) | 8 (4) | .1 |
| Composite outcome | 13 (16) | 30 (8) | 20 (9) | .07 |
Values are given as mean ± SD or n (%) unless otherwise indicated.
ABPR = abnormal blood pressure response to exercise (SBP increase <20 mm Hg or SBP decrease during exercise); AHA = American Heart Association; CMR = cardiac magnetic resonance; DBP = diastolic blood pressure; DT = deceleration time; E/A = ratio of early diastolic mitral flow velocity (E) to late diastolic mitral flow velocity (A); E/e = ratio of early diastolic mitral flow velocity (EO to early diastolic mitral septal annulus motion velocity; GLS = global longitudinal systolic strain; HCM = hypertrophic cardiomyopathy; HF = heart failure; HR = heart rate; ICD = implantable cardioverter-defibrillator; LGE = late gadolinium enhancement; LV = left ventricle; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVI = left ventricular end-systolic volume index; LVOT = left ventricular outflow tract; MAP = mean arterial pressure (MAP = DBP + 1/3 PP); METs = metabolic equivalents; NYHA = New York Heart Association; PP = pulse pressure (PP = SBP – DBP); RAS blockade = angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker; RPP = rate–pressure product; SBP = systolic blood pressure; SCD = sudden cardiac death; VF = ventricular fibrillation; VT = ventricular tachycardia.
Available N = 121.
available N = 535.
available N = 363.
Figure 2.
Distribution of rest systolic blood pressure (SBP) and rest diastolic blood pressure (DBP) in hypertrophic cardiomyopathy patients. Group 1: Rest SBP ≤110 mm Hg; group 2: rest SBP 111–140 mm Hg; group 3: rest SBP >140 mm Hg.
Group 1 had the highest proportion of patients with restrictive LV diastolic hemodynamics reflected by E/A ratio >2 and lowest E-wave deceleration time (Table 1). Group 3 exhibited the highest LV ejection fraction and LV mass. However, LV global longitudinal peak systolic strain, maximum septal thickness, rest and stress LVOT gradients, left atrial diameter, prevalence, and quantity of LV scar (reflected by LV-LGE) were similar among the 3 groups.
Heart rate at baseline and after vasodilator stress were similar in the 3 groups, but SBP, DBP, and MAP were significantly lower at both timepoints in group 1 compared to groups 2 and 3 (Table 2). As a result, group 1 demonstrated the lowest RPP at rest and after vasodilator stress. Corrected rest MBF values (MBFrest × 10,000/RPPrest) were in the normal range compared to previously published data from healthy individuals17 and were similar in the 3 groups. The main differences observed were in response to vasodilator stress, with group 1 demonstrating the lowest hyperemic (stress) MBF and highest SDS reflecting greater severity of inducible ischemia. Corrected MFR was lowest in group 1, but the differences were not statistically significant. Rest and stress CVR were similar in the 3 groups.
Table 2.
Hemodynamic and PET characteristics of HCM patient subgroup (N = 127) stratified by SBP∗
| Group 1 (≤110 mm Hg) (N = 12) | Group 2 (110–140 mm Hg) (N = 70) | Group 3 (>140 mm Hg) (N = 45) | P value | |
|---|---|---|---|---|
| Hemodynamics prevasodilator (rest) | ||||
| Rest HR (bpm) | 63 ± 7 | 65 ± 13 | 63 ± 9 | .6 |
| Rest SBP (mm Hg) | 106 ± 3 | 128 ± 7 | 159 ± 17 | <.001 |
| Rest DBP (mm Hg) | 56 ± 6 | 66 ± 7 | 78 ± 12 | <.001 |
| Rest PP (mm Hg) | 50 ± 7 | 62 ± 8 | 82 ± 14 | <.001 |
| Rest MAP (mm Hg) | 72 ± 4 | 86 ± 6 | 105 ± 12 | <.001 |
| Rest RPP (bpm∗mm Hg) | 6621 ± 606 | 8366 ± 1778 | 10,075 ± 1662 | <.001 |
| Hemodynamics at peak vasodilator (stress) | ||||
| Vasodilator stress HR (bpm) | 98 ± 16 | 98 ± 17 | 94 ± 15 | .5 |
| Vasodilator stress SBP (mm Hg) | 107 ± 11 | 134 ± 22 | 159 ± 24 | <.001 |
| Vasodilator stress DBP (mm Hg) | 55 ± 8 | 65 ± 10 | 74 ± 9 | <.001 |
| Vasodilator stress PP (mm Hg) | 51 ± 8 | 68 ± 17 | 85 ± 18 | <.001 |
| Vasodilator stress MAP (mm Hg) | 72 ± 8 | 88 ± 13 | 102 ± 13 | <.001 |
| Vasodilator stress RPP (bpm∗mm Hg) | 10,400 ± 1618 | 13,102 ± 3368 | 14,984 ± 3496 | <.001 |
| ECG during vasodilator (stress) | N=14 | N=72 | N=42 | |
| Ischemic ST-T changes | 5 (36) | 22 (31) | 11 (26) | .8 |
| Amplitude of ST depression (mV) | 0.1 ± 0.08 | 0.07 ± 0.09 | 0.05 ± 0.07 | .2 |
| Myocardial blood flow parameters | ||||
| Rest MBF (mL/g/min) | 0.81 ± 0.27 | 0.92 ± 0.28 | 1.06 ± 0.49 | .04 |
| Corrected rest MBF† (mL/g/min) | 1.15 ± 0.36 | 1.16 ± 0.48 | 1.01 ± 0.21 | .2 |
| Vasodilator stress MBF (mL/g/min) | 1.77 ± 0.53 | 2.01 ± 0.61 | 2.28 ± 0.77 | .03 |
| MFR | 2.28 ± 0.37 | 2.33 ± 0.77 | 2.56 ± 0.94 | .3 |
| Corrected MFR‡ (mL/g/min) | 1.82 ± 0.71 | 2.03 ± 0.89 | 2.11 ± 0.87 | .6 |
| Rest CVR (mm Hg/mL/g/min) | 100.89 ± 39.13 | 102.18 ± 30.28 | 111.67 ± 36.59 | .3 |
| Vasodilator stress CVR (mm Hg/mL/g/min) | 45.70 ± 18.51 | 48.76 ± 20.96 | 51.85 ± 24.51 | .6 |
| CVR change with vasodilator (%) | 57 ± 12 | 52 ± 17 | 51 ± 16 | .5 |
| SDS | 9.4 ± 5.6 | 4.7 ± 4.3 | 4.5 ± 5.4 | .006 |
CVR = coronary vascular resistance; ECG = electrocardiography; MBF = myocardial blood flow; MFR = myocardial flow reserve; PET = positron emission tomography; SDS = summed difference score; other abbreviations as in Table 1.
SBP obtained before PET examination.
Corrected rest MBF = MBFrest × 10,000/ RPPrest.
Corrected MFR = Stress MBF/corrected rest MBF.
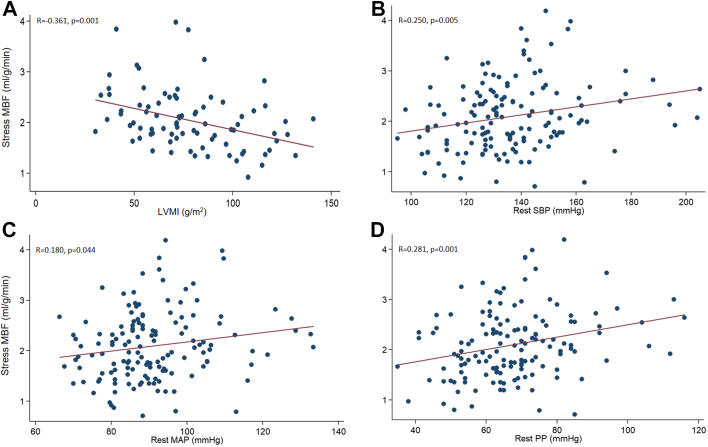
Because coronary perfusion pressure, cardiac work, and metabolic demand influence myocardial perfusion,19 we examined the correlation of MBF obtained by PET with hemodynamic and LV structural features (Table 3). Both rest and hyperemic MBF were positively correlated with age, rest pulse pressure (PP), mitral E and A waves, and E/e′ ratio. As expected, rest MBF was positively correlated with rest heart rate; rest MBF also was positively correlated with provoked peak LVOT gradient. Furthermore, hyperemic MBF was negatively correlated with LV mass and positively correlated with rest SBP and MAP. Inducible ischemia (SDS) was negatively correlated with age, SBP, MAP, PP at rest/vasodilator stress, mitral A wave, and rest and provoked LVOT gradients. Taken together, our univariate analysis in HCM patients suggests an inverse correlation between LV mass and hyperemic (stress) MBF (Figure 3A), and a positive correlation of rest SBP, rest MAP, and rest PP with hyperemic MBF (Figures 3B–3D).
Table 3.
Pearson correlations between hemodynamic parameters, LV structural parameters, and MBF in HCM subgroup with PET imaging (n = 127)
| Rest MBF | Stress MBF | MFR | SDS | |
|---|---|---|---|---|
| Age (y) | 0.277∗ | 0.226∗ | –0.021 | –0.259∗ |
| Rest hemodynamic parameters | ||||
| Heart rate (bpm) | 0.258∗ | 0.156 | –0.135 | 0.026 |
| Systolic blood pressure (mm Hg) | 0.146 | 0.250∗ | 0.176 | –0.218† |
| Mean arterial pressure (mm Hg) | 0.083 | 0.180† | 0.147 | –0.179† |
| Diastolic blood pressure (mm Hg) | 0.015 | 0.091 | 0.100 | –0.118 |
| Pulse pressure (mm Hg) | 0.194† | 0.281∗ | 0.168 | –0.217† |
| Vasodilator stress hemodynamic parameters | ||||
| Heart rate (bpm) | 0.159 | 0.136 | –0.031 | 0.167 |
| Systolic blood pressure (mm Hg) | 0.104 | 0.121 | 0.074 | –0.225† |
| Mean arterial pressure (mm Hg) | 0.021 | 0.100 | 0.104 | –0.217† |
| Diastolic blood pressure (mm Hg) | –0.084 | 0.062 | 0.129 | –0.185† |
| Pulse pressure (mm Hg) | 0.194† | 0.135 | 0.030 | –0.209† |
| Echocardiographic parameters | ||||
| Mitral E wave | 0.233∗ | 0.310∗ | 0.001 | –0.174 |
| Mitral A wave | 0.310∗ | 0.342∗ | 0.044 | –0.283∗ |
| E/A | –0.178† | –0.081 | 0.024 | 0.102 |
| e′ | –0.077 | –0.025 | 0.045 | 0.017 |
| E/e′ | 0.245∗ | 0.284∗ | –0.018 | –0.174 |
| Left atrial diameter | –0.156 | –0.160 | –0.022 | –0.091 |
| Rest LVOTG (mm Hg) | 0.137 | 0.131 | 0.028 | –0.179† |
| Provoked LVOTG (mm Hg) | 0.204† | 0.159 | 0.025 | –0.242∗ |
| CMR parameters | ||||
| LVEF | 0.066 | 0.087 | –0.150 | –0.027 |
| Left ventricular mass index (g/m2) | –0.140 | –0.361∗ | –0.078 | 0.209 |
| LGE percentage (% of LV mass) | –0.213 | –0.053 | 0.110 | 0.110 |
Figure 3.
Scatter plots of stress myocardial blood flow (MBF) and left ventricular mass index (LVMI) (A), rest systolic blood pressure (SBP) (B), rest mean arterial blood pressure (MAP) (C), and rest pulse pressure (PP) (D). In hypertrophic cardiomyopathy, left ventricular mass is negatively correlated with hyperemic (stress) MBF, whereas rest (prevasodilator) SBP, MAP, and PP are positively correlated with hyperemic MBF.
To investigate the possibility of indication bias introduced by the PET examination, we compared the clinical and imaging characteristics of HCM patients who underwent PET imaging (n = 127) with the remainder of the population (n = 563). HCM patients who underwent PET imaging were younger and more symptomatic, but echocardiographic and BP parameters were similar (Supplemental Table 2).
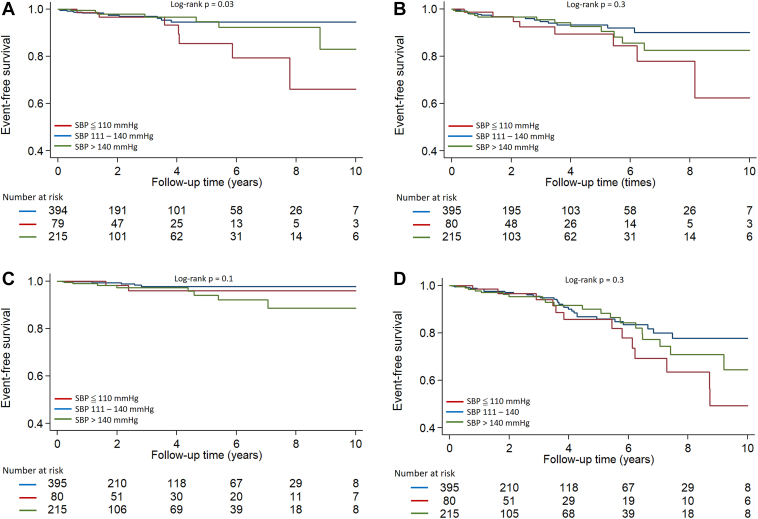
After average follow-up of 3.1 ± 2.7 years, 154 patients underwent myectomy and 36 underwent alcohol septal ablation for treatment of LV obstruction. There were 27 VT/VF events, 39 HF, and 15 all-cause deaths in this HCM cohort. Patients in group 1 had the highest incidence of VT/VF (Table 1 and Figure 4A), but the incidences of HF, all-cause death, and the composite outcome were similar across the 3 groups (Figures 4B–4D).
Figure 4.
Kaplan Meier curves of ventricular tachycardia (VT/ventricular fibrillation (VF) (A), heart failure (HF) (B), all-cause death (C), and composite outcome (D) in hypertrophic cardiomyopathy patients. Group 1: Rest SBP ≤110 mm Hg; group 2: rest SBP 111–140 mm Hg; group 3: rest SBP >140 mm Hg. Group 1 patients had the highest incidence of VT/VF; however, HF, all-cause death, and the composite outcome were similar across the 3 groups.
SBP ≤110 mm Hg was associated with a higher risk of VT/VF than SBP 111–140 mm Hg (hazard ratio 3.0; 95% confidence interval 1.2–7.3; P = .02). After adjusting for age, sex, HCM type, and history of ICD implantation, rest SBP ≤110 mm Hg continued to be independently associated with VT/VF (hazard ratio 2.6; 95% confidence interval 1.0–6.7; P = .04) (Table 4).
Table 4.
Association of SBP with cardiovascular outcomes in HCM patients (n = 690)
| Group 2 (111–140 mm Hg) |
Group 1 (≤110 mm Hg) |
Group 3 (> 40 mm Hg) |
|||
|---|---|---|---|---|---|
| (Reference) | HR (95% CI) | P value | HR (95% CI) | P value | |
| VT/VF | |||||
| Unadjusted HR | Reference | 3.0 (1.2–7.3) | .02 | 1.1 (0.4–2.7) | .9 |
| Model 1 | Reference | 2.9 (1.1–7.2) | .03 | — | — |
| Model 2 | Reference | 2.6 (1.0–6.7) | .04 | — | — |
| Heart failure | |||||
| Unadjusted HR | Reference | 2.0 (0.9–4.6) | .1 | 1.3 (0.7–2.7) | .4 |
| All-cause death | |||||
| Unadjusted HR | Reference | 1.7 (0.3–8.6) | .5 | 3.0 (1.0–9.1) | .06 |
| Composite outcome | |||||
| Unadjusted HR | Reference | 1.7 (0.9–3.3) | .1 | 1.2 (0.7–2.1) | .5 |
Model 1: adjusted for age, sex.
Model 2: Model 1 + HCM type, history of ICD implantation.
CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
Discussion
The main result of this study is the association of SBP ≤110 mm Hg with ventricular arrhythmias and greater severity of coronary microvascular ischemia in symptomatic HCM patients. This is contrary to our original hypothesis that HCM patients with HTN would have the greatest severity of CMD and worse outcomes. The association of SBP ≤110 mm Hg with ventricular arrhythmias could result from lower coronary perfusion pressure leading to microvascular ischemia. Because coronary perfusion pressure is the pressure gradient between aortic diastolic pressures and left ventricular end-diastolic pressure (LVEDP),20 group 1 patients with the lowest SBP and MAP and the highest LVEDP (reflected by high proportion patients with restrictive diastolic hemodynamics) would be expected to have the lowest coronary perfusion pressures. It should be noted that aortic diastolic BP is dynamic and changes all the time during the entire diastolic phase. Coronary perfusion pressure is actually the pressure gradients between “a static DBP + dynamic PP” and LVEDP, but not the gradients between DBP and LVEDP. As such, it is reasonable that we found a significant correlation of stress MBF with PP but not with DBP (Table 3).
Myocardial perfusion is determined by coronary perfusion pressure and autoregulation.19 High myocardial O2 extraction of 70%–80% at rest necessitates augmentation of MBF to meet the metabolic demands imposed by LV hypertrophy, increase in heart rate, contractility, preload, and/or afterload.20 Resting coronary blood flow is primarily determined by myocardial demand, which explains our observed positive association of rest MBF with heart rate (Table 2). Pathologic LVH, as in the case of HCM, increases myocardial O2 demand, which is met by vasodilation of the coronary microcirculation.21 Thus, rest MBF is preserved in HCM,22 and blunting of hyperemic MBF8 drives the reduction in MFR observed in HCM/pathologic LVH.8
Pathology studies in HCM reveal myocyte hypertrophy, replacement/interstitial fibrosis, and coronary microvascular remodeling characterized by medial hypertrophy, reduction in luminal diameter2 of intramural coronary arteries/arterioles, and decreased capillary density, as well as perivascular fibrosis.23,24 Vasodilator infusion induces arteriolar dilation and increases heart rate and myocardial O2 demand, which are met by increases in cardiac output and coronary blood flow. Concentric coronary microvascular remodeling would reduce microcirculatory compliance, and LV hypercontractility would increase intramyocardial pressure,25 necessitating a higher driving pressure (SBP, DBP, MAP) to maintain coronary perfusion in the setting of higher heart rates and O2 demand. This could explain why group 3 patients with the highest SBP, DBP, and MAP had the highest hyperemic MBF (Table 2).
Despite LV coronary blood flow occurring mainly in diastole, we observed an association of SBP (not DBP) with hyperemic MBF (Table 2). The mechanism could be higher SBP producing greater pulsatility and larger shear forces that promote dilation of prearterioles,12 leading to increases in MBF and coronary perfusion. This could explain why group 1 patients who had the lowest SBP, DBP, and MAP also had the lowest hyperemic MBF. Exhaustion of autoregulation at higher coronary perfusion pressures leading to myocardial ischemia in the setting of low diastolic aortic pressures could explain the higher SDS scores in Group 1.19 Our results of the beneficial effects of higher SBP, DBP, and MAP in HCM are supported by a previous study in patients with HTN, which suggested that DBP in the mid-80s may be needed to maintain coronary perfusion and prevent myocardial ischemia in the setting of severe LVH.20
Group 1 had the highest proportion of patients with restrictive LV diastolic filling, which could contribute to mechanical reduction in hyperemic MBF by increasing myocardial and extravascular compressive forces26 and reducing the diastolic “suction wave”27 that promotes myocardial perfusion. It also is possible that group 1 patients had diffuse interstitial and perivascular fibrosis and/or greater medial hypertrophy, which would reduce compliance of the microvasculature, thus limiting the maximal cross-sectional area achieved during maximum coronary vasodilation. Cardiac fibrosis would also increase the risk for reentrant ventricular arrhythmias by reducing the velocity of impulse propagation and increasing the dispersion of depolarization and repolarization.28 In our study, the prevalence and amount of replacement fibrosis (reflected by LV-LGE) were similar in the 3 groups, and no association was observed between LV replacement fibrosis and MBF. However, LV interstitial fibrosis was not quantified, so we are unable to ascertain the contribution of interstitial and perivascular fibrosis to the higher prevalence of ventricular arrhythmias and lower hyperemic MBF observed in group 1.
We observed an inverse association between LV mass and hyperemic MBF, which has been described previously in HCM.22 Lack of vascular proliferation leads to reduction in maximum perfusion per gram of hypertrophied myocardium, even if maximum hyperemic absolute flow (mL/min) remains unchanged.19 To our surprise, we observed a positive association between hyperemic MBF and mitral E, A waves, and E/e′ ratio, and no association with LV obstruction. The positive association with diastolic function indices could be from vasodilator-induced augmentation of cardiac output leading to increases in mitral E- and A-wave amplitude. Because LV myocardial perfusion primarily occurs in diastole and is influenced by LV cavity and intramyocardial pressures,25,29 lack of association between LV obstruction and hyperemic MBF, as well as highest hyperemic MBF values in group 3 with the highest LVEF (reflecting higher contractility and intramyocardial pressures), suggests that coronary perfusion pressure is the main driver of myocardial perfusion in HCM hearts.
Pathology studies in patients with aortic stenosis/LVH showing lack of concentric microvascular remodeling30 suggest that LV obstruction per se does not promote vascular smooth muscle cell (VSMC) hypertrophy. It is possible that group 1 patients have greater degrees of VSMC remodeling and/or dysfunction compared to group 2 and 3 patients, leading to reduction of hyperemic MBF. Basic studies are needed to examine the effect of disease stage and sarcomeric protein gene mutations on intramural coronary arteries/arterioles, and VSMC phenotype and function.
Previous studies have demonstrated higher mortality in hospitalized patients with heart failure with preserved ejection fraction (HFpEF) and SBP <120 mm Hg,31 as well as in patients with heart failure with reduced ejection fraction (HFrEF) and SBP <130 mm Hg.32 With regard to the mechanisms underlying the association between SBP and cardiovascular outcomes, it has been suggested that lower cardiac output leads to lower SBP.12 This is supported by our results of group 3 patients (SBP >140 mm Hg) having the highest cardiac index and hyperemic MBF (Table 1). However, unlike previous studies showing an association between HTN and adverse outcomes, our study suggests that HCM patients with higher SBP have higher hyperemic MBF and less coronary microvascular ischemia. This leads us to hypothesize that agents such as cardiac myosin ATPase inhibitors33, 34, 35 that improve LV diastolic function and decrease LV hypercontractility without reducing BP could augment coronary perfusion pressure and thus prevent microvascular ischemia and ventricular arrhythmias.
Study limitations
First, we examined the association of SBP with cardiovascular outcomes in the entire patient group (n = 690), and the association of SBP with PET parameters in a small subgroup (n = 127) that underwent PET imaging. The small patient number and low event rate in the PET subgroup precluded assessment of the association between MBF and cardiovascular outcomes. Second, we did not measure plasma levels of N-terminal pro–brain natriuretic peptide (NT-proBNP) and hence are unable to examine the association between NT-proBNP, HTN, and ventricular arrhythmias in this cohort.36 Third, for the stratification process, we used SBP values obtained before subjects underwent treadmill exercise testing. Hence, it is possible that SBP used to stratify patients was higher than that recorded at home. Because almost every patient would likely have higher office SBPs compared to their home SBPs, we do not expect this difference to affect the direction of our conclusions. Lastly, in contrast to previous studies in patients with HFpEF, HFrEF, HTN, and CAD, which reported an association of SBP with HF and all-cause mortality,31,32,37,38 here we demonstrate an association between SBP and VT/VF. However, we were unable to adjust for all clinical variables (included in the European Society of Cardiology39 and American Heart Association guidelines10 for sudden cardiac death risk stratification) in our model because of the small number of events per group. Our results are supported by a previous machine learning study in this HCM cohort, which evaluated 93 clinical variables (including those used for sudden cardiac death risk stratification in the European Society of Cardiology/American Heart Association guidelines) and revealed an association between lower rest SBP (P = .001) with a higher risk for VT/VF (HCM-VAr-Risk Model),9 but not with atrial fibrillation (HCM-AF-Risk Model) or HF (HCM-HF-Risk Model).40
Conclusion
Lower coronary perfusion pressure in HCM patients with SBP ≤110 mm Hg underlies reduction in hyperemic MBF, coronary microvascular ischemia, and ventricular arrhythmias. Strategies aimed at maintaining coronary perfusion pressure could prevent coronary microvascular ischemia and fatal ventricular arrhythmias in HCM.
Acknowledgments
We thank Glenn Lie and Gunnar Hansen (GE Ultrasound, Horten, Norway) for providing the strain analysis software, and Susan Phillip and the Johns Hopkins Echocardiography laboratory for their support. We thank Emily Brown and Rebecca McClellan from the HCM-COE for their help with genetic counseling and clinical genotyping of HCM patients.
Funding Sources
This work was supported by the JTB (John Taylor Babbitt) Foundation and startup funds from the UCSF Division of Cardiology to M. Roselle Abraham. Styliani Vakrou and Ioannis Ventoulis were supported by grants from the Hellenic Society of Cardiology. Hulya Yalcin was supported by a Fulbright Fellowship (Bureau of Educational and Cultural Affairs, United States Department of State).
Disclosures
All authors declare no conflict of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Informed consent was obtained for use of medical records for research purposes.
Ethics Statement
The HCM Registry is approved by the Institutional Review Boards of the Johns Hopkins Hospital and the University of California San Francisco (UCSF). The research in this study was conducted according to the Helsinki Declaration guidelines on human research.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2023.07.009.
Appendix. Supplementary Data
Supplemental Figure 1.
References
- 1.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J., Wolfson J.K., Epstein S.E., Roberts W.C. Intramural ("small vessel") coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986;8:545–557. doi: 10.1016/s0735-1097(86)80181-4. [DOI] [PubMed] [Google Scholar]
- 3.Foa A., Agostini V., Rapezzi C., et al. Histopathological comparison of intramural coronary artery remodeling and myocardial fibrosis in obstructive versus end-stage hypertrophic cardiomyopathy. Int J Cardiol. 2019;291:77–82. doi: 10.1016/j.ijcard.2019.03.060. [DOI] [PubMed] [Google Scholar]
- 4.Luo Q., Chen J., Zhang T., Tang X., Yu B. Retrospective analysis of clinical phenotype and prognosis of hypertrophic cardiomyopathy complicated with hypertension. Sci Rep. 2020;10:349. doi: 10.1038/s41598-019-57230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez A., Ravassa S., Lopez B., et al. Myocardial remodeling in hypertension. Hypertension. 2018;72:549–558. doi: 10.1161/HYPERTENSIONAHA.118.11125. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W., Brown J.M., Bajaj N.S., et al. Hypertensive coronary microvascular dysfunction: a subclinical marker of end organ damage and heart failure. Eur Heart J. 2020;41:2366–2375. doi: 10.1093/eurheartj/ehaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flint A.C., Conell C., Ren X., et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 8.Camici P.G., Olivotto I., Rimoldi O.E. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol. 2012;52:857–864. doi: 10.1016/j.yjmcc.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya M., Lu D.Y., Kudchadkar S.M., et al. Identifying ventricular arrhythmias and their predictors by applying machine learning methods to electronic health records in patients with hypertrophic cardiomyopathy (HCM-VAr-Risk Model) Am J Cardiol. 2019;123:1681–1689. doi: 10.1016/j.amjcard.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 11.Canepa M., Sorensen L.L., Pozios I., et al. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:1182–1189. doi: 10.1016/j.amjcard.2013.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J., Yuan J., Li B. SBP is superior to MAP to reflect tissue perfusion and hemodynamic abnormality perioperatively. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.705558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Spiewak M., Malek L.A., Misko J., et al. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol. 2010;74:e149–e153. doi: 10.1016/j.ejrad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Corona-Villalobos C.P., Saha S., Pozios I., et al. Exercise-QTc is associated with diffuse interstitial fibrosis reflected by lower approximated T1 relaxation time in hypertrophic cardiomyopathy patients. J Electrocardiol. 2017;50:484–490. doi: 10.1016/j.jelectrocard.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Valenta I., Antoniou A., Marashdeh W., et al. PET-measured longitudinal flow gradient correlates with invasive fractional flow reserve in CAD patients. Eur Heart J Cardiovasc Imaging. 2017;18:538–548. doi: 10.1093/ehjci/jew116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chareonthaitawee P., Kaufmann P.A., Rimoldi O., Camici P.G. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–161. doi: 10.1016/s0008-6363(01)00202-4. [DOI] [PubMed] [Google Scholar]
- 18.Uren N.G., Melin J.A., De Bruyne B., et al. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–1788. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 19.Duncker D.J., Koller A., Merkus D., Canty J.M., Jr. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis. 2015;57:409–422. doi: 10.1016/j.pcad.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruickshank J.M. Clinical importance of coronary perfusion pressure in the hypertensive patient with left ventricular hypertrophy. Cardiology. 1992;81:283–290. doi: 10.1159/000175818. [DOI] [PubMed] [Google Scholar]
- 21.Bache R.J. Effects of hypertrophy on the coronary circulation. Prog Cardiovasc Dis. 1988;30:403–440. doi: 10.1016/0033-0620(88)90005-9. [DOI] [PubMed] [Google Scholar]
- 22.Petersen S.E., Jerosch-Herold M., Hudsmith L.E., et al. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023. [DOI] [PubMed] [Google Scholar]
- 23.Johansson B., Morner S., Waldenstrom A., Stal P. Myocardial capillary supply is limited in hypertrophic cardiomyopathy: a morphological analysis. Int J Cardiol. 2008;126:252–257. doi: 10.1016/j.ijcard.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Nijenkamp L., Bollen I.A.E., Niessen H.W.M., et al. Sex-specific cardiac remodeling in early and advanced stages of hypertrophic cardiomyopathy. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westerhof N. Physiological hypotheses—intramyocardial pressure. A new concept, suggestions for measurement. Basic Res Cardiol. 1990;85:105–119. doi: 10.1007/BF01906964. [DOI] [PubMed] [Google Scholar]
- 26.Vogt M., Motz W., Strauer B.E. Coronary haemodynamics in hypertensive heart disease. Eur Heart J. 1992;13(Suppl D):44–49. doi: 10.1093/eurheartj/13.suppl_d.44. [DOI] [PubMed] [Google Scholar]
- 27.Davies J.E., Whinnett Z.I., Francis D.P., et al. Evidence of a dominant backward-propagating "suction" wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation. 2006;113:1768–1778. doi: 10.1161/CIRCULATIONAHA.105.603050. [DOI] [PubMed] [Google Scholar]
- 28.Hurtado-de-Mendoza D., Corona-Villalobos C.P., Pozios I., et al. Diffuse interstitial fibrosis assessed by cardiac magnetic resonance is associated with dispersion of ventricular repolarization in patients with hypertrophic cardiomyopathy. J Arrhythm. 2017;33:201–207. doi: 10.1016/j.joa.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan L., Namani R., Choy J.S., Kassab G.S., Lee L.C. Transmural distribution of coronary perfusion and myocardial work density due to alterations in ventricular loading, geometry and contractility. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.744855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConkey H.Z.R., Marber M., Chiribiri A., et al. Coronary microcirculation in aortic stenosis. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faselis C., Lam P.H., Zile M.R., et al. Systolic blood pressure and outcomes in older patients with HFpEF and hypertension. Am J Med. 2021;134:e252–e263. doi: 10.1016/j.amjmed.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arundel C., Lam P.H., Gill G.S., et al. Systolic blood pressure and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:3054–3063. doi: 10.1016/j.jacc.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohde J.A., Roopnarine O., Thomas D.D., Muretta J.M. Mavacamten stabilizes an autoinhibited state of two-headed cardiac myosin. Proc Natl Acad Sci U S A. 2018;115:E7486–E7494. doi: 10.1073/pnas.1720342115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saberi S., Cardim N., Yamani M., et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation. 2021;143:606–608. doi: 10.1161/CIRCULATIONAHA.120.052359. [DOI] [PubMed] [Google Scholar]
- 35.Spertus J.A., Fine J.T., Elliott P., et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2467–2475. doi: 10.1016/S0140-6736(21)00763-7. [DOI] [PubMed] [Google Scholar]
- 36.Wu G., Liu J., Wang S., et al. N-terminal pro-brain natriuretic peptide and sudden cardiac death in hypertrophic cardiomyopathy. Heart. 2021;107:1576–1583. doi: 10.1136/heartjnl-2020-317701. [DOI] [PubMed] [Google Scholar]
- 37.Kalkman D.N., Brouwer T.F., Vehmeijer J.T., et al. J curve in patients randomly assigned to different systolic blood pressure targets: an experimental approach to an observational paradigm. Circulation. 2017;136:2220–2229. doi: 10.1161/CIRCULATIONAHA.117.030342. [DOI] [PubMed] [Google Scholar]
- 38.Vidal-Petiot E., Ford I., Greenlaw N., et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–2152. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 39.Authors/Task Force Members. Elliott P.M., Anastasakis A., et al. 2014 ESC GUIDELINES on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya M., Lu D.Y., Ventoulis I., et al. Machine learning methods for identifying atrial fibrillation cases and their predictors in patients with hypertrophic cardiomyopathy: the HCM-AF-Risk Model. CJC Open. 2021;3:801–813. doi: 10.1016/j.cjco.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.