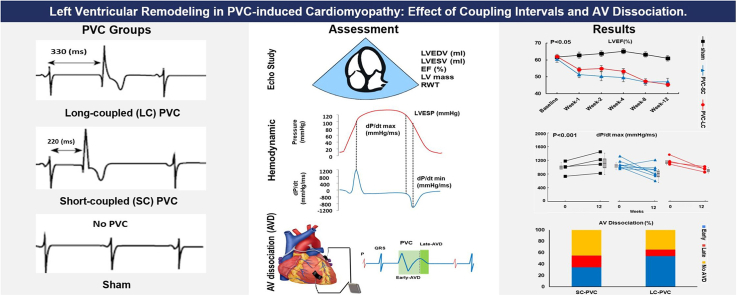
Abstract
Background
Left ventricular dyssynchrony (LVD) and postextrasystolic potentiation (PESP) associated with premature ventricular contractions (PVCs) may play a role in the development of premature ventricular contraction–induced cardiomyopathy (PVC-CM). Long-coupled (LC) PVCs have a greater LVD than short-coupled (SC) PVCs, whereas SC-PVCs have a stronger PESP than LC-PVCs.
Objective
The purpose of this study was to compare SC-PVCs and LC-PVCs to evaluate the roles of LVD, PESP, and atrioventricular dissociation (AVD) in the development of PVC-CM.
Methods
Thirty-six canines underwent pacemaker implantation to induce bigeminal right ventricular apical epicardial PVCs (50% burden) for 12 weeks. Telemetry assessed PVC burden and AVD. Animals were grouped as SC-PVC (coupling interval [CI] 200–220ms), LC-PVC (CI 330 ms), or sham (control). Echocardiographic changes, AVD, and hemodynamics were monitored for 12 weeks.
Results
PVC burden was similar between SC-PVC and LC-PVC groups but was statistically higher in the SC-PVC group (50% vs 47.5%; P = .028). After 12 weeks, left ventricular ejection fraction (LVEF) significantly decreased in both SC-PVC and LC-PVC groups (47.1% ± 1.4% and 45.5% ± 2%, respectively) compared to sham group (61% ± 1.6%; P <.001). Overall AVD was similar between SC-PVC and LC-PVC groups, and there was no significant correlation between AVD and reduction in LVEF at 12 weeks (r = 0.09, P = .5; and r = 0.06, P = .8, respectively). Additionally, both SC-PVC and LC-PVC groups experienced substantial declines in max and min dP/dt after 12 weeks compared to baseline.
Conclusion
Neither PVC CI nor AVD played an independent role in the development or severity of PVC-CM. LVD and PESP make equal relative contributions to the development of PVC-CM.
Keywords: Premature ventricular contractions, Cardiomyopathy, Left ventricular remodeling, Heart failure, Coupled interval, Atrioventricular dissociation
Graphical abstract
Key Findings.
-
▪
The development and degree of left ventricular (LV) systolic dysfunction were similar with long-coupled (LC) and short-coupled (SC) premature ventricular contraction (PVCs).
-
▪
Mean heart rate was significantly higher in SC-PVCs than LC-PVCs.
-
▪
Atrioventricular dissociation (AVD) during PVC did not predict the development of PVC cardiomyopathy regardless of coupling interval.
-
▪
Whereas the incidence of early AVD was significantly higher in LC-PVC compared to SC-PVC, the occurrence of overall AVD was not significantly different in SC-PVC compared to LC-PVC.
-
▪
LV contractility (max dP/dt) and lusitropy (min dP/dt) were decreased in both SC- and LC-PVC groups.
Introduction
Currently, the presence of frequent premature ventricular contractions (PVCs) in the setting of an unexplained cardiomyopathy (CM) prompts therapeutic suppression of PVCs in order to improve or resolve left ventricular (LV) systolic dysfunction.1,2 Although high PVC burden is the strongest predictor for developing premature ventricular contraction–induced cardiomyopathy (PVC-CM), why some patients with high-burden PVCs do not develop CM is unclear.3 Therefore, other PVC features may play additional roles in the development of PVC-CM. PVCs have acute intra- and extraventricular effects.4 Left ventricular dyssynchrony (LVD) and postextrasystolic potentiation (PESP) are acute intraventricular effects of PVCs and are related to PVC coupling interval (CI) in a dichotomous fashion. Both features have been shown to play a role in the development of PVC-CM.5,6 A PVC with longer CI results in a more pronounced LVD,7 whereas a PVC with a shorter CI has a more prominent PESP.8 In addition, atrioventricular dissociation (AVD), an acute extraventricular effect of PVCs, has been implicated as a factor in the development of PVC-CM.4 Animal models of chronic PVCs and PVC-CM are ideal for understanding the impact of LVD, PESP, and AVD in the development of PVC-CM because it is challenging to adjust multiple variables besides CI, as well as the limited ability to assess AVD on the standard electrocardiogram (inability to identify P waves in the QT interval) in the clinical setting. The goal of this preclinical study was to compare the severity of PVC-CM and AVD in short-coupled (SC) and long-coupled (LC) PVCs to better differentiate the roles of LVD, PESP, and AVD in the development of PVC-CM.
Methods
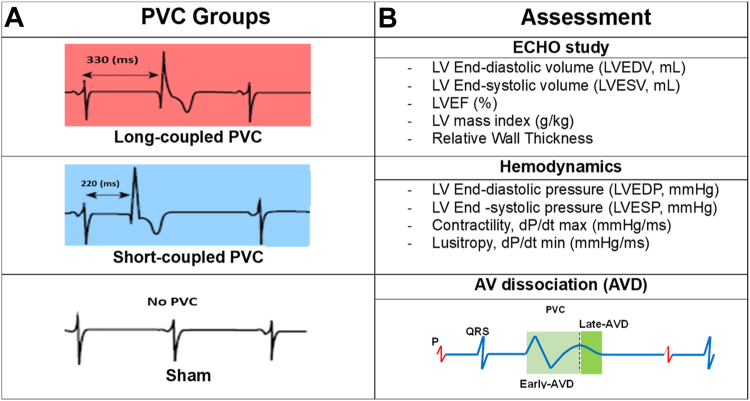
The Institutional Animal Care and Use Committee (IACUC) approved all procedures per the provisions of the USDA Animal Welfare Act Regulations and Standards, PHS Policy, the Guide for the Care and Use of Laboratory Animals, and the VA Policy. Figure 1 shows a summary of the methodology used in the study. Thirty-six mongrel female canines (age >9 months; weight 21 kg) underwent implantation of a Myopore™ (Greatbatch Medical, Alden, NY) epicardial bipolar lead in the right ventricular (RV) apex connected to a modified pacemaker (via left thoracotomy) to reproduce RV apical epicardial PVCs in a bigeminal pattern (50% burden) for 12 weeks as previously described.9,10 A telemetry device also was implanted during survival surgery to assess PVC burden. In some subjects, AVD was quantified by summed atrial and ventricular electrograms. After a 2-week surgical recovery, animals were randomly assigned to 1 of 3 groups: (1) SC-PVC with CI 200–220 ms; (2) LC-PVC with CI 330 ms; or (3) sham (disabled PVCs) to serve as control. Long and short PVC CI were designated based on LVD and PESP previously reported.7
Figure 1.
Summary of methodology. A: Long-coupled PVC (330 ms), short-coupled PVC (220 ms), and sham groups (No PVC). B: Assessments for 12-week study including echocardiographic study (ECHO), hemodynamics, and atrioventricular (AV) dissociation (AVD). AVD was determined as early or late if the P wave fell within the early (light green) or late (dark green) window, respectively. LV = left ventricle; LVEF = left ventricular ejection fraction; PVC = premature ventricular contraction.
To identify differences between PVC CIs (SC vs LC), all animals from each group underwent (1) echocardiography to assess changes in LV remodeling throughout the 12-week protocol, whereas some underwent (2) chronic recording of atrial and ventricular intracardiac electrograms to assess AVD and heart rate (HR) throughout the 12-week PVC protocol, and (3) acute perioperative hemodynamic measurements before and after the 12-week PVC protocol. To limit the use of animals, some of the animals participated in multiple assessments.
Echocardiographic evaluation
LV function, volume, and mass were evaluated by echocardiography in all 36 canines (Figure 1). Echocardiography was performed with a 5-MHz probe using a commercial system (Vivid-7, Vingmed–General Electric, Fairfield, CT) at baseline and weeks 1, 4, 8, and 12 to assess left ventricular systolic ejection fraction (LVEF). All animals in the SC-PVC (n = 15), LC-PVC (n = 9), and sham (n = 12) groups underwent echocardiographic assessment. All echocardiographic studies were performed at least 15 minutes after the pacing algorithm was disabled. Echocardiographic analysis was performed offline using EchoPAC software (EchoPAC v204, GE, Horton, Norway) as previously described.11 LVEF was quantified by using the biplane method of disks (modified Simpson method). LV relative wall thickness (RWT) was calculated as RWT = 2 ∗ posterior wall thickness/left ventricular end-diastolic diameter (LVEDD). Two different methods were used to calculate LV mass—(1) linear method (Cube formula): LV mass = 0.8 ∗ 1.04 (IVSEDD + LVEDD + PWEDD)3 – LVEDD3 + 0.6 g,12 with parameters of LVEDD, interventricular septum end-diastolic dimension (IVSEDD), and posterior wall end-diastolic dimension (PWEDD), respectively; and (2) area–length method: LV mass = 1.05 ∗ 5/6(A1∗[LV length + t] – A2 ∗ LV length), where t = √(A1/π) – √(A2/π) represents mean wall thickness, and A1 and A2 are epicardial and endocardial cross-sectional areas, respectively, obtained in the short-axis view at the papillary muscle level (with the papillary muscles considered part of the LV cavity).13 LV cavity length is measured as the distance from the apex to the mid-mitral annulus plane in the apical 4-chamber view. LV mass index was normalized for variances in animal size (LV mass/kg body weight). Images were reviewed by cardiologists blinded to the randomization arm. Intra- and intergroup comparisons were performed at each timepoint. PVC-CM was defined as LVEF <50%.
AVD and HR assessment
AVD assessment required implantation of a DSI radiotelemetry device (Data Sciences Inc., Minneapolis, MN) with a bipolar electrode sutured to the left atrial (LA) appendage and RV outflow tract with Prolene 6-0 to record local atrial and ventricular electrograms for assessment of AVD during PVCs. Twenty animals underwent AVD assessment (11 SC-PVC, 9 LC-PVC), and 29 animals were assessed for HR changes (14 SC-PVC, 7 LC-PVC, 8 sham). AVD was not assessed in sham animals because they did not have spontaneous PVCs. AVD was categorized as absent (no P wave during QT interval), early (P wave present between QRS and peak of the T wave of PVC), or late AVD (P wave between peak and end of T wave of PVC) (Figure 1). This classification was based on previous clinical data, which identified a significant/prominent A wave (>10 mm Hg) on pulmonary capillary wave pressure tracing during hemodynamic testing in patients with PVC.14 AVD for four 1-minute segments for 24 hours (6 AM, 12 PM, 6 PM, and 12 AM) was assessed at weeks 1, 4, and 12 of bigeminal PVCs. Ponemah software (Data Sciences Inc.) was used for HR analysis to calculate the minimum, maximum, and average HR, and PVC burden.
Hemodynamic evaluation
A limited number of animals (8 SC-PVC, 4 LC-PVCs, 4 sham) provided hemodynamic data. With animals under general anesthesia with isoflurane 1.5%–2%, invasive hemodynamic assessment was performed on canines during survival and terminal (week 12) surgeries. Hemodynamic evaluation consisted of measurements of left ventricular end-diastolic pressure (LVEDP), left ventricular end-systolic pressure (LVESP), contractility (dP/dt max), and lusitropy (dP/dt min) using an impedance-based multipolar catheter (Ventricath 507 5F, Millar Inc., Houston, TX) introduced into the LV through a carotid artery cutdown (Figure 1). These measurements were taken at baseline and at 12 weeks during sinus rhythm after the premature pacing algorithm was disabled for at least 5 minutes in animals with SC- and LC-PVCs.
Statistical analysis
Statistical analysis was performed using SigmaPlot Software 14.0 (Systat Software Inc., San Jose, CA). The normality of distributions was tested using the Shapiro-Wilk method and the Brown-Forsythe test for equal variances. Normally distributed variables are given as mean ± SEM. Groups of normally distributed data with equal variances were compared using 1-way analysis of variance (ANOVA) with the Bonferroni post hoc test. When examining the influence of 2 categorical independent variables on a continuous dependent variable, we performed 2-way ANOVA for repeated measures with the Holm-Sidak method to adjust for multiple comparisons within the model. Multiple comparisons were performed within the groups for assumed and not assumed equal variance by the Student and Welch t test, respectively. P <.05 was considered significant.
Results
LV remodeling
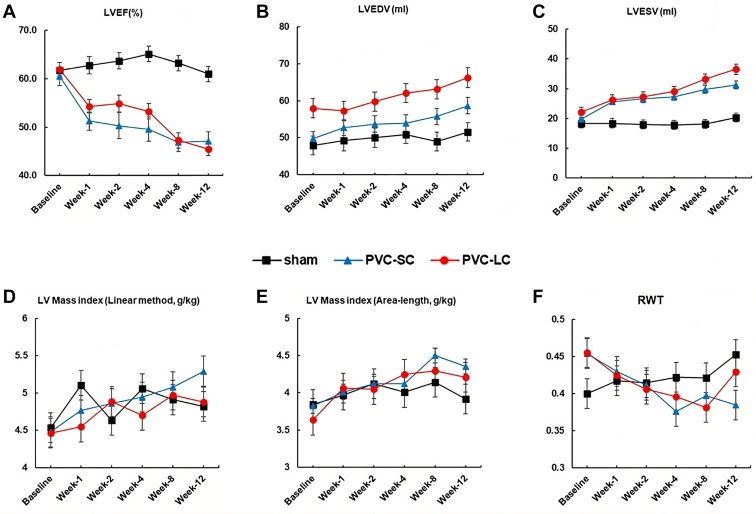
Detailed echocardiographic data for all groups (sham, SC, and LC-PVC) are given in Table 1 and Supplemental Table 1. In contrast to sham (61.0% ± 1.6%), LVEF significantly declined at 12 weeks in the SC- and LC-PVC groups (60.5% ± 1.4% vs 47.1% ± 1.4% and 62.0% ± 1.9% vs 45.5% ± 2.0%, respectively; ANOVA P <.001) compared to its baseline, but no significant difference was found between SC-PVC and LC-PVC (Figure 2A). Decline in LVEF in both SC- and LC-PVC was noted 1 week after PVCs were initiated. After 12 weeks, left ventricular end-diastolic volume (LVEDV) in LC- (66.3 ± 2.7) and SC-PVC (58.7 ± 2.2) increased markedly compared to sham (51.6 ± 2.5) (ANOVA P = .034 and P <.001, respectively). Subsequently, left ventricular end-systolic volume (LVESV) in LC- and SC-PVC (36.5 ± 1.8 and 31.3 ± 1.3, respectively) was significantly higher than in the sham group (20.3 ± 1.5) (ANOVA P <.001). LVEDV and LVESV progressively increased throughout the 12-week study period (Figures 2B and 2C, and Table 1). Similarly, LV mass index in the SC-PVC and LC-PVC groups significantly increased at week 8 compared to baseline, as assessed by the linear and area–length methods (Figures 2D and 2E, and Table 1). Measured LV mass index by the area–length method at 12 weeks was significantly higher in the SC-PVC group (4.4 ± 0.1) compared to the sham group (3.9 ± 0.2) (ANOVA P = .031). Significant decline in RWT was observed in the SC-PVC group compared to the sham group after the 12-week protocol (0.38 ± 0.02 vs 0.46 ± 0.02, respectively; ANOVA P = .008) (Figure 2F and Table 1).
Table 1.
LV volume and mass over time
| Sham | n | PVC-SC | n | PVC-LC | n | |
|---|---|---|---|---|---|---|
| LVEF (%) | ||||||
| Baseline | 61.8 ± 1.6 | 12 | 60.5 ± 1.4 | 15 | 62.0 ± 1.9 | 9 |
| Week 1 | 62.8 ± 1.8 | 10 | 51.3 ± 1.4 | 15∗† | 54.3 ± 1.9 | 9∗† |
| Week 2 | 63.7 ± 1.7 | 11 | 50.3 ± 1.7 | 15∗† | 54.9 ± 2.7 | 9∗† |
| Week 4 | 65.2 ± 1.6 | 12 | 49.6 ± 1.6 | 15∗† | 53.3 ± 2.5 | 9∗† |
| Week 8 | 63.2 ± 1.6 | 12 | 46.9 ± 1.6 | 15∗† | 47.3 ± 1.9 | 9∗† |
| Week 12 | 61.0 ± 1.6 | 12 | 47.1 ± 1.4 | 15∗† | 45.5 ± 2.0 | 8∗† |
| LVEDV (mL) | ||||||
| Baseline | 47.9 ± 2.5 | 12 | 49.8 ± 1.9 | 15 | 58.0 ± 2.6 | 9∗‡ |
| Week 1 | 49.2 ± 2.7 | 10 | 52.7 ± 2.2 | 15 | 57.2 ± 2.6 | 9∗ |
| Week 2 | 50.0 ± 2.6 | 11 | 53.7 ± 2.2 | 15 | 59.8 ± 2.6 | 9∗‡ |
| Week 4 | 50.9 ± 2.5 | 12 | 54.0 ± 2.2 | 15 | 62.1 ± 2.6 | 9∗‡ |
| Week 8 | 49.0 ± 2.5 | 12 | 55.8 ± 2.2 | 15∗ | 63.1 ± 2.6 | 9∗‡ |
| Week 12 | 51.6 ± 2.5 | 12 | 58.7 ± 2.2 | 15∗† | 66.3 ± 2.7 | 8∗‡ |
| LVESV (mL) | ||||||
| Baseline | 18.3 ± 1.5 | 12 | 19.8 ± 1.3 | 15 | 22.1 ± 1.7 | 9 |
| Week 1 | 18.3 ± 1.6 | 10 | 25.7 ± 1.3 | 15∗† | 26.2 ± 1.7 | 9∗ |
| Week 2 | 18.0 ± 1.5 | 11 | 26.5 ± 1.3 | 15∗† | 27.2 ± 1.7 | 9∗ |
| Week 4 | 17.8 ± 1.5 | 12 | 27.3 ± 1.3 | 15∗† | 29.1 ± 1.7 | 9∗† |
| Week 8 | 18.2 ± 1.5 | 12 | 29.7 ± 1.3 | 15∗† | 33.2 ± 1.7 | 9∗† |
| Week 12 | 20.3 ± 1.5 | 12 | 31.3 ± 1.3 | 15∗† | 36.5 ± 1.8 | 8∗†‡ |
| LV MI (L) (g/kg) | ||||||
| Baseline | 4.5 ± 0.2 | 12 | 4.5 ± 0.2 | 15 | 4.5 ± 0.2 | 9 |
| Week 1 | 5.1 ± 0.2 | 10 | 4.8 ± 0.2 | 15 | 4.5 ± 0.2 | 9 |
| Week 2 | 4.6 ± 0.2 | 11 | 4.9 ± 0.2 | 15 | 4.9 ± 0.2 | 9† |
| Week 4 | 5.1 ± 0.2 | 12 | 4.9 ± 0.2 | 15 | 4.7 ± 0.2 | 9 |
| Week 8 | 4.9 ± 0.2 | 12 | 5.1 ± 0.2 | 15† | 5.0 ± 0.2 | 9† |
| Week 12 | 4.8 ± 0.2 | 12 | 5.3 ± 0.2 | 15† | 4.9 ± 0.2 | 8 |
| LV MI (AL) (g/kg) | ||||||
| Baseline | 3.8 ± 0.2 | 12 | 3.8 ± 0.1 | 15 | 3.6 ± 0.2 | 9 |
| Week 1 | 4.0 ± 0.2 | 10 | 4.0 ± 0.1 | 15 | 4.1 ± 0.2 | 9† |
| Week 2 | 4.1 ± 0.2 | 11 | 4.1 ± 0.1 | 15 | 4.0 ± 0.2 | 9 |
| Week 4 | 4.0 ± 0.2 | 12 | 4.1 ± 0.1 | 15 | 4.2 ± 0.2 | 9† |
| Week 8 | 4.1 ± 0.2 | 12 | 4.5 ± 0.1 | 15† | 4.3 ± 0.2 | 9† |
| Week 12 | 3.9 ± 0.2 | 12 | 4.4 ± 0.1 | 15∗† | 4.2 ± 0.2 | 8† |
| RWT | ||||||
| Baseline | 0.40 ± 0.02 | 12 | 0.45 ± 0.02 | 15∗ | 0.46 ± 0.02 | 9 |
| Week 1 | 0.42 ± 0.02 | 10 | 0.43 ± 0.02 | 15 | 0.42 ± 0.02 | 9 |
| Week 2 | 0.41 ± 0.02 | 11 | 0.41 ± 0.02 | 15 | 0.40 ± 0.02 | 9† |
| Week 4 | 0.42 ± 0.02 | 12 | 0.38 ± 0.02 | 15† | 0.40 ± 0.02 | 9† |
| Week 8 | 0.42 ± 0.02 | 12 | 0.40 ± 0.02 | 15 | 0.38 ± 0.02 | 9† |
| Week 12 | 0.46 ± 0.02 | 12 | 0.38 ± 0.02 | 15∗† | 0.43 ± 0.02 | 8 |
Values are given as mean ± SEM unless otherwise indicated.
Multiple comparison test (P <.05): ∗vs sham; †vs baseline; ‡vs PVC-SC.
AL = area–length method; L = linear method; LV = left ventricle; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; MI = mass index; PVC-LC = premature ventricular contraction long-coupled; PVC-SC = premature ventricular contraction short-coupled; RWT = relative wall thickness.
Figure 2.
Left ventricular volumes and mass measurement over time. A: Left ventricular ejection fraction (LVEF). B: Left ventricular end-diastolic volume (LVEDV). C: Left ventricular end-systolic volume (LVESV). D: Left ventricular (LV) mass index (linear method). E: LV mass index (area–length method). F: Relative wall thickness (RWT). Values are given as mean ± SEM. PVC-LC = premature ventricular contraction long-coupled; PVC-SC = contraction short-coupled.
PVCs, HR, and AVD
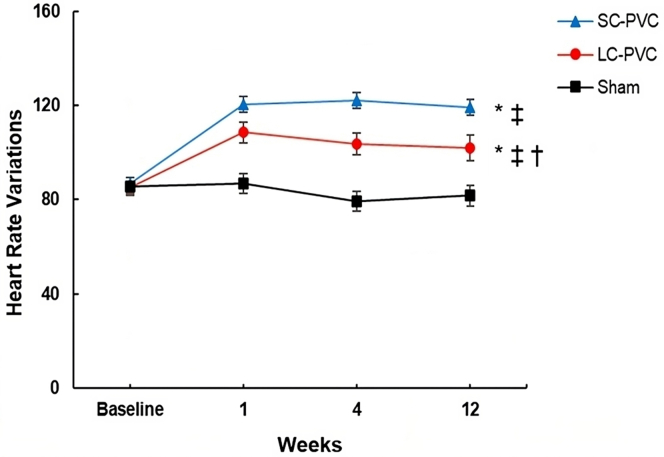
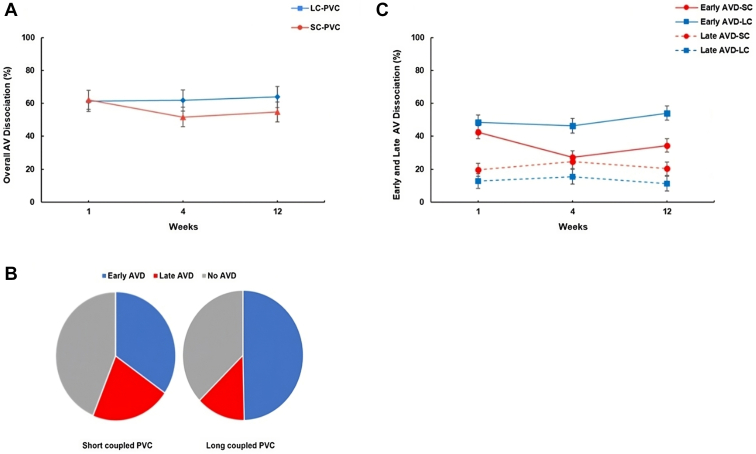
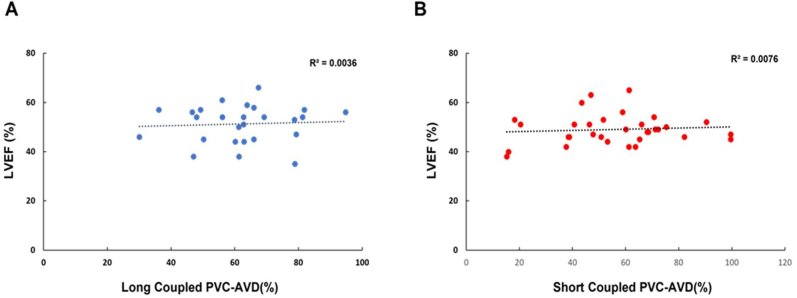
HR and AVD are summarized in Supplemental Table 2 and Table 2. Overall, PVC burden in SC-PVC was similar but statistically higher compared to LC-PVC (50% vs 47.5 %, respectively; ANOVA P = .028). HR was higher in both SC- and LC-PVC groups compared to the sham group throughout the 12-week PVC protocol. HR was also significantly higher in SC-PVC compared to LC-PVC at week 4 (122.2 ± 3.4 bpm vs 103 ± 3.9 bpm, respectively) and week 12 (119 ± 3.4 bpm vs 101 ± 3.1 bpm, respectively; ANOVA P <.001) (Figure 3 and Supplemental Table 2). Overall AVD (early and late) was similar between SC- and LC-PVCs throughout the 12-week study period (Figure 4A). After 12 weeks, SC-PVCs had 35% early and 21% late AVD, without AVD in the remaining 44% of PVC beats (Figure 4B and Table 2). LC-PVCs had 50% and 13% early and late AVD, respectively, without AVD in the remaining 37% of PVC beats (Figure 4B). There was significantly greater early AVD in LC-PVCs compared to SC-PVCs (50% vs 35%; ANOVA P <.001), whereas late AVD was more prevalent in SC-PVCs compared to LC-PVCs (22% vs 13%; ANOVA P = .018) (Figure 4C and Table 2). Neither PVC group demonstrated a significant correlation between AVD and reduction in LVEF at 12 weeks (r = 0.09, P = .5; and r = 0.06, P = .8, respectively) (Supplemental Figure 1).
Table 2.
Early and late AVD over time within different PVC coupling intervals
| Week 1 | n | Week 4 | n | Week 12 | n | |
|---|---|---|---|---|---|---|
| Early AVD-SC | 42.5 ± 3.98∗† | 11 | 27.24 ± 3.98† | 11 | 34.46 ± 4.18∗† | 11 |
| Early AVD-LC | 48.54 ± 4.4∗† | 9 | 46.4 ± 4.4∗†‡ | 9 | 54.1 ± 4.4∗†‡ | 9 |
| Late AVD-SC | 19.6 ± 3.98 | 11 | 24.53 ± 3.98 | 11 | 20.31 ± 4.18 | 11 |
| Late AVD-LC | 12.78 ± 4.4 | 9 | 15.39 ± 4.4 | 9 | 11.2 ± 4.4 | 9 |
Values are given as mean ± SEM unless otherwise indicated.
Multiple comparison test (P <.05): ∗vs Late AVD-SC; †vs Late AVD-LC; ‡vs Early AVD-SC.
AVD = atrioventricular dissociation; Early AVD = P wave present between QRS and the peak of the T wave of premature ventricular contraction (PVC); Late AVD = P wave between peak and end of T wave of PVC; LC = long-coupled PVC; SC = short-coupled PVC.
Figure 3.
Heart rate changes in sham, short-coupled premature ventricular contraction (SC-PVC), and long-coupled premature ventricular contraction (LC-PVC) groups over the course of 12 weeks. Values are given as mean ± SEM. Multiple comparison test (P <.05): ∗vs sham; †vs PVC-SC; ‡vs baseline.
Figure 4.
Atrioventricular dissociation (AVD) in short-coupled (SC) and long-coupled (LC) premature ventricular contractions (PVCs). A: Progression of AVD throughout the 12-week protocol in SC- and LC-PVC. B: Distribution of AVD (early vs late) and No AVD during SC- and LC-PVC. C: Early and Late AVD in SC- and LC-PVC. AV = atrioventricular.
Hemodynamics
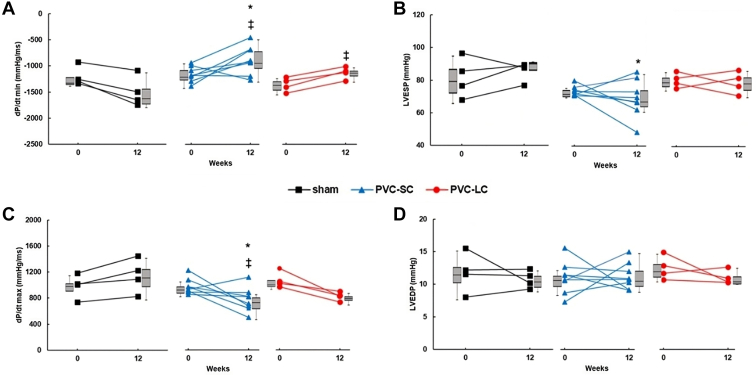
Hemodynamic assessment is summarized in Table 3 and Figure 5. LVEDP was similar among sham, SC-PVC, and LC-PVC at 12 weeks (Figure 5A). In contrast, LVESP in SC-PVC substantially decreased compared to the sham group (69.4 ± 4 vs 85.7 ± 3, respectively; P <.05) (Figure 5B). Contractility (dP/dt max) significantly declined from baseline in both SC- and LC-PVC (768.2 ± 65 mm Hg/s from 973.6 ± 42.1, and 820.8 ± 68 m Hg/s from 1070.7 ± 60.7 mmHg/s, respectively; P <.05) (Figure 5C); however, the decrease in contractility was only significant in SC-PVC compared to the sham group (768.2 ± 65 mm Hg/s vs 1147 ± 130.3 mm Hg/s, respectively; P <.05). Lusitropy (dP/dt min) declined from baseline in SC-PVC and LC-PVC groups (–880.6 ± 95.6 mm Hg/s from –1159 ± 52.7 mm Hg/s, and –1125.25 ± 58.7 mm Hg/s from –1353.5 ± 67.5 mm Hg/s, respectively; P <.05) (Figure 5D) and was significantly reduced in SC-PVC compared to sham (–880.6 ± 95.6 mm Hg/s vs –1492.5 ± 146.4 mm Hg/s, respectively; P <.05).
Table 3.
Hemodynamic changes
| Sham (n = 4) | PVC-SC (n = 8) | PVC-LC (n = 4) | |
|---|---|---|---|
| LVEDP (mm Hg) | |||
| Baseline | 11.8 ± 1.5 | 11 ± 0.8 | 12.5 ± 0.9 |
| Week 12 | 10.7 ± 0.6 | 11.2 ± 0.7 | 11 ± 0.5 |
| LVESP (mm Hg) | |||
| Baseline | 81.6 ± 6.1 | 73.8 ± 1 | 80.7 ± 2.2 |
| Week 12 | 85.7 ± 3 | 69.4 ± 4∗ | 79.3 ± 3.3 |
| dP/dt max (mm Hg/s) | |||
| Baseline | 984.8 ± 92.3 | 973.6 ± 42.1 | 1070.7 ± 60.7 |
| Week 12 | 1147 ± 130.3 | 768.2 ± 65∗† | 820.8 ± 68† |
| dP/dt min (mm Hg/s) | |||
| Baseline | –1201.2 ± 93.1 | –1159.4 ± 52.7 | –1353.5 ± 67.5 |
| Week 12 | –1492.5 ± 146.4 | –880.6 ± 95.6∗† | –1125.25 ± 58.7† |
Values are given as mean ± SEM.
Multiple comparison test (P <.05): ∗vs sham; †vs baseline.
dP/dt max = left ventricular contractility; dP/dt min = lusitropy; LVEDP = left ventricular end-diastolic pressure; LVESP = left ventricular end-systolic pressure; other abbreviations as in Table 1.
Figure 5.
Hemodynamic changes and left ventricular pressure changes in sham and short-coupled (SC) and long-coupled (LC) premature ventricular contraction (PVC) groups at baseline and after 12 weeks. A: Lusitropy (dP/dt min). B: Left ventricular end-systolic pressure (LVESP). C: Left ventricular contractility (dP/dt max). D: Left Ventricular end-diastolic pressure (LVEDP). Values are given as mean ± SEM. Boxes next to individual values represent median + interquartile range. Multiple comparison test (P <.05): ∗vs sham; ‡vs baseline.
Discussion
The results of our PVC canine study are consistent with those of other translational and clinical studies in which high-burden PVCs frequently result in CM.7,9,15 Several studies have attempted to elucidate the influence of PVC features such as duration, CI, PVC origin, and QRS width on the development of PVC-CM,14,16, 17, 18, 19 because high PVC burden does not always lead to CM in the clinical setting.6,20,21 Currently, high PVC burden, epicardial PVC origin, and broad QRS duration are the most consistently reported PVC features associated with a higher incidence of PVC-CM.15, 16, 17, 18 In contrast, published data on the association between PVC-CI and PVC-CM are inconsistent. A study by Del Carpio Munoz et al18 did not identify a significant correlation between PVC-CI and LV systolic dysfunction. However, Sun et al22 showed a higher incidence of PVC-CM in patients with SC-PVCs. Similarly, a clinical study showed that interpolated PVCs are more likely to cause PVC-CM.19 Unfortunately, assessment of interpolated PVCs was performed manually and may be of limited clinical utility because these data are not provided in current commercially available Holter monitor reports. The discordant results of these clinical studies may be explained by numerous factors, including observational study design, nonstandardized techniques for CI assessment, small and clinically heterogeneous patient samples, inconsistent PVC burdens, and variable PVC origins.7 To the best of our knowledge, our present study is the first to use a prospective translational approach to address some of these limitations and clarify the influence of PVC CI on the development of PVC-CM.
Our main study findings include the following: (1) the development and degree of LV systolic dysfunction were similar with long and short PVC-CI; (2) LVEDV and LVESV increased in both SC- and LC-PVC groups compared to sham group, whereas (3) calculated RWT declined over the 12-week protocol and LV mass increased in SC- and LC-PVC, consistent with eccentric hypertrophy as previously reported11; (4) HR was significantly higher in SC-PVCs than LC-PVCs; (5) AVD during PVC did not predict the development of PVC-CM regardless of CI, but (6) the incidence of early AVD was significantly higher in LC-PVC compared to SC-PVC, and the occurrence of overall AVD was not significantly different in SC-PVC compared to LC-PVC; and (7) contractility (max dP/dt) and lusitropy (min dP/dt) were decreased in SC- and LC-PVC groups.
Insight into mechanisms of PV-CM in SC- and LC-PVC groups
LVD vs PESP
LVD and PESP have been implicated in altered intracellular calcium handling and structural and electrical remodeling promoting contractile dysfunction.1,5,6 Preclinical studies have demonstrated that SC-PVCs are associated with greater PESP and lesser LVD, independent of PVC origin (RV, RV outflow tract, or LV).7,8,23 More recently, preclinical studies have shown that PESP and LVD can predict PVC-CM.5,6 In contrast, LVD is significantly greater whereas PESP is lesser in LC-PVC regardless of PVC origin.7 In our study, we elucidated the relative contributions of PESP and LVD to the development of LV systolic dysfunction and adverse LV remodeling in PVC-CM. Based on our echocardiographic findings, SC and LC bigeminal PVCs did not differentially impact the incidence or severity of PVC-CM in this canine model. However, RWT was not changed significantly within or between groups (SC-PVC, LC-PVC, and sham), LV mass index was elevated in both SC- and LC-PVC groups. Thus, we can conclude that LVD and PESP likely make equal relative contributions to the development of PVC-CM. Our findings are consistent with most clinical studies, in which PVC CI has not been found to determine or significantly change the risk of developing PVC-CM.
AVD
AVD is thought to depend on PVC prematurity, baseline HR, and the rate of retrograde AV conduction.2 Kuroki et al14 performed an elegant clinical study to determine pulmonary capillary wedge pressure (PCWP) augmentation (LA pressure) based on AVD during PVCs. They demonstrated that PCWP augmentation during PVC (CI 350 ms) depended on the CI of atrial contraction (PVC-to-atrial contraction interval), with the most prominent at atrial CI 120 ms, with an increase in PCWP >10 mm Hg with atrial CI from 40 to 300 ms.14 Because the QT interval of PVCs in canines is up to 300 ms, we defined early AVD as the presence of a P wave between QRS and the peak of the T wave of PVC, and late AVD as a P wave between the peak and end of the T wave. Based on this classification, we noted early AVD commonly occurred in both SC- and LC-PVCs. Interestingly, early AVD was greater in LC-PVC compared to SC-PVC, which is expected because LC-PVCs are more likely to occur at the time of intrinsic sinus depolarization/atrial contraction. However, overall AVD (early and late) was similar in both SC- and LC-PVCs, and there was no correlation between early, late, and overall AVD and LV systolic dysfunction, suggesting that AVD may not be a predictor of the development of PVC-CM.
Hemodynamics
Takada et al23 showed that as the PVC CIs become shorter in an isolated canine heart, stroke volume in acute PVCs progressively decreases. We measured hemodynamic changes after PVC-CM developed in both SC- and LC-PVCs in a long-term study (12 weeks) (Figure 5). Measured LVEDP in both SC- and LC-PVCs showed no significant difference compared to the sham group. This finding is consistent with our previously reported study.11 Direct measurement of contractility is not feasible and therefore is calculated indirectly based on changes in other functional parameters under carefully controlled experimental conditions. Despite its limitations due to reliance on preload, afterload, and HR, the peak first derivative of systolic LV pressure (dP/dt max) has been validated as a sensitive indicator of contractility changes in properly controlled experimental settings.24 In our study, we observed a significant decline in dP/dt max in both SC- and LC-PVC groups compared to their respective baselines (Figure 5). Even though LVESP showed a decreasing trend over the 12-week experiment in the SC-PVC group, we are unable to draw significant conclusions because of the small number of animals.
Study limitations
(1) Early vs late AVD: We arbitrarily divided AVD into early and late. Although we could not demonstrate a correlation between early, late, and overall AVD (Pearson correlation), we cannot completely exclude that AVD does not predispose to the development of PVC-CM due to similar overall AVD in the SC-and LC-PVC groups. Moreover, no concurrent LA pressure (PCWP) monitoring was conducted during ambulatory PVCs to compare the effects of early and late AVD on PCWP. Canine species do not seem to have retrograde VA conduction during PVCs; thus, we can speculate that AVD is more prominent in this species than observed in the clinical setting. Further studies are required to clarify the impacts of early and late AVD on LA pressure, stroke volume changes, and cardiac output. (2) Single PVC origin (epicardial RV): We assessed the impact of 2 PVC CIs (200 and 330 ms) and their triggers on LV systolic function from RV epicardial origin. It is possible that we would observe different patterns of LVD and subsequent susceptibility to LV dysfunction, and hemodynamic changes associated with endocardial origin PVCs with different CIs. The effect of different PVC origin is outside the scope of this study. However, based on our previous studies comparing LVD based on epicardial PVC origin and CI, PVC location had little effect on LVD compared to CI. We cannot exclude that there would be a difference in severity of PVC-CM with different PVC origins; however, we expect it would be unrelated to LVD because our previous studies demonstrated little effect of acute PVCs from different PVC origins. We postulate that these findings are applicable to the clinical setting because of similarity of the endocardial His–Purkinje conduction system between humans and canines. Clinical data have demonstrated that epicardial PVCs and wider QRS duration are PVC features predictive of PVC-CM. Future studies should explore the variations in LVD with different CI values originating from different endocardial locations because they could provide further insights into the mechanisms and effects of PVC location on LV systolic dysfunction. (3) Structurally normal canine heart: Whether PVC CI influences the development of PVC-CM when superimposed on concurrent heart disease is unclear. Further studies are needed to explore the effects of variable PVC CIs on cardiac hemodynamics and AVD in different CM models. (4) Female sex model: The primary reason to include female sex was to minimize the number of canines (sensitive animal species due to social ethical considerations) because addressing sex differences would have required a much larger sample size. The impact of sex on the development of PVC-induced CM is unclear. A comprehensive study by Latchamsetty et al25 suggested that male gender could potentially serve as an independent risk factor for CM development. Conversely, Sirichand et al26 discovered that women exhibited a higher incidence of symptomatic PVCs, whereas the occurrence of idiopathic ventricular tachycardia was similar across both genders. Because an asymptomatic status might delay diagnosis and subsequently contribute to CM development, women could be less susceptible as a result of receiving earlier treatment.3
Conclusion
In an animal model of PVC-CM, the development of LV systolic dysfunction was similar between SC- and LC-PVCs despite differences in LVD and PESP (LC-PVC primarily with LVD than PESP and SC-PVC primarily with more PESP than LVD). Because previous preclinical studies have demonstrated that LVD and PESP, independently, play a role in the development of PVC-CM, these findings suggest that LVD and PESP may make equal relative contributions to the development of PVC-CM. Although overall AVD was similar among different PVC CIs, early AVD was more pronounced in LC-PVCs than SC-PVCs. Early, late, or overall AVD did not correlate with the development of PVC-CM regardless of PVC-CI. Our findings suggest that neither PVC CI nor AVD plays a significant independent role in the development or severity of PVC-CM.
Acknowledgments
Funding Sources
This work was supported by National Institutes of Health (NIH) Grant Number 1R01HL139874-01 (PI: Jose F. Huizar) and VA Merit Grant BX-004861-01 (PI: Jose F. Huizar).
Disclosures
Jose F. Huizar reports research support from Abbott. Alex Y. Tan reports research support from veterans affair (VA) Merit, Abbott, Medtronic Inc. (MDT), and Biotronik, Inc. (BTK). Kenneth A. Ellenbogen reports research support from Boston Scientific (BS), Biosense Webster (BW), MDT, St. Jude Medical (SJM), NIH; being a consultant for BS, SJM, AtriCure, and Medtronic; and receiving honoraria from MDT, BS, BTK, BW, and AtriCure. All other authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Ethics Statement
The Institutional Animal Care and Use Committee (IACUC) approved all procedures per the provisions of the USDA Animal Welfare Act Regulations and Standards, PHS Policy, the Guide for the Care and Use of Laboratory Animals, and the VA Policy.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2023.07.008.
Appendix. Supplementary Data
Supplemental Material.
References
- 1.Huizar J.F., Ellenbogen K.A., Tan A.Y., Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2328–2344. doi: 10.1016/j.jacc.2019.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel G., Cho S., Ghayoumi A., et al. Prognostic significance of PVCs and resting heart rate. Ann Noninvasive Electrocardiol. 2007;12:121–129. doi: 10.1111/j.1542-474X.2007.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panizo J.G., Barra S., Mellor G., Heck P., Agarwal S. Premature ventricular complex-induced cardiomyopathy. Arrhythmia Electrophysiol Rev. 2018;7:128–134. doi: 10.15420/aer.2018.23.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huizar J.F., Tan A.Y., Kaszala K., Ellenbogen K.A. Clinical and translational insights on premature ventricular contractions and PVC-induced cardiomyopathy. Prog Cardiovasc Dis. 2021;66:17–27. doi: 10.1016/j.pcad.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters T.E., Rahmutula D., Szilagyi J., et al. Left ventricular dyssynchrony predicts the cardiomyopathy associated with premature ventricular contractions. J Am Coll Cardiol. 2018;72:2870–2882. doi: 10.1016/j.jacc.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Kowlgi G.N., Ramirez R.J., Kaszala K., et al. Post-extrasystolic potentiation as a predictor of premature ventricular contraction-cardiomyopathy in an animal model. Europace. 2020;22:813–820. doi: 10.1093/europace/euaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potfay J., Kaszala K., Tan A.Y., et al. Abnormal left ventricular mechanics of ventricular ectopic beats: insights into origin and coupling interval in premature ventricular contraction induced cardiomyopathy. Circ Arrhythmia Electrophysiol. 2015;8:1194–1200. doi: 10.1161/CIRCEP.115.003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper M.W. Postextrasystolic potentiation: do we really know what it means and how to use it? Circulation. 1993;88:2962–2971. doi: 10.1161/01.cir.88.6.2962. [DOI] [PubMed] [Google Scholar]
- 9.Huizar J.F., Kaszala K., Potfay J., et al. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythmia Electrophysiol. 2011;4:543–549. doi: 10.1161/CIRCEP.111.962381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Eltit J.M., Kaszala K., et al. Cellular mechanism of premature ventricular contraction–induced cardiomyopathy. Heart Rhythm. 2014;11:2064–2072. doi: 10.1016/j.hrthm.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrado J., Kowlgi G.N., Ramirez R.J., et al. Eccentric hypertrophy in an animal model of mid- and long-term premature ventricular contraction–induced cardiomyopathy. Heart Rhythm O2. 2021;2:80–88. doi: 10.1016/j.hroo.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devereux R.B., Alonso D.R., Lutas E.M., et al. Assessment hypertrophy to necropsy findings—FYI. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Victor M.A., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki K., Tada H., Seo Y., et al. Prediction and mechanism of frequent ventricular premature contractions related to haemodynamic deterioration. Eur J Heart Fail. 2012;14:1112–1120. doi: 10.1093/eurjhf/hfs095. [DOI] [PubMed] [Google Scholar]
- 15.Baman T.S., Lange D.C., Ilg K.J., et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Carballeira Pol L., Deyell M.W., Frankel D.S., et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11:299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Yokokawa M., Kim H.M., Good E., et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–1464. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Del Carpio Munoz F., Syed F.F., Noheria A., et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011;22:791–798. doi: 10.1111/j.1540-8167.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 19.Olgun H., Yokokawa M., Baman T., et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–1049. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Bogun F., Crawford T., Reich S., et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Takemoto M., Yoshimura H., Ohba Y., et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y., Blom N.A., Yu Y., et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging. 2003;19:295–299. doi: 10.1023/a:1025418531853. [DOI] [PubMed] [Google Scholar]
- 23.Takada H., Takeuchi S., Ando K., et al. Experimental studies on myocardial contractility and hemodynamics in extrasystoles. Jpn Circ J. 1970;34:419–430. doi: 10.1253/jcj.34.419. [DOI] [PubMed] [Google Scholar]
- 24.Sarazan R.D., Kroehle J.P., Main B.W. Left ventricular pressure , contractility and dP/dt max in nonclinical drug safety assessment studies. J Pharmacol Toxicol Methods. 2012;66:71–78. doi: 10.1016/j.vascn.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Latchamsetty R., Yokokawa M., Morady F., et al. Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACC Clin Electrophysiol. 2015;1:116–123. doi: 10.1016/j.jacep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Sirichand S., Killu A.M., Padmanabhan D., et al. Incidence of idiopathic ventricular arrhythmias: a population-based study. Circ Arrhythmia Electrophysiol. 2017;10:1–10. doi: 10.1161/CIRCEP.116.004662. [DOI] [PMC free article] [PubMed] [Google Scholar]