Abstract
Background
Lymphadenomas are rare benign tumors of the major salivary glands that are further classified as sebaceous and non-sebaceous. No association with viruses has been reported so far. Little is known about the mechanisms that allow lymphadenomas to undergo malignant transformation. Among these rare instances, there has never been a malignant transformation to Epstein–Barr virus (EBV)-associated lymphoepithelial carcinoma.
Methods
Clinical data of the reported case were retrieved from the patient’s electronic medical record. Hematoxylin & eosin-stained slides, immunohistochemical tests, and in situ hybridization performed for routine diagnostic purposes were reviewed.
Results
We report a salivary gland sebaceous lymphadenoma in which the luminal components were mostly replaced by malignant epithelial cells with markedly atypical nuclear features. Presence of EBV was demonstrated in all components by EBER. The morphological and immunohistochemical findings were consistent with a lymphoepithelial carcinoma arising from a sebaceous lymphadenoma.
Conclusion
We report the first case of an Epstein–Barr virus-associated lymphoepithelial carcinoma arising from a sebaceous lymphadenoma.
Keywords: Salivary gland tumor, Parotid tumor, Lymphadenoma, Lymphoepithelial carcinoma, Epstein–Barr virus
Introduction
Sebaceous and non-sebaceous lymphadenomas (LAD) are rare benign tumors of the major salivary glands first described in 1960 by McGravan, et al. [1]. Little is known about the mechanisms that allow LADs to undergo malignant transformation. Among these rare instances, transformation to lymphoepithelial carcinoma (LEC) has never been reported. LECs are histologically similar to nasopharyngeal non-keratinizing squamous cell carcinoma (NPC), and they are associated with Epstein–Barr virus (EBV) in endemic areas [2]. We report the first case of an EBV-associated lymphoepithelial carcinoma arising from a sebaceous LAD.
Materials and Methods
Clinical data of the reported case were retrieved from the patient’s electronic medical record. Hematoxylin & eosin-stained slides, immunohistochemical tests, and in situ hybridization performed for routine diagnostic purposes were reviewed and diagnosis was reconfirmed in accordance with the WHO classification of head and neck tumors [3–5].
Results
Case Report
We report the case of a 51-year-old non-smoking male without significant medical history who presented with a left preauricular mass. Imaging revealed a 1.3-cm well-delimited lesion of the superficial parotid lobe without metastasis. No nasopharyngeal or other suspicious masses were found on head and neck CT scan or PET scan (Fig. 1). A superficial parotidectomy was performed.
Fig. 1.
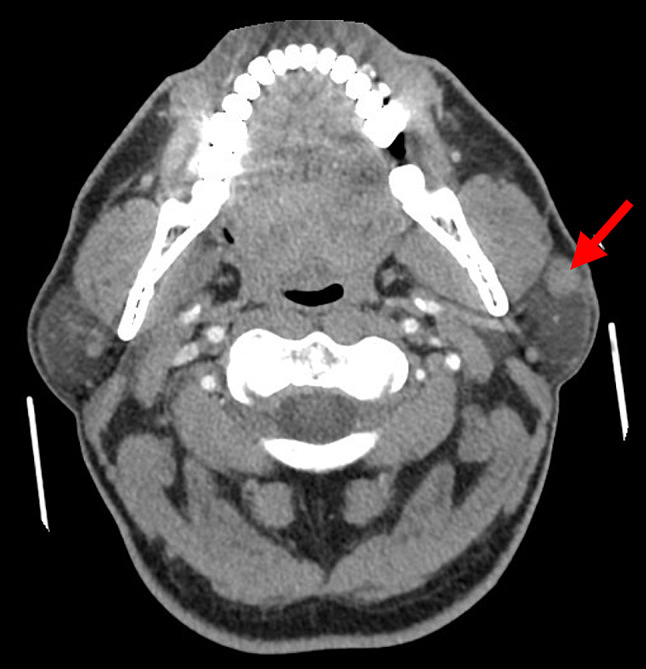
Computed tomography scan shows a solid and well-demarcated lesion of the left superficial parotid lobe measuring 1.3 cm in diameter (arrow)
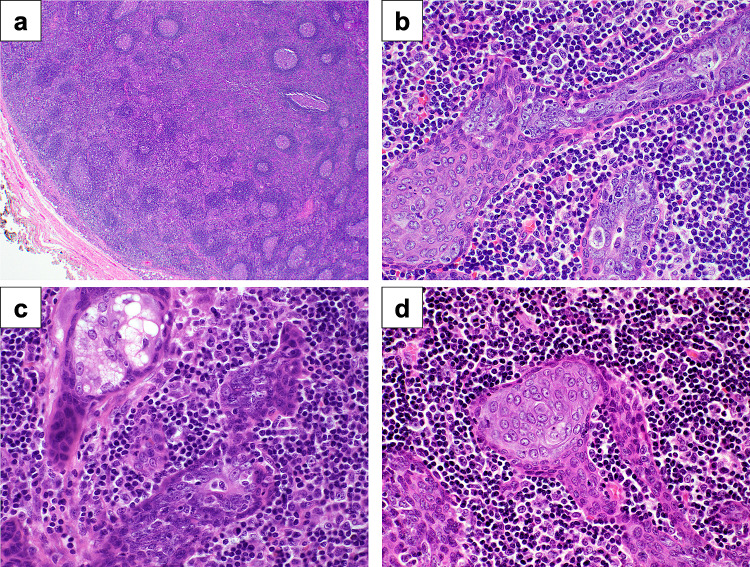
Grossly, the lesion consisted of a beige 1.4-cm well-encapsulated nodule without extraparenchymal extension. Microscopically, the tumor was well demarcated and it was composed of epithelial tubules and trabeculae within a background of lymphoid tissue exhibiting germinal centers. The epithelial foci consisted of peripheral monotonous basaloid cells without atypia. In the center of these foci, the epithelial cells showed focal squamous and sebaceous differentiation, consistent with the epithelial component of a sebaceous lymphadenoma. However, in most of the lesion, the luminal component was replaced by malignant epithelial cells with indistinct cytoplasmic borders, markedly enlarged nuclei, prominent nucleoli, and a high nuclear-to-cytoplasmic ratio (Fig. 2). Lymphovascular invasion and perineural invasion were absent.
Fig. 2.
a The tumor is well demarcated and is composed of epithelial cells within a lymphoid stroma (H&E 20X), b Monotonous abluminal cells surround the luminal carcinoma component (H&E 100X), c Sebaceous lymphadenoma in relation to malignant component (H&E 100X), d Squamous differentiation (H&E 100X)
Immunohistochemistry and in situ Hybridization
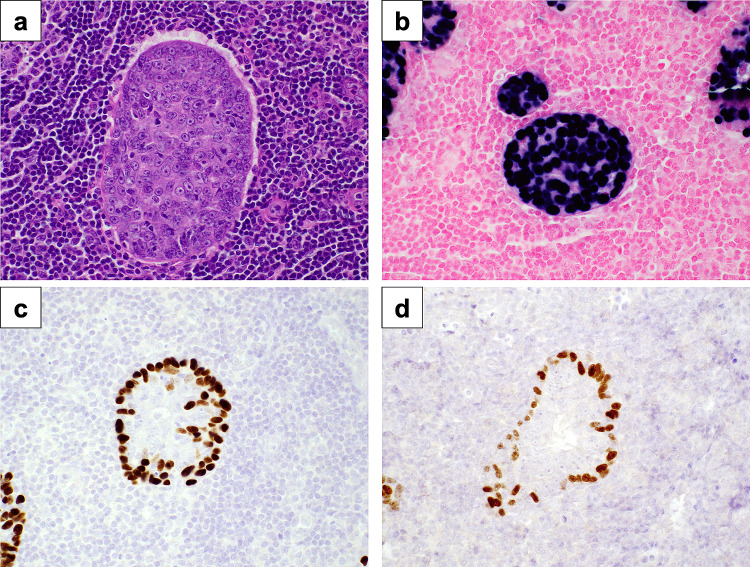
By immunohistochemistry, the peripheral and the luminal components of the lymphadenoma showed an intercalated duct phenotype and the cells were positive for cytokeratin 7, p40, p63, and SOX10. In contrast, the central malignant cells were weakly positive for cytokeratin 7 and Ki67 showed a high proliferation index of up to 60%, while p40, p63, and SOX10 were negative. The presence of EBV was demonstrated in both the malignant and benign lymphadenoma components with an EBV in situ hybridization probe (EBER) (Fig. 3). Smooth muscle actin and p16 were negative. The changes were consistent with a lymphoepithelial carcinoma, EBV-related, arising from a sebaceous lymphadenoma.
Fig. 3.
a Tumor (H&E, 400X), b Epstein–Barr Virus in situ hybridization shows positivity in the luminal carcinoma and abluminal lymphadenoma components, c The abluminal component shows positivity for p40, d SOX10 is expressed in abluminal cells
Follow-Up
The patient was treated with local adjuvant radiation therapy and no suspicious lesion was identified on follow-up imaging. There was no evidence of disease after 24 months.
Discussion
LADs are rare benign tumors of the major salivary glands that are further subclassified according to the presence or absence of sebaceous differentiation. Sebaceous LADs occur in patients over 50 years old with a predilection for the parotid glands [6]. Histologically, LADs are characterized by a well-circumscribed proliferation of epithelial cells accompanied by a dense background of reactive lymphocytes [3]. The epithelial component shows anastomosing cords and nests of basaloid cells that can be solid or cystic with a varying degree of squamous differentiation [6].
Even though the vast majority of LADs are benign and do not recur after complete surgical excision, cases of carcinoma ex LAD have been described. Including the present case, there are only 12 reports of LAD with malignant transformation, all of which are summarized in Table 1. Mean age of presentation is 64 years old with a slight male preponderance [6–14]. Except for two patients who died of unrelated causes, all the patients were alive and showed no evidence of recurrence after follow-up periods ranging between 4 months and 6 years [6–14]. Malignant transformation predominantly arises in sebaceous LADs. Reported subtypes of carcinoma ex sebaceous LAD include sebaceous lymphadenocarcinoma, basal cell adenocarcinoma, and sebaceous carcinoma [6, 14]. The only case of malignancy arising in a non-sebaceous LAD was an undifferentiated carcinoma [7].
Table 1.
Reported cases of lymphadenoma with malignant transformation
| Author | Patient | Clinical | Diagnosis of malignant component (Histology) | EBV | Treatment | Course |
|---|---|---|---|---|---|---|
| Kara et al. [7] | F 54 | R parotid. 2 cm. Present for 12 months, enlarging over past 3 months | Undifferentiated carcinoma from non-sebaceous LAD | – | Excision and postoperative locoregional radiotherapy | NED at 10 months |
| Seethala et al. [6] | F 74 | R parotid. 1.5 cm | Basal cell adenocarcinoma from Sebaceous LAD | N/A | N/A | N/A |
| Seethala et al. [6] | M 76 | R parotid. 5.5 cm | Sebaceous carcinoma | N/A | N/A | N/A |
| Linhartova [8] | F 68 | Parotid. 5 cm. Slow growth over 2.5 years | Sebaceous carcinoma from sebaceous LAD | N/A | Excision and postoperative radiotherapy | NED at 6 years |
| Gnepp and Brannon [9] | M 7th decade | Periparotid lymph node. Asymptomatic. Present for 1 month | Sebaceous lymphadenocarcinoma from sebaceous LAD | N/A | Excision and superficial parotidectomy | NED at 14 months |
| Gnepp and Brannon [9] | M 7th decade | Parotid. Asymptomatic. Present for at least 20 years | Sebaceous lymphadenocarcinoma from sebaceous LAD | N/A | Excision and superficial parotidectomy | Died of other causes at 18 months. (cardiovascular disease) |
| Croitoru, et al. [10] | M 55 | L parotid. 6 cm. Present for 3 years. Rapidly enlarging over 3 months | Sebaceous lymphadenocarcinoma from sebaceous LAD | N/A | Excision and postoperative radiotherapy | NED at 4 months |
| Ahn and Park [11] | F 36 | L parotid. 1.2 cm. Present for at least 10 years. Enlarging over 4 months | Sebaceous lymphadenocarcinoma from sebaceous LAD | N/A | Superficial parotidectomy and postoperative radiotherapy | NED at 24 months |
| Claudius et al. [12] | M 87 | L parotid. 2.7 cm. Reddish violet plaques on left cheek and neck. Infraauricular mass. Palpable cervical lymph nodes | Sebaceous lymphadenocarcinoma from sebaceous LAD with cervical lymph node metastasis and lymphangiosis carcinomatosa | N/A | Skin excision, neck dissection, and postoperative radiotherapy | Died of other causes at 8 months (respiratory failure) |
| Vazmitsel et al. [13] | N/A | Parotid | Sebaceous lymphadenocarcinoma arising within Sebaceous LAD | N/A | N/A | N/A |
| Hao, et al.[14] | F 82 | R parotid. 2.8 cm. Rapidly enlarging over 4 months | Sebaceous lymphadenocarcinoma with lymph node metastasis | – | Extended resection and neck dissection | NED at 10 months |
| Reported case | M 51 | L Parotid | Lymphoepithelial carcinoma arising in sebaceous lymphadenoma | + | Superficial parotidectomy and postoperative radiotherapy | NED at 12 months |
R right; L left; LAD lymphadenoma; NED no evidence of disease
LAD has never been reported in association with LEC, which is a rare EBV-associated undifferentiated carcinoma characterized by a syncytial growth pattern of malignant epithelial cells accompanied by a dense background of non-neoplastic lymphocytes [3]. LEC typically occurs in the sixth decade without sex predilection [3]. Most cases occur in North American Inuit people and in EBV-endemic regions such as South-East Asia, Japan, and North Africa [3]. Salivary LEC is undistinguishable from nasopharyngeal non-keratinizing squamous cell carcinoma, EBV-associated, and from LECs of other sites of the upper aero-digestive tract [15]. Treatment consists of surgical resection with neck dissection followed by adjuvant radiation therapy [2]. Nodal metastases are observed in up to 40% of cases, while 3-year and 5-year overall survival are reported to be 93% and 86%, respectively [16].
Our case represents the first instance of a LEC arising in a sebaceous LAD. Both the carcinoma and the LAD components were EBV-positive by in situ hybridization. Until now, sebaceous LADs have never been associated with viruses such as EBV and human papilloma virus (HPV) [6, 7, 17–20]. Our differential diagnosis included metastatic NPC, but no nasopharyngeal lesion or other suspicious masses were found on imaging and clinical examination. Also, in our case, the carcinomatous component was confined within the tubules and trabeculae of the lymphadenoma, which argues against a metastasis. The presence of EBV and absence of myoepithelial cells ruled out other basaloid salivary carcinomas such as sebaceous lymphadenocarcinoma, basal cell adenocarcinoma, epithelial myoepithelial carcinoma, adenoid cystic carcinoma, and sebaceous carcinoma.
In summary, we report a novel case of EBV-associated LEC arising in a sebaceous LAD. In addition, the LEC component remained confined in the LAD, which may suggest a more indolent course than traditional LEC. Our patient shows no evidence of disease recurrence after 24 months, which mirrors the absence of recurrence in other reported cases of carcinoma ex LAD [6–14]. Additional cases are required to truly evaluate the clinical evolution of EBV-associated LEC arising in LAD.
Author Contributions
JW performed literature review and wrote the manuscript. JB supervised the project, substantially edited the manuscript, and provided images. OG, KA, and RRS reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The authors have no relevant financial or non-financial interests to disclose. This study was not supported by any funding.
Data Availability
All material included in manuscript. No supplementary data to provide.
Declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGavran MH, Bauer WC, Ackerman LV. Sebaceous lymphadenoma of the parotid salivary gland. Cancer. 1960;13:1185–1187. doi: 10.1002/1097-0142(196011/12)13:6<1185::aid-cncr2820130605>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Wenig BM. Lymphoepithelial-like carcinomas of the head and neck. Semin Diagn Pathol. 2015;32(1):74–86. doi: 10.1053/j.semdp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Grandis J, Slootweg P, editors. WHO classification of head and neck tumours. 4. Lyon: IARC; 2017. [Google Scholar]
- 4.WHO Classification of Tumours Editorial Board. Head and neck tumours [Internet; beta version ahead of print]. Lyon (France): International Agency for Research on Cancer; 2022 [cited 2022 Aug 16]. (WHO classification of tumours series, 5th ed.; vol. 9). Available from: https://tumourclassification.iarc.who.int/chapters/52.
- 5.Skalova A, Hyrcza MD, Leivo I. Update from the 5th edition of the world health organization classification of head and neck tumors: salivary glands. Head Neck Pathol. 2022;16(1):40–53. doi: 10.1007/s12105-022-01420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seethala RR, Thompson LD, Gnepp DR, Barnes EL, Skalova A, Montone K, et al. Lymphadenoma of the salivary gland: clinicopathological and immunohistochemical analysis of 33 tumors. Mod Pathol. 2012;25(1):26–35. doi: 10.1038/modpathol.2011.135. [DOI] [PubMed] [Google Scholar]
- 7.Kara H, Sonmez S, Bagbudar S, Gulluoglu M, Basaran B. Malignant transformation of parotid gland non-sebaceous lymphadenoma: case report and review of literature. Head Neck Pathol. 2020;14(4):1123–1128. doi: 10.1007/s12105-020-01133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linhartova A. Sebaceous glands in salivary gland tissue. Arch Pathol. 1974;98(5):320–324. [PubMed] [Google Scholar]
- 9.Gnepp DR, Brannon R. Sebaceous neoplasms of salivary gland origin. Report of 21 cases. Cancer. 1984;53(10):2155–70. doi: 10.1002/1097-0142(19840515)53:10<2155::AID-CNCR2820531026>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Croitoru CM, Mooney JE, Luna MA. Sebaceous lymphadenocarcinoma of salivary glands. Ann Diagn Pathol. 2003;7(4):236–239. doi: 10.1016/s1092-9134(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 11.Ahn SH, Park SY. Sebaceous lymphadenocarcinoma of parotid gland. Eur Arch Otorhinolaryngol. 2006;263(10):940–942. doi: 10.1007/s00405-006-0087-x. [DOI] [PubMed] [Google Scholar]
- 12.Claudius K, Ginzkey C, Gattenlohner S, Muller J, Demmer P, Brocker EB. A red cheek as first clinical sign of a sebaceous lymphadenocarcinoma of the parotid gland with lymphangiosis carcinomatosa and lymph node metastases. Am J Dermatopathol. 2011;33(4):e50–e53. doi: 10.1097/DAD.0b013e3181edabf5. [DOI] [PubMed] [Google Scholar]
- 13.Vazmitsel M, Esebua M, Layfield LJ. Cytologic features of sebaceous, lymphadenoma, and sebaceous lymphadenocarcinoma: differential diagnostic considerations. Diagn Cytopathol. 2020;48(5):424–429. doi: 10.1002/dc.24357. [DOI] [PubMed] [Google Scholar]
- 14.Hao FY, Wang YL, Li SM, Xue LF. Sebaceous lymphadenocarcinoma of the parotid gland: a case report. World J Clin Cases. 2020;8(22):5751–5757. doi: 10.12998/wjcc.v8.i22.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saw D, Lau WH, Ho JH, Chan JK, Ng CS. Malignant lymphoepithelial lesion of the salivary gland. Hum Pathol. 1986;17(9):914–923. doi: 10.1016/s0046-8177(86)80641-4. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Zhu G, Wang Y, Wang Y, Chen T, Ji Q. A clinical analysis of 37 cases with lymphoepithelial carcinoma of the major salivary gland treated by surgical resection and postoperative radiotherapy: a single institution study. Med Oncol. 2014;31(5):957. doi: 10.1007/s12032-014-0957-9. [DOI] [PubMed] [Google Scholar]
- 17.Yeo MK, Kim DM, Kim JM. An atypical nonsebaceous lymphadenoma with diffuse lymphoepithelial differentiation. Indian J Pathol Microbiol. 2016;59(4):521–523. doi: 10.4103/0377-4929.191810. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Chan JK, Chow CW, Orell SR. Lymphadenoma: a report of three cases of an uncommon salivary gland neoplasm. Histopathology. 2002;41(4):342–350. doi: 10.1046/j.1365-2559.2002.01528.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang KT, Chadha NK, Leung R, Shago M, Phillips MJ, Thorner PS. Lymphadenoma: case report of a rare salivary gland tumor in childhood. Pediatr Dev Pathol. 2010;13(4):331–337. doi: 10.2350/09-08-0701-CR.1. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Song JS, Roh JL, Choi SH, Nam SY, Kim SY, et al. Increased immunoglobulin G4-positive plasma cells in lymphadenoma of the salivary gland: an immunohistochemical comparison among lymphoepithelial lesions. Appl Immunohistochem Mol Morphol. 2018;26(6):420–424. doi: 10.1097/PAI.0000000000000461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All material included in manuscript. No supplementary data to provide.