Abstract
Background
In super obesity, Roux-en-Y gastric bypass (RYGB) may be insufficient why some surgeons advocate biliopancreatic diversion with duodenal switch (BPD/DS), a more malabsorptive procedure. There is a paucity of evidence regarding results beyond 10 years, especially after BPD/DS. The aim of this randomized controlled trial was to compare the long-term outcome of BPD/DS, and RYGB in patients with super obesity, i.e., body mass index (BMI) > 50 kg/m2.
Methods
This is a 13- to 17-year follow-up study of a single-center, single-blinded randomized trial in which 47 patients (BMI > 48 and eligible for bariatric surgery) were randomized 1:1 to BPD/DS and RYGB (25 men, 24 BPD/DS, 39.1 ± 9.9 years, BMI 54.5 ± 6.1 kg/m2). The primary outcome was weight loss. The study was financed by Swedish governmental funding of clinical research (ALF). Trial registration number: ISRCTN10940791.
Results
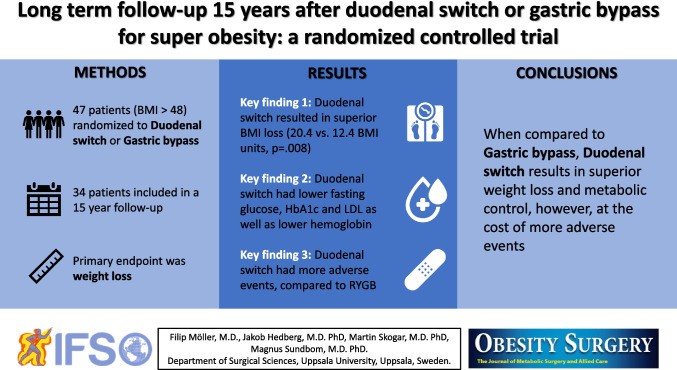
Thirty-four (18 BPD/DS) of the living 42 patients (81.0%) participated. BPD/DS resulted in higher BMI loss (20.4 ± 7.9 vs. 12.4 ± 8.6, p = .008) and higher percent of total body weight loss (37.5% ± 12.2 vs. 22.8% ± 14.8, p = .004). BPD/DS was associated with lower fasting glucose, glycated hemoglobin (HbA1c), and low-density lipoprotein (LDL) as well as lower hemoglobin. Adverse events were more common after BPD/DS (2.7 vs. 0.9 per patient, p = .004). The global assessment tool BAROS (Bariatric Analysis and Reporting Outcome System) demonstrated superior scores for BPD/DS (p = .047).
Conclusion
When compared to RYGB, BPD/DS results in superior weight loss and metabolic control as well as superior BAROS score, however, at the cost of more adverse events.
Graphical Abstract
Keywords: Roux-en-Y gastric bypass, Biliopancreatic diversion with duodenal switch, Long-term results, Obesity, Weight loss, Diabetes mellitus, BAROS, Bariatric surgery
Introduction
Obesity and obesity-related diseases are rapidly increasing worldwide [1]. In addition to big health risks and negative impact on quality of life [2], obesity is a major economic burden with 69 billion dollars spent on health care in the USA alone in 2013 [3]. In 2017, 42.4% of US adults had obesity (Body Mass Index, BMI > 30) and 9.2% were having severely obesity (BMI > 40) [4, 5]. Patients with super obesity (BMI > 50) have been increasing disproportionally and pose an added challenge due to more serious comorbidities and technical difficulties during surgery [6, 7].
There is currently no consensus on the preferred surgical procedure in patients with super obesity. Roux-en-Y gastric bypass (RYGB), often referred to as golden standard among bariatric procedures, has excellent results on weight-loss and comorbidities. RYGB can lead to insufficient weight loss in patients with super obesity [8, 9], and therefore, some surgeons advocate a more malabsorptive procedure, known to result in persistent weight loss and superior metabolic control [10–14]. These procedures include the biliopancreatic diversion, introduced by Scopinaro [15], and the biliopancreatic diversion with duodenal switch (BPD/DS), a modification by Hess [16] based on a bile-reflux reducing procedure by DeMeester [17]. However, BPD/DS is a technically more complex operation than RYGB and requires a more rigid follow-up program due to the risk of malnutritional deficiencies and various gastrointestinal adverse effects such as diarrhea, acid reflux, and foul-smelling flatus [12, 18]. In 2004–2007, we randomized 47 patients with super obesity to BPD/DS or RYGB. At three years, the expected results were found, i.e., greater weight loss and superior glucose homeostasis in BPD/DS, at the cost of diarrhea [19]. In a previous study including all our patients with super obesity (n = 211), BPD/DS was also found to have a superior score (4.7 vs. 4.0, p < 0.05) [11], when analyzed with the validated and well-established Bariatric Analysis and Reporting Outcome System (BAROS) [20, 21]. The global assessment tool BAROS judges weight loss, changes in comorbidities and quality of life, while points are deducted for complications and reoperations [22].
The aim of this study was to compare the long-term outcome of BPD/DS and RYGB beyond 10 years in a randomized controlled trial on super obesity by assessing weight loss, comorbidities, adverse events, quality of life, and biochemical profiles as well as patient-rated gastrointestinal symptoms and overall satisfaction.
Methods
Study Design and Participants
All 47 patients from our original single-center, single-blinded, randomized trial [19] (25 men, age 39.1 ± 9.9 years, BMI 54.5 ± 6.1 kg/m2) were eligible to join this long-term follow-up. Details of the study design have been described earlier [19], but in short, from 2004 to 2007, 99 patients referred to us for bariatric surgery with BMI > 48 were assessed. Inclusion criteria were BMI > 48, above 18 years of age and being referred to us for bariatric surgery, exclusion criteria were language difficulties, previous problems with diarrhea and suspected inflammatory bowel disease. 90 patients deemed eligible for randomization, nine of which were excluded on medical grounds or language difficulties. The 47 patients who accepted participation were stratified by gender and BMI (> 53 or < 53 kg/m2) and were randomly assigned 1:1 between BPD/DS and RYGB. A sample size of 80 patients was calculated necessary (30% improved weight loss, 5% significance, and 80% power) but increasing numbers of patients declining inclusion led to premature closure of inclusion. The type of procedure was unknown to the participants and ward staff until 2 days after the surgery. All patients provided informed written consent.
Surgery and Postoperative Follow-up
Our operative technique has been described previously [19]. In short, both procedures were performed through an upper midline incision. BPD/DS constituted of a 36-Fr sleeve gastrectomy and a 150-cm alimentary limb, emptying into a 100-cm common limb, while the remaining small bowel formed the biliary limb. In RYGB, a small gastric pouch was anastomosed to a 120-cm Roux limb, using a 50-cm biliary limb. Postoperatively, patients were started on multivitamin supplementation (iron 15 mg, calcium 240 mg, vitamin A 600 µg, vitamin D3 750 µg, vitamin E 60 mg) and vitamin B12 injections. Following the second year, RYGB patients had annual checks-ups at their primary care physician, while BPD/DS patients continued their follow-up at the Department of Metabolic Medicine at our hospital.
Outcomes
The primary outcomes were weight loss, and the secondary outcomes were change in comorbidities, adverse effects, biochemical profiles, patient-rated quality of life and gastrointestinal symptoms, overall satisfaction and the calculated BAROS score.
Long-term Follow-up
Thirteen to seventeen (mean 15.4) years after the initial surgery, the included patients were asked to complete a questionnaire regarding current weight and comorbidities, present medication, subsequent surgeries, and gastrointestinal symptoms. In addition, they rated their overall satisfaction with the procedure (very satisfied, satisfied, dissatisfied, and very dissatisfied), and if they would recommend their procedure to a friend seeking bariatric surgery. For BAROS scoring, we included a translated version of the Moorhead-Ardelt Quality of life Questionnaire (MAQ), grading six aspects of quality of life on a 10-point Likert scale [21]. Weight was self-reported and is presented as change in body mass index (BMI) as well as percent total body weight loss (%TBWL). The following comorbidities were analyzed; diabetes; hypertension/cardiovascular disease, sleep apnea, and dyslipidemia. Presence of a comorbidity was defined as use of medication for the specific condition, or Continuous Positive Airway Pressure (CPAP) for sleep apnea, and remission was defined as complete cessation of medication or CPAP. Information regarding adverse effects and subsequent surgeries were collected from the questionnaires as well as from medical records. International Classification of Diseases 10th Revision (ICD-10) was used to aid identifying complications from medical records. The gastrointestinal symptoms (vomiting, reflux, dumping, abdominal pain, diarrhea, soling and foul-smelling gases) were rated by the patient as occurring daily, weekly, monthly, or yearly. According to the BAROS scoring tool; weight loss, improvement in medical conditions and quality of life were given up to 3 points each, while 1 and 0.2 points were deducted for major or minor complications and adverse events, respectively [22, 23]. The outcome of the procedure was classified as failure (≤ 1 point), fair (> 1 to 3 points), good (> 3 to 5 points), very good (> 5 to 7 points), or excellent (> 7 to 9 points) [22, 23]. Because of the long-term focus, we also calculated a BAROS score without including complications, as BAROS is mainly designed for perioperative complications, not for assessment of additional surgeries nor adverse effects several years after the bariatric procedure. Finally, biochemical profiles concerning hemoglobin, vitamin B12, folate, albumin, fasting glucose, hemoglobin A1c (HbA1c), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were analyzed.
Statistics
Regarding the frequency of gastrointestinal symptoms, the result was dichotomized into often (once per week or more) or seldom (less than once a week). For between-group comparisons of continuous variables, independent samples t-test was used for normally distributed data and the Mann–Whitney U test for non-normally distributed data. Chi2 test or Fischer’s exact test was used for categorical variables when comparing between groups, while McNemar test was used for comparisons within groups. A p value of < 0.05 was considered statistically significant. IBM® SPSS® 28 was used for the statistical analysis.
Ethics
This study has been approved by the regional ethical review board (Dnr: 2014/318).
Trial Registration
The trail has been registered at ISRCTN.com (Registration number: ISRCTN10940791).
Funding
The study was financed by Swedish governmental funding of clinical research (ALF).
Results
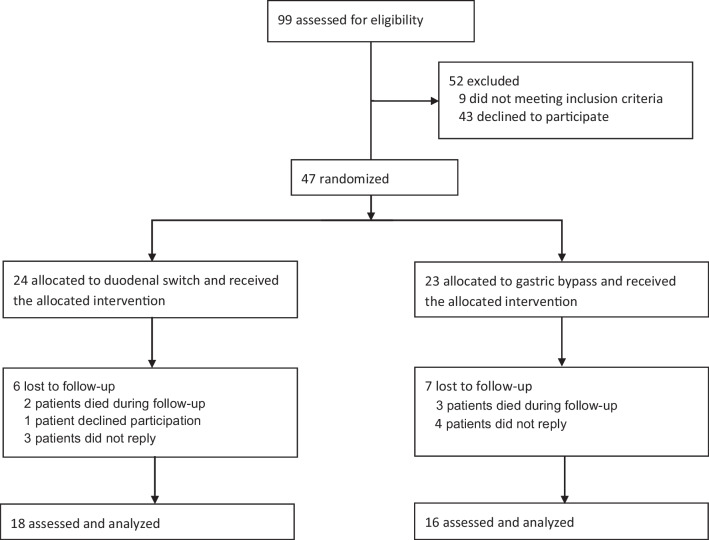
Of the initial 47 patients (24 BPD/DS, 23 RYGB), 42 patients were still alive and 34 (81%) (18 BPD/DS, 16 RYGB) accepted participation and returned our questionnaires (Fig. 1). The median follow-up time from surgery was 15 years (range 13 to 17 years). At follow-up, mean age (38.3 ± 8.6 vs. 38.5 ± 9.3) and proportion of men (61.1%, n = 11 vs. 56.3%, n = 9) did not differ between the two groups (Table 1). In a sensitivity analysis, baseline characteristics (gender, type of surgery, and preoperative BMI) did not differ between the responding patients (n = 34) and those lost to follow-up (n = 13).
Fig. 1.
Participant flow through the study
Table 1.
Anthropometric data, comorbidities, and biochemical profile at baseline and long-term follow-up
| BPD/DS (n = 18) | RYGB (n = 16) | ||||||
|---|---|---|---|---|---|---|---|
| Preop | Long-term | p value1 | Preop | Long-term | p value1 | p value2 | |
| BMI kg/m2, mean ± SD | 54.2 ± 7.1 | 33.8 ± 7.3 | < .001b | 53.8 ± 5.7 | 41.4 ± 8.6 | < .001b | .008a |
| Age, mean ± SD | 38.3 ± 8.6 | 38.5 ± 9.3 | .945a | ||||
| Men, % (n) | 61.1 (11) | 56.3 (9) | 1.000c | ||||
| Hypertension, % (n) | 44.4 (8) | 22.2 (4) | .219d | 31.3 (5) | 37.5 (6) | 1.00d | .457c |
| Hyperlipidemia, % (n) | 22.2 (4) | 11.1 (2) | .625d | 6.3 (1) | 6.3 (1) | 1.00d | 1.00c |
| Diabetes, % (n) | 22.2 (4) | 0 (0) | .125d | 18.8 (3) | 12.5 (2) | 1.00d | .214c |
| Sleep apnea, % (n) | 22.2 (4) | 5.6 (1) | .250d | 12.5 (2) | 0 (0) | .500d | 1.000c |
| Hemoglobin3, mean ± SD | 145.3 ± 10.1 | 122.4 ± 18.8 | < .001b | 140.6 ± 8.0 | 140.1 ± 6.2 | .237b | .002a |
| Albumin3, mean ± SD | 39.7 ± 2.2 | 36.3 ± 5.2 | .013b | 38.7 ± 2.4 | 38.2 ± 3.1 | .656b | .274a |
| HbA1c4, mean ± SD | 31.4 ± 10.4 | 30.8 ± 7.1 | .839b | 31.7 ± 12.5 | 41.8 ± 4.9 | .031b | < .001a |
| Glucose4, mean ± SD | 6.2 ± 1.4 | 5.1 ± 0.7 | .001b | 5.4 ± 0.8 | 6.2 ± 0.9 | .003b | < .001a |
| LDL4, mean ± SD | 3.0 ± 0.8 | 1.7 ± 0.7 | < .001b | 3.2 ± 0.8 | 2.8 ± 0.7 | .032b | < .001a |
| HDL4, mean ± SD | 1.1 ± 0.2 | 1.4 ± 0.7 | .108b | 1.1 ± 0.2 | 1.3 ± 0.4 | .087b | .541a |
| Triglycerides4, mean ± SD | 2.0 ± 1.0 | 0.9 ± 0.2 | < .001b | 2.0 ± 1.0 | 1.4 ± 0.8 | .148b | .063a |
1Within groups, i.e., before versus after surgery. 2Between groups in the long-term follow-up. 3g/l. 4mmol/l. aIndependent samples t-test. bPaired samples t-test. cFischer’s exact test. dMcNemar’s test. Preop, preoperative; Long-term, after 13–17 years; BMI, body mass index; BPD/DS, biliopancreatic diversion with duodenal switch; RYGB, Roux-en-Y gastric bypass; HbA1c, glycated hemoglobin; LDL, low density lipoprotein; HDL, high density lipoprotein; SD, standard deviation. Bold indicates statistical significance
Weight Loss
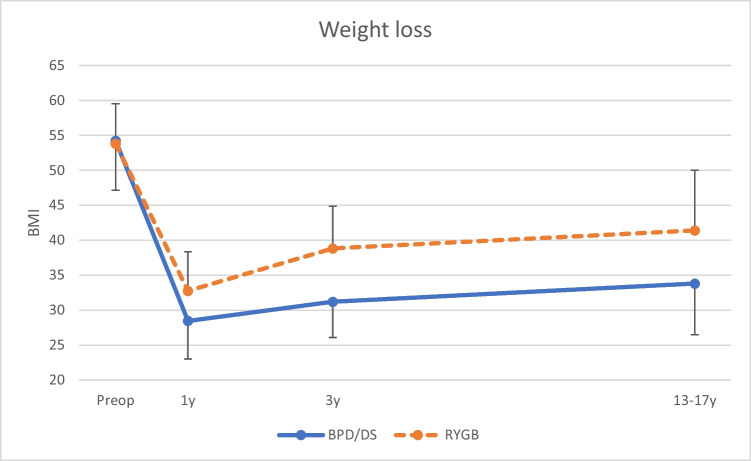
The BPD/DS group had a significantly higher weight loss. The BMI at follow-up was 33.8 ± 7.3 vs. 41.4 ± 8.6, which corresponded to a total BMI loss of 20.4 ± 7.9 vs. 12.4 ± 8.6 and %TBWL of 37.5% ± 12.2 vs. 22.8% ± 14.8, for BPD/DS and RYGB, respectively (all p < 0.01) (Table 1 and Fig. 2). Notably, 53.3% (n = 8) of the patients in the RYGB group had a BMI > 40 and were thus still classified as having severe obesity.
Fig. 2.
Weight loss, demonstrated as BMI at the actual time points. BMI, body mass index; BPD/DS, biliopancreatic diversion with duodenal switch; RYGB, Roux-en-Y gastric bypass; Preop, preoperative; 1y, 1 year postoperative; 3y, 3 years postoperative; 13–17y, 13–17 years postoperative
Comorbidities
There was no difference in comorbidities between the two groups at follow up. However, no BPD/DS-operated patient had diabetes mellitus at long-term follow-up (Table 1).
Adverse Events and Mortality
Adverse events were more common in the BPD/DS group (2.7 vs. 0.9 per patient, p < 0.004) with anemia, vitamin/mineral deficiency, and symptomatic cholelithiasis, with or without biliary events, being the most common. Subsequent surgeries were numerically more common after BPD/DS (0.9 vs. 0.3 surgeries per patient, p = 0.070) but showed no statistical significance (Table 2). Three BPD/DS patients had significant hypoalbuminemia, leading to reversal surgery in one patient. Furthermore, two BPD/DS patients underwent emergency surgery due to small bowel perforation and one due to large bowel perforation (perforated diverticulitis). Despite this, the long-term mortality did not differ (2 BPD/DS vs. 3 RYGB). One patient in the BPD/DS group died of postoperative pulmonary embolism and one patient in the RYGB group died of severe swine flu; no data was available for the other mortalities.
Table 2.
Registered number of adverse events, additional surgeries, and mortality during follow-up
| BPD/DS (n = 18) | RYGB (n = 16) | p value | Total (n = 34) | |
|---|---|---|---|---|
| Early postoperative complications | ||||
| Postoperative bleeding | 1 | 0 | 1.000a | 1 |
| Postoperative biliary leak | 1 | 0 | 1.000a | 1 |
| Postoperative abscess | 1 | 0 | 1.000a | 1 |
| Reoperation without pathology | 0 | 1 | .471a | 1 |
| Postoperative MI | 1 | 0 | 1.000a | 1 |
| Additional surgery | ||||
| Cholecystectomy | 6 | 1 | .090a | 7 |
| Bowel perforation | 3 | 0 | .230a | 3 |
| Bowel obstruction | 0 | 1 | .471a | 1 |
| Incisional hernia repair | 4 | 1 | .340a | 5 |
| Reversal due to resistant hypoalbuminemia | 1 | 0 | 1.000a | 1 |
| Deficiencies | ||||
| Anemia | 12 | 4 | .020a | 16 |
| Vitamin/mineral deficiency | 8 | 4 | .297a | 12 |
| Hypoalbuminemia | 3 | 1 | .604a | 4 |
| Non-surgical conditions | ||||
| Peptic ulcer disease/esophagitis | 1 | 0 | 1.000a | 1 |
| Alcohol abuse | 3 | 2 | 1.000a | 5 |
| Severe depression | 1 | 2 | .591a | 3 |
| Total number (per patient) | 48 (2.7) | 15 (0.9) | .004b | 61 (1.9) |
| Total additional surgeries (per patient) | 16 (0.9) | 4 (0.3) | .070b | 20 (0.6) |
| Patients with any adverse event (%) | 17 (94) | 9 (56) | .014a | 26 (76) |
| 30-day mortality (n = 47) | 0 | 1 | 1 | |
| Long-term mortality (n = 47) | 2 | 3 | 5 | |
aFischer’s exact test. bMann-Whitney U test. BPD/DS, biliopancreatic diversion with duodenal switch; RYGB, Roux-en-Y gastric bypass; MI, myocardial infarction. Bold indicates statistical significance
Gastrointestinal Symptoms
Reflux was more common in the BPD/DS group (22.2% vs. 0%, p = 0.043) whereas no differences were seen among the remaining symptoms: vomiting, dumping, abdominal pain, diarrhea, soiling, and foul smelling flatus.
Overall Satisfaction and Quality of Life
A high proportion of patients were satisfied or very satisfied with their procedure (BPD/DS: 72% and RYGB: 56%) and would recommend it to a friend seeking bariatric surgery (BPD/DS: 77.7% and RYGB: 66.6%), however without statistical significance between the two groups (p > 0.999 and p = 0.712, respectively). Quality of life (MAQ) was rated 0.50 ± 1.11 for BPD/DS and 0.24 ± 1.55 for RYGB (Table 3).
Table 3.
BAROS scores
| BPD/DS (n = 18) | RYGB (n = 16) | p value | |
|---|---|---|---|
| Weight loss sub-score | 2.22 ± 0.81 | 1.31 ± 1.14 | .011a |
| Comorbidity sub-score | 0.83 ± 1.29 | 0.25 ± 1.18 | .182a |
| Quality of life (MAQ) | 0.50 ± 1.11 | 0.24 ± 1.55 | .575a |
| Complication sub-score | − 0.58 ± 0.42 | − 0.46 ± 0.45 | .446a |
| BAROS score | 2.98 ± 2.32 | 1.34 ± 2.30 | .047a |
| BAROS w/o complication sub-score | 3.55 ± 2.43 | 1.80 ± 2.20 | .035a |
aIndependent samples t-test. BAROS, Bariatric Analysis and Reporting Outcome System; BPD/DS, biliopancreatic diversion with duodenal switch; RYGB, Roux-en-Y gastric bypass; MAQ, Moorhead-Ardelt Quality of life Questionnaire. Bold indicates statistical significance
Biochemical Profile
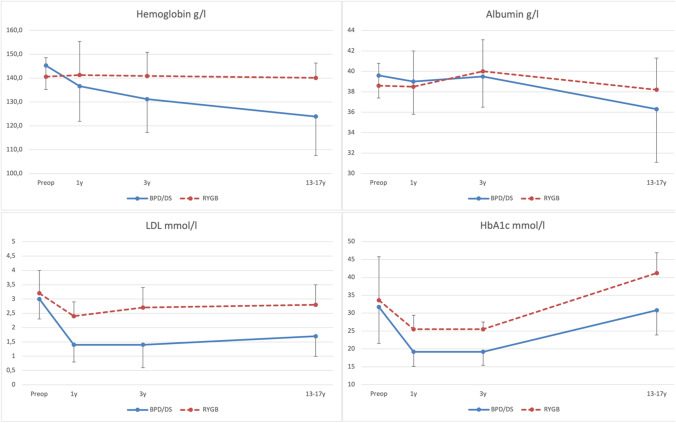
There was no difference in biochemical profiles at baseline. At 13–17 years postoperatively, the BPD/DS group had lower hemoglobin (122.4 ± 18.8 vs. 140.1 ± 6.2, p = 0.002), lower fasting glucose (5.1 ± 0.7 vs. 6.2 ± 0.9, p < 0.001), lower HbA1c (30.8 ± 7.1 vs. 41.8 ± 4.9, p < 0.001), and lower LDL (1.7 ± 0.7 vs. 2.8 ± 0.7, p < 0.001) compared to RYGB. Notably, fasting glucose was higher following RYGB compared to baseline (6.2 ± 0.9 vs. 5.4 ± 0.8, p = 0.003). No significant difference in albumin was seen between the two groups (36.3 ± 5.2 vs. 38.2 ± 3.1, p = 0.274) (Fig. 3).
Fig. 3.
Biochemical profiles. BPD/DS, biliopancreatic diversion with duodenal switch; RYGB, Roux-en-Y gastric bypass; Preop, preoperative; 1y, 1 year postoperative; 3y, 3 years postoperative; 13–17y, 13–17 years postoperative; LDL, low density lipoprotein; HbA1c, glycated hemoglobin
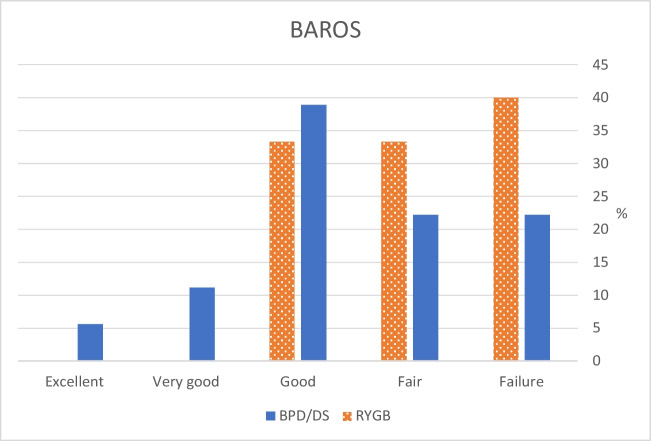
BAROS Score
A superior BAROS score was seen in the BPD/DS group (2.98 vs. 1.34, p = 0.047), also with the complication sub-score excluded (3.55 vs. 1.80, p = 0.035) (Table 3 and Fig. 4).
Fig. 4.
Classification of the overall result based on the total BAROS score. BAROS, Bariatric Analysis and Reporting Outcome System; BPD/DS, biliopancreatic diversion with duodenal switch; RYGB, Roux-en-Y gastric bypass
Discussion
This is the first randomized study with follow-up data beyond 10 years comparing RYGB and BPD/DS in patients with super obesity. We could demonstrate that BPD/DS resulted in a sustained superior weight loss, better metabolic control and a superior BAROS score, however, at the cost of a higher rate of nutritional and surgical adverse events.
Superior short-term weight loss in BPD/DS vs. RYGB has been demonstrated in other randomized and non-randomized studies [10, 11, 18, 19, 24]. A slight weight regain is common after all types of bariatric surgery but the fact that half (53.3%) of our RYGB-patients still have a BMI above 40, i.e., still making them candidates for bariatric surgery, is challenging. This problem has been identified by others [25, 26] and is of great concern as a BMI above 40 reduces life expectancy by 8–10 years [27]. Both procedures have well-documented effects on comorbidities. In the present study, with similar rates of diabetes preoperatively, no relapses were found in BPD/DS, while half of the diabetes-free RYGB-patients suffered relapse of their diabetes. Although this finding did not reach statistical significance in this study, superior effect on diabetes has previously been demonstrated by Marinari et al. (100% remission rate) [28] and by Süsstrunk et al. (92.8% complete or partial remission rate) [29], while our former meta-analysis based on 16 studies with single-center comparisons (874 DS and 1149 RYGB) failed to do so [18, 24]. In accordance with other randomized and non-randomized studies, no difference could be seen regarding resolution of hypertension and hyperlipidemia [10, 11, 18, 19, 24].
BPD/DS patients suffered from more adverse effects with an average of 2.7 issues per patient compared to 0.9 with RYGB. Some of the complications were considered severe with one patient requiring reversal surgery due to resistant hypoalbuminemia, two patients requiring emergency surgery due to small bowel perforation and one patient requiring emergency surgery due to perforated diverticulitis. The exact etiology to the bowel perforations is hard to establish, but in one of the small bowel perforations, malnutrition appeared to be a contributing factor. A higher number of consequent surgeries was seen for BPD/DS (0.9 vs. 0.3 surgeries per patient) with cholecystectomy being the most common but failed to reach statistical significance. The increased number of cholecystectomies is presumably due to a combination of higher weight loss and reduced bile re-absorption in BPD/DS. Performing a routine simultaneous cholecystectomy during BPD/DS has been subject to debate, some surgeons have performed it based on surgical judgment while others find it unnecessary [30] as the risk of biliary events is low [31]. It is however notable that 6 out of 18 (33%) BPD/DS-patients later underwent cholecystectomy. The somewhat high incidence of surgically addressed incisional hernias is related to the open approach might not translate well to current practice since 2017 with laparoscopic approach being the method of choice. The higher incidence of complications and subsequent surgeries after BPD/DS have been confirmed in other studies [10, 11, 18, 24]. However, even though the complication rate was higher in BPD/DS, the overall mortality was similar between the groups. Gastrointestinal symptoms were similar between groups except for reflux, which was more common in the BPD/DS group. The increased reflux rate is strongly associated to the gastric sleeve component [32]. Although not reaching statistical significance, we could note that dumping was numerically more common after RYGB, while diarrhea and foul-smelling flatus was seen after BPD/DS, all in accordance with previous studies [18, 19]. We believe that it is important to discuss these differences during preoperative counseling.
Regarding quality of life, the result was similar between the groups even though the BPD/DS group suffered from more adverse effects and gastrointestinal symptoms. We, however, report rather low quality of life scores compared to other studies [33, 34]. Population characteristics such as cultural factors, expectations as well as the long follow-up time, resulting in gradual weight regain might explain these findings.
As expected, the BPD/DS group had superior glucose hemostasis as seen in other studies [10, 18, 19, 24, 35] and increased risk for anemia (12 vs. 4 patients), with a mean hemoglobin reduction of 23 g/l from baseline to the long-term follow up. Although one BPD/DS-patient was reversed due to resistant hypoalbuminemia, no significant difference in albumin was seen between the two groups, both having mean values within the normal range. Hypoalbuminemia and other metabolic deficits, especially in fat-soluble vitamins, are otherwise common after BPD/DS [18, 36]. We have occasionally resorted to use intramuscular cholecalciferol injections in BPD/DS-patients with treatment resistant hypovitaminosis D [37]. Single-Anastomosis Duodenal Switch (SADI-S) had been recently proposed as an alternative to BPD/DS with some authors claiming similar or slightly lower weight loss but also lower morbidity [38–40]. However, more randomized studies are needed to fully elicit the differences.
BAROS is a well-established tool [20], however, seldom used in long-term studies. In the present study, the total BAROS score was still rated “good” or better in 56% of BPD/DS patients compared to only 31% of RYGB patients. The difference is mainly due to the superior weight loss sub-score in BPD/DS. The present average BAROS score (BPD/DS: 2.98 and RYGB: 1.34) is however lower than in other studies, especially those with shorter follow-up. As mentioned previously, we demonstrated a score of 4.7 vs. 4.0 at 4 years postoperatively in a larger cohort of patients having identical care [11], while Patel et al. reported a BAROS score of 3.3 for laparoscopic RYGB at 10 years [34]. At the same time point, Askari et al. demonstrated that 53.2% of LRYGB patients had a “good” or better result [33]. Thus, the BAROS scores seem to decrease with time and some of the differences compared to our study may be attributed to inclusion of patients with less severe obesity, cultural factors affecting quality of life scorings, and observer bias when scrutinizing complications, and differing methodology.
The multiple benefits of BPD/DS outlined above must be weighed against the increased risk of complications and the need for a rigorous follow-up regimen. Some complications are severe and further consume a significant amount of health-care resources aside for patient morbidity. However, relapse of comorbidities and weight gain are as well very problematic as it creates health-related problems further on in life, reducing life expectancy [27]. A two-step approach with an initial sleeve gastrectomy and, in selected cases, addition of the duodenal switch has been proposed by some authors to reduce problems in patients with potential inferior follow up [18, 41]. A second-stage duodenal switch might also be valuable in patients with insufficient weight loss after sleeve gastrectomy, despite having good adherence to the follow-up regimen [41]. Nevertheless, the present long-term results have encouraged us to continue using BPD/DS in our clinical practice.
Strengths and Limitations
This is the first randomized study comparing RYGB and BPD/DS with follow-up data extending beyond 10 years. Despite the rather small number of patients, we could verify the expected benefits of the two procedures and establish the long-term superiority of BPD/DS concerning BAROS score, weight loss, glucose homeostasis, and HbA1c, at the cost of more complications. However, the small number of patients could make the study under-powered in finding statistical significance for some trends that were noted. Premature closure of inclusion to the study was due to the increasing numbers of patients declining randomization in favor of BPD/DS. Among other potential limitations, the remission of comorbid diseases is somewhat crude as it is based on the use of pharmacological therapy and not exact diagnostic criteria for each disease. We did unfortunately not have access to vitamin and mineral status, nor the cause of death for some of the deceased patients. Weight loss was self-reported which might underestimate the weight at follow-up. However, in a study of 179 overweight bariatric surgery candidates, the BMI misestimation was only negative 0.59% BMI and did not show statistical significance [42].
Moreover, the use of open surgery, standard at our center for patients with super obesity at the time of randomization (2004–2007) explains the rather large number of incisional hernias, while laparoscopic approach, with its well established advantages [43], is standard today since 2016. Furthermore, BAROS scoring can be problematic as the subtraction of points for complications is very observer dependent, and the fact that early postoperative complications lose their significance as time passes. Indeed, several authors have raised critique regarding this instrument [44]. Considering that the operated population ages with time, and in turns develops comorbidities as the general populations does, the comorbidity scores will unenviably migrate toward failure. Some trends could be noted regarding the comorbidities, especially diabetes mellitus, but unfortunately, this study lacked power to show statistical significance.
Conclusion
In conclusion, we could verify that BPD/DS results in superior weight loss and metabolic control, at the cost of more complications, when compared to RYGB in patients with super obesity at 13–17 years after surgery. In addition, the global assessment tool BAROS demonstrated superior scores in the BPD/DS group.
Funding
Open access funding provided by Uppsala University. The study was financed by Swedish governmental funding of clinical research (ALF).
Data Availability
Aggregated data supporting this study are available upon reasonable request. Please contact filip.moller@surgsci.uu.se.
Declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Key Points
• Optimal surgical treatment for patients with super obesity, BMI > 50 kg/m2 is still a matter of debate.
• We present long-term data (13–17 years) from an RCT between BPD/DS and RYGB.
• BPD/DS resulted in superior weight loss, metabolic control, and BAROS score.
• However, at the cost of more surgical and medical adverse events, compared to RYGB.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Overweight & Obesity Statistics. National Institute of Diabetes and Digestive and Kidney Diseases. Updated September, 2021. https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity. Accessed December 20, 2022.
- 2.Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7(5):273–89. doi: 10.1111/cob.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YC, Pamplin J, Long MW, Ward ZJ, Gortmaker SL, Andreyeva T. Severe obesity in adults cost state medicaid programs nearly $8 billion in 2013. Health Aff (Millwood) 2015;34(11):1923–31. doi: 10.1377/hlthaff.2015.0633. [DOI] [PubMed] [Google Scholar]
- 4.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 5.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121(7):492–6. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37(6):889–91. doi: 10.1038/ijo.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakarla VR, Nandipati K, Lalla M, Castro A, Merola S. Are laparoscopic bariatric procedures safe in superobese (BMI >/=50 kg/m2) patients? An NSQIP data analysis. Surg Obes Relat Dis. 2011;7(4):452–8. doi: 10.1016/j.soard.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 8.MacLean LD, Rhode BM, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg. 2000;231(4):524–8. doi: 10.1097/00000658-200004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DinizMde F, Passos VM, Barreto SM, Linares DB, de Almeida SR, Rocha AL, et al. Different criteria for assessment of Roux-en-Y gastric bypass success: does only weight matter? Obes Surg. 2009;19(10):1384–92. doi: 10.1007/s11695-008-9669-6. [DOI] [PubMed] [Google Scholar]
- 10.Skogar ML, Sundbom M. Weight loss and effect on co-morbidities in the long-term after duodenal switch and gastric bypass: a population-based cohort study. Surg Obes Relat Dis. 2020;16(1):17–23. doi: 10.1016/j.soard.2019.09.077. [DOI] [PubMed] [Google Scholar]
- 11.Skogar ML, Sundbom M. Duodenal switch is superior to gastric bypass in patients with super obesity when evaluated with the bariatric analysis and reporting outcome system (BAROS) Obes Surg. 2017;27(9):2308–16. doi: 10.1007/s11695-017-2680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marceau P, Biron S, Marceau S, Hould FS, Lebel S, Lescelleur O, et al. Long-term metabolic outcomes 5 to 20 years after biliopancreatic diversion. Obes Surg. 2015;25(9):1584–1593. doi: 10.1007/s11695-015-1599-5. [DOI] [PubMed] [Google Scholar]
- 13.Prachand VN, Davee RT, Alverdy JC. Duodenal switch provides superior weight loss in the super-obese (BMI > or =50 kg/m2) compared with gastric bypass. Ann Surg. 2006;244(4):611–9. doi: 10.1097/01.sla.0000239086.30518.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prachand VN, Ward M, Alverdy JC. Duodenal switch provides superior resolution of metabolic comorbidities independent of weight loss in the super-obese (BMI > or = 50 kg/m2) compared with gastric bypass. J Gastrointest Surg. 2010;14(2):211–220. doi: 10.1007/s11605-009-1101-6. [DOI] [PubMed] [Google Scholar]
- 15.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66(9):618–20. doi: 10.1002/bjs.1800660906. [DOI] [PubMed] [Google Scholar]
- 16.Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8(3):267–282. doi: 10.1381/096089298765554476. [DOI] [PubMed] [Google Scholar]
- 17.DeMeester TR, Fuchs KH, Ball CS, Albertucci M, Smyrk TC, Marcus JN. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206(4):414–26. doi: 10.1097/00000658-198710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Risstad H, Sovik TT, Engstrom M, Aasheim ET, Fagerland MW, Olsen MF, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015;150(4):352–361. doi: 10.1001/jamasurg.2014.3579. [DOI] [PubMed] [Google Scholar]
- 19.Hedberg J, Sundbom M. Superior weight loss and lower HbA1c 3 years after duodenal switch compared with Roux-en-Y gastric bypass–a randomized controlled trial. Surg Obes Relat Dis. 2012;8(3):338–43. doi: 10.1016/j.soard.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Wolf AM, Falcone AR, Kortner B, Kuhlmann HW. BAROS: an effective system to evaluate the results of patients after bariatric surgery. Obes Surg. 2000;10(5):445–450. doi: 10.1381/096089200321593940. [DOI] [PubMed] [Google Scholar]
- 21.Moorehead MK, Ardelt-Gattinger E, Lechner H, Oria HE. The validation of the Moorehead-Ardelt Quality of Life Questionnaire II. Obes Surg. 2003;13(5):684–692. doi: 10.1381/096089203322509237. [DOI] [PubMed] [Google Scholar]
- 22.Oria HE, Moorehead MK. Updated Bariatric Analysis and Reporting Outcome System (BAROS) Surg Obes Relat Dis. 2009;5(1):60–6. doi: 10.1016/j.soard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Oria HE, Moorehead MK. Bariatric analysis and reporting outcome system (BAROS) Obes Surg. 1998;8(5):487–499. doi: 10.1381/096089298765554043. [DOI] [PubMed] [Google Scholar]
- 24.Hedberg J, Sundstrom J, Sundbom M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obes Rev. 2014;15(7):555–63. doi: 10.1111/obr.12169. [DOI] [PubMed] [Google Scholar]
- 25.Suter M, Calmes JM, Paroz A, Romy S, Giusti V. Results of Roux-en-Y gastric bypass in morbidly obese vs superobese patients: similar body weight loss, correction of comorbidities, and improvement of quality of life. Arch Surg. 2009;144(4):312–8. doi: 10.1001/archsurg.2009.19. [DOI] [PubMed] [Google Scholar]
- 26.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prospective Studies C. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marinari GM, Murelli F, Camerini G, Papadia F, Carlini F, Stabilini C, et al. A 15-year evaluation of biliopancreatic diversion according to the Bariatric Analysis Reporting Outcome System (BAROS) Obes Surg. 2004;14(3):325–328. doi: 10.1381/096089204322917828. [DOI] [PubMed] [Google Scholar]
- 29.Süsstrunk J, Kraljević M, Schneider R, Peterli R. Long-term outcome after biliopancreatic diversion type duodenal switch: a single-center experience with up to 20 years follow-up. Br J Surg. 2022;109(Supplement_3). [DOI] [PubMed]
- 30.Bardaro SJ, Gagner M, Consten E, Inabnet WB, Herron D, Dakin G, et al. Routine cholecystectomy during laparoscopic biliopancreatic diversion with duodenal switch is not necessary. Surg Obes Relat Dis. 2007;3(5):549–53. doi: 10.1016/j.soard.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Sucandy I, Abulfaraj M, Naglak M, Antanavicius G. Risk of biliary events after selective cholecystectomy during biliopancreatic diversion with duodenal switch. Obes Surg. 2016;26(3):531–537. doi: 10.1007/s11695-015-1786-4. [DOI] [PubMed] [Google Scholar]
- 32.Elias K, Hedberg J, Sundbom M. Prevalence and impact of acid-related symptoms and diarrhea in patients undergoing Roux-en-Y gastric bypass, sleeve gastrectomy, and biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2020;16(4):520–7. doi: 10.1016/j.soard.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Askari A, Dai D, Taylor C, Chapple C, Halai S, Patel K, et al. Long-term outcomes and quality of life at more than 10 years after laparoscopic Roux-en-Y gastric bypass using bariatric analysis and reporting outcome system (BAROS) Obes Surg. 2020;30(10):3968–73. doi: 10.1007/s11695-020-04765-0. [DOI] [PubMed] [Google Scholar]
- 34.Patel K, Askari A, Mamidanna R, Jain V, Adil T. Long-term BAROS scores and independent obesity-related co-morbidity predictors of failure after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2020;16(12):1954–60. doi: 10.1016/j.soard.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 35.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397(10271):293–304. doi: 10.1016/S0140-6736(20)32649-0. [DOI] [PubMed] [Google Scholar]
- 36.Skogar ML, Sundbom M. Early complications, long-term adverse events, and quality of life after duodenal switch and gastric bypass in a matched national cohort. Surg Obes Relat Dis. 2020;16(5):614–9. doi: 10.1016/j.soard.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Hultin H, Stevens K, Sundbom M. Cholecalciferol injections are effective in hypovitaminosis D after duodenal switch: a randomized controlled study. Obes Surg. 2018;28(10):3007–11. doi: 10.1007/s11695-018-3307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebellí JP, Lazzara C, de Gordejuela AGR, Nora M, Pereira AM, Sánchez-Pernaute A, et al. Duodenal switch vs. single-anastomosis duodenal switch (SADI-S) for the treatment of grade IV obesity: 5-year outcomes of a multicenter prospective cohort comparative study. Obes Surg. 2022;32(12):3839–46. doi: 10.1007/s11695-022-06317-0. [DOI] [PubMed] [Google Scholar]
- 39.Surve A, Cottam D, Belnap L, Richards C, Medlin W. Long-term (> 6 years) outcomes of duodenal switch (DS) versus single-anastomosis duodeno-ileostomy with sleeve gastrectomy (SADI-S): a matched cohort study. Obes Surg. 2021;31(12):5117–26. doi: 10.1007/s11695-021-05709-y. [DOI] [PubMed] [Google Scholar]
- 40.Yashkov Y, Bordan N, Torres A, Malykhina A, Bekuzarov D. SADI-S 250 vs Roux-en-Y duodenal switch (RY-DS): results of 5-year observational study. Obes Surg. 2021;31(2):570–9. doi: 10.1007/s11695-020-05031-z. [DOI] [PubMed] [Google Scholar]
- 41.Biertho L, Theriault C, Bouvet L, Marceau S, Hould FS, Lebel S, et al. Second-stage duodenal switch for sleeve gastrectomy failure: a matched controlled trial. Surg Obes Relat Dis. 2018;14(10):1570–9. doi: 10.1016/j.soard.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 42.White MA, Masheb RM, Burke-Martindale C, Rothschild B, Grilo CM. Accuracy of self-reported weight among bariatric surgery candidates: the influence of race and weight cycling. Obesity (Silver Spring) 2007;15(11):2761–2768. doi: 10.1038/oby.2007.328. [DOI] [PubMed] [Google Scholar]
- 43.Reoch J, Mottillo S, Shimony A, Filion KB, Christou NV, Joseph L, et al. Safety of laparoscopic vs open bariatric surgery: a systematic review and meta-analysis. Arch Surg. 2011;146(11):1314–1322. doi: 10.1001/archsurg.2011.270. [DOI] [PubMed] [Google Scholar]
- 44.Nicareta JR, de Freitas AC, Nicareta SM, Nicareta C, Campos AC, Nassif PA, et al. Baros Method Critical Analysis(Bariatric Analysis and Reporting System) Arq Bras Cir Dig. 2015;28(Suppl 1):73–8. doi: 10.1590/S0102-6720201500S100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Aggregated data supporting this study are available upon reasonable request. Please contact filip.moller@surgsci.uu.se.