Key Points
-
•
Haplo-HSCT results in encouraging disease-free survival despite significant hemorrhagic cystitis in the MAC arm.
-
•
Early cessation of CNI is feasible in haplo-HSCT, with most patients discontinuing immunosuppression at 12 months.
Visual Abstract
Abstract
Haploidentical hematopoietic stem cell transplant (haplo-HSCT) using posttransplant cyclophosphamide (PTCy) is appropriate for those who lack matched donors. Most studies using PTCy have been retrospective making conclusions difficult. ANZHIT-1 was a phase 2 study conducted at 6 Australian allogeneic HSCT centers. The primary end points were disease-free and overall survival at 2 years after HSCT. The reduced-intensity conditioning (RIC) included fludarabine, cyclophosphamide, and 200 cGy total body irradiation, and the myeloablative conditioning (MAC) was IV fludarabine and busulfan. PTCy, MMF and a calcineurin inhibitor (CNI) were used for graft-versus-host disease (GVHD) prophylaxis. CNIs were weaned and ceased by day +120 in eligible patients on day 60. Patients (n = 78) with hematological malignancies were included in the study, with a median follow-up of 732 days (range, 28-1728). HSCT was RIC in 46 patients and MAC in 32 patients. Disease-free survival probability at 2 years was 67.5% (95% [CI], 53.2-85.6) for MAC recipients and 68.3% (95% CI, 56.3-83.01) for RIC recipients. Transplant-related mortality (TRM) on day 100 and year 1 was 4.9% (95% CI, 1.6-15.3) and 17.9% (95% CI, 8.8-36.5), respectively, in the MAC group compared with 3.1% (95% CI, 0.8.1-12) and 11.6% (95% CI, 6-22.4), respectively, in the RIC group. The median time for elective cessation of CNI was day 142.5 days, with no excess chronic GVHD (cGVHD) or mortality. Of the evaluable patients, 71.6% discontinued immunosuppression 12 months after transplant. This prospective haplo-HSCT trial using PTCY demonstrated encouraging survival rates, indicating that early CNI withdrawal is feasible and safe.
Introduction
Allogeneic stem cell transplant (HSCT) is a potentially curative procedure for a wide range of hematological conditions. The donor choice for transplant is classically determined by the availability of a HLA-matched sibling or an unrelated donor. However, a large number of patients, based on HLA heterogeneity, cannot access these donor sources, as demonstrated by an analysis of the National Marrow Donor Program in 2014.1 Thankfully, partially HLA-mismatched related (haploidentical) donors can be identified for the vast majority of these patients, particularly among those with a non-white background.1 Historically, haploidentical hematopoietic stem cell transplant (haplo-HSCT) has been complicated because of excessive graft-versus-host disease (GVHD), transplant-related mortality (TRM), and poor overall survival (OS) due to major HLA mismatch.2
The advent of posttransplant cyclophosphamide (PTCy) in 2008, however, substantially mitigated GVHD,3 making haplo-HSCT increasingly safer and more widely used. PTCy has been demonstrated to significantly reduce both acute GVHD (aGVHD) and chronic GVHD (cGVHD) in both the haploidentical4 and matched settings.5,6 More recently, the potent effects of PTCy have allowed for successful transplantation, even in patients receiving mismatched allografts.7 Consequently, the use of haplo-HSCT has markedly increased globally over the last 10 years, whereas the use of other alternative sources, such as cord blood, has decreased significantly.8 This trend has also been highlighted in annual surveys from the European Society for Blood and Marrow Transplantation (EBMT), in which haplo-HSCT has increased threefold from 2008 to 2018.9
Despite this increased activity in haplo-HSCT, there are few multicenter prospective trials assessing the optimal regimen for the growing number of patients with haplo-HSCT in need of allografting. The original Baltimore protocol3 has been the standard for reduced-intensity conditioning (RIC) regimens over the last decade and has been widely adopted. However, the use of bone marrow as a stem cell source and the relatively high rate of relapse (as reported by the same group10) suggests that refinement is warranted. In the myeloablative conditioning (MAC) setting, there are no accepted standard regimens, and very few studies have assessed a specific regimen prospectively. The vast majority of studies examining the use of haplo-HSCT have been retrospective and through co-operative groups using multiple conditioning regimens and both stem cell sources,11 thus making the interpretation of outcomes challenging.
Here, we present a prospective, multicenter study of haplo-HSCT using predefined MAC and RIC and GVHD regimens, with the stratification of patients based on the HCT-comorbidity index12 (HCT-CI) and age. The ANZHIT-1 study used the Baltimore regimen for RIC recipients and fludarabine busulfan regimen for MAC recipients. The study was overseen by an independent safety data monitoring committee, with a primary objective of overall and disease survival at 2 years. Peripheral blood was the stem cell source in all patients. Given the high rate of relapse in some studies,10 there were predefined criteria for ceasing immunosuppression early, when feasible, given the suggestion that earlier cessation with bone marrow as the donor source may lead to a reduced incidence of relapse.13
Methods
Study design and patients
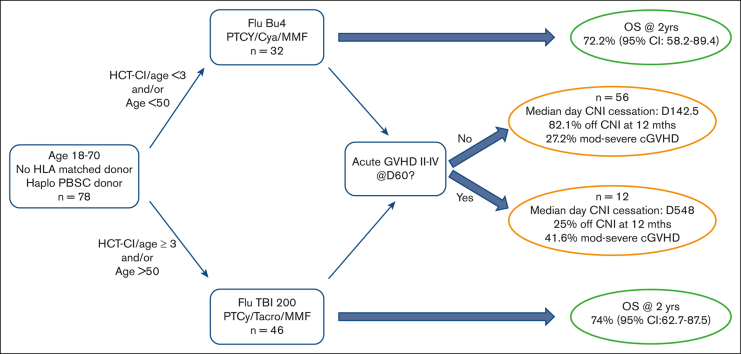
This was a human research ethics committee–approved prospective, multicenter, phase 2 study of peripheral blood haplo-HSCT (the ANZHIT-1 study). All recipients were patients without an HLA-matched sibling or 10/10 HLA-matched unrelated donors in all available unrelated donor registries. Haploidentical donors were identified using appropriate HLA serological and molecular typing. Molecular typing for HLA-A, -B, -C, HLA-DRB1, and HLA-DQB genes was performed for all donor–recipient pairs. However, the definition of haploidentical was based on the matching of 3 or 4 of HLA-A, -B, and -DRB1 antigens in first-degree relatives. The study was registered in the Australian/New Zealand Clinical Trials Register before enrollment of the first patient (ACTRN 12617000151336). ANZHIT-1 was conducted between 2015 and 2020 at 6 Australian allogeneic HSCT centers, with St Vincent’s Hospital, Sydney, being the lead site Human Research Ethics Committee (HREC 15/100). All the patients underwent peripheral blood HSCT from haploidentical family members after obtaining written informed consent. Allocation of patients to the MAC or RIC regimen was at the discretion of the attending physician, but protocol guidelines suggested that patients with HCT-CI < 3 and/or age < 50 years receive MAC and those with HCT-CI ≥ 3 and/or age > 50 years receive RIC. Data were collected in an online electronic case report form through the Australasian Bone Marrow Transplant Recipients Registry.
Power calculations for the study were applied to the MAC arm, given the lack of prospective data using fludarabine busulfan in the haplo-HSCT setting. The hypothesis was that the MAC regimen would reduce the relapse rate without increased toxicity and improve disease-free survival from 50% to 75%. Based on the single-stage design by A’Hern, a sample size of 24 patients will have >80% power, with 95% confidence to exclude a 50% disease-free survival rate in favor of the more favorable 75% rate.
This study was conducted in compliance with the Declaration of Helsinki and any subsequent amendments, the ICH Guidelines for Good Clinical Practice (CPMP/ICH/135/95) annotated with TGA comments (July 2000), the NHMRC National Statement on Ethical Conduct in Research involving Humans (2007) and any applicable local guidelines. All the patients were fully informed before signing the approved informed consent forms. Serious adverse events were reported to the local human research ethics committee per ICH Good Clinical Practice guidelines as an untoward medical occurrence in a trial participant. These serious adverse events were graded based on the Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
Treatment schedule
Peripheral blood stem cells were collected after mobilization with granulocyte colony–stimulating factor from haploidentical donors, as per the institutional guidelines. The dose of CD34+ cells transplanted into the peripheral blood stem cell product was recommended, although not mandated, from a minimum of 2 × 106/kg to a maximum of 5 × 106/kg body weight.
Patients who underwent MAC peripheral blood stem cell transplant received fludarabine 40 mg/m2 per day on days −5, −4, −3, and −2 and IV busulfan 3.2 mg/kg on days −5, −4, −3, and −2. GVHD prophylaxis consisted of PTCy 50 mg/kg on days +3 and +4, cyclosporin 3 mg/kg per day, commencing on day +5, IV, until engraftment and then orally. Mycophenolate 15 mg/kg, IV or orally, was administered from day +5 to day +35.
Patients who underwent RIC allogeneic transplant received fludarabine 30 mg/ m2 per day on days −6, −5, −4, −3, and −2, IV cyclophosphamide 14.5 mg/kg per day on days −6 and −5, and total body irradiation 200 cGy on day -1. GVHD prophylaxis consisted of PTCy 50 mg/kg on days +3 and +4, tacrolimus in 2 divided daily oral doses to target blood levels from 5 to 15 ng/ml, commenced on day +5. Mycophenolate 15 mg/kg, IV or orally, was used from day +5 to day +35.
As per the protocol, tacrolimus and cyclosporine administration was tapered on day +60; and if there was no evidence of grade 2-4 aGVHD, relapse, death, or low chimerism, calcineurin inhibitors (CNIs) could be electively weaned according to institutional guidelines from day +60 to day 120, provided there was no evidence of GVHD. Optional donor lymphocyte infusions commencing at a CD3 cell dose of 0.5 × 106 per kg body weight at day +120 could be administered to those deemed to be at high risk for relapse by the treating physician. Donor-specific antibodies were managed with IV rituximab 375 mg/m2 weekly × 4 (days 35, 28, 21, and 14) and plasma exchange on days −1 and 0.
Supportive care was performed per institutional guidelines, but all patients were advised to receive adequate Pneumocystis jirovecii, Aspergillus, and herpetic prophylaxis along with immunosuppression. Cytomegalovirus (CMV) prophylaxis was not used, but weekly CMV and Epstein-Barr virus (EBV) polymerase chain reaction monitoring was advised from day +21 to day +100. Letermovir was not used for CMV prophylaxis because it was not available in the Pharmaceutical Benefits Scheme in Australia during the study. Pre-emptive therapy for CMV or EBV reactivation was at the discretion of individual centers based on their own policies and guidelines.
Definitions and end points
The primary objective of this study was to assess the probability of disease-free survival (DFS) and OS at 2 years in both the MAC and RIC cohorts. OS was defined as the time in days until death from any cause. DFS was defined as the time from the transplant to the first disease progression/relapse or death (whichever occurred first). GVHD, relapse-free survival (GRFS) was defined as the time to any of the following end points, as previously described14: alive with no relapse, no aGVHD (grade 3-4), and no cGVHD requiring systemic treatment. aGVHD was assessed using the Center for International Blood and Marrow Transplant Research (CIBMTR) criteria,15 and cGVHD was assessed using the National Institutes of Health consensus document.16
The time to GRFS was determined as the earliest among the times to aGVHD (grade 2-4), relapse, or death. Safety, measured based on the TRM, was defined as death due to complications from the transplant procedure after stem cell infusion that were not due to the underlying disease.
Stopping rules
The study was overseen by a safety data monitoring committee (SDMC) consisting of a statistician and 2 hematologists who were not investigators at any site involved in ANZHIT-1. The committee met every 3 months to review safety, as measured based on the TRM. An overall 100-day TRM of 30% observed in the MAC arm would require closure of the trial in that arm. The stopping rule for the IV fludarabine/busulfan MAC arm was that if after 12 patients had been observed for 180 days, the number of patients experiencing TRM exceeded 7, consideration would be given to closing that arm of the study.
Statistical analysis
OS and DFS were analyzed using the Kaplan-Meier method at 12 months and then yearly. TRM at day 100 and 1 year was calculated using the cumulative incidence function, with relapse or death (other than TRM) as competing risks, with point estimates reported on day +100 and 1 year. Neutrophil engraftment was defined as the first date of the first of 3 consecutive absolute neutrophil counts ≥0.5 × 109/L on different days. Platelet engraftment was defined as the date of the first of 3 consecutive laboratory values ≥20 × 109/L obtained on different days when no platelet transfusion was given within the preceding 7 days. The time to successful engraftment was analyzed using a cumulative incidence function, with relapse and death as competing risks. Disease relapse was estimated using cumulative incidence functions, with death as a competing risk. Multivariate analysis of predictive factors associated with OS, DFS, and TRM was conducted using Cox regression. Several relevant covariates were considered for the univariate and multivariate analyses of DFS, OS, GRFS, and TRM. Covariates analyzed included conditioning regimen, CMV status, age, underlying disease (acute myeloid leukemia [AML] vs others), disease risk index (DRI) (low/intermediate vs high/very high), previous autologous transplant, CD34 cell dose, CD3 dose, donor age and relationship, HCT-CI (> or <3), time to elective cessation of CNI, aGVHD grade (1/2 vs 3/4), and cGVHD (none/mild vs moderate/severe). For each outcome of interest, univariate models were fitted using SAS PROC PHREG, and a hazard ratio was obtained with 95% confidence limits and a P-value. All terms with a P < .2 or a hazard ratio [HR] < 0.8 or > 1.2 were considered for inclusion in a multivariate model. The final MV model for each outcome was chosen based on automated selection procedures, best subsetapproach together with clinical judgment, and model fit statistics (AIC).
P ≤ .05 was considered significant. All analyses were performed using SAS version 9.4 or higher, and R version 4.0.3 (2020-10-10).
Results
Patient characteristics
Seventy-eight patients (52 male and 26 female) were enrolled in ANZHIT-1, with patient demographics outlined in Table 1. The median age was 38 years (range, 18-59) in the MAC arm and 59 years (range, 20-69) in the RIC arm. The median follow-up was 732 days (range, 28-1728). Thirty-nine patients had AML (21 in CR1 and 18 in CR2), 12 had acute lymphoblastic leukemia (8 in CR1 and 4 in CR2), 14 non-Hodgkin or Hodgkin lymphoma (1 in CR1 and all others in CR >2), 4 had myelodysplastic syndrome, and 9 had other hematological malignancies (supplemental Table 1). Most patients received RIC (n = 46%-59%), whereas the remainder received MAC (n = 32%-41%). Most patients were in remission at the time of transplant, with low/intermediate DRI in 87.5% of MAC and 82.2% of RIC recipients. HCT-CI was ≥3 years in none of the MAC recipients and 21.7% of the RIC recipients. The median age of the donors was 28.5 years (range, 14-65) for MAC recipients and 34 years (range, 21-68) for RIC recipients. Transplant donors were siblings in 50%, offspring in 43.6%, and parents in 5.3% of the cases. The median infused CD34 dose was 5 × 106 CD34 cells per kg (range, 2.7-8.4) in both the MAC and RIC cohorts.
Table 1.
Baseline patient demographics
| MAC | RIC | |
|---|---|---|
| N | 32 | 46 |
| Male, N (%) | 21 (65.6) | 31 (67.4) |
| Female, N (%) | 11 (34.4) | 15 (32.6) |
| Age (med) (y) (Min-Max) |
38.0 (18-59) | 59.0 (20-69) |
| Diagnoses | ||
| AML | 14 (43.8) | 25 (54.3) |
| CR1 | 8 | 13 |
| CR2 | 6 | 11 |
| ALL | 9 (28.1) | 3 (6.5) |
| CR1 | 7 | 1 |
| CR2 | 2 | 2 |
| CML | 1 (3.1) | 0 (0.0) |
| MDS | 3 (9.3) | 2 (4.3) |
| NHL | 3 (9.3) | 7 (15.2) |
| CR1 | 0 | 1 |
| CR2 | 1 | 1 |
| >CR2 | 2 | 5 |
| HL | 0 | 4 (8.7) |
| CR1 | — | 0 |
| CR2 | — | 2 |
| >CR2 | — | 2 |
| MPD/MM/other | 2 (6.2) | 5 (10.9) |
| DRI | ||
| Low | 4 (12.5) | 11 (24.4) |
| Intermediate | 24 (75) | 26 (57.8) |
| High | 4 (12.5) | 8 (17.8) |
| HCT-CI | ||
| <3 | 32(100) | 36 (78.3) |
| ≥3 | 0(0) | 10(21.7) |
| Karnofsky performance status (%) | ||
| ≥90 | 83.4 | 60.8 |
| <90 | 17.6 | 39.2 |
| Previous auto HSCT | 3 (9.4) | 6 (13) |
| CMV serology | ||
| Positive | 25 (78.1) | 39 (84.8) |
| Negative | 7 (21.9) | 7 (15.2) |
| Donor age (med) (y) (min-max) |
28.5 (14-65) | 34.5 (21-68) |
| Donor relation | ||
| Offspring | 7 | 27 |
| Sibling | 22 | 17 |
| Parent | 3 | 1 |
ALL, acute lymphoblastic leukemia; Max; maximum; MDS, myelodysplastic syndrome; Min, minimum; MM; med, median; MPD, myeloproliferative disease.
OS and DFS
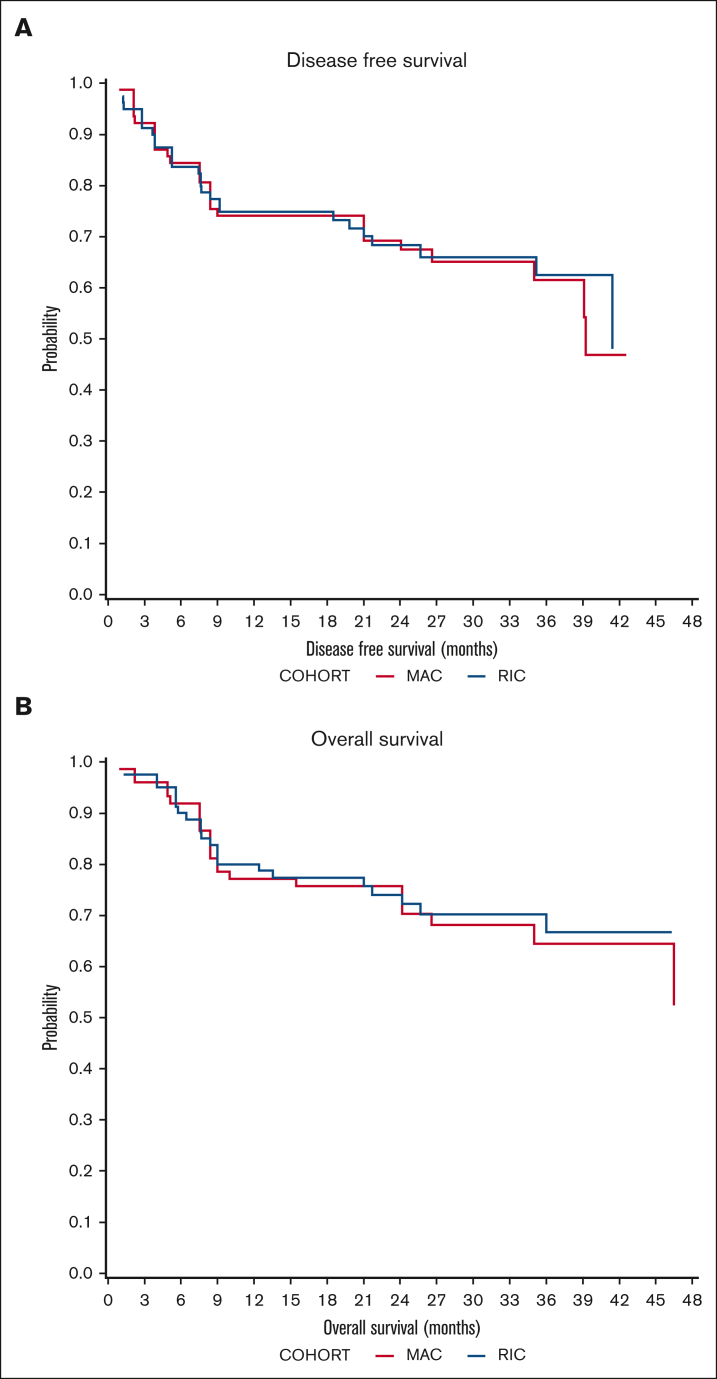
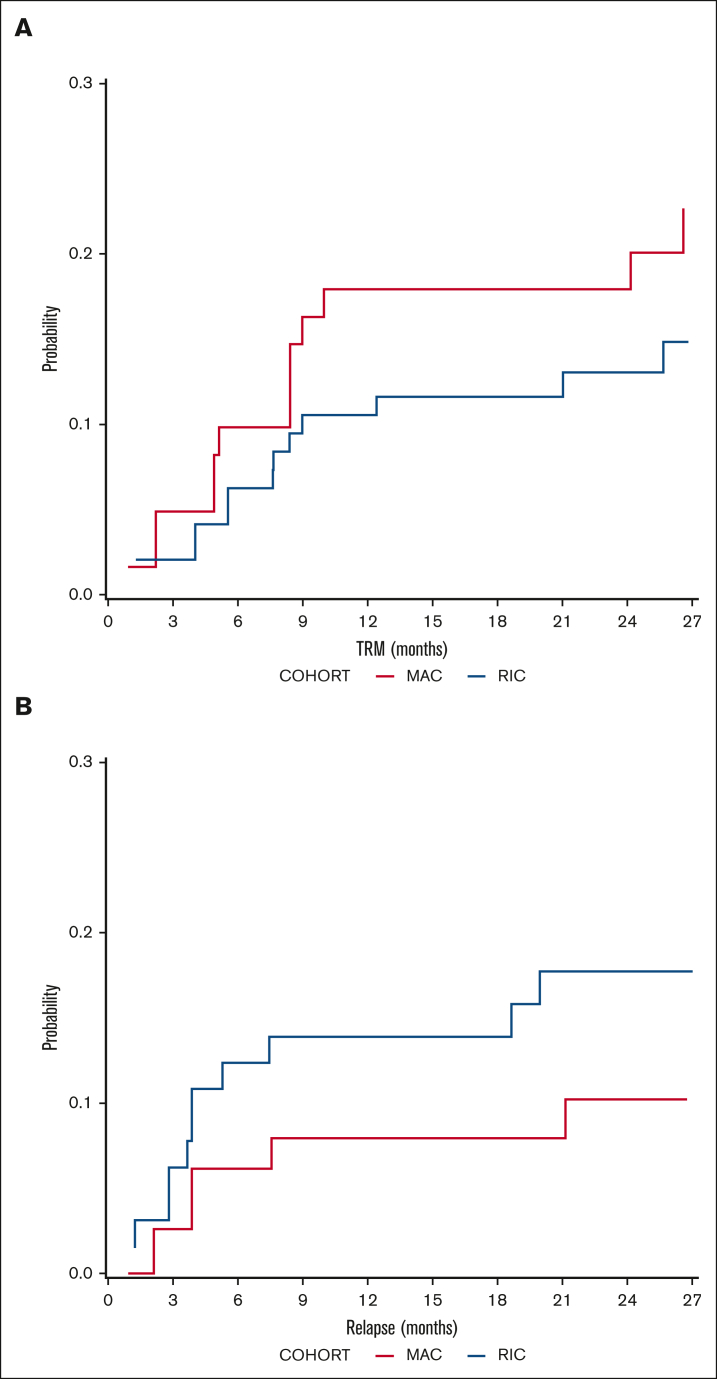
DFS probability at 2 years was 67.5% (95% confidence interval [CI], 53.2-85.6) for MAC recipients and 68.3% (95% CI, 56.3-83.01) for RIC recipients (Figure 1). The OS probability at 2 years was 72.2% (95% CI, 58.2-89.4) for MAC recipients and 74% (95% CI, 62.7-87.5) for RIC recipients (Figure 1). The incidence of disease relapse was higher in the RIC cohort (13.9% at 12 months, increasing to 17.7% at 24 months) than in the MAC cohort (7.9% at 12 months, then increasing to 10.2% by 24 months), but the difference between cohorts was not statistically significant (P = .285; Figure 2).
Figure 1.
Survival outcomes. (A) Probability of DFS in MAC (red) and RIC (blue) recipients. (B) Probability of OS.
Figure 2.
Relapse and transplant mortality outcomes. Cumulative incidence of (A) TRM and (B) relapse in MAC (red) and RIC (blue) recipients.
Subset analysis: AML
The major indication for haplo-HSCT was AML, with 39 of 78 (50%) patients accrued; thus, a subgroup analysis was performed. The DFS probability at 2 years was 76.1% (95% CI, 57.4-100) for MAC recipients and 72.3% (95% CI, 56.8-91.9) for RIC recipients (P = .77). The OS probability at 2 years was 85.5% (95% CI, 68.8-100) for MAC recipients and 70.9% (95% CI, 55.1-91.2) for RIC recipients (P = .31). Kaplan-Meir curves for the AML subset are available in supplemental Figure 3. AML was also included in the univariate and multivariate analyses, and this demonstrated no significant relationship with outcomes such as DFS/OS/GRFS or TRM.
Engraftment and early toxicity
The median neutrophil and platelet engraftment times were 18 days (range, 12-92) and 28 days (range, 13-262) respectively, with no difference between the MAC and RIC cohorts. Neutrophil engraftment occurred by day 30 in 87.7% of MAC recipients and 93.6% of RIC recipients.
Cytokine release syndrome occurred in 27 of 78 (34.6%) recipients but was grade 1 in 25 of 27 patients, with 1 case of grade 2 and 3 each. A patient with grade 3 cytokine release syndrome at day 4 required tociluzimab with full recovery.
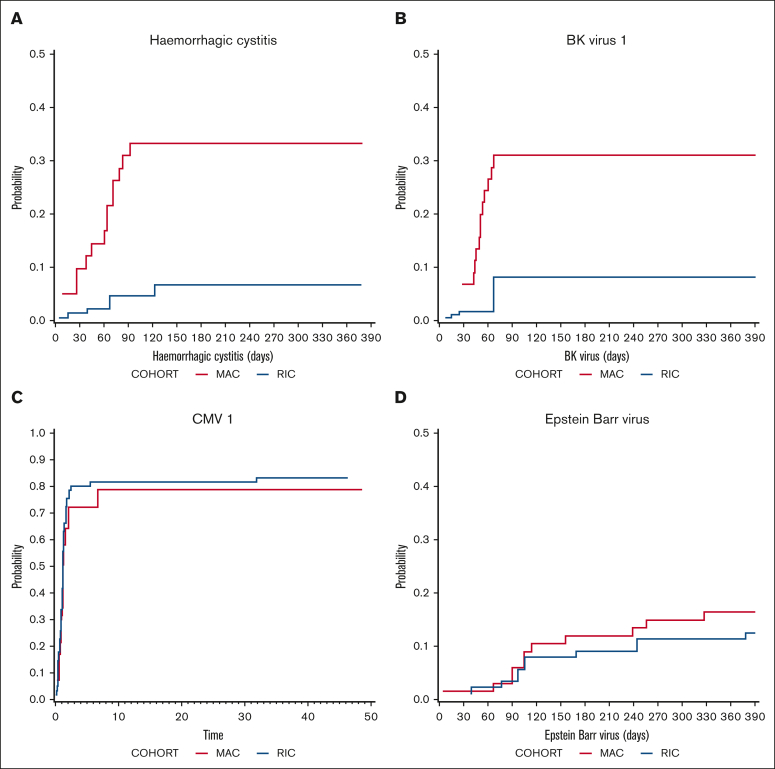
The cumulative incidence of hemorrhagic cystitis was significantly higher in MAC recipients (33.2%) than in RIC recipients (6.6%) on day 120 (P = .007; Figure 3) and occurred at a median of 61 days (range, 5-92) after transplantation. According to CTCAE version 5, hemorrhagic cystitis was grade 3 in 5 patients, grade 1 in 5 patients, and not recorded in 4 patients. Consistent with this finding, BK virus reactivation was more common in MAC recipients (P = .013; Figure 3) and occurred at a median of 52.5 days (range, 15-785). CMV viremia was common, occurring in 78.9% of MAC and 83.4% of RIC CMV+ recipients at 12 months, with no statistical difference between the cohorts (Figure 3). Twelve months after transplantation, the cumulative incidence of CMV disease (as opposed to viremia) occurred in 8.1% (95% CI, 2.5-26.6) of patients receiving MAC and 7.6% (95% CI, 2.9-19.7) of patients receiving RIC. Veno-occlusive disease occurred in 9.5% of MAC recipients and 2.2% of RIC recipients on day 180 (P = .176). EBV reactivation occurred in 15.6% of MAC and 13% of RIC recipients at 12 months (Figure 3). Of the 78 patients, 15 (19.2%) had a positive EBV polymerase chain reaction result at any time point after transplantation. EBV viremia was detected at a median day of 156 (range, 5-710). Only 1 of the 78 patients developed PTLD and was successfully treated with rituximab. There were no deaths from PTLD.
Figure 3.
Infectious morbidity. Cumulative incidence of (A) Hemorrhagic cystitis (P = .007), (B) BK viremia (P = .013), (C) CMV viremia (P = .604), and (D) Epstein-Barr viremia in MAC (red) and RIC (blue) recipients.
The incidence of TRM on day 100 was 4.9% (95% CI, 1.6-15.3) in the MAC cohort and 3.1% (95% CI, 0.8-12) in the RIC cohort. This increased to 17.9% (95% CI, 8.8-36.5) at 12 months in the MAC cohort and 11.6% (95% CI, 6.0-22.4) in the RIC cohort. This difference in TRM between cohorts was not statistically significant (P = .365; Figure 2). The details of all cases of TRM are available in supplemental Table 3. Independent SDMC was met on 6 occasions between November 2017 and May 2019. The stopping rules were not met, and the study was allowed to continue accrual until April 2020.
GVHD and GRFS
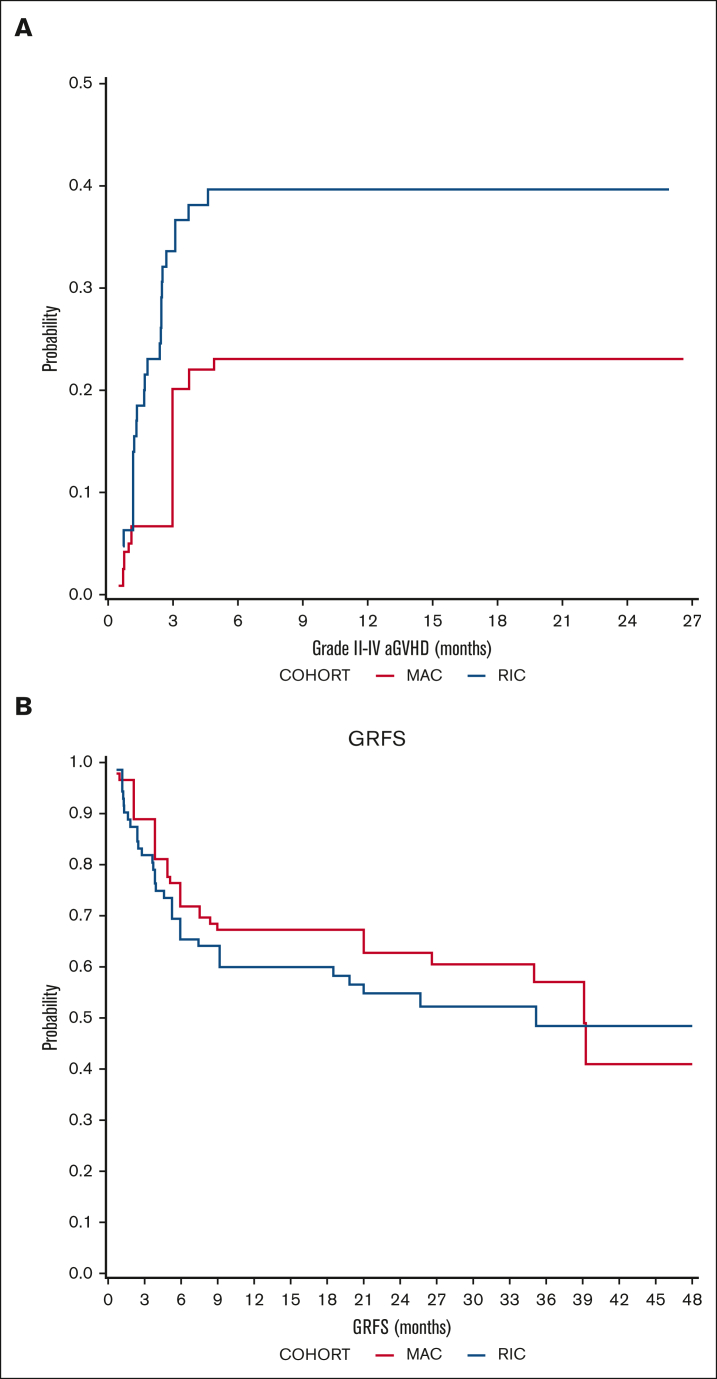
The cumulative incidence of aGVHD grade 2-4 on day 100 was 20. 1% (95% CI, 9.5-42.5) in the MAC cohort and 35.1% (95% CI, 24.3-50.6) in the RIC cohort, but the difference between the cohorts was not statistically significant (P = .152; Figure 4). Grade 3-4 aGVHD incidence was higher in the RIC cohort (19.2% vs 3.2% at 6 months; P = .076).
Figure 4.
GVHD outcomes. Cumulative incidence of (A) aGVHD (2-4) and (B) probability of GRFS in MAC (red) and RIC (blue) recipients.
Overall, cGVHD at 1 year occurred in 48% and 47.2% of the evaluable MAC and RIC recipients, respectively. Moderate-to-severe cGVHD, according to the National Institutes of Health consensus criteria, occurred in 12% and 27.7% of evaluable MAC and RIC recipients, respectively, at 1 year. Consequently, the probability of GRFS at 24 months was 62.80% (95% CI, 48.6-81.1) in the MAC cohort and 54.88% (95% CI, 42.2-71.3) in the RIC cohort (Figure 4).
Withdrawal of immunosuppression
Six patients ceased CNI before day 120 because of disease recurrence or low chimerism, and 3 patients died of TRM before day 120, leaving n = 9 patients excluded from the elective CNI cessation analysis. Thus, there were 69 remaining evaluable patients. This leaves 2 cohorts of patients as per supplemental Figure 2: a group of 12 patients who experienced early grade 2-4 GVHD before day 60 and the remaining 56 patients who did not develop aGVHD before day 60 and were, thus, eligible for early elective CNI cessation as per the protocol. Patients in the elective cessation group (n = 56) discontinued CNI on a median day 142.5 (range, 47-1255), whereas the early aGVHD group (n = 12) discontinued it on a median day 548 (range, 45-816). There was no significant difference in the OSs between the 2 groups (P = .42; data not shown). Moderate-to-severe cGVHD occurred in 41.6% of the early aGVHD cohort and 27.2% of the elective CNI cessation cohort. Of the eligible patients (n = 69), 71.6% discontinued immunosuppression at 12 months after transplantation (25% patients with aGVHD 2-4 before day 60 and 82.1% patients without aGHVD 2-4 before day 60).
DLIs
Six patients received a donor lymphocyte infusion (DLI) of 0.5 × 106/kg at a median of 123.5 days (range, 105-601) after transplant. Graft failure or loss of chimerism was the indication of DLI in 3 of 6 patients: early molecular disease recurrence was the indication in 1 patient, 1 patient received DLI for poor engraftment with full donor chimerism, and the other was pre-emptive because of high-risk disease (ALL CR2). Only 2 of 6 patients were alive at a median of 898.5 days (range, 156-1096). The cause of death was GVHD in 2 patients and infection/graft failure in 2 patients. More details are provided in supplemental Table 5.
Multivariate analysis of prognostic factors
The full results of the multivariate models are shown in Table 2. The univariate analyses are provided in supplemental Tables 6-9.
Table 2.
Multivariate analyses for DFS, OS, GRFS, and TRM
| Variable |
DFS |
||
|---|---|---|---|
| HR | 95% CI | P | |
| Previous autologous HSCT: no/yes |
1.69 | 0.4-7.17 | .473 |
| DRI: High/very high vs intermediate/low |
2.33 | 0.98-5.55 | .056 |
| Variable |
OS |
||
|---|---|---|---|
| HR | 95% CI | P | |
| DRI: High/very high vs intermediate/low |
2.46 | 1.02-5.95 | .046 |
| Previous autologous HSCT: yes/no |
2.91 | 0.39-21.6 | .296 |
| Variable |
GRFS |
||
|---|---|---|---|
| HR | 95% CI | P | |
| DRI: High/very high vs intermediate/low |
2.36 | 1.1-5.06 | .028 |
| Variable |
TRM |
||
|---|---|---|---|
| HR | 95% CI | P | |
| MAC vs RIC | 1.92 | — | .197 |
| CD3 dose infused: Less than median vs greater than or equal to median |
0.34 | — | .063 |
For DFS, a 2-factor multivariate model was selected, with previous autologous transplant and DRI as the variables. A high or very high DRI was associated with an adverse DFS, although this was not significant (HR, 2.33; 95% CI, 0.98-5.55; P = .056).
For OS, a 2-factor model was selected with previous autologous transplant and DRI as the variables. A high or very high DRI was associated with a statistically significant adverse OS (HR, 2.46; 95% CI, 1.02-5.95; P = .046).
For GRFS, a 1-factor model was selected with DRI was selected. A high or very high DRI was associated with a statistically significant adverse OS (HR, 2.36;95% CI, 1.1-5.06; P = .028).
For TRM, a 2-factor model was selected, which included the CD3 dose–infused and MAC vs RIC regimens. A lower infused CD3 dose was associated with nonstatistically significant improved TRM (HR, 0.34; P = .063).
Discussion
This prospective study of predefined conditioning regimens has demonstrated promising 2-year survival rates from 72% to 74%, using peripheral blood as the stem cell source. This result is encouraging, given that a study of transplant outcomes using sibling and matched unrelated donors over the same period from majority of the same centers demonstrated a 2-year OS of 63.2%.17 The strength of the data in this study lies in the fact that it was a prospective, multicenter design with stringent stopping rules overseen by the SDMC. The ANZHIT-1 study confirmed that peripheral blood as a stem cell source is both feasible and safe, with acceptable GVHD rates, despite retrospective studies suggesting a higher GVHD rate using peripheral blood.18,19 Lack of toxicity, as measured based on the TRM, in this analysis, was reassuring, with day-100 rates of 4.9% (95% CI, 1.6-15.3) in the MAC cohort and 3.1% (95% CI, 0.8-12) in the RIC cohort. In contrast, relapse remains a major challenge in the RIC cohort, as previously demonstrated.10
However, delayed toxicity and morbidity were significant, particularly with hemorrhagic cystitis and BK viremia in the MAC cohort. Infectious morbidity was prominent in the ANZHIT-1 study, with CMV viremia occurring in 77% or 81% of the patients who tested as seropositive. This high rate of CMV viremia has previously been reported by CIBMTR20 and appears to be unique to the PTCy setting. Unfortunately, letermovir was not available during the time of this study, but it may be worth exploring in future studies as a prophylactic agent. Patients also experienced significant nonCMV viral infections with EBV and BK/JC viruses, which were especially prominent. To our knowledge, this is the first prospective analysis of these viruses in a haploidentical setting and complements the recent retrospective work of CIBMTR in this field.21
A novel finding from this analysis is that immunosuppression can be withdrawn early despite the use of peripheral blood as a stem cell source. The median time to withdrawal of CNIs was day 147 in those who did not experience aGVHD 2 or 4 by day 60. This result compares favorably with those of studies using PTCy GVHD prophylaxis in both matched22 or haploidentical settings,13 in which cessation of immunosuppression is often delayed until day 180 or later, although these studies used bone marrow as the stem cell source. Similarly, Bejanyan et al23 demonstrated the median time to withdrawal of immunosuppression on day 255 after haplo-HSCT, using sirolimus and mycophenolate. Importantly, there were no adverse outcomes in the 56 patients who electively weaned from their calcineurin inhibitor from day 60, with no excess GVHD or mortality in this group. Importantly, 82.1% of this cohort remained without immunosuppressed at 12 months, with 27.2% suffering from moderate-to-severe cGVHD, suggesting that early elective cessation of CNI is feasible and safe, provided there is no early aGVHD 2 or 4 before day 60. It is possible that ceasing immunosuppression early may have beneficial effects on infectious morbidity, renal function, and relapse, although this would have to be analyzed in a randomized setting. Multivariate analysis did not demonstrate a beneficial survival effect on relapse, with the withdrawal of immunosuppression before day 120; however, the study was not powered to make such comparisons.
Despite the early withdrawal of immunosuppression in this study, the rates of aGVHD and cGVHD were comparable with those of other studies that also used peripheral blood as the stem cell source, probably because of the potent protective effects of PTCy. The rates of aGVHD in ANZHIT-1 were comparable with those of several registry-based studies of haplo-HSCT, with day-100 aGVHD 2 or 4 from 20% to 35.1%. This compares favorably with a recent propensity score analysis by Huselton et al,24 in which aGVHD 2-4 was from 29.6% to 35.8%. Similarly, a CIBMTR analysis demonstrated an aGVHD 2-4 rate ranging from 29% to 33% for haploidentical patients.25 In contrast, the rate of aGVHD 3-4 in ANZHIT-1 was unexpectedly high in the RIC cohort, at 19.2% at 6 months. Most of these patients had grade 3 aGVHD, and the vast majority were alive (6 of 8) at the last follow-up, consistent with only mild lower gut GVHD but significant skin and liver GVHD. Similarly, the overall cGVHD rate at 1 year in ANZHIT-1 is slightly higher than those showed by both Gooptu et al25 and Huselton et al,24 but most of these patients suffered from mild cGVHD, which did not require ongoing immunosuppression, as confirmed by the vast majority of patients being off immunosuppression by 12 months.
Most haplo-HSCT studies are registry-based and retrospective. Numerous retrospective studies have demonstrated equivalent survival rates using PTCy-based haploidentical transplants and HLA-matched donors.26, 27, 28 Haploidentical transplant using bone marrow has also been assessed in 2 randomized studies, confirming the superior outcomes of cord stem cell transplant.29,30 Based on this evidence, haplo-HSCT is rapidly becoming the standard treatment option for the management of hematological malignancies. Thus, it has become increasingly important to formally investigate the optimal conditioning and GVHD strategies for this procedure in prospective studies. Surprisingly, to our knowledge, there are only a handful of prospective PTCy studies in haplo-HSCT, with very few being multicenter. Symons et al demonstrated a 57% OS rate at 2 years in a single-center prospective study of 96 children and adults using bone marrow as the stem cell source.31 Relapse was higher in the Symons study than this study, at 35% (a finding confirmed in registry-based retrospective studies,19 possibly suggesting a greater graft-versus-leukemia effect from peripheral blood). Solomon et al demonstrated an encouraging 78% 2-year OS in 30 patients receiving fludarabine TBI1200cGy haploidentical transplant using peripheral blood stem cells32 in a single-center cohort. The same unit used fludarabine, with varying doses of busulfan, in 20 patients.33 Notably, the rate of BK virus–associated cystitis was high in this myeloablative busulfan study, affecting 75% of patients, a finding similar to that of this study, suggesting that myeloablation may predispose to this complication.
There are several limitations to this study. In particular, with only 78 patients, the study did not have the statistical power to adequately assess multiple prognostic factors in the multivariate analyses of survival or TRM. Adverse outcomes in patients with high or very high DRI were the only consistent finding from the multivariate analysis, suggesting that more novel strategies are required for these patients. Most of the patients in this study were in the intermediate DRI group; thus, the relapse rate might have been lower than that in some other studies. Nevertheless, this group of patients benefited from a procedure with a relatively low TRM, in keeping with the trend that has been occurring in Australia and New Zealand over the last 15 years.34 Despite the low TRM, later toxicities, such as cystitis and veno-occlusive disease, were prominent in the MAC group, and relapse remains a concern with the RIC regimen.
In summary, AZNHIT-1 demonstrated encouraging survival outcomes in patients without access to a matched donor. Further refinement of the procedure will be undertaken in a future study (the ANZHIT-2 study), which will involve the use of treosulfan, which may cause less veno-occlusive disease. It may also be possible to increase the total body irradiation dose for conditioning, to reduce relapse and toxicity. Eventually, phase 3 studies assessing changes in conditioning and GVHD PTCy regimens will be required to optimize the outcomes of patients undergoing this procedure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors acknowledge the expertise and diligence of the safety and data monitoring committee of the ANZHIT-1 trial: Glen Kennedy, Mark Hertzberg, Val Gebski, and Louise Christophersen.
The authors also thank Ken Bradstock for his guidance and experience during initial protocol development. The care of patients in this study by nursing and junior medical staff is appreciated and acknowledged. The authors acknowledge the funding from the Arrow Bone Marrow Transplant Foundation.
Authorship
Contribution: J.M., J.K., D.G., A.B., and M.G. conceived the study; J.M., N.H., S.T., A.S., and D.A. analyzed and interpreted the data; J.M. wrote the manuscript; and all authors interpreted and edited the manuscript and approved the final manuscript.
Footnotes
This study was presented at the 62nd annual meeting of the American Society of Hematology (5-8 December 2020).
Data are available on request from the corresponding author, John Moore (jmoore@stvincents.com.au).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 3.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanakry CG, O'Donnell PV, Furlong T, et al. Multi-institutional study of posttransplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorentino F, Labopin M, Bernardi M, et al. Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation Comparable outcomes of haploidentical, 10/10 and 9/10 unrelated donor transplantation in adverse karyotype AML in first complete remission. Am J Hematol. 2018;93(10):1236–1244. doi: 10.1002/ajh.25231. [DOI] [PubMed] [Google Scholar]
- 8.Niederwieser D, Baldomero H, Bazuaye N, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022;107(5):1045–1053. doi: 10.3324/haematol.2021.279189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passweg JR, Baldomero H, Chabannon C, et al. European Society for Blood and Marrow Transplantation EBMT Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56(7):1651–1664. doi: 10.1038/s41409-021-01227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. doi: 10.1182/blood-2015-01-623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brissot E, Labopin M, Ehninger G, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104(3):524–532. doi: 10.3324/haematol.2017.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasamon YL, Fuchs EJ, Zahurak M, et al. Shortened-duration tacrolimus after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2018;24(5):1022–1028. doi: 10.1016/j.bbmt.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 16.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Othman J, Greenwood M, Moore J, et al. Unrelated donor transplant recipients given thymoglobuline have superior GRFS when compared to matched related donor recipients undergoing transplantation without ATG. Biol Blood Marrow Transplant. 2020;26(10):1868–1875. doi: 10.1016/j.bbmt.2020.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Ruggeri A, Labopin M, Bacigalupo A, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124(7):1428–1437. doi: 10.1002/cncr.31228. [DOI] [PubMed] [Google Scholar]
- 19.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldsmith SR, Abid MB, Auletta JJ, et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood. 2021;137(23):3291–3305. doi: 10.1182/blood.2020009362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A, Dandoy CE, Chen M, et al. Post-transplantation cyclophosphamide is associated with an increase in non-cytomegalovirus herpesvirus infections in patients with acute leukemia and myelodysplastic syndrome. Transplant Cell Ther. 2022;28(1):48.e1–48.e10. doi: 10.1016/j.jtct.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanakry CG, Bolanos-Meade J, Kasamon YL, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood. 2017;129(10):1389–1393. doi: 10.1182/blood-2016-09-737825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bejanyan N, Pidala JA, Wang X, et al. A phase 2 trial of GVHD prophylaxis with PTCy, sirolimus, and MMF after peripheral blood haploidentical transplantation. Blood Adv. 2021;5(5):1154–1163. doi: 10.1182/bloodadvances.2020003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huselton E, Slade M, Trinkaus KM, DiPersio JF, Westervelt P, Romee R. Propensity score analysis of conditioning intensity in peripheral blood haploidentical hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24(10):2047–2055. doi: 10.1016/j.bbmt.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gooptu M, Romee R, St Martin A, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138(3):273–282. doi: 10.1182/blood.2021011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagler A, Kanate AS, Labopin M, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin for graft-versus-host disease prevention in haploidentical transplantation for adult acute lymphoblastic leukemia. Haematologica. 2021;106(6):1591–1598. doi: 10.3324/haematol.2020.247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambinder A, Jain T, Tsai HL, Horowitz MM, Jones RJ, Varadhan R. HLA-matching with PTCy: a reanalysis of a CIBMTR dataset with propensity score matching and donor age. Blood Adv. 2022;6(14):4335–4346. doi: 10.1182/bloodadvances.2022007741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs EJ, O'Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021;137(3):420–428. doi: 10.1182/blood.2020007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz J, Montoro J, Solano C, et al. Prospective randomized study comparing myeloablative unrelated umbilical cord blood transplantation versus HLA-haploidentical related stem cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2020;26(2):358–366. doi: 10.1016/j.bbmt.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Symons HJ, Zahurak M, Cao Y, et al. Myeloablative haploidentical BMT with posttransplant cyclophosphamide for hematologic malignancies in children and adults. Blood Adv. 2020;4(16):3913–3925. doi: 10.1182/bloodadvances.2020001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon SR, Sizemore CA, Sanacore M, et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015;21(7):1299–1307. doi: 10.1016/j.bbmt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12):1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Kliman D, Tran S, Kennedy G, et al. The improvement in overall survival from unrelated donor transplantation in Australia and New Zealand is driven by a reduction in non-relapse mortality: a study from the ABMTRR. Bone Marrow Transplant. 2022;57(6):982–989. doi: 10.1038/s41409-022-01683-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.