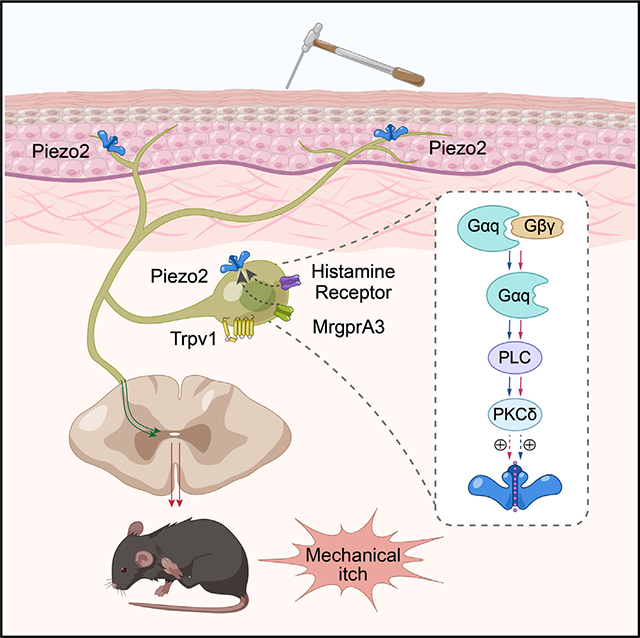
SUMMARY
Although touch and itch are coded by distinct neuronal populations, light touch also provokes itch in the presence of exogenous pruritogens, resulting in a phenomenon called alloknesis. However, the cellular and molecular mechanisms underlying the initiation of pruritogen-induced mechanical itch sensitization are poorly understood. Here, we show that intradermal injections of histamine or chloroquine (CQ) provoke alloknesis through activation of TRPV1- and MrgprA3-expressing prurioceptors, and functional ablation of these neurons reverses pruritogen-induced alloknesis. Moreover, genetic ablation of mechanosensitive Piezo2 channel function from MrgprA3-expressing prurioceptors also dampens pruritogen-induced alloknesis. Mechanistically, histamine and CQ sensitize Piezo2 channel function, at least in part, through activation of the phospholipase C (PLC) and protein kinase C-δ (PKCδ) signaling. Collectively, our data find a TRPV1+/MrgprA3+ prurioceptor-Piezo2 signaling axis in the initiation of pruritogen-induced mechanical itch sensitization in the skin.
In brief
Lu et al. showed that mechanosensitive Piezo2 expressed by the TRPV1+/MrgprA3+ pruriceptors contributes to pruritogen-induced alloknesis. Specifically, their results uncover a pruritogen-activated PLC-PKCδ signaling pathway that sensitizes Piezo2 channel function to produce hypersensitivity in MrgprA3+ pruriceptors in response to mechanical stimulation.
Graphical Abstract
INTRODUCTION
Alloknesis (or mechanical itch sensitization) is defined as an abnormal sensory state where innocuous mechanical stimuli (such as that from clothing) evoke nocuous itch sensation in the settings of pruritogen stimulation and chronic itch. Although the phenomenon is well known clinically, mouse models of pruritogen-induced mechanical itch were not established until the year of 2012.1 Recent exciting studies have begun to identify critical spinal cord inhibitory neurons and excitatory neurons in the modulation and transduction of mechanical itch signaling.2–5 Moreover, alloknesis can also be promoted by a loss of inhibitory Piezo2-Merkel cell signaling as well as inhibitory CD26-mediated dipeptidylpeptidase IV (DPPIV) enzyme activity in the skin.6,7 However, the cellular and molecular mechanisms mediating mechanical itch in the skin are incompletely understood.
Recent RNA sequencing studies have revealed that a subpopulation of TRPV1-expressing (TRPV1+) neurons express Mas-related G protein-coupled receptor member A3 (MrgprA3) and serve as a direct target for histamine and chloroquine (CQ) to mediate chemical-induced itch sensation in mice.8,9 Moreover, it was reported that MrgprA3-TRP channel signaling initiates itch sensation but that MrgprA3-P2X3 signaling produces pain sensation, suggesting that MrgprA3 signaling may function as a polymodal signal integrator to allow the diversification of somatosensation.8,10,11 Interestingly, although in vivo electrophysiological recordings showed that MrgprA3+ neurons respond to mechanical stimuli,12,13 the molecular basis of the mechanosensitivity in the MrgprA3+ neurons remains unknown, and the physiological/pathological relevance of MrgprA3+ neurons in mechanosensation also needs to be determined.
Piezo2 is a bona fide mechanosensitive ion channel and mediates the rapidly adapting (RA) mechanically activated (MA) currents in response to mechanical stimuli in various cell types.14–18 In addition to its expression in Merkel cells and thickly myelinated slowly adapting type I (SAI) fibers, Piezo2 channel expressed by thinly myelinated Ad and unmyelinated C afferents mediates mechanical hypersensitivity in response to tissue inflammation and peripheral nerve injury.14,19–21 In contrast, whether the Piezo2 channel is involved in chemical itch and/or mechanical itch is not well understood, and the cellular basis of sensory neuron-expressed Piezo2 in the development of itch sensation remains largely unexplored.
Here, we show that TRPV1+/MrgprA3+ neurons are required for mechanical itch induced by both histamine and CQ. Importantly, alloknesis-associated mechanohypersensitivity in the TRPV1+/MrgprA3+ neurons relies on the sensitization of the Piezo2 channel via Gαq/phospholipase C (PLC)/protein kinase C-δ (PKCδ) signaling downstream of the activation of histamine receptors and MrgprA3, initiating mechanical itch but not acute chemical itch. Collectively, our data demonstrate that MrgprA3+ prurioceptor-Piezo2 signaling is essential to the generation of pruritogen-induced alloknesis.
RESULTS
Chemogenetic activation of TRPV1+ neurons promotes mechanical itch
Large-scale single-cell RNA sequencing revealed three distinct subtypes of mechanosensitive nociceptive DRG neurons innervating the skin: the non-peptidergic polymodal C fibers expressing MrgprD, the peptidergic polymodal C fibers expressing TRPV1, and the low-threshold mechanosensitive C fibers (C-LTMRs) expressing tyrosine hydroxylase (TH) and vesicular glutamate transporter type 3 (VGlut3).22 To determine the classes of DRG neurons involved in the generation of mechanical itch, we first investigated if chemogenetic stimulation of distinct DRG subpopulations is sufficient to cause mechanical itch. We generated Trpv1-hM3Dq, Th-hM3Dq, and MrgprD-hM3Dq mice by crossing Trpv1Cre, ThCreER, and MrgprDCreERT with Rosa26CAG-ds-hM3Dq mice, respectively. Intradermal injections of the DREADD agonist clozapine N-oxide (CNO) into the nape of neck induced a robust, spontaneous scratching response (acute chemical itch) in the Cre+, but not Cre−, Trpv1-hM3Dq mice (Figures S1A and S1B). Interestingly, 30 min after the CNO injection, when the spontaneous scratching subdued, a light touch stimulation delivered by a 0.7 mN von Frey hair filament induced significantly increased mechanical itch in the Cre+ Trpv1-hM3Dq mice when compared with their Cre− littermates (Figure 1A), recapitulating the alloknesis phenotype produced by intradermal injections of pruritogens.1
Figure 1. TRPV1+/MrgprA3+ neurons are critically involved in the initiation of mechanical itch.
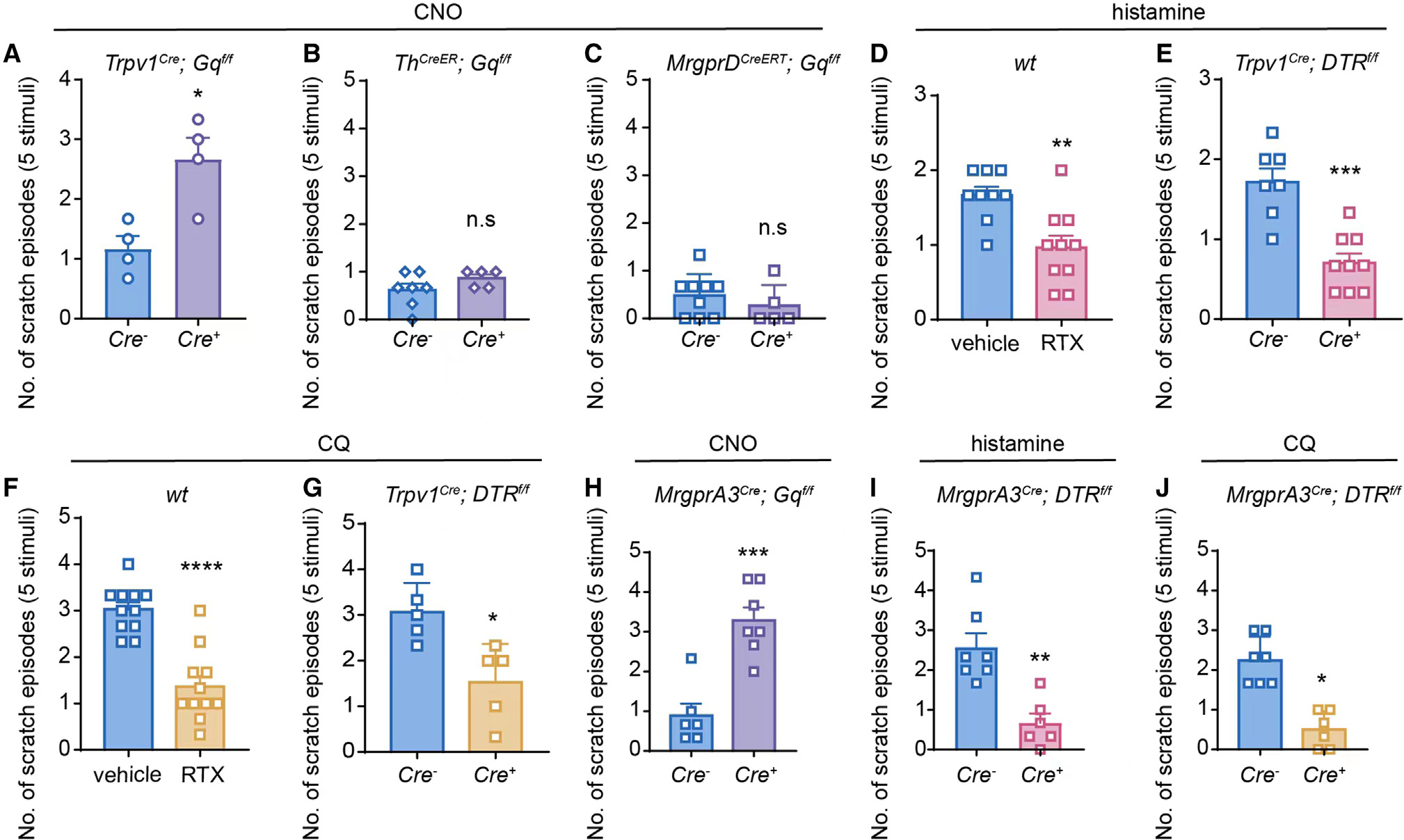
(A) CNO-induced alloknesis scores in Cre− and Cre+ Trpv1-hM3Dq mice. n = 4 for each group.
(B) CNO-induced alloknesis scores in Cre− (n = 7) and Cre+ (n = 5) Th-hM3Dq mice.
(C) CNO-induced alloknesis scores in Cre− (n = 9) and Cre+ (n = 5) MrgprD-hM3Dq mice.
(D) Histamine-induced alloknesis scores in RTX-treated WT mice (n = 10) and vehicle-treated WT mice (n = 9).
(E) Histamine-induced alloknesis scores in Cre− (n = 7) and Cre+ (n = 9) Trpv1-DTR mice.
(F) CQ-induced alloknesis scores in RTX-treated WT mice (n = 11) and vehicle-treated WT mice (n = 11).
(G) CQ-induced alloknesis scores in Cre− (n = 5) and Cre+ (n = 5) Trpv1-DTR mice.
(H) CNO-induced alloknesis scores in Cre− (n = 6) and Cre+ (n = 7) MrgprA3-hM3Dq mice.
(I) Histamine-induced alloknesis scores in Cre− (n = 7) and Cre+ (n = 6) MrgprA3-DTR mice.
(J) CQ-induced alloknesis scores in Cre− (n = 7) and Cre+ (n = 6) MrgprA3-DTR mice.
All data are expressed as mean ± SEM. n.s, not significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Unpaired two-tailed Student’s t test.
To ensure that the chemogenetic activation-induced scratching behavior in mice is not a nociceptive response, we employed the well-established cheek model, which provides a behavioral differentiation between itch and pain in mice. As expected, intradermal injections of CNO in the cheek induced a robust scratching response in the Cre+, but not Cre−, Trpv1-hM3Dq mice (Figure S1C), while there were no differences in wiping behavior between these two groups (Figure S1D), suggesting that chemogenetic activation of TRPV1+ neurons evokes alloknesis, but not allodynia, in mice. On the other hand, after Cre induction by intraperitoneal injections of tamoxifen for 5 consecutive days, neither Cre+ Th-hM3Dq nor MrgprD-hM3Dq mice showed increased mechanical itch in response to CNO treatment when compared with their respective Cre− littermates (Figures 1B and 1C). Collectively, these results demonstrate that TRPV1-lineage neurons, but not mechanosensitive primary afferents expressing Th or MrgprD, are critically involved in the initiation of mechanical itch.
Chemically or genetically induced ablation of TRPV1+ neurons reduces mechanical itch provoked by histamine or CQ
Although TRPV1+ DRG neurons are defined as a specific subset of peptidergic sensory neurons in adult mice, which is not overlapped with other C-LTMR markers such as VGlut3/TH and MrgprD,23,24 TRPV1 mRNA is transiently expressed in a wide range of DRG neurons during development, including TRPV1+ and TRPM8-expressing neurons as well as MrgprD-expressing neurons. Thus, there is a possibility that the Trpv1Cre line may non-selectively label other TRPV1− nociceptive DRG neurons in adult mice. To further confirm the role of TRPV1+ neurons in the generation of pruritogen-induced alloknesis, we employed loss-of-function studies either by ablating TRPV1+ nociceptors with intradermal injections of a super potent TRPV1 ligand resiniferatoxin (RTX) to wild type (WT) mice or by ablating TRPV1-lineage nociceptors with intraperitoneal injections of diphtheria toxin (DTX) to the Trpv1Cre; ROSA26DTR mice.
The ablation efficiency of TRPV1+ DRG neurons was confirmed by significantly reduced thermal pain behavior in mice and reduced calcium influx in response to CQ and capsaicin in live-cell calcium imaging assays using isolated DRG neurons (Figures S2A–S2D). As expected, acute scratching evoked by either histamine or CQ was significantly reduced in RTX-treated mice and DTX-treated Cre+ Trpv1Cre; ROSA26DTR mice when compared with their respective controls (Figures S3A–S3D). Surprisingly, in contrast to a previous study showing that CQ did not evoke alloknesis at a concentration of 200 nmol/10 μL,1 intradermal injections of CQ evoked mechanical itch in a bell-shaped manner, with the peak effect produced by a concentration of 50 nmol/30 μL (Figures S4A–S4D), suggesting that higher concentrations of CQ may lead to an inhibition and/or desensitization of alloknesis. Moreover, both histamine- and CQ-induced mechanical itch was markedly reduced in the RTX-treated WT mice and DTX-treated Cre+ Trpv1Cre; ROSA26DTR mice when compared with their respective controls (Figures 1D–1G), while silencing the TRPV1+ neurons by selective delivery of a charged membrane-impermeable sodium channel blocker lidocaine N-ethyl-lidocaine (QX-314)25 plus CQ also significantly reduced CQ- and histamine-induced mechanical itch (Figures S5A and S5B). Taken together, these results suggest that TRPV1+ C fibers are necessary and sufficient to produce pruritogen-induced mechanical itch.
MrgprA3+ pruriceptors mediate mechanical itch
A subpopulation of TRPV1+ and histamine receptor+ neurons express MrgprA3 and play important roles in histamine- and CQ-induced itch sensation.8,26,27 However, whether MrgprA3+ prurioceptors are involved in the pathogenesis of pruritogen-induced alloknesis remains unknown. To address this question, we expressed the excitatory Gq-DREADD in the MrgprA3+ neurons by crossing MrgprA3GFP-Cre mice with Rosa26CAG-ds-hM3Dq. Intradermal injections of CNO produced robust, spontaneous scratching in the Cre+, but not Cre−, MrgprA3-hM3Dq mice (Figures S6A and S6B), confirming functional expression of hM3Dq in the MrgprA3+ prurioceptors. Interestingly, mechanical itch responses induced by light mechanical stimulations were significantly increased in the Cre+, but not the Cre−, MrgprA3-hM3Dq mice (Figure 1H), recapitulating the mechanical itch response induced by intradermal injections of CQ. Corroborating with the gain-of-function experiments, genetic ablation of the MrgprA3+ neurons resulted in significantly reduced acute chemical itch (Figures S6C–S6E) and mechanical itch (Figure 1I and 1J) in the DTX-treated MrgprA3Cre; ROSA26DTR mice when compared with their littermate controls. Taken together, our results suggest that chemical-induced sensitization of MrgprA3+ neurons is critically involved in the development of pruritogen-induced mechanical itch.
Piezo2, but not TRPA1, is required for pruritogen-induced mechanical itch
Although it is commonly accepted that TRPA1 is expressed by a subset of TRPV1+ nociceptors and acts as a chemosensor mediating both inflammatory and neuropathic pain as well as pruritogen-induced chemical itch, the role of TRPA1 in mechanosensation has been controversial.28–31 We found that mechanical itch evoked by either histamine or CQ was comparable between the TRPA1+/+ and TRPA1−/− mice (Figure S7), suggesting that TRPA1 is dispensable in the development of pruritogen-induced alloknesis.
Although we have demonstrated that the epithelial Merkel cell-expressing Piezo2 channel modulates mechanical itch through driving Merkel cell-SA1 Aβ signaling,6 the role of the mechanosensitive Piezo2 channel expressed by the TRPV1+/MrgprA3+ C-type pruriceptors is not known. To address this, we first checked the expression of Piezo2 in TRPV1+/MrgprA3+ neurons using mRNA fluorescent in situ hybridization. Indeed, 43.9% of Piezo2 mRNA-expressing neurons overlapped with the TRPV1-GFP signal and 79.0% of TRPV1-GFP+ neurons expressed Piezo2 mRNA transcripts (Figures 2A and 2B), while 12.5% of Piezo2 mRNA-expressing neurons expressed MrgrpA3-GFP, and more than 80% of MrgprA3-GFP+ neurons displayed Piezo2 mRNA transcripts (Figures 2F and 2G). To determine if Piezo2 is functionally expressed by TRPV1+/MrgprA3+ neurons, we crossed Trpv1Cre and MrgprA3GFP-Cre; Ai9f/f mice with Piezo2f/f mice and performed whole-cell patch-clamp recording. Surprisingly, although the majority of MrgprA3+ neurons express Piezo2 mRNA transcripts, inward whole-cell MA currents were recorded in only 7 out of 20 TRPV1-GFP+ neurons and 12 out of 39 MrgprA3-GFP+ neurons (Figures 2C, 2D, 2H, and 2I), suggesting that the pruriceptor-expressed Piezo2 gene may combine high- and low-translation activities. Of note, only 2 out of 25 TRPV1+ neurons from Trpv1Cre; Piezo2f/f mice and none of 22 MrgprA3+ neurons from the MrgprA3Cre; Ai9f/f; Piezo2f/f mice responded to the same mechanical stimulation protocol (Figures 2E and 2J), suggesting that the Piezo2 channel confers mechanosensitivity to the TRPV1+/MrgprA3+ pruriceptors.
Figure 2. Functional expression of Piezo2 channels in TRPV1+ and MrgprA3+ neurons.
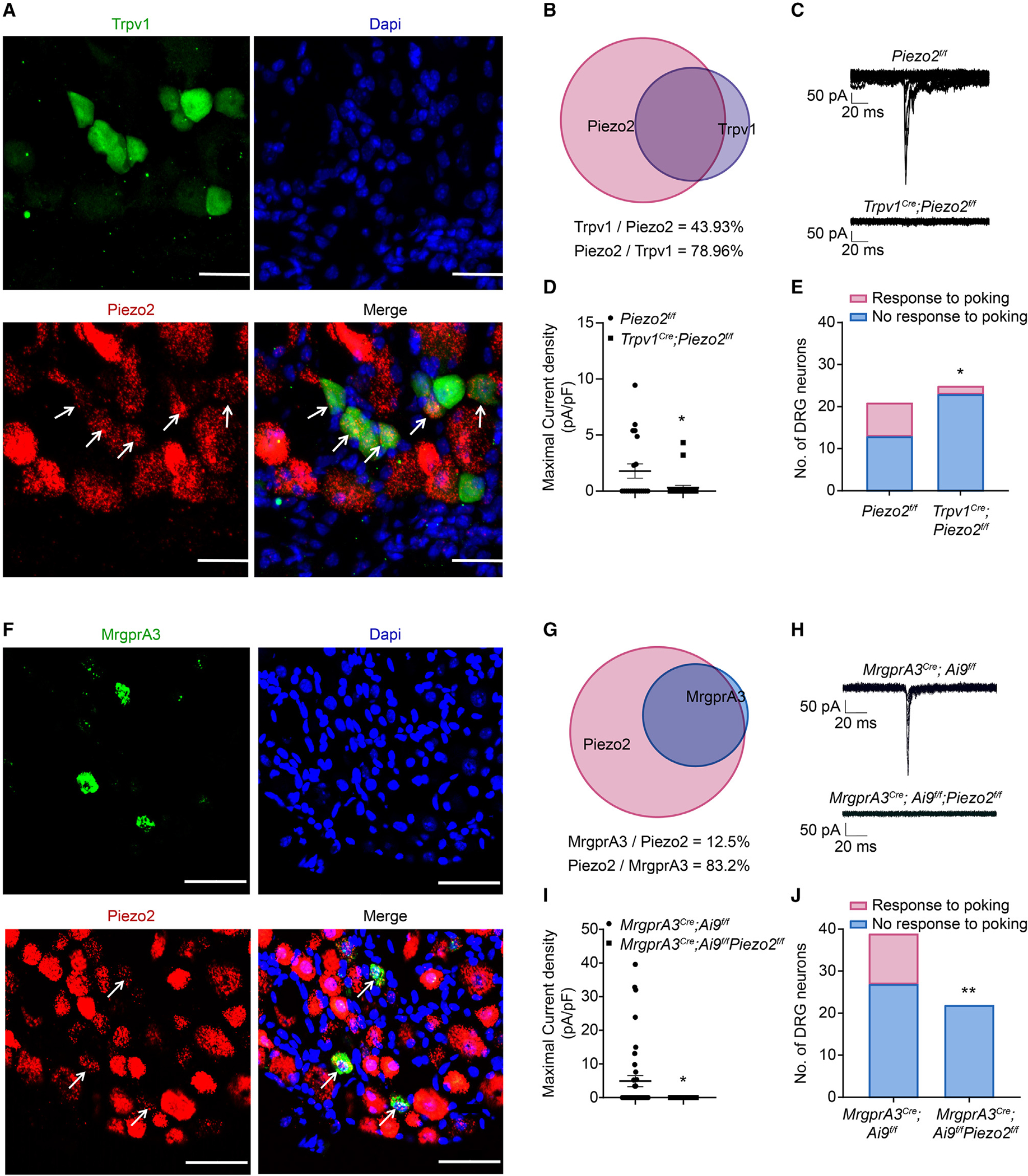
(A) Representative images of Piezo2 mRNA transcripts (red) and TRPV1-GFP (green) in DRG neurons isolated from Trpv1-EGFP mice. Arrows refer to double-labeled cells. n = 3–5 sections from 3 mice. Scale bar, 50 μm.
(B) The BioVenn diagram illustrates the overlap between TRPV1+ neurons and Piezo2 mRNA+ neurons.
(C) Representative traces of whole-cell MA currents in Piezo2f/f and Trpv1Cre; Piezo2f/f neurons.
(D) Current density of whole-cell MA currents in Piezo2f/f and Trpv1Cre; Piezo2f/f neurons.
(E) Summarized data of the percentage of mechanosensitive and mechanoinsensitive DRG neurons recorded in (D).
(F) Representative images of Piezo2 mRNA transcripts (red) and MrgprA3-GFP (green) in DRG neurons isolated from MrgprA3GFP-Cre mice. Arrows refer to double-labeled cells. n = 3–5 sections from 3 mice. Scale bar, 50 μm.
(G) The BioVenn diagram illustrates the overlap between MrgprA3+ neurons and Piezo2 mRNA+ neurons.
(H) Representative traces of whole-cell MA currents in MrgprA3Cre; Ai9f/f and MrgprA3Cre; Ai9f/f; Piezo2f/f neurons.
(I) Current density of whole-cell currents in MrgprA3Cre; Ai9f/f and MrgprA3Cre; Ai9f/f; Piezo2f/f neurons.
(J) Summarized data of the percentage of mechanosensitive and mechanoinsensitive DRG neurons recorded in (I).
*p < 0.05, **p < 0.01. Unpaired two-tailed Student’s t test for (D) and (I) and chi-squared test for (E) and (J).
To further investigate whether TRPV1+/MrgprA3+ pruriceptor-expressed Piezo2 is involved in the generation of pruritogen-induced mechanical itch, we injected histamine and CQ into the nape of the neck of the Trpv1Cre; Piezo2f/f mice and MrgprA3Cre; Piezo2f/f mice. Although the acute chemical itch induced by either histamine or CQ was comparable between the Cre− and Cre+ Trpv1Cre; Piezo2f/f mice and MrgprA3Cre; Piezo2f/f mice (Figures 3A, 3C, 3E, and 3G), alloknesis scores were markedly reduced in the Cre+ mice when compared with that in their respective Cre− littermates (Figures 3B, 3D, 3F, and 3H), suggesting that Piezo2 is a critical downstream mediator of MrgprA3 signaling in the generation of mechanical itch, but not acute chemical itch, in response to pruritogens in the TRPV1+/MrgprA3+ pruriceptors.
Figure 3. Conditional ablation of Piezo2 function from TRPV1 or MrgprA3 lineage neurons significantly attenuates pruritogen-induced mechanical itch without affecting chemical itch.

(A and B) Histamine-induced acute chemical itch (A) and mechanical itch (B) in Cre− (n = 8) and Cre+ (n = 10) Trpv1Cre; Piezo2f/f mice.
(C and D) CQ-induced acute chemical itch (C) and mechanical itch (D) in Cre− (n = 6) and Cre+ (n = 6) Trpv1Cre; Piezo2f/f mice.
(E and F) Histamine-induced acute chemical itch (E) and mechanical itch (F) in Cre− (n = 6) and Cre+ (n = 6) MrgprA3Cre; Piezo2f/f mice.
(G and H) CQ-induced acute chemical itch (G) and mechanical itch (H) in Cre− (n = 13) and Cre+ (n = 12) MrgprA3Cre; Piezo2f/f mice.
All data are expressed as mean ± SEM. n.s, not significant. *p < 0.05, **p < 0.01, ****p < 0.0001. Unpaired two-tailed Student’s t test.
PLC-PKCδ signaling sensitizes Piezo2 to mediate pruritogen-induced mechanical itch
Prior studies demonstrated that increased levels of inflammatory mediators in the inflamed or injured tissues sensitize Piezo2 function, leading to enhanced mechanical hypersensitivity,14,19,21 which involves multiple intracellular signaling pathways.20,32,33 Corroborating these findings, both CQ and histamine significantly increased the MA current density in tdTomato+ DRG neurons from the Cre+ MrgprA3Cre; Ai9f/f mice compared with vehicles (Figures 4A and 4B), suggesting that pruritogens sensitize Piezo2 function in the MrgprA3+ pruriceptors.
Figure 4. PLC-PKCδ signaling is critically involved in pruritogen-induced sensitization of mechanosensitive pruriceptors and mechanical itch.
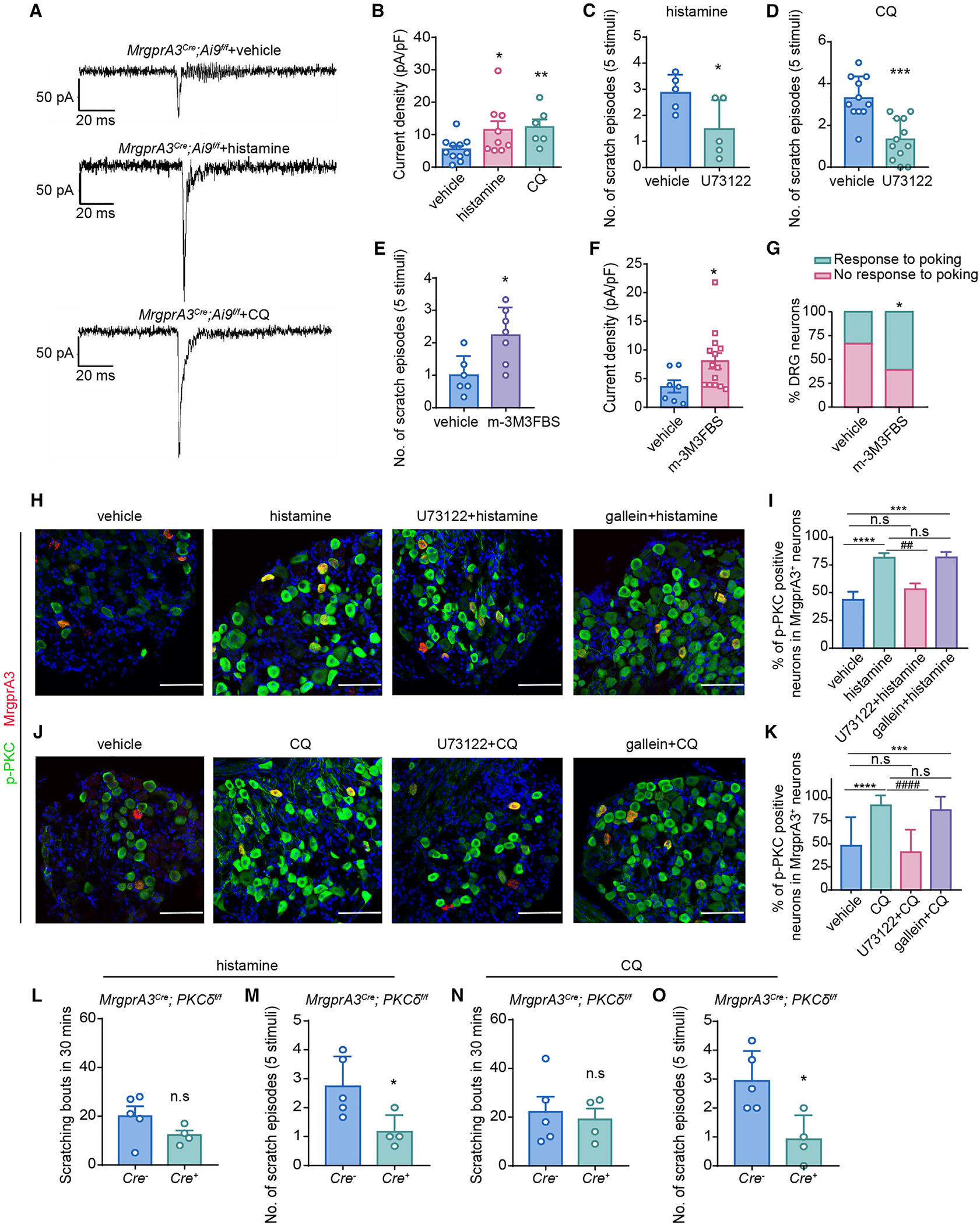
(A) Representative whole-cell MA current traces elicited by mechanical indentation on DRG neurons isolated from MrgprA3Cre; Ai9f/f mice perfused with vehicle, histamine, or CQ, respectively.
(B) Summarized data of the current density of mechanosensitive MrgprA3+ neurons in response to vehicle (n = 11), histamine (n = 9), and CQ (n = 6).
(C and D) Histamine-induced (C) and CQ-induced (D) alloknesis scores in mice treated with vehicle or PLC inhibitor U73122. n = 5 mice for each group in (C), n = 11 vehicle-treated and n = 12 U73122-treated mice in (D).
(E) m-3M3FBS-induced alloknesis score in WT mice. n = 6 for vehicle-treated and n = 7 for m-3M3FBS-treated mice.
(F) Current density of whole-cell MA currents in the presence of vehicle and 1 μM m-3M3FBS in neurons isolated from MrgprA3Cre;Ai9f/f mice.
(G) Bar graph summarized the percentage of mechanosensitive DRG neurons in presence of vehicle and 1 μM m-3M3FBS.
(H) Representative images of MrgprA3 mRNA transcripts (red) and p-PKC (green) staining in DRG neurons isolated from WT mice treated with vehicle, histamine,U73122 + histamine, and gallein + histamine, respectively. Scale bar, 50 μm.
(I) Quantification of the number of p-PKC-expressing cells in MrgprA3+ neurons in response to different treatments in (H). n = 3–5 sections from 3 mice for each group.
(J) Representative images of MrgprA3 mRNA transcripts (red) and p-PKC (green) staining in DRG neurons isolated from WT mice treated with vehicle, CQ,U73122 + CQ, and gallein + CQ, respectively. Scale bar, 50 μm.
(K) Quantification of the number of p-PKC-expressing cells in MrgprA3+ neurons in response to different treatments in (J). n = 3–5 sections from 3 mice for each group. All scale bars, 100 μm.
(L and M) Histamine-induced acute chemical itch (L) and mechanical itch (M) in Cre− (n = 5) and Cre+ (n = 4) MrgprA3Cre; PKCdf/f mice.
(N and O) CQ-induced acute chemical itch (N) and mechanical itch (O) in Cre− (n = 5) and Cre+ (n = 4) MrgprA3Cre; PKCδf/f mice.
Data are expressed as mean ± SEM. n.s, not significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ##p < 0.01, ####p < 0.0001. Unpaired two-tailed Student’s t test in (C)–(F) and (L)–(O), chi-squared test for (G), and one-way ANOVA in (B), (I), and (K).
MrgprA3 and histamine receptors could be dissociated into Gαq/11 and Gβγ subunits, mediating noxious sensation through coupling to downstream ion channels. To determine the signaling pathways involved in Piezo2 sensitization, we first employed gallein, a small-molecule inhibitor of Gβγ that has been shown to mediate TRPA1-dependent chemical itch.34,35 Surprisingly, pretreatment of gallein blocked neither the chemical itch nor the mechanical itch induced by CQ or histamine (Figure S8).
On the other hand, it was also reported that CQ-induced spontaneous scratching and neuronal activities were abolished in the PLCb3 knockout mice.36 To test whether PLC signaling contributes to MrpgrA3+ neuron sensitization in mechanical itch, we pretreated WT mice with the PLC inhibitor U73122 and the PLC agonist m-3M3FBS. U73122 significantly reduced histamine- and CQ-evoked mechanical itch (Figures 4C and 4D), while application of m-3M3FBS alone was sufficient to promote mechanical itch (Figure 4E). Consistent with these behavioral observations, 3M3FBS also significantly increased both Piezo2 current density and the percentage of mechanosensitive MrgprA3+ DRG neurons (Figures 4F and 4G), suggesting that PLC signaling is necessary and sufficient to promote mechanical itch.
Upon activation, PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG). While IP3 is critical to intracellular Ca2+ mobilization, the main effect of DAG is to activate the PKC enzyme, and phospho-PKC (p-PKC) represents a sensitive biomarker of neuronal activity.37–39 Indeed, immunofluorescent staining and western blot revealed an increased p-PKC protein expression in response to histamine- or CQ-induced activation of the MrgprA3+ neurons, which was further blocked by U73122, but not gallein (Figures 4H–4K and S10), indicating that PLC-PKC signaling is tightly correlated with pruritogen-induced sensitization of the MrgprA3+ neurons. Of note, the family of serine/threonine kinases comprises 11 PKC isoforms encoded by 9 genes.40 To determine the PKC isoforms involved in MrgprA3-dependent alloknesis, we first reanalyzed the single-cell RNA sequencing (RNA-seq) data of DRG neurons reported by Xing et al., showing that the PKCδ gene is the highest expressed PKC isoform in MrgprA3+ neurons (Figure S9A),27 which was also shown in our single-cell qRT-PCR data (Figure S9B). To determine the functional role of PKCδ in mechanical itch, we genetically ablated PKCd from the MrgprA3+ neurons by crossing the MrgprA3Cre mice with PKCδf/f mice. Interestingly, although pruritogen-induced acute chemical itch was comparable, mechanical itch evoked by CQ or histamine was significantly reduced in the PKCδcko mice when compared with their littermate controls (Figures 4L–4O). Taken together, our data demonstrate that intracellular PLC-PKCδ-Piezo2 signaling contributes to the enhanced mechanosensitivity of the MrgprA3+ neurons in pruritogen-induced mechanical itch sensitization.
DISCUSSION
Mechanical itch (alloknesis) is an exaggerated itch sensation elicited by innocuous mechanical stimuli, especially in the presence of exogenous pruritogens or in the setting of chronic itch. Although alloknesis was first described 30 years ago,41,42 the molecular and cellular mechanisms underlying pruritogen-induced alloknesis are not well understood. Here, we provided multiple lines of evidence showing that the TRPV1+/MrgprA3+ pruriceptors mediate mechanical itch following administration of CQ or histamine, which requires the function of the mechanosensitive Piezo2 channel. We further demonstrated that intracellular PLC-PKCδ signaling induced by CQ or histamine sensitizes Piezo2 channel function to produce mechanical itch (Figure S11). Our results place the TRPV1+/MrgprA3+ pruriceptors-Piezo2 signaling axis at the center of the mechanical itch signaling pathway driven by exogenously applied pruritogens.
Previous studies have shown multiple forms of mechanical itch under both physiological and pathological conditions. For instance, Ucn3-expressing excitatory interneurons as well as the neuropeptide Y1 receptor-expressing excitatory interneurons in the spinal cord are required for the transmission of mechanical itch occurring in specific locations,2,5 i.e., skin areas behind the ears. Interestingly, the Ucn3+ neurons receive inputs from TLR5+ Aβ LTMRs, which also express NF200 and contribute to the generation of touch and mechanical allodynia.5,25 In contrast to the excitatory role of TLR5+ Aβ LTMRs, our recent study showed that inhibitory inputs from Piezo2-expressing Merkel cells and innervating SAI-Aβ LTMRs suppress touch-evoked mechanical itch,6 suggesting that subpopulations of NF200+ Aβ fibers have distinct roles in mechanical itch signaling. Besides potential roles of Aβ LTMR subsets in mechanical itch, alloknesis is also associated with chronic skin inflammation such as that in mouse models of experimental dry skin and imiquimod-induced psoriasis as well as exogenously applied pruritogens such as histamine and endomorphins,1,7,43 which is mainly dependent on the abnormal activities of C pruriceptors, implicating a potential role of C-LTMRs in the development of mechanical itch sensitization. Indeed, C-LTMRs expressing VGlut3/TH and MrgprD were shown to play important roles in mechanical hypersensitivity. For example, VGlut3+/TH+ C-LTMRs mediate mechanical allodynia in the setting of neuropathic pain, while activation of MrgprD+ neurons evokes both acute itch and chronic itch associated with allergic contact dermatitis (ACD) in mice.12,44–47 However, our genetic studies showed that chemogenetic activation of MrgprD+ and VGlut3+/TH+ neurons does not evoke mechanical itch, suggesting that MrgprD+ non-peptidergic C polymodal nociceptors and VGlut3+/TH+ C-LTMRs are unlikely major players in pruritogen-induced mechanical itch in mice. Interestingly, a recent study demonstrated that the VGlut3-lineage neurons mediate spinal inhibition of pruritogen-evoked chemical itch,48 suggesting distinct roles of C-LTMRs in itch modulation.
It was recently reported that a subset of itch-sensing, neuron-expressing neuropeptide genes, somatostatin (Sst) and natriuretic polypeptide precursor B (Nppb), are critically involved in the development of mechanical itch. Specifically, Piezo1-expressing pruriceptors respond to histamine primarily through the Sst+Nppb+ neurons to promote mechanical itch and itch sensitization.49 Of note, Piezo2 was found to be expressed in a smaller percentage of Sst+Nppb+ neurons, while Piezo1 does not overlap with MrgprA3. This distribution pattern suggests that both Piezo1 and Piezo2 are critical mediators for mechanical itch sensitization through conferring mechanical sensitivity to distinct subpopulations of mechanosensitive pruriceptors.
Various ion channels downstream of GPCR signaling pathways contribute to distinct pruritogen-induced itch sensation. For instance, histamine elicits acute chemical itch sensation by activating downstream TRPV1 through PLCβ3 and PLA2/lipoxygenase,24,36,50,51 and CQ evokes TRPA1-dependent acute chemical itch through a signaling mechanism involving MrgprA3-coupled Gβγ signaling but not PLC.52 Moreover, inhibition of PKCδ significantly decreases histamine-induced acute chemical itch, as histamine treatment produces PKCδ phosphorylation leading to sensitization of the voltage-gated sodium channel Nav1.7 in pruriceptors.53,54 Interestingly, our results further showed that PLC-PKCδ signaling-mediated sensitization of the MrgprA3+ neuron-expressing Piezo2 channel plays a pivotal role in pruritogen-induced mechanical itch, but not acute chemical itch, revealing a signaling axis of PLC-PKCδ-Piezo2 in mechanical itch initiation, which is also separate from the roles of the EPAC1-Piezo2 and PKA/PKC-Piezo2 signaling axes in mechanical allodynia.19,33 Of note, although the mechanical itch responses induced by histamine and CQ were significantly attenuated in mice where PKCδ was conditionally ablated in the MrgprA3+ pruriceptors, we also observed high expression levels of Prkca and Prkce in the MrgprA3+ neurons, which is consistent with a recent report.55 Therefore, we cannot exclude the possibility that other PKC isoforms might also be involved in the pruritogen-induced sensitization of Piezo2 and the promotion of mechanical itch.
In summary, our findings show that activation of TRPV1+/MrgprA3+ DRG neurons mediates pruritogen-induced mechanical itch by sensitizing the mechanosensitive Piezo2 channel through PLC-PKCδ signaling in the skin. Identification of the MrgprA3+ C pruriceptors in mediating the pruritogen-induced mechanical itch highlights the importance of this small subset of TRPV1+ neurons in the generation of both mechanically stimulated and non-evoked chemical itch not only to the MrgprA3 receptor ligand CQ but also to other pruritogens such as histamine. Our data may also offer a therapeutic target for treating chronic itch.
Limitations of the study
There are a few limitations in this study: first, although the single-cell RT-PCR data in the study showed that Prkcd is the predominant protein kinase in MrgprA3+ neurons, we could not exclude the possibility that other PKC isoforms and/or other signaling pathways might also be involved in the pruritogen-induced sensitization of Piezo2 and the promotion of mechanical itch. Second, our work employed acute mouse models of pruritogen-induced mechanical itch; future work should be performed to determine whether TRPV1+/MrgprA3+ pruriceptors-Piezo2 signaling is also involved in the development of chronic itch-associated alloknesis. Last, while we showed inhibition of PLC-PKC signaling-mitigated mechanical itch in mice, it is challenging to inhibit the functions of MrgprA3 and Piezo2 because of a lack of specific blockers.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for reagents may be directed to and will be fulfilled by the lead contact, Hongzhen Hu (Hongzhen. Hu@wustl.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal procedures were performed using protocols approved by the Animal Studies Committee at Washington University School of Medicine and in compliance with the guidelines provided by the National Institute of Health and the International Association for the Study of Pain. Both adult male and female mice (8–12 weeks old) were used for all experiments and mice were randomly assigned to different experimental conditions. Mice were sex- and age-matched in all experiments and were group-housed in standard mouse housing cages at room temperature with unrestricted access to food and water on a 12 h light/12 h dark cycle.
For lineage tracing, Ai9 (B6;129S6-Gt (ROSA)26Sortm9(CAG-tdTomato)Hze/J) were crossed with MrgprA3GFP-Cre to induce tdTomato expression in MrgprA3+ cells. PCR primers used for genotyping are: Ai9 loxP Forward, CTG TTC CTG TAC GGC ATG G and Ai9 loxP Reverse, GGC ATT AAA GCA GCG TAT CC; Cre Forward, GGTTCGCAAGAACCTGATGG and Cre Reverse, GCCTTCTCTAC ACCTGCGG. The expected product sizes are 196 bps and 550 bps for Ai9flox/flox and Cre mice. Piezo2flox/flox mice were mated with Trpv1Cre and MrgprA3GFP-Cre mice to generate Piezo2 conditional knockout mice (Piezo2cKO). PCR primers used for genotyping are: Piezo2 loxP Forward, AGGCTCAGACTTGGAGATCCTGTAGCA and Piezo2 loxP Reverse, GACTCAGATTTTCCACATGG GGGTACTA. The expected product size is 196 bps for Piezo2flox/flox mice. PKCδflox/flox mice were mated with MrgprA3GFP-Cre mice to generate PKCδ conditional knockout mice (PKCδcKO). PCR primers used for genotyping are: PKCδ loxP Forward, GACAAGATTATCGGCCGCTG and PKCδ loxP Reverse, CAAACTGTGGGTTGTCAGAG. The expected product size is 356 bps for PKCδflox/flox mice. The Rosa26iDTR mice were crossed with Trpv1Cre and MrgprA3GFP-Cremice to obtain Trpv1Cre; iDTR and MrgprA3GFP-Cre; iDTR mice. PCR primers used for genotyping are: DTR loxP Forward, ATGAAGCTGCTGCCGTCGGTG and DTR loxP Reverse, GATCTGCCTCTTGCAAGTCAC. The expected product size is 250 bps for DTRflox/flox mice. For chemogenetic activation of DRG neurons, the transgenic mice were engineered by crossing Trpv1Cre, ThCreER, MrgprDCreERT and MrgprA3GFP-Cre mice with Gq-DREADD (B6N;129-Tg (CAG-CHRM3, mCitrine)1Ute/J)) mice, respectively. PCR primers used for genotyping are: Gq loxP Forward, CGCCACCATGTACCCATAC and Gq loxP Reverse, GTGGTACCGTCTGGAGAGGA. The expected product size is 204 bps for Gqflox/flox mice.
Primary mouse DRG neuronal culture
The DRGs from thoracic and lumbar levels were acutely isolated and cleaned of adhering connective tissue. Isolated ganglia were collected in ice-cold Ca2+ and Mg2+-free Hank’s buffered salt solution (HBSS, Gibco, USA). DRG neurons were enzymatically digested in dispase (4 U/mL, Gibco, USA) and collagenase type I (342 U/mL, Gibco, USA) dissolved in HBSS for 30 min at 37°C. After digestion, neurons were pelleted, suspended in neurobasal medium containing 2% B-27 supplement, 1% L-glutamine, 100 U·mL−1 penicillin plus 100 μg mL−1 streptomycin, and 50 ng mL−1 nerve growth factor, plated on a 5 mm coverslip coated with poly-l-lysine (10 mg mL−1) and cultured under a humidified atmosphere of 5% CO2/95% air at 37°C for 18–24 h before use.
METHOD DETAILS
Pruritogen-induced acute chemical itch
To test the acute itch behavior, the fur on the nape of the neck was shaved and mice were acclimated in the red transparent recording chamber for 5 days. On the testing day, mice were habituated in the behavioral testing apparatus for 1 hour and then histamine (50 μg; Sigma-Aldrich, St. Louis MO) or CQ (50 nmol; Sigma-Aldrich, St. Louis, MO) in 30 μL sterile saline was injected intradermally to the nape of the neck. Insulin syringes with 30G needle (UltiCare) were used for intradermal injection. Immediately after the injection, mice were videotaped for 30 min. After the recording, the videotapes were played back and the number of scratch bouts were counted over the 30-min recording period by an investigator blinded to the treatment. A scratching bout is defined as one or more rapid back-and-forth motion of the hindpaw directed to the site of injection, and ending with licking or biting of the toes and/or placement of the hindpaw on the floor.
Pruritogen-induced alloknesis
Mice were acclimated in a red recording chamber with a removable mesh cover for at least 5 days. 30 min after intradermal injection of respective pruritogens, mice received an innocuous mechanical stimulus for 1 second delivered using a von Frey filament (North Coast Medical, bending force: 0.7 mN) at five randomly selected points oriented radially 7 mm away from the injection site. Mice received 3 separate stimulations at each point with an interval of 10 seconds. The scratching response of hind paw toward the poking site was considered as a positive response. The scratching number at each point was averaged and then summed into the final alloknesis score for comparison. The stimulus at 5 points means 5 stimuli.
Drug administration
To induce robust Cre activity, tamoxifen (Sigma-Aldrich) was dissolved in corn oil and made fresh daily before use. Both Cre− and Cre+ mice received intraperitoneal injection of tamoxifen at 100 mg/kg body weight for 5 consecutive days. In vivo experiments were performed between 7 and 14 days after tamoxifen injection.
For the chemogenetic stimulation, Cre+ and Cre− mice of Trpv1; Gq-DREADD mice were intradermally injected with 50 μl 500 nM clozapine N-oxide CNO (Sigma-Aldrich), Cre+ and Cre− mice of Th; Gq-DREADD, MrgprD; Gq-DREADD and MrgprA3; Gq-DREADD mice were intradermally injected with 50 μl 3 mM CNO respectively. Immediately after the injection, mice were videotaped for 60 min. Alloknesis scores were evaluated 0.5 hours after CNO injections.
Mouse cheek model
The right cheek of mice was shaved and acclimated for at least two days before CNO injection. The naltrexone (10 mg/kg) or ibuprofen (150 mg/kg) was injected into each mouse by i.p, 30 minutes later mice were injected intradermally with 20 μL CNO (500 nM) in the right cheek. All mice were videotaped for one hour and the scratching/wiping bouts were counted.
Pharmacological silence of sensory fibers in the skin
Intradermal injection of 30 μL 0.2% QX-314+ 50 nmol CQ are employed to block the the C fibers, respectively. QX-314 (Sigma-Aldrich) and CQ (Sigma-Aldrich) were dissolved in isotonic saline. QX-314 (0.2%, 30 μL) was intradermally injected into the nape of the neck as a vehicle control. The effect of the Phospholipase C (PLC) activator m-3M3FBS (20 μM, 30 μL; Sigma-Aldrich), PLC antagonist U73122 (0.1 μM, 30 μL; Sigma-Aldrich) and the G protein βγ subunit inhibitor gallein (500 μM, 30 μL; Tocris Bioscience) and on scratching and alloknesis was tested.
In situ hybridization and immunohistochemistry
Non-isotopic in situ hybridization (ISH) on DRG sections prepared from MrgprA3GFP-Cre and Trpv1-EGFP mice were performed to detect Piezo2 mRNA expression. RNAscope Multiplex Fluorescent Reagent Kit V2 (Cat # 323100) and Piezo2 probes (400191) were purchased from ACDBio. The ISH/GFP double staining was performed as previously described.56 Briefly, after in situ hybridization for Piezo2 as instructed in the manual, DRG sections were incubated overnight at 4°C with chicken anti-GFP antibody (GFP-1020, Aves Labs, 1:1,000) and then incubated for 1 h at room temperature with donkey anti-chicken IgY (703-545-155, Alexa Fluor 488 conjugated, Jackson ImmuneResearch). Sections were then washed three times in PBS and mounted with DAPI for imaging.
To detect pruritogen-induced p-PKC expression in the MrgprA3+ neurons, wt mice were divided into eight groups for double staining. Four groups of mice were intradermally injected with vehicle (isotonic saline), CQ (50 nmol, 30 μL), U73122 (0.1 mM, 30 μL; 30 min prior to the CQ) + CQ and gallein (500 μM, 30 μL; 30 min prior to the CQ) + CQ respectively, and the other four groups were administered intradermally with vehicle (isotonic saline), histamine (50 μg, 30 μL), U73122 (0.1 μM, 30 mL; 30 min prior to the histamine) + histamine and gallein (500 μM, 30 μL; 30 min prior to the histamine) + histamine respectively. Thirty minutes after treatments, mice were deeply anesthetized with 2% isoflurane and were perfused intracardially with phosphate buffered saline (PBS, 0.1 M, pH 7.4) followed with fixative (4% paraformaldehyde, pH 7.4) at room temperature. Thoracic DRG were dissociated and fixed in fixative at 4°C for overnight, then stored in 30% sucrose in PBS at 4°C for 3 days. Samples were then embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Tissue-Tek, PA). Sections of approximately 12 μm on slides were performed for ISH with MrgprA3 probes (548161, ACDBio) firstly, then DRG sections were incubated overnight at 4°C with rabbit antibody to p-PKC (ab109539, Abcam, 1:500) and then incubated for 1 h at room temperature with goat anti-rabbit IgG H&L (ab150077, Alexa Fluor® 488, Abcam).
Both fluorescent and ISH signals were observed under the Nikon C2 Confocal Laser Microscope. Only DRG cells with clearly visible nuclei were quantified to prevent double-counting of the same cells in different sections. For quantification, 3–5 DRG sections from 3 mice were analyzed in each group.
Tail flick test
The ablation efficiency of the TRPV1+ neurons were determined by the tail flick test. The tail flick response to 51°C hot water was recorded. 20 s was set as the cut-off time.
Chemical ablation of TRPV1+ and MrgprA3+ neurons
For systemic DTX-mediated cell ablation, Trpv1Cre; iDTR and MrgprA3GFP-Cre; iDTR mice were treated with diphtheria toxin (DTX), according to the previous methods with a little modification.8 Briefly, 6 weeks old mice were injected intraperitoneally with DTX (50 mg/kg; Sigma-Aldrich) in 100 μL PBS at day 1, day 4, day7 and day 10. Mice were allowed for four weeks rest before behavioral experiments. For chemical ablation of Trpv1+ neurons, C57BL/6 mice at 4 weeks of age were treated with resiniferatoxin (RTX, Tocris Bioscience). RTX was prepared in 2% DMSO with 0.15% Tween 80 in PBS. Mice received subcutaneous injections of RTX in the flank on consecutive days with three increasing doses of RTX (30, 70, and 100 μg/kg). We performed behavioral experiments 4 to 6 weeks after RTX injection. Considering the potential variation of ablation efficiency in different approaches, mice displayed significantly increased latency to thermal pain response and less than 5% capsaicin-responding DRG neurons were kept for further analysis.
Mouse DRG neuron cultures
Mice were deeply anaesthetized with isoflurane and then exsanguinated. The spinal cord was removed and DRG from thoracic levels were dissected. Isolated ganglia were collected in ice-cold Ca2+ and Mg2+-free Hank’s buffered salt solution (HBSS, Gibco). DRG neurons were enzymatically digested in dispase (4 U/mL, Gibco) and collagenase type I (342 U/mL, Gibco) dissolved in HBSS for 30 min at 37°C. After digestion, neurons were pelleted, suspended in neurobasal medium containing 2% B-27 supplement, 1% L-glutamine, 100 U·mL1 penicillin plus 100 μg mL−1 streptomycin, and 50 ng mL−1 nerve growth factor, plated on a 5 mm coverslip coated with poly-l-lysine (10 μg mL−1) and cultured under a humidified atmosphere of 5% CO2/95% air at 37°C for 18–24 h before use.
Live-cell calcium imaging
Culture DRG neurons on cover slips were loaded with 4 μmol/L fura 2 (Invitrogen) for 60 min in the dark at 37°C. After a three-time wash with HBSS, the cells were imaged by a microscope. Fluorescence at 340 and 380 nm excitation wavelengths was recorded on an inverted Nikon Ti-E microscope equipped with 340, 360 and 380 nm excitation filter wheels using NIS-Elements imaging software (Nikon Instruments Inc., Melville, NY, USA). Fura-2 ratios (F340/F380) were used to reflect changes in intracellular Ca2+ upon stimulation. Values were obtained from 80 to 150 cells in time-lapse images from each coverslip. The standard extracellular solution for Ca2+ imaging in cultured DRG neurons contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM Na-HEPES, and 10 mM glucose (pH 7.3). Chloroquine (1 μM), capsaicin (300 nM) and KCl (100 mM) was dissolved respectively in standard extracellular solution.
Dissociated DRG neurons from Trpv1Cre and Trpv1Cre;Piezo2flox/flox mice were first screened for capsaicin sensitivity using calcium imaging to pick up the TRPV1+ neurons, and then labeled for following patch clamp recordings.
Whole-cell patch-clamp recordings
Whole-cell patch-clamp recordings were performed using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) at room temperature (22–24°C) on the stage of an inverted phasecontrast microscope equipped with a filter set for tdTomato visualization. Pipettes pulled from borosilicate glass (BF 150-86-10; Sutter Instrument Company, Novato, CA, USA) with a Sutter P-1000 pipette puller had resistances of 2–4 MΩ when filled with pipette solution containing 120 mM K+-gluconate, 30 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 2 mM MgATP, 11 mM EGTA, and 10 mM HEPES with pH 7.3. Cells were continuously perfused with standard extracellular solution containing 145 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose and 10 mM HEPES (pH was adjusted to 7.4 with NaOH). Cells were clamped to a holding potential of −80 mV and stimulated with a series of mechanical stimuli in 1 mm increments every 10 s, and the stimulus was applied for 100 ms. Step indentations were applied using a fire-polished glass pipette (tip diameter 2–3 μm) that was positioned at an angle of 70° to the surface of the cell. The pipette was controlled by a Piezo Servo Controller (E-625, Physik Instrument, Karlsruhe, Germany). Data were acquired using pClampex 10 (Molecular Devices, San Jose, CA). Currents were filtered at 2 kHz and digitized at 10 kHz. Values were given as mean ± SEM; n represents the number of measurements.
Western blot analysis
DRG isolated from thoracic levels were homogenized in ice-cold RIPA lysis and extraction buffer (Thermo Fisher Scientific) with protease inhibitor mix (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE gel electrophoresis and transferred to a PVDF membrane (Millipore Corp) which was blocked for 1 h at room temperature. The membrane was then incubated overnight at 4°C with monoclonal antibody against p-PKC (ab109539, Abcam, 1:1000) and as loading control antibody against GAPDH (ab8245, Abcam, 1:1000). Subsequently, PVDF membranes were incubated with goat anti-mouse IgG (sc2005, Santa Cruz Biotechnology, 1:3000) or donkey-anti-rabbit IgG H&L (ab205722, Abcam, 1:3000).
Single cell RT-PCR
To isolate MrgprA3+ neurons, DRGs from MrgprA3Cre;Ai9f/f mice were dissected and dissociated. DRG neurons were purified using a 15% BSA density gradient column. MrgprA3+ neuron cells were visually identified under a Nikon eclipse TE2000-S microscope and manually picked using a Sutter Instrument ROE285-4323 micromanipulator. Isolated MrgprA3+ neurons were ejected into PCR tubes containing 10 μL of lysis buffer and RNase inhibitor (4458236, Life Tech), and flash frozen on dry ice and stored at −80°C until cDNA synthesis.
cDNA was generated using Invitrogen Single Cell-to-CT TM Kit (4458236, Life Tech), according to the manufacturer’s protocol. RT-PCR was performed using 2 μL cDNA and TaqManTM Gene Expression Master Mix(4369016, Life Tech). qPCR Taqman probes (Thermo Fisher Scientific) used were Prkca: Assay ID Mm00440858_m1; Prkcb: Assay ID Mm00435749_m1, Prkcd: Assay ID Mm00440891_m1, Prkce: Assay ID Mm00440894_m1, Prkcg: Assay ID Mm00440861_m1 and Gapdh: Assay ID Mm99999915_g1. PCR reactions were run in triplicate.
QUANTIFICATION AND STATISTICAL ANALYSIS
Results are expressed as mean ± SEM. All histology, calcium imaging, and electrophysiology experiments were repeated using tissues from at least three different mice. Statistical analysis was performed in Prism 9 (GraphPad). A threshold of p < 0.05 was accepted as statistically different and p > 0.05 considered non-significant. For itch behavioral test, we did not observe any obvious sex differences in our experiments and data from both genders were pooled together for futher analysis with unpaired two-tailed Student’s t test (for two groups) or one-way ANOVA (for three or more groups). For statistical analysis of incidence of electrophysiological results, data were analyzed with Chi-square test.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Chicken polyclonal anti-GFP antibody | Aves Labs | Cat#GFP-1020; RRID: AB_2307313 |
| Rabbit monoclonal (EP2730Y) to PKC (phospho T514) | Abcam | Cat#ab109539; RRID: AB_10863532 |
| Goat polyclonal anti-rabbit IgG H&L Alexa Fluor 488 | Abcam | Cat#ab150077; RRID: AB_2630356 |
| Mouse monoclonal anti- GAPDH | Abcam | Cat#ab8245; RRID: AB_2107448 |
| Donkey polyclonal anti-chicken IgY Alexa Fluor 488 | Jackson ImmuneResearch | Cat#703-545-155; RRID: AB_2340375 |
| Goat polyclonal anti-mouse IgG, HRP conjugated | Santa Cruz Biotechnology | Cat#sc2005; RRID: AB_631736 |
| Donkey polyclonal anti-rabbit IgG H&L, HRP conjugated | Abcam | Cat#ab205722; RRID: AB_2904602 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Histamine | Sigma-Aldrich | Cat# H7125 |
| Chloroquine | Sigma-Aldrich | Cat# C6628 |
| Capsaicin | Sigma-Aldrich | Cat# M2028 |
| Tamoxifen | Sigma-Aldrich | Cat# T5648 |
| CNO | Sigma-Aldrich | Cat# C0832 |
| Naltrexone | Sigma-Aldrich | Cat# 1453504 |
| Ibuprofen | Sigma-Aldrich | Cat#I1892 |
| QX-314 | Sigma-Aldrich | Cat# 552233 |
| U73122 | Sigma-Aldrich | Cat# U6756 |
| Gallein | Tocris Bioscience | Cat# 3090/50 |
| DAPI | Invitrogen | Cat# E140588 |
| Optimal cutting temperature (OCT) compound | SAKURA Tissue-Tek | Cat# 4583 |
| Diphtheria toxin (DTX) | Sigma-Aldrich | Cat#D0564 |
| Resiniferatoxin (RTX) | Tocris Bioscienc | Cat#1137 |
| Dispase | Gibco | Cat# DN25 |
| Collagenase type I | Gibco | Cat#17018029 |
| poly-l-lysine | Sigma-Aldrich | Cat#P8920 |
| Nerve growth factor | Sigma-Aldrich | Cat#N8133 |
| Neurobasal medium | Gibco | Cat#21103049 |
| Fura 2 | Invitrogen | Cat#F1201 |
| RIPA lysis and extraction buffer | Thermo Fisher Scientific | Cat#89900 |
| protease inhibitor mix | Thermo Fisher Scientific | Cat#A32965 |
| RNase inhibitor | Life Tech | Cat#4458236 |
| TaqManTM Gene Expression Master Mix | Life Tech | Cat#4369016 |
| B-27 supplement | Gibco | Cat#17504044 |
| L-glutamine | Gibco | Cat#25030081 |
| Western HRP Substrate | Millipore | Cat# WBLUR0100 |
|
| ||
| Critical commercial assays | ||
|
| ||
| RNAscope Multiplex Fluorescent Reagent Kit V2 | ACD | Cat #323100 |
| Single Cell-to-CT TM Kit | Thermo Fisher Scientific | Cat #4458237 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX:000664 |
| Mouse: ThCreER | The Jackson Laboratory | JAX:008532 |
| Mouse: MrgprDCreERT | The Jackson Laboratory | JAX: 031286 |
| Mouse: B6N;129-Tg(CAG-CHRM3, mCitrine)1Ute/J(Gq-DREADD) | The Jackson Laboratory | JAX:026220 |
| Mouse: ROSA26iDrR | The Jackson Laboratory | JAX:007900 |
| Mouse: B6;129S6-Gt (ROSA)26Sortm9(CAG-tdTomato)Hze/J (Ai9) | The Jackson Laboratory | JAX:007909 |
| Mouse: Piezo2flox/flox | The Jackson Laboratory | JAX: 027720 |
| Mouse: Trpa1-KO | The Jackson Laboratory | JAX: 006401 |
| Mouse: PKCδflox/flox | RIKEN BRC | Strain #: 06462 |
| Mouse: Trpv1-EGFP | MMRRC | 033029-UCD |
| Mouse: Trpv1Cre | Mark Hoon, NIH | N/A |
| Mouse: MrgprA3GFP-Cre | Xinzhong Dong, Johns Hopkins University, HHMI | N/A |
|
| ||
| Oligonucleotides | ||
|
| ||
| RNAscope® Probe- MrgprA3 | ACD | Cat #548161 |
| RNAscope® Probe- Piezo2 | ACD | Cat #400191 |
| qPCR Taqman probes for Prkca | Thermo Fisher Scientific | Assay ID Mm00440858_m1 |
| qPCR Taqman probes for Prkcb | Thermo Fisher Scientific | Assay ID Mm00435749_m1 |
| qPCR Taqman probes for Prkcd | Thermo Fisher Scientific | Assay ID Mm00440891_m1 |
| qPCR Taqman probes for Prkce | Thermo Fisher Scientific | Assay ID Mm00440894_m1 |
| qPCR Taqman probes for Prkcg | Thermo Fisher Scientific | Assay ID Mm00440861_m1 |
| qPCR Taqman probes for Gapdh | Thermo Fisher Scientific | Assay ID Mm99999915_g1 |
|
| ||
| Software and algorithms | ||
|
| ||
| Prism 9 | GraphPad | https://www.graphpad.com/ |
| Adobe Photoshop CS6 | Adobe | https://www.adobe.com/ |
|
| ||
| Other | ||
|
| ||
| von Frey filament | North Coast Medical | Cat #NC12775 |
| Piezo Servo Controller | Physik Instrument | E-625 |
Highlights.
TRPV1+/MrgprA3+ neurons are critical to the initiation of mechanical itch
TRPV1+/MrgprA3+ neurons are required for pruritogen-induced mechanical itch sensitization
Piezo2 channel mediates pruritogen-induced mechanical itch sensitization
Sensitization of Piezo2 by PLC-PKCδ signaling underlies pruritogen-induced alloknesis
ACKNOWLEDGMENTS
We thank Dr. Mark Hoon for sharing the Trpv1Cre mice and Dr. Xinzhong Dong for sharing the MrgprA3GFP-Cre mouse line. This work is supported by National Institutes of Health grants R01AA027065, R01AR077183, and R01DK103901 to H.H. and R01NS106289 to G.F.W.
INCLUSION OF DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
Footnotes
DECLARATION OF INTERESTS
B.S.K. has served as a consultant for AbbVie, Almirall S.A., Amagma, Argenx, Astra Zeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim Corporation, Bristol-Myers Squibb, Cara Therapeutics, Daewoong Pharmaceutical, Eli Lilly and Company, Guidepoint Global, Janssen Pharmaceuticals, Incyte Corporation, Kiniksa Pharmaceuticals, LectureLinx, LEO Pharma, Maruho, Novartis, OM Pharma, Pfizer, Sanofi Genzyme, Shaperon, Third Rock Ventures, and Trevi Therapeutics; is a stockholder of Recens Medical and Locus Biosciences; and serves on the scientific advisory boards for Abrax Japan, Granular Therapeutics, Recens Medical, National Eczema Association, Cell Reports Medicine, and Journal of Allergy and Clinical Immunology. B.S.K. is an inventor on a patent/patent application (WO2017143014A1) held/submitted by Washington University that covers the use of JAK inhibitors for chronic pruritus.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112283.
REFERENCES
- 1.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, and Carstens E (2012). Mouse model of touch-evoked itch (alloknesis). J. Invest. Dermatol. 132, 1886–1891. 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acton D,Ren X,DiCostanzo S,Dalet A,Bourane S,Bertocchi I,Eva C,and Goulding M(2019).SpinalneuropeptideY1receptor-expressingneuronsform anessentialexcitatorypathwayformechanicalitch.CellRep.28,625–639.e6. 10.1016/j.celrep.2019.06.033. [DOI] [Google Scholar]
- 3.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, and Goulding M (2015). Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554. 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Gao XF, Zhou Y, Liu BL, Liu XY, Zhang Y, Barry DM, Liu K, Jiao Y, Bardoni R, et al. (2020). A spinal neural circuitry for converting touch to itch sensation. Nat. Commun. 11, 5074. 10.1038/s41467-020-18895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan H, Fatima M, Li A, Lee H, Cai W, Horwitz L, Hor CC, Zaher N, Cin M, Slade H, et al. (2019). Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron 103, 1135–1149.e6. 10.1016/j.neuron.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng J, Luo J, Yang P, Du J, Kim BS, and Hu H (2018). Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 360, 530–533. 10.1126/science.aar5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komiya E, Tominaga M, Hatano R, Kamikubo Y, Toyama S, Sakairi H, Honda K, Itoh T, Kamata Y, Tsurumachi M, et al. (2022). Peripheral endomorphins drive mechanical alloknesis under the enzymatic control of CD26/DPPIV. J. Allergy Clin. Immunol. 149, 1085–1096. 10.1016/j.jaci.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Han L,Ma C,Liu Q,Weng HJ,Cui Y,Tang Z,Kim Y,Nie H,Qu L,Patel KN, et al. (2013). A subpopulation of nociceptors specifically linked to itch. Nat.Neurosci.16,174–182. 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, Chesler AT, and Hoon MA (2019). Nppb neurons are sensors of mast cell-induced itch. Cell Rep. 26, 3561–3573.e4. 10.1016/j.celrep.2019.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharif B, Ase AR, Ribeiro-da-Silva A, and Séguéla P (2020). Differential coding of itch and pain by a subpopulation of primary afferent neurons. Neuron 106, 940–951.e4. 10.1016/j.neuron.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 11.von Buchholtz LJ, Ghitani N, Lam RM, Licholai JA, Chesler AT, and Ryba NJP (2021). Decoding cellular mechanisms for mechanosensory discrimination. Neuron 109, 285–298.e5. 10.1016/j.neuron.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, and LaMotte RH (2014). Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain 137, 1039–1050. 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, Zhao Y, Xie Z, Zang K, Sviben S, Hu X, Fitzpatrick JAJ, Wen L, Liu Y, Wang T, et al. (2022). Miswiring of Merkel cell and pruriceptive C fiber drives the itch-scratch cycle. Sci. Transl. Med. 14, eabn4819. 10.1126/scitranslmed.abn4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR, and Patapoutian A (2018). The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 10, eaat9897. 10.1126/scitranslmed.aat9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, Li Y, Lei J, Li X, and Xiao B (2019). Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 573, 225–229. 10.1038/s41586-019-1505-8. [DOI] [PubMed] [Google Scholar]
- 16.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, et al. (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626. 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, and Patapoutian A (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 18, 1756–1762. 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, Ogata T, Daou I, Stowers LT, Bö nnemann CG, et al. (2020). PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 588, 290–295. 10.1038/s41586-020-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, and Patapoutian A (2012). Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2, 511–517. 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nencini S, Morgan M, Thai J, Jobling AI, Mazzone SB, and Ivanusic JJ (2021). Piezo2 knockdown inhibits noxious mechanical stimulation and NGF-induced sensitization in A-delta bone afferent neurons. Front. Physiol. 12, 644929. 10.3389/fphys.2021.644929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szczot M,Liljencrantz J,Ghitani N,Barik A,Lam R,Thompson JH,Bharucha-Goebel D,Saade D,Necaise A,Donkervoort S,et al.(2018).PIEZO2 mediatesinjury-inducedtactilepaininmiceandhumans.Sci.Transl.Med.10, eaat9892. 10.1126/scitranslmed.aat9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153. 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 23.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, and Anderson DJ (2009). Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA 106, 9075–9080. 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra SK, and Hoon MA (2013). The cells and circuitry for itch responses in mice. Science 340, 968–971. 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, and Ji RR (2015). Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 21, 1326–1331. 10.1038/nm.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, and Bautista DM (2013). The ion channel TRPA1 is required for chronic itch. J. Neurosci. 33, 9283–9294. 10.1523/jneurosci.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y, Chen J, Hilley H, Steele H, Yang J, and Han L (2020). Molecular signature of pruriceptive MrgprA3(+) neurons. J. Invest. Dermatol. 140, 2041–2050. 10.1016/j.jid.2020.03.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, and Blackshaw LA (2011). TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J. Physiol. 589, 3575–3593. 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain MZ, Ando H, Unno S, and Kitagawa J (2022). TRPA1s act as chemosensors but not as cold sensors or mechanosensors to trigger the swallowing reflex in rats. Sci. Rep. 12, 3431. 10.1038/s41598-022-07400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwan KY,Glazer JM,Corey DP,Rice FL,andStucky CL(2009).TRPA1 modulatesmechanotransductionincutaneoussensoryneurons.J.Neurosci. 29,4808–4819. 10.1523/jneurosci.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lennertz RC,Kossyreva EA,Smith AK,andStucky CL(2012).TRPA1mediatesmechanicalsensitizationinnociceptorsduringinflammation.PLoSOne 7, e43597. 10.1371/journal.pone.0043597. [DOI] [Google Scholar]
- 32.Del Rosario JS, Yudin Y, Su S, Hartle CM, Mirshahi T, and Rohacs T (2020). Gi-coupled receptor activation potentiates Piezo2 currents via Gβγ. EMBO Rep. 21, e49124. 10.15252/embr.201949124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, et al. (2013). A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 4, 1682. 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, et al. (2014). The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology 147, 1417–1428. 10.1053/j.gastro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, Lyman K, Olsen ASB, Wong JF, Stucky CL, et al. (2015). HTR7 mediates serotonergic acute and chronic itch. Neuron 87, 124–138. 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, and Han SK (2009). TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 106, 11330–11335. 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeley M, Kelleher D, and Long A (2011). Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites. Cell. Signal. 23, 753–762. 10.1016/j.cellsig.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Hopper RA, Forrest CR, Xu H, Zhong A, He W, Rutka J, Neligan P, and Pang CY (2000). Role and mechanism of PKC in ischemic preconditioning of pig skeletal muscle against infarction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R666–R676. 10.1152/ajpregu.2000.279.2.R666. [DOI] [PubMed] [Google Scholar]
- 39.Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, and Trimmer JS (2006). Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J. Neurosci. 26, 13505–13514. 10.1523/jneurosci.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cocco L, Follo MY, Manzoli L, and Suh PG (2015). Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 56, 1853–1860. 10.1194/jlr.R057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyer G, Ulmer FJ, Schmitz J, and Handwerker HO (1995). Histamine-induced itch and alloknesis (itchy skin) in atopic eczema patients and controls. Acta Derm. Venereol. 75, 348–352. 10.2340/0001555575348352. [DOI] [PubMed] [Google Scholar]
- 42.Simone DA, Alreja M, and LaMotte RH (1991). Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens. Mot. Res. 8, 271–279. 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 43.Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, and Akiyama T (2016). Mouse model of imiquimod-induced psoriatic itch. Pain 157, 2536–2543. 10.1097/j.pain.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Pichon CE, and Chesler AT (2014). The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front. Neuroanat. 8, 21. 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, and Edwards RH (2009). Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655. 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, and Dong X (2012). Mechanisms of itch evoked by b-alanine. J. Neurosci. 32, 14532–14537. 10.1523/jneurosci.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steele HR,Xing Y,Zhu Y,Hilley HB,Lawson K,Nho Y,Niehoff T,andHan L(2021).MrgprC11(+)sensoryneuronsmediateglabrousskinitch.Proc.Natl.Acad.Sci.USA 118. 10.1073/pnas.2022874118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakai K, Sanders KM, Lin SH, Pavlenko D, Funahashi H, Lozada T, Hao S, Chen CC, and Akiyama T (2020). Low-threshold mechanosensitive VGLUT3-lineage sensory neurons mediate spinal inhibition of itch by touch. J. Neurosci. 40, 7688–7701. 10.1523/jneurosci.0091-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill RZ, Loud MC, Dubin AE, Peet B, and Patapoutian A (2022). PIEZO1 transduces mechanical itch in mice. Nature 607, 104–110. 10.1038/s41586-022-04860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jian T, Yang N, Yang Y, Zhu C, Yuan X, Yu G, Wang C, Wang Z, Shi H, Tang M, et al. (2016). TRPV1 and PLC participate in histamine H4 receptor-induced itch. Neural Plast. 2016, 1682972. 10.1155/2016/1682972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim BM, Lee SH, Shim WS, and Oh U (2004). Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci. Lett. 361, 159–162. 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Than JYXL, Li L, Hasan R, and Zhang X (2013). Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J. Biol. Chem. 288, 12818–12827. 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Ding M, Wu Y, Xue S, Ji Y, Zhang P, Zhang Z, Cao Z, and Zhang F (2022). Histamine sensitization of the voltage-gated sodium channel Nav1.7 contributes to histaminergic itch in mice. ACS Chem. Neurosci. 13, 700–710. 10.1021/acschemneuro.2c00012. [DOI] [PubMed] [Google Scholar]
- 54.Valtcheva MV, Davidson S, Zhao C, Leitges M, and Gereau RW 4th. (2015).ProteinkinaseCδmediateshistamine-evokeditchandresponsesinpruriceptors.Mol.Pain 11,1. 10.1186/1744-8069-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, and Ginty DD (2020). The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398. 10.1038/s41586-019-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. (2009). Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365. 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.


