Highlights
-
•
Endogenous H2S is an emerging novel cancer modulator in different pathological conditions and a possible diagnostic and prognostic marker in cancer.
-
•
Inhibition of endogenous H2S could suppress the viability, proliferation, migration, and invasion of human EC cells.
-
•
PAG, AOAA, and L-Asp suppressed EC xenograft tumor growth and the combination group exhibited more potent inhibitory effects on tumor growth.
Keywords: Esophageal cancer, Endogenous H2S, Apoptosis, Pyroptosis, Signaling pathway
Abstract
Background
Hydrogen sulfide (H2S) has been identified as the third gaseous signaling molecule. Endogenous H2S plays a key role in the progression of various types of cancer. However, the effect of endogenous H2S on the growth of esophageal cancer (EC) remains unknown.
Methods
In this study, three kinds of H2S-producing enzymes inhibitors, DL-propargylglycine (PAG, inhibitor of cystathionine-γ-lyase), aminooxyacetic acid (AOAA, inhibitor of cystathionine-β-synthase), and L-aspartic acid (L-Asp, inhibitor of 3-mercaptopyruvate sulfurtransferase) were used to determine the role of endogenous H2S in the growth of EC9706 and K450 human EC cells.
Results
The results indicated that the combination (PAG+AOAA+L-Asp) group showed higher inhibitory effects on the viability, proliferation, migration, and invasion of EC cells than PAG, AOAA, and L-Asp group. Inhibition of endogenous H2S promoted apoptosis via activation of mitogen-activated protein kinase pathway in EC cells. Endogenous H2S suppression triggered pyroptosis of EC cells by activating reactive oxygen species-mediated nuclear factor-κB signaling pathway. In addition, the combine group showed its more powerful growth-inhibitory effect on the growth of human EC xenograft tumors in nude mice without obvious toxicity.
Conclusion
Our results indicate that inhibition of endogenous H2S production can significantly inhibit human EC cell growth via promotion of apoptosis and pyroptosis. Endogenous H2S may be a promising therapeutic target in EC cells. Novel inhibitors for H2S-producing enzymes can be designed and developed for EC treatment.
Background
Esophageal cancer (EC), which has the sixth highest mortality rate and patients with this advanced illness have a fewer than 25% five-year overall survival rate, is the seventh most dangerous cancer in the world [1], [2], [3]. Although multimodality therapies have remarkably improved during the past decades, the prognosis of EC remains dismal. Therefore, multidisciplinary therapy has been strongly recommended to improve the prognosis [4]. Hydrogen sulfide (H2S) has been known as the third gasotransmitter [5], [6], [7], [8]. It is well known that endogenous H2S is essential as a mediator in a variety of physiological and pathological conditions [9]. Owing to its vascular relaxant and angiogenesis effects, H2S has shown its key player in modulating cancer development and progression in recent studies [10], [11], [12], [13], [14], [15]. However, the effect of endogenous H2S on esophageal cancer remains unknown and its potential mechanism is lacking. Hence, the current study aimed to explore the regulatory role of endogenous H2S in EC cells.
Currently, three kinds of H2S-synthesizing enzymes have been identified as being involved in H2S production: cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST). Recent studies have shown that the three enzymes exhibit a tissue-specific expression pattern [16,17]. The aggressiveness of various solid tumors, including hepatoma cells [12], colon cancer [10,18], prostate cancer [19], and breast cancer [16], has been associated with high amounts of CSE and CBS expression. And 3-MST expression were found in human neoplastic cells lines, melanoma cell lines, colon cancer cell lines, lung adenocarcinoma lines and urothelial cancer cell lines [17]. Together, the expression levels and functional correlations of CBS, CSE and 3-MST have been identified in different cancer cells, but their roles in EC are not yet to be clarified.
In this study, we aim to determine the roles of H2S-synthesizing enzymes in the growth of EC cells. In summary, in vitro and in vivo studies deepen our understanding of the crucial functions of endogenous H2S in EC progression and provide novel insights for anti-cancer intervention.
Methods and materials
Cell culture
Human Esophageal Cancer Cells (EC9706, K450) were purchased from Shanghai Jining Biosciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin (Gibco), and 100 µg/ml streptomycin (Gibco). The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Five groups were included in the experiment as follows: control group, DL-propargylglycine (PAG, CSE inhibitor) group, aminooxyacetic acid (AOAA, CBS inhibitor) group, L-aspartic acid (L-Asp, 3-MST inhibitor) group, and the combination (PAG+AOAA+L-Asp) group. All drugs were used at the concentrations of 1, 2.5, 5, and 10 mM for 24 h. Administration of phosphate buffer saline (PBS) for 24 h was considered as the control group. Then the cells were visualized under a CKX41 microscope (Olympus, Tokyo, Japan). And 10 mM PAG, AOAA, and L-Asp were used in further experiments.
Measurement of H2S levels
H2S levels in EC9706 and K450 cells were detected using the enzyme-linked immunosorbent assay kit (LanpaiBio, Shanghai, China) as previously described [20].
Cell proliferation assay
The cell proliferation was performed by 5-Ethynyl-2′-deoxyuridine (EdU) assay using Cell-Light EdU DNA Cell Proliferation Kit (RiboBio, Shanghai, China).
Cell viability assay
Cell viability was assessed using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit (MTS; Promega, Madison, WI, USA). Then the absorbance was determined at 490 nm.
Colony formation assay
6 × 102 cells/plate were grown for 10 to 14 days on 35-mm dishes until individual clones could be identified. After washed with PBS buffer, the colonies were fixed with methanol and stained with crystal violet. Then the colonies containing more than 50 cells were counted.
Wound healing assay
The cells per well were seeded in 6-well plates and cultured in medium with 10% FBS. When the cells reached 90% confluence, scratch wounds were made with the use of a sterile 200 μL pipette tip and the cell monolayer was put in a serum-free medium. At 0 h, 12 h, and 24 h, the scratched area was identified and photographed. The areas of the wounds were measured by using ImageJ Software.
Soft agar assay
Soft agar assay was performed as we previously described [21].
Transwell assay
Cell migration and invasion assays were assessed by Transwell. In 24-well chamber, cells were placed into the upper compartment with serum-free medium and medium containing 20% FBS was added to the lower wells as a chemoattractant. The difference between cell migration and invasion assay was whether the bottom of the insert chamber was pre-coated with or without matrigel. For the migration and invasion assays, 5 × 106 cells were incubated for 24 h and 48 h, respectively. Then the cells migrated to the lower surface of the insert dish were fixed with 100% methanol for 15 min, stained with 0.1% crystal violet for 10 min, and then imaged under a microscope.
Western blot
EC cells were treated, harvested, and lysed with a protease inhibitor (Beyotime, Shanghai, China). Using sodium dodecyl sulphate-polyacrylamide gel electrophoresis, equal amount of protein was separated and then transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% nonfat milk for 2 h followed by incubation overnight at 4°C with a primary antibody of anti-E-cadherin antibody (1:1000, CST, Danvers, MA, USA), anti-N-cadherin (1:1000, CST), anti-matrix metalloproteinase-9 (MMP-9) (1:1000, CST), anti-Vimentin (1:1000, CST), anti-Snail (1:1000, CST), anti-Slug (1:1000, CST), anti-phospho (p)-extracellular signal-regulated protein kinase (ERK) (1:1000, Proteintech, Chicago, IL, USA), anti-ERK (1:1000, Proteintech), anti-p-c-Jun N-terminal kinase (JNK) (1:1000, Proteintech), anti-JNK (1:1000, Proteintech), anti-p-p38 (1:1000, Proteintech), anti-p38 (1:1000, Proteintech), anti-nod-like receptor pyrin domain-containing protein 3 (NLRP3) (1:1000, Proteintech), anti-cleaved gasdermin D (GSDMD) (1:1000, Proteintech), anti-interleukin (IL)-1β (1:1000, Proteintech), anti-cleaved caspase (cas)-1 (1:1000, Proteintech), anti-IL-18 (1:1000, Proteintech), anti-p50 (1:1000, Proteintech), anti-p-p65 (1:1000, Proteintech), anti-p65 (1:1000, Proteintech), anti-p-IκBα (1:1000, Proteintech), anti-IκBα (1:1000, Proteintech), anti-B-cell lymphoma-2 (Bcl-2) (1:1000, Proteintech), anti-Bcl-2-associated X protein (Bax) (1:1000, Proteintech), anti-B-cell lymphoma-extra large (Bcl-xl) (1:1000, Proteintech), anti-Bcl-xl/Bcl-2-associated death promoter (Bad) (1:1000, Proteintech), anti-cleaved cas-3 (1:1000, Proteintech), anti-cleaved cas-9 (1:1000, Proteintech), anti-cleaved poly adenosine diphosphate-ribose polymerase (PARP) (1:1000, Proteintech), anti-cytochrome C (Cyt C) (1:1000, Proteintech), and anti-β-actin (1:5000, CST). After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. The bands were detected using an enhanced chemiluminescence system (Thermo, Rockford, IL, USA). The bands were semi-quantified with ImageJ software.
Cell apoptosis assay
Apoptosis was measured using TdT-mediated dUTP-biotin nick end labeling (TUNEL) assay. TUNEL-positive cells were imaged with a fluorescent microscope and the percentage was calculated using ImageJ software. The Annexin V Apoptosis Detection Kit (KeyGen Biotech, Nanjing, Jiangsu, China) was used for Annexin/PI analyses by a flow cytometer (CytoFLEX S, Beckman, CA, USA).
Reactive oxygen species (ROS) detection
Cellular ROS detection was performed by the dihydroethidium assay kit (Beyotime).
Xenograft nude mouse model
Animal studies were approved by the Committee of Medical Ethics and Welfare for Experimental Animals of Henan University School of Medicine (HUSOM-2019-167). Nude male mice (BALB/C-nu/nu) of four-week-old were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Then, 60 mice were divided into ten groups (n = 6/group) for the administration of EC9706 and K450 cells (5 × 106 cells in 200 μl PBS) with 10 mM PAG, AOAA, L-Asp, PAG+AOAA+L-Asp and PBS subcutaneously injected near the tumor for 28 days respectively. The body weight and tumor volume of the mice were determined every 4 days. The volume (V) was calculated with the formula (V = 1/2 × length × width2). After 28 days the mice were euthanized, and tumor, heart, liver, spleen, lung, kidney, and brain were weighted. The tumor growth inhibition rate (IR) was calculated as IR = [(A - B)/A] × 100%, where A and B was the average tumor weight of the control group and treatment group, respectively.
Immunohistochemistry (IHC)
The paraffin-embedded tissue sections were used for hematoxylin and eosin (HE) staining. IHC was conducted using 4-μm-thick paraffin-embedded tumor sections. The primary antibodies included anti-Ki67 (CST), anti-cluster of differentiation 31 (CD31) (CST), anti-cleaved cas-3, anti-NLRP3, anti-E-cadherin were diluted and then incubated at 4°C overnight. The slides were carefully washed before being incubated with HRP conjugates using diaminobenzidine detection. Tumor tissues were observed using a Zeiss Axioskop 2 plus microscope. Then, the microvessel density (MVD) was calculated, and the proliferation index, apoptotic index, NLRP3 positive cells, and E-cadherin positive cells were determined by the ratios of the positively stained cells to the total number.
Statistical analysis
The data are provided as the mean ± standard error of the mean (SEM). The difference between indicated groups was evaluated by one-way analysis of variance using SPSS 19.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Suppression of endogenous H2S inhibits the viability and proliferation of human EC cells
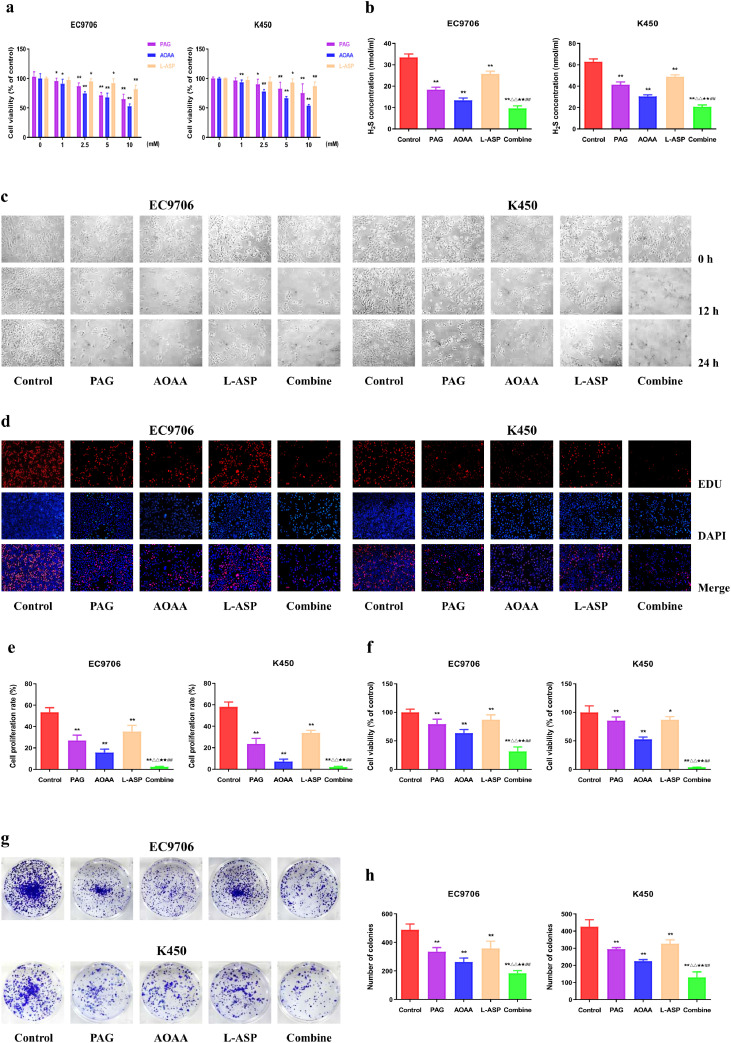
To assess the potential involvement of endogenous H2S in the development and progression of EC, the EC cells were treated with 1-10 mM PAG, AOAA, and L-Asp. As a result, the viability of EC9706 and K450 cells was dose-dependently reduced, suggesting that suppression of CSE, CBS, and 3-MST could inhibit the growth of human EC cells (Fig. 1a). Thus, in the next experiments we selected the concentration of 10 mM PAG, AOAA, and L-Asp as the ideal inhibition concentration. Furthermore, the decrease of H2S level was conformed after pretreated with the three H2S-producing enzymes inhibitors (Fig. 1b). The proliferative capacity of EC9706 and K450 cells was declined in PAG, AOAA, and L-Asp group when compared to the control group while the combine group exhibited the highest inhibitory effects (Fig. 1c–f). There were similar trends in the clonogenicity ability of EC9706 and K450 cells (Fig. 1g, h). Overall, these data imply that suppression of endogenous H2S level may inhibit the viability and proliferation of human EC cells.
Fig. 1.
Effects of PAG, AOAA, and L-Asp on the viability and proliferation of human EC cells. (a) The MTS assay was used to determine the percentage of viable cells after treated with different concentration of PAG, AOAA, L-Asp alone or in combination. The cell viability of each group without PAG, AOAA, and L-Asp treatment was normalized as 100% and considered to be the control group. (b) The levels of H2S after administration alone (10 mM PAG, 10mM AOAA, or 10mM L-Asp) or in combination were detected in EC cells. (c) Phase-contrast microscopy was used to observe the morphology of the indicated cells; original magnification × 200. (d) DNA replication activities of EC cells in each group were examined by EdU assay; original magnification × 200. (e) The proliferation rate of each group was analyzed. (f) The MTS assay was used to determine the percentage of viable cells. The cell viability of the control group was normalized as 100%. (g) The clonogenic capacity was determined in EC cells. (h) The number of colonies was calculated. The experiments were performed in triplicates. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 compared with the control group; △△P < 0.01 compared with PAG group; ★★P < 0.01 compared with AOAA group; ##P < 0.01 compared with L-Asp group.
The endogenous H2S mediates the migration and invasion of human EC cells
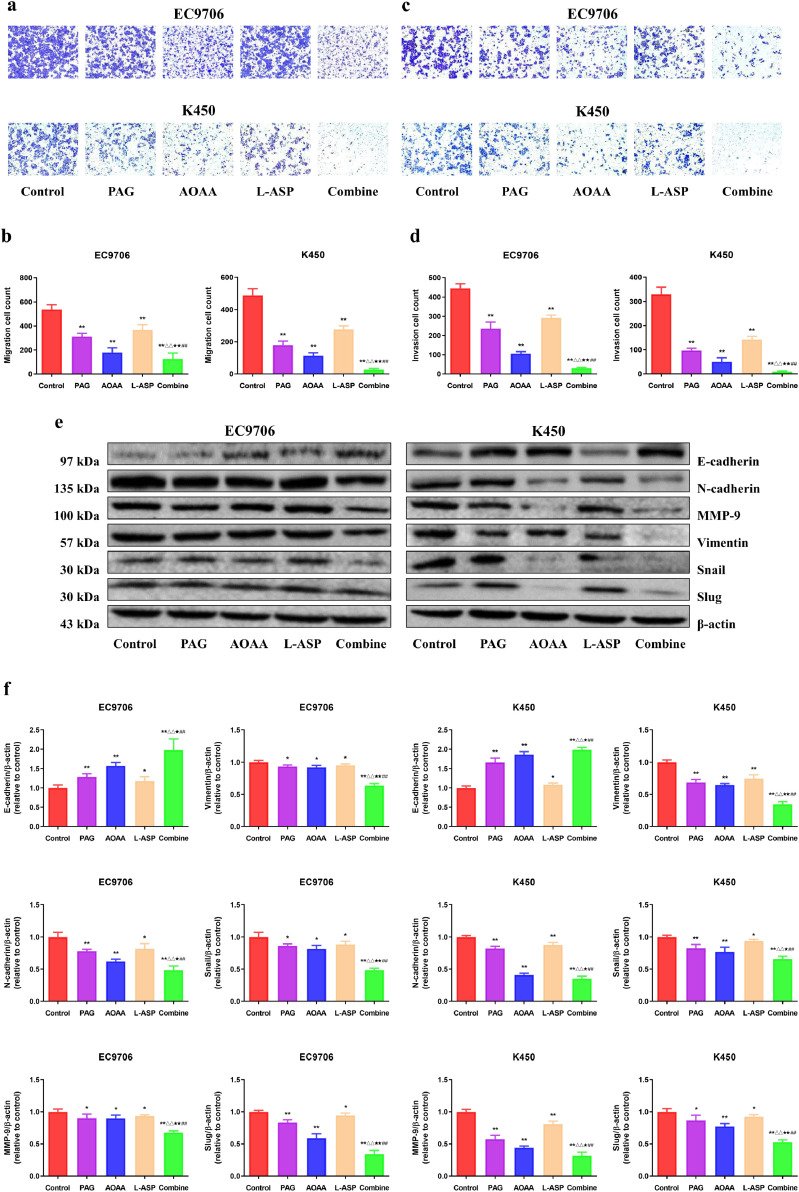
Next, we assessed the influence of PAG, AOAA, and L-Asp on the migration and invasion of EC cells. Compared with the control group, the migration and invasion were decreased in PAG, AOAA, and L-Asp group, whereas the combine group showed the most striking effect (Fig. 2a–d). In addition, the results of the wound healing and colony formation exhibited the similar trends (Fig. S1). Moreover, the expression levels of epithelial-mesenchymal transition (EMT)-related proteins were determined by western blotting. The level of E-cadherin exhibited an elevation trend and the expressions of N-cadherin, MMP-9, Vimentin, snail, and slug were decreased compared with the control group. (Fig. 2e, f). These findings support the conclusion that endogenous H2S exerts an important effect on the suppression of EC cell migration and invasion.
Fig. 2.
Effects of PAG, AOAA, and L-Asp on the migration and invasion of human EC cells. (a) Transwell assay was performed to assess the migration of EC cells; original magnification × 200. (b) The number of the migrated cells was calculated. (c) Transwell assay was performed to assess the invasion of EC cells; original magnification × 200. (d) The number of the invasive cells was calculated. (e) Western blotting analysis for the expression of E-cadherin, N-cadherin, MMP-9, Vimentin, snail, and slug in EC9706 and K450 cells. β-actin was used as the loading control. (f) The relative intensity of E-cadherin, N-cadherin, MMP-9, Vimentin, snail, and slug by densitometry scanning are shown, normalized to the corresponding β-actin level. The experiments were performed in triplicates. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 compared with the control group; △△P <0.01 compared with PAG group; ★P < 0.05, ★★P < 0.01 compared with AOAA group; ##P < 0.01 compared with L-Asp group.
Suppression of endogenous H2S induces apoptosis via mitogen-activated protein kinase (MAPK) pathway in human EC cells
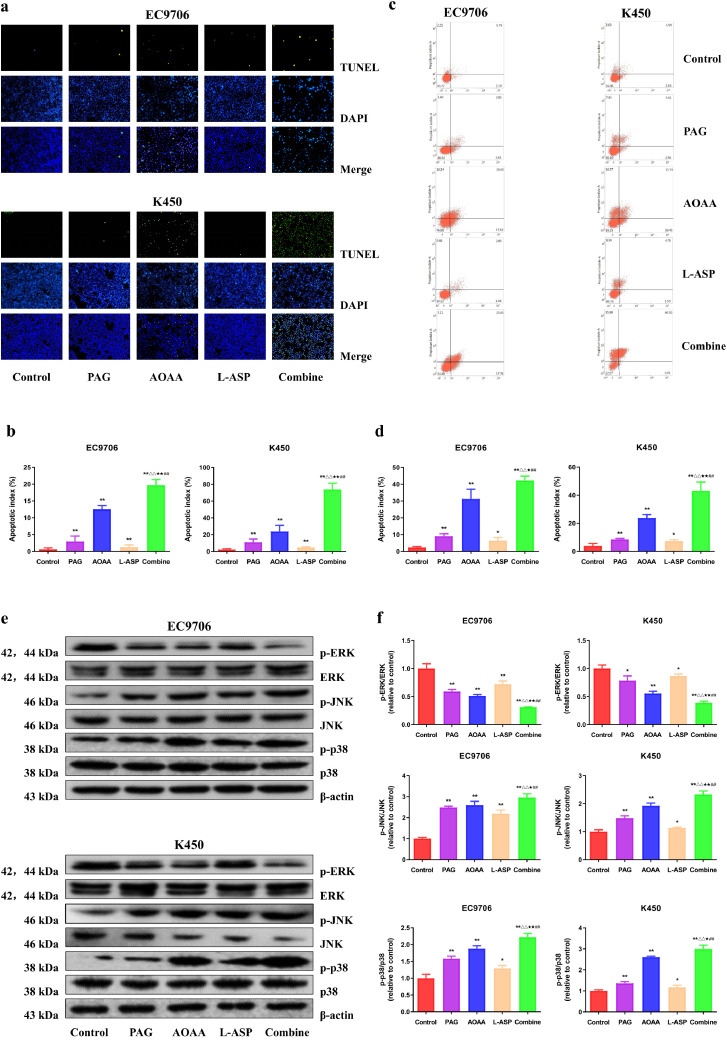
To investigate the potential role of endogenous H2S in the regulation of apoptosis, we performed the TUNEL and AnnexinV/PI assays on EC9706 and K450 cells. As shown in Fig. 3a–d, in comparison with the control group, the apoptotic index was higher in PAG, AOAA, and L-Asp group while the combination group showed the highest apoptotic index. The levels of Cyt C, Bax, Bad, cleaved caspase-3, 9, PARP in human EC cells exhibited similar trends. In addition, the reverse trends were observed in the expressions of Bcl-2 and Bcl-xl (Fig. S2). MAPKs, which include ERK, JNK, and p38, play key roles in cellular apoptosis. Both JNK and p38 were activated whereas ERK was inactivated by administration alone (PAG, AOAA, and L-Asp group) or in combination; yet the effects were more pronounced by the combined administration (Fig. 3e, f). In sum, these data suggest that suppression of endogenous H2S can induce apoptosis via MAPK pathway in human EC cells.
Fig. 3.
Effects of PAG, AOAA, and L-Asp on the apoptosis of human EC cells. (a) The apoptotic level was measured by TUNEL staining; original magnification × 200. (b) The apoptotic index was calculated. (c) The apoptotic level was detected by flow cytometry. (d) The result of flow cytometry was determined. (e) Western blotting analysis for the expression of p-ERK1/2, ERK1/2, p-JNK, JNK, p-p38, and p38 in EC9706 and K450 cells. β-actin was used as the loading control. (f) The densitometry analyses of p-ERK1/2, ERK1/2, p-JNK, JNK, p-p38, and p38 were performed, normalized to the corresponding β-actin level. The experiments were performed in triplicates. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 compared with the control group; △△P < 0.01 compared with PAG group; ★P < 0.05, ★★P < 0.01 compared with AOAA group; ##P < 0.01 compared with L-Asp group.
Suppression of endogenous H2S activates pyroptosis through ROS-nuclear factor-κB (NF-κB) signaling pathway in human EC cells
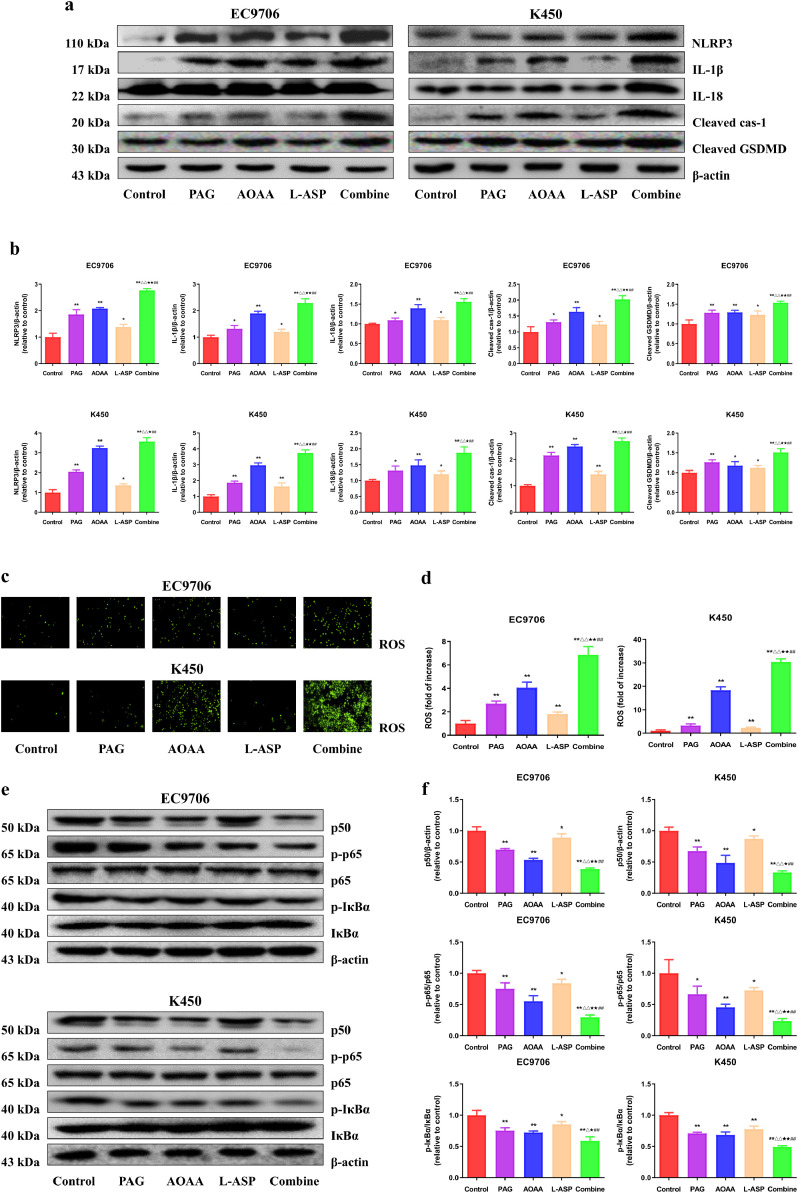
To clarify the effect of impairing endogenous H2S formation on pyroptosis, we firstly detected the expression levels of pyroptosis-related proteins. As shown in Fig. 4a, b, compared with the control group, the levels of NLRP3, cleaved GSDMD, IL-1β, cleaved cas-1 and IL-18 were markedly up-regulated in PAG, AOAA, L-Asp, and combination group. Additionally, the combination group had significantly higher response than the three monotherapy groups. It has been proved that ROS is one of the key factors which can trigger the inflammatory response caused by the NLRP3 inflammasome [22]. Consistently, in our study, the ROS levels were significantly upregulated in PAG, AOAA, L-Asp, and combination group compared with the control group (Fig. 4c, d). Meanwhile, the results revealed that either administration alone (PAG, AOAA, and L-Asp group) or in combination had inhibitory effects on the expression levels of p50, p-p65, and p-IκBα (Fig. 4e, f), suggesting that suppression of endogenous H2S could develop anti-cancer effect at least partly through ROS- NF-κB signaling pathway.
Fig. 4.
Effects of PAG, AOAA, and L-Asp on the pyroptosis of human EC cells. (a) Western blotting analysis for the expression of NLRP3, IL-1β, IL-18, cleaved GSDMD, and cleaved cas-1 in EC9706 and K450 cells. (b) The densitometry analyses of NLRP3, IL-1β, IL-18, cleaved GSDMD, and cleaved cas-1 in EC9706 and K450 cells, normalized to the corresponding β-actin level. (c, d) Representative images and quantification of the intracellular ROS production was detected using the fluorescent probes DHE; original magnification × 100. (e) Western blotting analysis for the expression of p50, p-p65, p65, p-IκBα and IκBα in EC9706 and K450 cells. (f) The densitometry analyses of p50, p-p65, p65, p-IκBα and IκBα in EC9706 and K450 cells, normalized to the corresponding β-actin level. The experiments were performed in triplicates. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 compared with the control group; △P < 0.05, △△P < 0.01 compared with PAG group; ★P < 0.05, ★★P < 0.01 compared with AOAA group; ##P < 0.01 compared with L-Asp group.
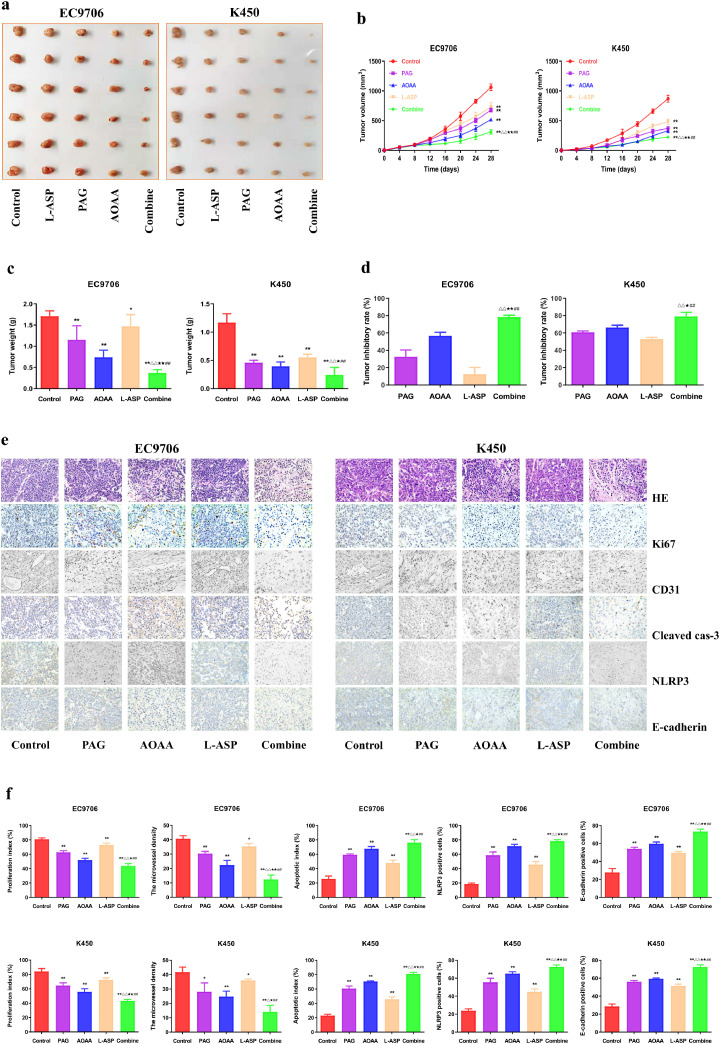
Suppression of endogenous H2S inhibits the angiogenesis and growth of human EC xenograft tumors
EC9706 and K450 cells were successfully adopted to establish the nude mouse tumor models. Compared with the control group, the tumor volumes and weights were dramatically decreased in PAG, AOAA, and L-Asp group. In addition, the tumor volume and tumor weight were lower but the tumor inhibitory rate was higher in the combination group than those in PAG, AOAA, and L-Asp group (Fig. 5a–d). As shown in Fig. 5e, f, HE staining results revealed that suppression of endogenous H2S was negatively correlation to EC progression. Furthermore, inhibition of tumor growth was evidenced by staining for a decrease in Ki67 and CD31 staining, as well as the increase in cleaved cas-3, NLRP3, and E-cadherin staining.
Fig. 5.
Effects of PAG, AOAA, and L-Asp on the growth of human EC xenograft tumors in nude mice. (a) Representative xenografts dissected from different groups of nude mice were shown. (b) The tumor volumes of human EC xenograft tumors were measured every 4 days. (c, d) The tumors were weighed and the inhibition rates of tumor growth were calculated. (e) Representative photographs of HE, Ki67, CD31, Cleaved cas-3, NLRP3, and E-cadherin staining in human EC xenograft tumors; original magnification × 200. (f) The proliferation rate, MVD, apoptotic index, NLRP3 and E-cadherin positive cells were calculated. Data are presented as mean ± SEM (n=6). *P < 0.05, **P < 0.01 compared with the control group; △P < 0.05, △△P < 0.01 compared with PAG group; ★P < 0.05, ★★P < 0.01 compared with AOAA group; ##P < 0.01 compared with L-Asp group.
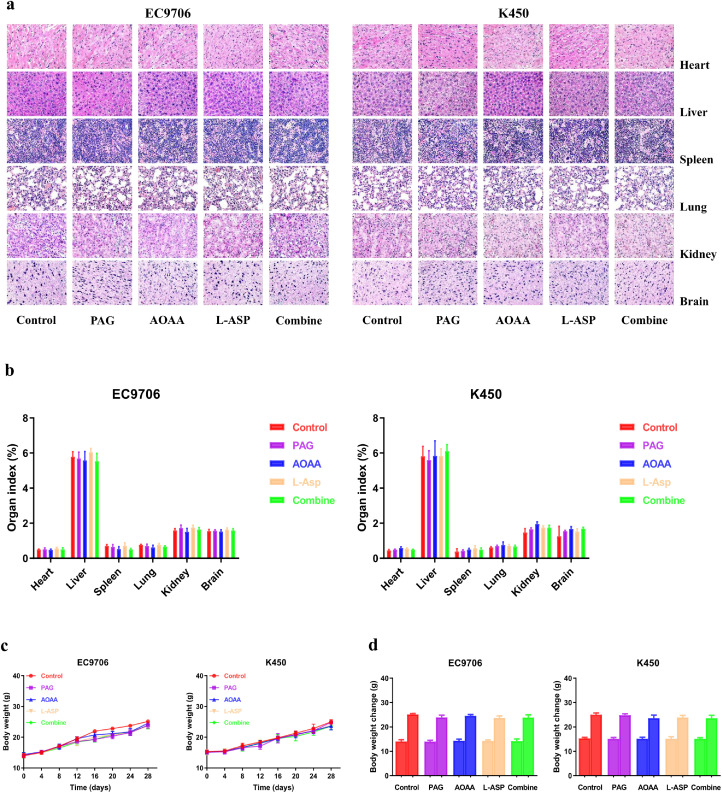
Suppression of endogenous H2S has no significant toxicity
Further, we determined the effects of suppression of endogenous H2S on the histology of other organs. We did not observe abnormalities in other organs, such as heart, liver, spleen, lung, kidney, and brain in PAG, AOAA, L-Asp and the combined group-treated mice, suggesting no adverse effects (Fig. 6a, b). Moreover, there was no obvious difference in body weight and organ index among groups (Fig. 6c, d). Taken together, the data demonstrate that inhibition of endogenous H2S can dramatically suppress the angiogenesis and growth of human EC xenograft tumors without significant toxicity.
Fig. 6.
Effects of PAG, AOAA, and L-Asp on the toxicity in nude mice. (a) Representative figures of the heart, liver, spleen, lung, kidney, and brain in nude mice. (b) The organ index was calculated. (c) The body weight change curve of each group during the experiment. (d) The body weight of each group on the first day (day 0) and the last day (day 28). Data are presented as mean ± SEM (n=6).
Discussion
Endogenous H2S is an emerging novel cancer modulator in different pathological conditions and a possible diagnostic and prognostic marker in cancer. Recently, it was found that H2S-synthesizing enzymes (CBS, CSE, 3-MST) were dramatically altered in tumor tissues, indicating a potential role in the process of carcinogenesis [23]. However, the function of endogenous H2S in EC remains unclear. Thereby, in current study, we observed the effects of synthesizing enzymes inhibitors of H2S: PAG (CBS inhibitor), AOAA (CSE inhibitor), and L-Asp (3-MST inhibitor) on EC9706 and K450 EC cells. The findings demonstrated that the proliferation, viability, migration, and invasion of EC9706 and K450 cells were reduced in PAG, AOAA, and L-Asp group when compared to the control group. Whereas the combine group showed higher inhibitory effects. Many studies have revealed that EMT plays a decisive role in tumor progression and metastasis [24], [25], [26]. In this study, we observed that synthesizing enzymes inhibitors of H2S increased the expression levels of epithelial markers (such as E-cadherin) and decreased the expression levels of mesenchymal markers (such as N-cadherin, Vimentin, Snail, Slug, MMP-9) in EC cells. These findings suggest that synthesizing enzymes inhibitors of H2S negatively regulates the EMT process and thereby significantly weakens tumor cell migration and invasion in vitro. So far, it has been revealed that H2S can inhibit the EMT process through decreased phospho-p38 expression [27]. Therefore, the antimetastatic effects of the three inhibitors on EC cells mediated by inhibition of the EMT process in this study are consistent with the previously reported works. In sum, these data suggest that inhibition of endogenous H2S could suppress the viability, proliferation, migration, and invasion of human EC cells.
Apoptosis, type Ⅰ cell death, is an important process to maintain tissue and cell homeostasis [28]. The intrinsic mitochondrial pathway and extrinsic stimulation of death receptors are two apoptotic signaling pathways [29], [30], [31]. The activation of Bax and caspase could cause morphological changes and mitochondrial dysfunction, thus promoting mitochondrial apoptosis. Our findings revealed that administration alone (PAG, AOAA, and L-Asp group) or in combination had exhibited higher Bad/Bcl-xl and Bax/Bcl-2 ratios, as well as Cyt C, cleaved caspase-3, cleaved caspase-9 and cleaved PARP levels than the control group, indicating that inhibition of endogenous H2S induced apoptosis in human EC cells. Three most important MAPK family members that are associated with cell apoptosis are ERK, JNK, and p38 [32]. The activation of ERK is generally a survival signal, whereas the activation of p38/JNK is a type of apoptotic signal pathway [33]. The effects of H2S-produced enzymes on apoptosis were further determined by assessing the roles of ERK, JNK and p38 in EC9706 and K450 cells. The data revealed that inhibition of endogenous H2S could induce apoptosis via MAPK pathway in human EC cells.
Pyroptosis is a non-traditional type of programmed cell death characterized by the pore formation on the plasma membrane, which causes cell enlargement and plasma membrane disruption [34], [35], [36]. Therefore, pyroptosis induction might be a novel strategy for treating cancer [37,38]. The in vitro and in vivo results revealed that inhibition of endogenous H2S could induce EC cell pyroptotic death evidenced by the elevated levels of NLRP3, cleaved GSDMD, IL-1β, cleaved cas-1, and IL-18. The similar trend was observed in the levels of ROS. Many studies have indicated that low levels of ROS are essential for a range of physiological functions, such as signal transduction and cell growth [[39], [40]]. However, ROS over-production could cause redox imbalance and oxidative stress, thus affecting a number of cellular functions, such as apoptosis, necroptosis and pyroptosis [25,35,36]. It has been shown that in colon cancer cells AOAA can increase the intracellular ROS induced by oxaliplatin [41]. Similarly, our results indicated that PAG, AOAA, and L-Asp increased ROS levels. Alternatively, H2S has been demonstrated to be involved in many inflammatory states including the NF-κB pathway [42,43]. NF-κB, an inflammatory oncogenic pathway, plays key roles in angiogenesis and proliferation and is constitutively activated in a number of human cancers [44], [45], [46]. Of note, NF-κB activation contributes to the development and progression of esophageal squamous cell carcinoma [47]. Our results demonstrated that suppression of H2S producing enzymes dramatically decreased the expression levels of p-IκBα, p-p65, and p50, thereby inhibiting NF-κB signaling pathway in EC. The results indicate that PAG, AOAA, and L-Asp can promote pyroptosis through ROS-NF-κB signaling pathway in human EC cells.
We further examined the effect of endogenous H2S on the growth of human EC xenograft tumors. The results suggested that PAG, AOAA, and L-Asp suppressed EC xenograft tumor growth and the combination group exhibited more potent inhibitory effects on tumor growth. Furthermore, there were no differences in organ and body weight, as well in the morphologies of heart, liver, spleen, lung, kidney, and brain, indicating that administration was not associated with any significant toxicity. The extraordinary tumor-inhibiting properties of PAG, AOAA, and L-Asp were further confirmed by IHC analysis for proliferation (Ki67), angiogenesis (CD31), apoptosis (cleaved cas-3), pyroptosis (NLRP3), and EMT (E-cadherin) of xenografted tumors from control, PAG, AOAA, L-Asp, and the combination group mice. Taken together, our data demonstrated that inhibition of endogenous H2S could suppress the angiogenesis and growth of human EC xenograft tumors without significant toxicity.
All small-molecule inhibitors suffer from possible lack of specificity. Of note is that in our study PAG, AOAA, L-Asp, and the combination group had not produced any effect in matched healthy control mice, suggesting the inhibitors has possible specific effects under the conditions. Nevertheless, the utilization of pharmacological agents (in general, and also in particular in the current set of experiment) may be complicated by non-specific (“off-target”) effects. Therefore, further studies remain to be conducted in the future to further validate the findings. To investigate the role of CBS, CSE or 3-MST in EC cancer development and progression, different strategies such as gene silencing or knockout using siRNA, shRNA, or CRISPR can be employed.
Conclusions
Our results indicated that inhibition of endogenous H2S production could inhibit the growth of human EC cells via promoting apoptosis and pyroptosis both in vitro and in vivo. Endogenous H2S might be a promising therapeutic target in human EC cells. Novel inhibitors for H2S-generating enzymes can be designed and developed for EC treatment.
Ethics approval and consent to participate
Animal studies were approved by the Committee of Medical Ethics and Welfare for Experimental Animals of Henan University School of Medicine (HUSOM-2019-167). All methods were performed in accordance with the relevant guidelines and regulations and this study is reported in accordance with ARRIVE guidelines.
Authors’ contributions
D.D.W., X.Y.J. and H.X.L. wrote the main manuscript text. H.G.W., D.W., M.S., A.A., M.R.J., Y.X.Z., C.B.C., H.W.Q., H.J.C., T.L. and S.J.H. prepared Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 and supplementary Figs. 1, 2. All authors read and approved the final manuscript.
Consent to publish
Confirm.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest related to this work.
Acknowledgments
Acknowledgement
None.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81802718, U1504817), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), the Natural Science Foundation of Education Department of Henan Province, China (No. 21A310003), the Foundation of Science & Technology Department of Henan Province, China (Nos. 222102310490, 222102310495), the Foundation of Science & Technology Department of Kaifeng City, Henan Province, China (No. 2203001), and the Natural Science Foundation of Education Department of Henan Province, China (No. 23A310012).
Footnotes
Supplementary material associated with this article can be found, in the online version, doi:10.1016/j.tranon.2023.101770.
Contributor Information
Hong-Xia Liu, Email: liuhx@henu.edu.cn.
Xin-Ying Ji, Email: 10190096@vip.henu.edu.cn.
Dong-Dong Wu, Email: 10190117@vip.henu.edu.cn.
Appendix. Supplementary materials
Data availability
All data generated or analyzed in this study were included in this article.
References
- 1.Pennathur A., Gibson M.K., Jobe B.A., Luketich J.D. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Z.Y., Yang P.L., Li R.Y., Liu L.X., Xu X.E., Liao L.D., et al. STAT3beta disrupted mitochondrial electron transport chain enhances chemosensitivity by inducing pyroptosis in esophageal squamous cell carcinoma. Cancer Lett. 2021;522:171–183. doi: 10.1016/j.canlet.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Ghoneum M.H., Badr E.N., Abdel F.S., Pan D., Tolentino L. Hydroferrate fluid, MRN-100, provides protection against chemical-induced gastric and esophageal cancer in Wistar rats. Int. J. Biol. Sci. 2015;11:295–303. doi: 10.7150/ijbs.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Tang H., Fang Y., Tan L., Yin J., Shen Y., et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. Jama Surg. 2021;156:444–451. doi: 10.1001/jamasurg.2021.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R. Hydrogen sulfide: the third gasotransmitter in biology and medicine. Antioxid. Redox. Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 6.Wu D., Wang J., Li H., Xue M., Ji A., Li Y. Role of hydrogen sulfide in ischemia-reperfusion injury. Oxid. Med. Cell Longev. 2015;2015 doi: 10.1155/2015/186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowicka E., Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol. Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 8.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 9.Wu D.D., Wang D.Y., Li H.M., Guo J.C., Duan S.F., Ji X.Y. Hydrogen sulfide as a novel regulatory factor in liver health and disease. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/3831713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo C., Coletta C., Chao C., Modis K., Szczesny B., Papapetropoulos A., et al. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen S., Kawahara B., Gupta D., Tsai R., Khachatryan M., Roy-Chowdhuri S., et al. Role of cystathionine beta-synthase in human breast cancer. Free Radic. Biol. Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Pan Y., Ye S., Yuan D., Zhang J., Bai Y., Shao C. Hydrogen sulfide (H2S)/cystathionine gamma-lyase (CSE) pathway contributes to the proliferation of hepatoma cells. Mutat. Res. 2014:763–764. doi: 10.1016/j.mrfmmm.2014.03.002. 10-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y.H., Huang J.T., Chen W.L., Wang R.H., Kao M.C., Pan Y.R., et al. Dysregulation of cystathionine gamma-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20:e45986. doi: 10.15252/embr.201845986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin P., Zhao C., Li Z., Mei C., Yao W., Liu Y., et al. Sp1 is involved in regulation of cystathionine gamma-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal. 2012;24:1229–1240. doi: 10.1016/j.cellsig.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Augsburger F., Randi E.B., Jendly M., Ascencao K., Dilek N., Szabo C. Role of 3-mercaptopyruvate sulfurtransferase in the regulation of proliferation, migration, and bioenergetics in murine colon cancer cells. Biomolecules. 2020;10 doi: 10.3390/biom10030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youness R.A., Gad A.Z., Sanber K., Ahn Y.J., Lee G.J., Khallaf E., et al. Targeting hydrogen sulphide signaling in breast cancer. J. Adv. Res. 2021;27:177–190. doi: 10.1016/j.jare.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augsburger F., Szabo C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2020;154 doi: 10.1016/j.phrs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Fan K., Li N., Qi J., Yin P., Zhao C., Wang L., et al. Wnt/beta-catenin signaling induces the transcription of cystathionine-gamma-lyase, a stimulator of tumor in colon cancer. Cell Signal. 2014;26:2801–2808. doi: 10.1016/j.cellsig.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Pei Y., Wu B., Cao Q., Wu L., Yang G. Hydrogen sulfide mediates the anti-survival effect of sulforaphane on human prostate cancer cells. Toxicol. Appl. Pharmacol. 2011;257:420–428. doi: 10.1016/j.taap.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Wu D., Li M., Tian W., Wang S., Cui L., Li H., et al. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci. Rep. 2017;7:5134. doi: 10.1038/s41598-017-05457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu D.D., Gao Y.R., Li T., Wang D.Y., Lu D., Liu S.Y., et al. PEST-containing nuclear protein mediates the proliferation, migration, and invasion of human neuroblastoma cells through MAPK and PI3K/AKT/mTOR signaling pathways. BMC Cancer. 2018;18:499. doi: 10.1186/s12885-018-4391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv M., Li Y., Ji M.H., Zhuang M., Tang J.H. Inhibition of invasion and epithelial-mesenchymal transition of human breast cancer cells by hydrogen sulfide through decreased phospho-p38 expression. Mol. Med. Rep. 2014;10:341–346. doi: 10.3892/mmr.2014.2161. [DOI] [PubMed] [Google Scholar]
- 25.Zheng F., Han J., Lu H., Cui C., Yang J., Cui Q., et al. Cystathionine beta synthase-hydrogen sulfide system in paraventricular nucleus reduced high fatty diet induced obesity and insulin resistance by brain-adipose axis. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3281–3291. doi: 10.1016/j.bbadis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Czikora A., Erdelyi K., Ditroi T., Szanto N., Juranyi E.P., Szanyi S., et al. Cystathionine beta-synthase overexpression drives metastatic dissemination in pancreatic ductal adenocarcinoma via inducing epithelial-to-mesenchymal transformation of cancer cells. Redox. Biol. 2022;57 doi: 10.1016/j.redox.2022.102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M., Yan J., Cao X., Hua P., Li Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1alpha activation. Biochem. Pharmacol. 2020;172 doi: 10.1016/j.bcp.2019.113775. [DOI] [PubMed] [Google Scholar]
- 28.Li W., Zhang L., Guo B., Deng J., Wu S., Li F., et al. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFkappaB/c-Myc signaling in female esophageal carcinoma. Mol. Cancer. 2019;18:22. doi: 10.1186/s12943-019-0949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G.Y., Lu D., Duan S.F., Gao Y.R., Liu S.Y., Hong Y., et al. Hydrogen sulfide alleviates lipopolysaccharide-induced diaphragm dysfunction in rats by reducing apoptosis and inflammation through ROS/MAPK and TLR4/NF-kappaB signaling pathways. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/9647809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Q., Yang B., Han J.G., Zhang M.M., Liu W., Zhang X., et al. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Lett. 2019;455:60–72. doi: 10.1016/j.canlet.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Green D.R., Llambi F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak A.W., Yoon G., Lee M.H., Cho S.S., Shim J.H., Chae JI. Picropodophyllotoxin, an epimer of podophyllotoxin, causes apoptosis of human esophageal squamous cell carcinoma cells through ROS-mediated JNK/P38 MAPK pathways. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21134640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu J., Soroka D.N., Zhu Y., Sang S. Induction of apoptosis and cell-cycle arrest in human colon-cancer cells by whole-grain alkylresorcinols via activation of the p53 pathway. J. Agric. Food Chem. 2018;66:11935–11942. doi: 10.1021/acs.jafc.8b04442. [DOI] [PubMed] [Google Scholar]
- 34.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fink S.L., Cookson B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen I., Miao E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia X., Wang X., Cheng Z., Qin W., Lei L., Jiang J., et al. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death. Dis. 2019;10:650. doi: 10.1038/s41419-019-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vande W.L., Lamkanfi M. Pyroptosis. Curr. Biol. 2016;26:R568–R572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Wu D., Zhong P., Wang Y., Zhang Q., Li J., Liu Z., et al. Hydrogen Sulfide attenuates high-fat diet-induced non-alcoholic fatty liver disease by inhibiting apoptosis and promoting autophagy via reactive oxygen species/phosphatidylinositol 3-kinase/AKT/Mammalian target of rapamycin signaling pathway. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.585860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan L., Wei W., Zheng Y., Niu L., Chen X., Huang D., et al. Deferoxamine suppresses esophageal squamous cell carcinoma cell growth via ERK1/2 mediated mitochondrial dysfunction. Cancer Lett. 2018;432:132–143. doi: 10.1016/j.canlet.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Yue T., Zuo S., Bu D., Zhu J., Chen S., Ma Y., et al. Aminooxyacetic acid (AOAA) sensitizes colon cancer cells to oxaliplatin via exaggerating apoptosis induced by ROS. J. Cancer. 2020;11:1828–1838. doi: 10.7150/jca.35375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo C., Liang F., Shah M.W., Yan X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014;725:70–78. doi: 10.1016/j.ejphar.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Zhi L., Ang A.D., Zhang H., Moore P.K., Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J. Leukoc. Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 44.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 45.Lin C., Song L., Gong H., Liu A., Lin X., Wu J., et al. Nkx2-8 downregulation promotes angiogenesis and activates NF-kappaB in esophageal cancer. Cancer Res. 2013;73:3638–3648. doi: 10.1158/0008-5472.CAN-12-4028. [DOI] [PubMed] [Google Scholar]
- 46.Lin C., Song L., Liu A., Gong H., Lin X., Wu J., et al. Overexpression of AKIP1 promotes angiogenesis and lymphangiogenesis in human esophageal squamous cell carcinoma. Oncogene. 2015;34:384–393. doi: 10.1038/onc.2013.559. [DOI] [PubMed] [Google Scholar]
- 47.Gong H., Song L., Lin C., Liu A., Lin X., Wu J., et al. Downregulation of miR-138 sustains NF-kappaB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin. Cancer Res. 2013;19:1083–1093. doi: 10.1158/1078-0432.CCR-12-3169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study were included in this article.