Abstract
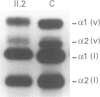
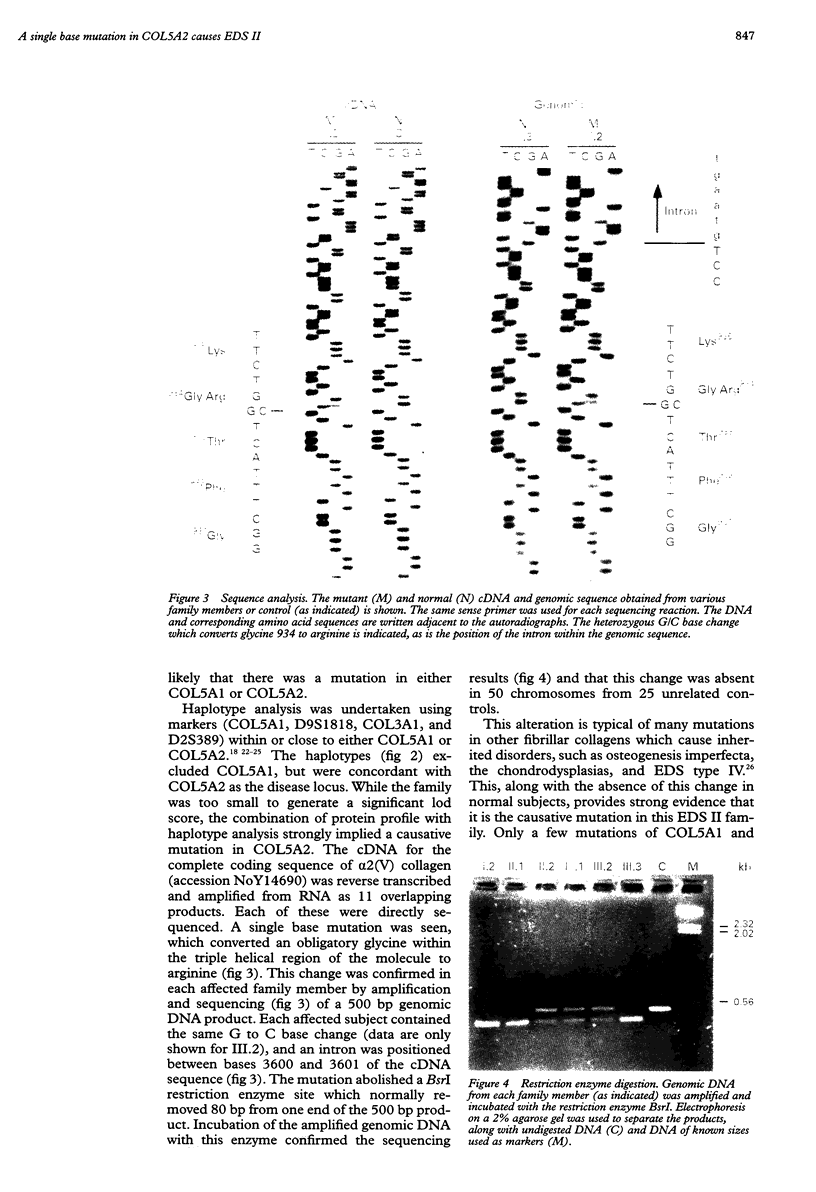
Ehlers-Danlos syndrome (EDS) is a heterogeneous group of connective tissue disorders. Recently mutations have been found in the genes for type V collagen in a small number of people with the most common forms of EDS, types I and II. Here we characterise a COL5A2 mutation in an EDS II family. Cultured dermal fibroblasts obtained from an affected subject synthesised abnormal type V collagen. Haplotype analysis excluded COL5A1 but was concordant with COL5A2 as the disease locus. The entire open reading frame of the COL5A2 cDNA was directly sequenced and a single base mutation detected. It substituted a glycine residue within the triple helical domain (G934R) of alpha2(V) collagen, typical of the dominant negative changes in other collagens, which cause various other inherited connective tissue disorders. All three affected family members possessed the single base change, which was absent in 50 normal chromosomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrikopoulos K., Liu X., Keene D. R., Jaenisch R., Ramirez F. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995 Jan;9(1):31–36. doi: 10.1038/ng0195-31. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Fitch J. M., Babiarz J. P., Doane K. J., Linsenmayer T. F. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990 Apr;95(Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Broek D. L., Madri J., Eikenberry E. F., Brodsky B. Characterization of the tissue form of type V collagen from chick bone. J Biol Chem. 1985 Jan 10;260(1):555–562. [PubMed] [Google Scholar]
- Burch G. H., Gong Y., Liu W., Dettman R. W., Curry C. J., Smith L., Miller W. L., Bristow J. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997 Sep;17(1):104–108. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., El Adli F. A., Kaitila I. I., Hollister D. W. Fetal membrane collagens: identification of two new collagen alpha chains. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2579–2583. doi: 10.1073/pnas.73.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R., McGinniss M. J., Kasch L. M., Tsipouras P., Antonarakis S. E. Physical mapping by PFGE localizes the COL3A1 and COL5A2 genes to a 35-kb region on human chromosome 2. Genomics. 1990 Oct;8(2):407–410. doi: 10.1016/0888-7543(90)90302-b. [DOI] [PubMed] [Google Scholar]
- Danielson K. G., Baribault H., Holmes D. F., Graham H., Kadler K. E., Iozzo R. V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997 Feb 10;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Elstow S. F., Weiss J. B. Extraction, isolation and characterization of neutral salt soluble type V collagen from fetal calf skin. Coll Relat Res. 1983 May;3(3):181–193. doi: 10.1016/s0174-173x(83)80002-8. [DOI] [PubMed] [Google Scholar]
- Fichard A., Kleman J. P., Ruggiero F. Another look at collagen V and XI molecules. Matrix Biol. 1995 Jul;14(7):515–531. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- Garofalo S., Metsäranta M., Ellard J., Smith C., Horton W., Vuorio E., de Crombrugghe B. Assembly of cartilage collagen fibrils is disrupted by overexpression of normal type II collagen in transgenic mice. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3825–3829. doi: 10.1073/pnas.90.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D. S., Northrup H., Au K. S., McAllister K. A., Francomano C. A., Wenstrup R. J., Marchuk D. A., Kwiatkowski D. J. COL5A1: fine genetic mapping and exclusion as candidate gene in families with nail-patella syndrome, tuberous sclerosis 1, hereditary hemorrhagic telangiectasia, and Ehlers-Danlos Syndrome type II. Genomics. 1995 Feb 10;25(3):737–739. doi: 10.1016/0888-7543(95)80021-d. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9(4):300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Li Y., Lacerda D. A., Warman M. L., Beier D. R., Yoshioka H., Ninomiya Y., Oxford J. T., Morris N. P., Andrikopoulos K., Ramirez F. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995 Feb 10;80(3):423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- Linsenmayer T. F., Gibney E., Igoe F., Gordon M. K., Fitch J. M., Fessler L. I., Birk D. E. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993 Jun;121(5):1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant J. K., Hahn R. A., Linsenmayer T. F., Birk D. E. Reduction of type V collagen using a dominant-negative strategy alters the regulation of fibrillogenesis and results in the loss of corneal-specific fibril morphology. J Cell Biol. 1996 Dec;135(5):1415–1426. doi: 10.1083/jcb.135.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Brewton R. G., Mayne P. M., Baker J. R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993 May 5;268(13):9381–9386. [PubMed] [Google Scholar]
- McLaughlin J. S., Linsenmayer T. F., Birk D. E. Type V collagen synthesis and deposition by chicken embryo corneal fibroblasts in vitro. J Cell Sci. 1989 Oct;94(Pt 2):371–379. doi: 10.1242/jcs.94.2.371. [DOI] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalickova K., Susic M., Willing M. C., Wenstrup R. J., Cole W. G. Mutations of the alpha2(V) chain of type V collagen impair matrix assembly and produce ehlers-danlos syndrome type I. Hum Mol Genet. 1998 Feb;7(2):249–255. doi: 10.1093/hmg/7.2.249. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Oliver J. E., McCarron S., Harrison J. B., Greenspan D. S., Pope F. M. An exon skipping mutation of a type V collagen gene (COL5A1) in Ehlers-Danlos syndrome. J Med Genet. 1996 Nov;33(11):940–946. doi: 10.1136/jmg.33.11.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. J., Yates J. R., Williams R., Payne S. J., Pope F. M., Scott J. D., Snead M. P. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in alpha 1 (XI) collagen. Hum Mol Genet. 1996 Sep;5(9):1339–1343. doi: 10.1093/hmg/5.9.1339. [DOI] [PubMed] [Google Scholar]
- Robertson I. Keratoconus and the Ehlers-Danlos syndrome: a new aspect of keratoconus. Med J Aust. 1975 May 3;1(18):571–573. doi: 10.5694/j.1326-5377.1975.tb111590.x. [DOI] [PubMed] [Google Scholar]
- Schuler G. D., Boguski M. S., Stewart E. A., Stein L. D., Gyapay G., Rice K., White R. E., Rodriguez-Tomé P., Aggarwal A., Bajorek E. A gene map of the human genome. Science. 1996 Oct 25;274(5287):540–546. [PubMed] [Google Scholar]
- Tseng S. C., Smuckler D., Stern R. Comparison of collagen types in adult and fetal bovine corneas. J Biol Chem. 1982 Mar 10;257(5):2627–2633. [PubMed] [Google Scholar]
- Wenstrup R. J., Langland G. T., Willing M. C., D'Souza V. N., Cole W. G. A splice-junction mutation in the region of COL5A1 that codes for the carboxyl propeptide of pro alpha 1(V) chains results in the gravis form of the Ehlers-Danlos syndrome (type I). Hum Mol Genet. 1996 Nov;5(11):1733–1736. doi: 10.1093/hmg/5.11.1733. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]