Abstract
Background
Over 70% of the patients with hepatocellular carcinoma (HCC) are diagnosed at an advanced stage and lose the opportunity for radical surgery. Combination therapy of tyrosine kinase inhibitors (TKIs) and anti-programmed cell death protein-1 (PD-1) antibodies has achieved a high tumor response rate in both the first-line and second-line treatment of advanced HCC. However, few studies have prospectively evaluated whether TKIs plus anti-PD-1 antibodies could convert unresectable intermediate-advanced HCC into resectable disease.
Methods
This single-arm, phase II study enrolled systemic therapy-naïve adult patients with unresectable Barcelona Clinic Liver Cancer stage B or C HCC. Patients received oral lenvatinib one time per day plus intravenous anti-PD-1 agents every 3 weeks (one cycle). Tumor response and resectability were evaluated before the fourth cycle, then every two cycles. The primary endpoint was conversion success rate by investigator assessment. Secondary endpoints included objective response rate (ORR) by independent imaging review (IIR) assessment per modified RECIST (mRECIST) and Response Evaluation Criteria in Solid Tumors, V.1.1 (RECIST 1.1), progression-free survival (PFS) and 12-month recurrence-free survival (RFS) rate by IIR per mRECIST, R0 resection rate, overall survival (OS), and safety. Biomarkers were assessed as exploratory objectives.
Results
Of the 56 eligible patients enrolled, 53 (94.6%) had macrovascular invasion, and 16 (28.6%) had extrahepatic metastasis. The median follow-up was 23.5 months. The primary endpoint showed a conversion success rate of 55.4% (31/56). ORR was 53.6% per mRECIST and 44.6% per RECIST 1.1. Median PFS was 8.9 months, and median OS was 23.9 months. Among the 31 successful conversion patients, 21 underwent surgery with an R0 resection rate of 85.7%, a pathological complete response rate of 38.1%, and a 12-month RFS rate of 47.6%. Grade ≥3 treatment-related adverse events were observed in 42.9% of patients. Tumor immune microenvironment analysis of pretreatment samples displayed significant enrichment of CD8+ T cells (p=0.03) in responders versus non-responders.
Conclusion
Lenvatinib plus anti-PD-1 antibodies demonstrate promising efficacy and tolerable safety as conversion therapy in unresectable HCC. Pre-existing CD8+ cells are identified as a promising biomarker for response to this regimen.
Trial registration number
Chinese Clinical Trial Registry, ChiCTR1900023914.
Keywords: Immunotherapy; Drug Therapy, Combination; Immune Checkpoint Inhibitors; Liver Neoplasms
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Most patients with hepatocellular carcinoma (HCC) are diagnosed at an advanced stage, making radical surgery impossible. Combining tyrosine kinase inhibitors and anti-programmed cell death protein-1 (PD-1 antibodies has shown promise in treating advanced HCC, but the effectiveness of this combination in converting unresectable intermediate-advanced HCC into resectable disease is uncertain due to limited research.
WHAT THIS STUDY ADDS
This phase II clinical trial demonstrated that combining lenvatinib with anti-PD-1 antibodies is a safe and effective conversion therapy regimen for unresectable HCC, and the patients with a higher percentage of CD8+ T cells respond better to this regimen.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Conversion therapy with lenvatinib plus PD-1 inhibitors represents a promising treatment option for unresectable HCC. Further clinical trials are necessary to validate these findings and may leverage the knowledge generated by this study.
Introduction
Hepatocellular carcinoma (HCC) is a major cause of cancer-related deaths worldwide.1 Surgical treatment is the optimal treatment strategy for long-term survival, with a 5-year survival rate of 50–70% in patients with early-stage HCC.2 3 Unfortunately, most patients with HCC are diagnosed at intermediate to advanced stages and are not candidates for surgical resection; hence systemic therapy remains the mainstay of care. Despite significant improvements in systemic therapy with the advent of novel drugs, the prognosis for patients with advanced or unresectable HCC remains grim.4
Conversion therapy refers to the application of interventions, such as locoregional or systemic treatment, that allow patients with initially unresectable malignancies to be amenable to curative-intent resection.5 Locoregional therapy primarily includes transarterial chemoembolization, hepatic artery infusion chemotherapy, selective internal radiotherapy, and external beam radiotherapy, which is usually appropriate for patients without extrahepatic metastases to improve the feasibility of liver resection. The conversion success rate of these locoregional therapy approaches in locally advanced HCC ranges mainly between 5% and 30%.5 Historically, systemic treatments for advanced HCC have been linked to comparatively limited objective response rates (ORRs), such as sorafenib, a Food and Drug Administration-approved first-line systemic drug with an ORR of approximately 10%.6 Few patients with advanced HCC have undergone curative surgery following systemic therapies, resulting in the unappreciation of systemic treatment in the conversion therapy setting.6
Excitingly, systemic treatments for advanced HCC have evolved quickly in recent years. Novel systemic treatments, particularly antiangiogenic=-agents combined with anti-programmed cell death protein-1/ligand-1 (anti-PD-1/L1) antibodies, present encouraging ORRs, such as lenvatinib+nivolumab with an ORR of 54.2%,7 lenvatinib+pembrolizumab with an ORR of 36.0%, and bevacizumab+atezolizumab with an ORR of 30.0%,8 9 which makes it necessary to reassess the value of systemic therapy in the conversion therapy setting. Additionally, the latest data from the phase 3 LEAP-002 trial (NCT03713593) demonstrated that lenvatinib combined with pembrolizumab provides a survival advantage (median progression-free survival (PFS) 8.2 vs 8.0 months; median overall survival (OS) 21.1 vs 19.0 months) over lenvatinib plus placebo for patients with advanced HCC, although the differences are not significant.10 Thus, even if patients do not successfully convert following lenvatinib plus anti-PD-1 therapy, they can still opt for a standard second-line regimen with almost no loss in terms of survival benefit. However, few studies have systematically evaluated lenvatinib plus PD-1 blockade in the conversion therapy setting for unresectable intermediate-advanced HCC.
Here, we aimed to prospectively assess the efficacy and safety of lenvatinib plus PD-1 inhibitors as conversion therapy in patients with unresectable intermediate-advanced HCC as well as the potential survival benefit of patients undergoing curative surgery following successful conversion treatment.
Methods
Patients
Patients were recruited from July 1, 2019, to February 28, 2021. The primary inclusion criteria were as follows: (1) histologically or cytologically confirmed HCC or clinically diagnosed HCC per the criteria of the American Association for the Study of Liver Diseases; (2) unresectable stage B or C based on the Barcelona Clinic Liver Cancer (BCLC) staging system due to one or more of the following factors: main portal vein or inferior vena cava invasion, extrahepatic metastasis, inadequate surgical margin, insufficient remnant liver volume after resection (<45% for patients with chronic liver disease and <35% for those without), or inability to reconstruct vascular structure; (3) not amenable to local–regional therapy, or disease progression after surgery and/or local–regional therapy; (4) at least one measurable target lesion based on investigator assessment using Response Evaluation Criteria in Solid Tumors, V.1.1 (RECIST 1.1); (5) age of 18–75 years; (6) Child-Pugh class A or B; (7) Eastern Cooperative Oncology Group performance status of 0 or 1; and (8) no prior systemic therapy, including but not limited to immunotherapy and targeted therapy with tyrosine kinase inhibitors (TKIs). The complete eligibility criteria are listed in the study protocol (online supplemental appendix 1, pp 34–39).
jitc-2023-007366supp001.pdf (6.3MB, pdf)
The trial complied with the principles of Good Clinical Practice and the Declaration of Helsinki and was registered as a prospective, non-randomized, single-institution phase II trial in the Chinese Clinical Trial \][POIUYTRDESAWq/egistry.
Study design
The study consisted of three major phases: (1) conversion therapy, (2) surgical resection, and (3) postoperative management. The CONSORT (Consolidated Standards of Reporting Trials) diagram and study design are depicted in figure 1.
Figure 1.
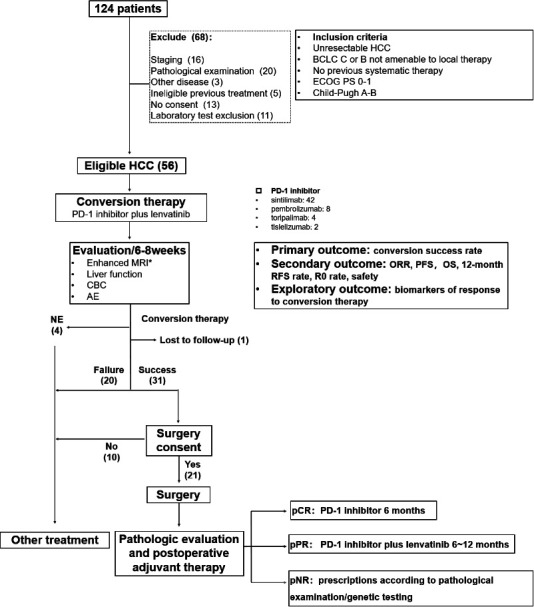
CONSORT (Consolidated Standards of Reporting Trials) diagram and study design. AE, adverse event; BCLC, Barcelona Clinic Liver Cancer; CBC, complete blood count; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; NE, not estimable; ORR, objective response rate; OS, overall survival; pCR, pathological complete response (no viable tumor cells in the resection specimens, including completely resected primary tumors, tumor thrombosis, and lymph nodes); PD-1, programmed cell death protein-1; PFS, progression-free survival; PS, performance status; pNR, pathological non-response (> 50% viable tumor cells in the primary tumor or appearance of new lesions); pPR, pathological partial response (≤50% viable tumor cells in the primary tumor).
All eligible patients received lenvatinib (12 mg/day for bodyweight ≥60 kg or 8 mg/day for bodyweight <60 kg) orally one time per day and a PD-1 inhibitor intravenously on day 1 of a 21-day treatment cycle. Resectability was assessed by the investigator every 6–8 weeks (the first assessment before the fourth cycle). If the resectability criteria were fulfilled within 48 weeks, the conversion was considered successful. The patients who converted successfully and underwent curative-intent surgery were given adjuvant therapy based on postoperative pathology. Follow-up visits occurred 4 weeks after surgery, then every 12 weeks.
The study procedure and the criteria for successful conversion were described in detail in the online supplemental methods.
jitc-2023-007366supp002.pdf (1.8MB, pdf)
Endpoints
The primary endpoint was conversion success rate assessed per the criteria for successful conversion. Secondary endpoints included ORR, PFS (time from the onset of conversion therapy to progression, recurrence, or death), and 12-month recurrence-free survival (RFS, time from the curative-intent surgery to recurrence or death) rate estimated by independent imaging review (IIR) per HCC-specific modified RECIST (mRECIST), ORR by IIR per RECIST 1.1, R0 resection rate, OS (time from the onset of conversion therapy to death), and safety. Biomarker analyses for predicting the response to conversion therapy were considered exploratory endpoints.
Clinical and radiographic assessment, safety evaluation, and multiplex immunofluorescence (mIF) were described in the online supplemental methods.
Statistical analyses
A total of 48 patients would provide 80% power to detect a conversion success rate of 45% with a one-sided alpha of 0.05 under the null hypothesis that the conversion success rate of the locoregional therapy is 22%. 5 11 Data were expressed as median values (minimum–maximum or 95% interval) or as numbers (%). Continuous variables were compared using the Student’s t-test or non-parametric Wilcoxon signed-rank test. Categorical variables were compared by the χ2 test or Fisher’s exact test. Univariate and multivariate Cox regression analyses were applied to analyze the correlations between risk factors and survival. Survival plots were prepared using the Kaplan-Meier method. All analyses were conducted using SPSS V.23 (SPSS Statistics V.23, IBM, Armonk, New York, USA). A two-tailed p<0.05 was considered statistically different.
Results
Demographic characteristics of the study population
A total of 124 patients were registered for screening. After excluding 68 patients who did not meet the eligibility criteria, 56 were enrolled (figure 1), and their clinicopathological data were collected (online supplemental table 1). Baseline demographic and disease characteristics are detailed in table 1. The median age was 54.5 years (range, 28–73 years), and most were men (n=50, 89.3%), had Child-Pugh A (n=55, 98.2%), were at BCLC stage C (n=54, 94.6%), and had macrovascular invasion (n=53, 94.6%). There were 16 (28.6%) patients with extrahepatic metastasis. The etiology of HCC included hepatitis B virus (HBV) (n=49, 87.5%) and hepatitis C virus (HCV) (n=5, 8.9%), and 35 (62.5%) patients had an alpha-fetoprotein (AFP) level ≥400 ng/mL.
Table 1.
Baseline clinical characteristics of 56 patients with HCC
| Clinical characteristics | Lenvatinib plus PD-1 inhibitors |
| Age, years | 54.5 (28–73) |
| ≥60 years | 17 (29.3) |
| Sex | |
| Male | 50 (89.3) |
| Female | 6 (10.7) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 52 (92.9) |
| 1 | 4 (7.1) |
| Child-Pugh score | |
| A | 55 (98.2) |
| B | 1 (1.8) |
| Barcelona Clinic Liver Cancer staging | |
| B | 2 (3.6) |
| C | 54 (96.4) |
| China Liver Cancer staging | |
| IIb | 2 (3.6) |
| IIIa | 38 (67.9) |
| IIIb | 16 (28.6) |
| Alpha-fetoprotein concentration ≥400 ng/mL* | 35 (62.5) |
| Presence of macrovascular invasion, extrahepatic metastasis, or both | 54 (96.4) |
| Macrovascular invasion | 53 (94.6) |
| Portal vein tumor thrombus | 47 (83.9) |
| Metastasis | 16 (28.6) |
| Lymph node | 13 (23.2) |
| Lung | 3 (5.4) |
| Hepatitis B virus infection† | 49 (87.5) |
| Hepatitis C virus infection‡ | 5 (8.9%) |
| Alcohol consumption§ | 22 (39.3) |
| Smoke status | 30 (53.6) |
| Prior local therapy for hepatocellular carcinoma | 3 (5.4) |
Data presented as median (minimum–maximum) or no. (%).
*There was one patient with missing alpha-fetoprotein concentration level (P045).
†Two patients with missing hepatitis B virus infection status (P003 and P045).
‡Three patients with missing hepatitis C virus infection status (P003, P033, and P039).
§One patient with missing alcohol consumption (P051).
¶Two patients with missing smoke status (P039 and P51).
HCC, hepatocellular carcinoma; PD-1, programmed cell death protein-1.
Treatment
Four conversion therapy drug regimens were employed, including lenvatinib+sintilimab (n=42), lenvatinib+pembrolizumab (n=8), lenvatinib+toripalimab (n=4), and lenvatinib+tislelizumab (n=2). Patients who achieved conversion success were then treated with surgery or other treatments per the patient’s wishes. For patients undergoing resection after conversion success, those with a pathological complete response (pCR; no viable tumor cells in the resection specimens) and pathological partial response (pPR; ≤50% viable tumor cells in the primary tumor) were initiated with the PD-1 inhibitor monotherapy for ≥6 months and conversion therapy regimen for 6–12 months, respectively. Patients with a pathological non-response (pNR; >50% viable tumor cells in the primary tumor or appearance of new lesions) were treated with the regimes recommended by a multidisciplinary team per the results of the pathological examination and/or genetic testing.
Response and conversion to resection
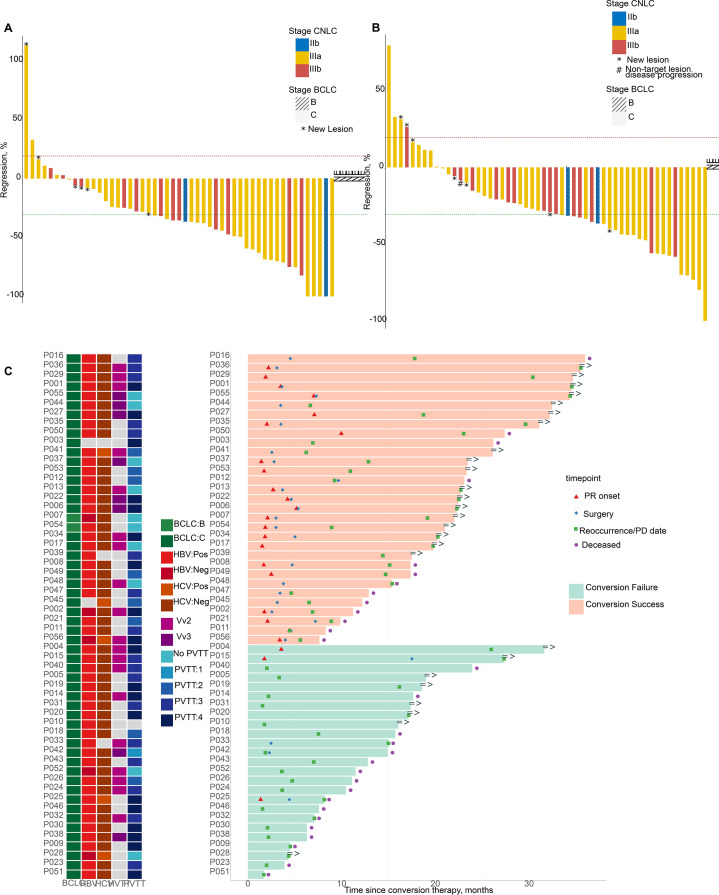
The efficacy of combination therapy was evaluated by investigators and IIR per mRECIST and RECIST 1.1 before the fourth cycle and then every two to three cycles, as presented in online supplemental table 2. One patient died, and one was lost to follow-up before the first evaluation. Three patients could not be evaluated by mRECIST due to no blood flow signals detected by imaging during the treatment. The following results for tumor assessments were based on IIR per mRECIST except for ORR per mRECIST and RECIST 1.1. ORR was 53.6% (30/56) per mRECIST and 44.6% (25/56) per RECIST 1.1 (figure 2A,B). The median duration of response was 17.1 months (95% CI 11.4 to 20.0) (figure 2C). In the 51 patients who completed clinical evaluation per mRECIST, 31 patients, including 4 with complete response, 21 with partial response, and 6 with stable disease, met the successful conversion criteria, yielding a conversion success rate of 55.4% (31/56).
Figure 2.
Response of 56 patients with HCC to conversion therapy with lenvatinib and anti-PD-1 antibodies (A and B) Waterfall plot of the best percentage change in target-lesion size from baseline by IIR assessment according to mRECIST (A) and RECIST 1.1 (B). (C) Swimmer plot of overall survival in 56 patients with HCC receiving lenvatinib plus PD-1 inhibitors. BCLC, Barcelona Clinic Liver Cancer; CNLC, China Liver Cancer; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HVTT, hepatic vein tumor thrombosis; IIR, independent imaging review; mRECIST, modified RECIST; NE, not estimable; PD, progressive disease; PD-1, programmed cell death protein-1; PR: partial response; PVTT: portal vein tumor thrombus; RECIST 1.1, Response Evaluation Criteria in Solid Tumors V.1.1.
Surgical resection of tumors
Among the 31 conversion success patients, 10 refused surgery, and 21 received surgical intervention. The perioperative information is shown in online supplemental table 3. The median interval between initiation of conversion therapy and surgery was 109 days (range, 77–219 days), and the median interval between lenvatinib withdrawal and surgery was 5 days (range, 2–7 days). Four patients (19.4%) experienced post-hepatectomy liver failure grade A. Three patients (14.3%) developed postoperative complications, with one grade I and two grade III. Of the 21 patients, 18 achieved R0 resection, and three underwent R1 resection. Pathological evaluation revealed eight with a pCR, nine with a pPR, and four with a pNR.
Survival
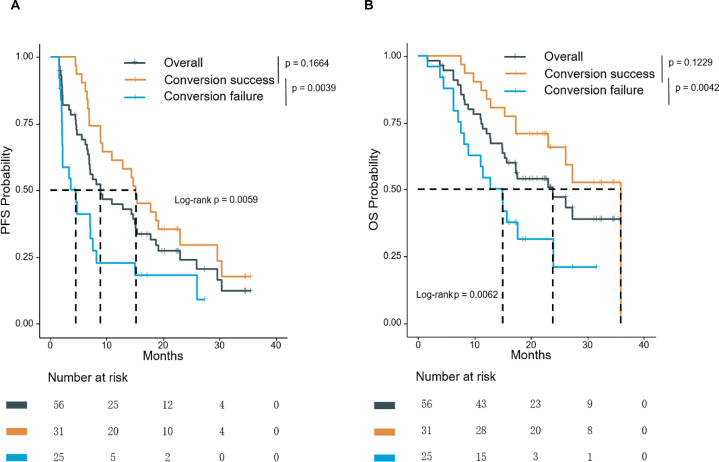
As of July 16, 2022, the median follow-up time was 23.5 months (95% CI 22.3 to 32.5). For 56 patients who received conversion therapy, the median PFS was 8.9 months (95% CI 6.9 to 15.4), with a 12-month PFS rate of 46.2% (95% CI 25.7% to 66.7%); and median OS was 23.9 months (95% CI 15.7 to not reached (NR)) with a 12-month OS rate of 72.8% (95% CI 61.9% to 85.5%) (figure 3A,B). The conversion success group had significantly longer PFS (median PFS, 15.1 vs 4.5 months, p=0.004) and OS (median OS, 36.0 vs 14.9 months, p=0.004) than the conversion failure group (figure 3A,B). Cox regression analyses identified successful conversion as an independent prognostic factor for favorable PFS (HR 0.29, 95% CI 0.15 to 0.57, p<0.001) and OS (HR 0.31, 95% CI 0.15 to 0.66, p=0.002) (online supplemental table 4). High baseline AFP level and multiple foci were identified as independent prognostic factors for poor PFS but not OS. Incidentally, we compared the benefit of surgery with other treatments after successful conversion despite the small sample size. Although there was no significant difference in the survival advantages of surgery versus other therapies, the long-term benefit may be higher for patients who experienced resection (online supplemental figure 1).
Figure 3.
Kaplan-Meier analysis of PFS (A) and OS (B) in the overall (n=56), conversion success (n=31), and conversion failure population (n=25). PFS was assessed by IIR according to mRECIST. IIR, independent imaging review; mRECIST, modified Response Evaluation Criteria in Solid Tumors. OS, overall survival; PFS, progression-free survival.
Postoperative recurrence and survival
The median postoperative follow-up for the 21 conversion resection patients was 22.1 months (95% CI 19.0 to NR). Median RFS was 11.6 months (95% CI 4.3 to NR), with a 12-month RFS rate of 47.6% (95% CI 30.4 to 74.6) (online supplemental figure 2). Median postoperative OS was 31.4 months (95% CI 12.6 to NR). As expected, achieving R0 resection had a significant impact on prolonging RFS (median RFS, 14.7 vs 1.6 months, p<0.001) and postoperative OS (median postoperative OS, 31.4 vs 3.6 months, p<0.001) compared with R1 resection (online supplemental figure 3C,D). Additionally, patients with postconversion AFP ≥400 ng/mL had significantly shorter RFS (median RFS, 1.6 vs 12.4 months, p=0.001) and postoperative OS (median postoperative OS, 8.7 vs 31.4 months, p<0.001) than those with AFP<400 ng/mL (online supplemental figure 3A,B).
Toxicity events
Details of treatment-related adverse events (TRAEs) during conversion and adjuvant therapy are summarized in (online supplemental table 5). Overall, 50 patients (89.3%) experienced TRAEs, and 24 (42.9%) developed grade 3 to 5 TRAEs. The most common grade 3 TRAEs were hypertension (10.7%, n=6) and periodontal disease (10.7%, n=6). Immune-related myocarditis was the only grade 4 TRAE. Two patients who experienced grade 5 adverse events died (patient #025 and patient #051). Patient #025 did not meet the criteria for successful conversion, but opted for resection. On postoperative day 63 during adjuvant therapy, the patient developed wheezing and was admitted to the local hospital, followed by respiratory failure, and eventually died of multiple organ failure on postoperative day 112. Owing to the COVID-19 pandemic quarantine, this patient presented with discomfort and could not come to our hospital for examination and treatment. Therefore, we cannot rule out the possibility of TRAEs as a cause of death. Patient #051 died before the first imaging evaluation. Only one treatment cycle was administered, during which no TRAE was observed that led to treatment interruption or discontinuation. But this patient discontinued treatment for personal reasons and died 2 months later. The cause of death was unknown and might be related to the study treatment.
Correlation of the fractions of tumor-infiltrating immune cells with the efficacy of conversion therapy
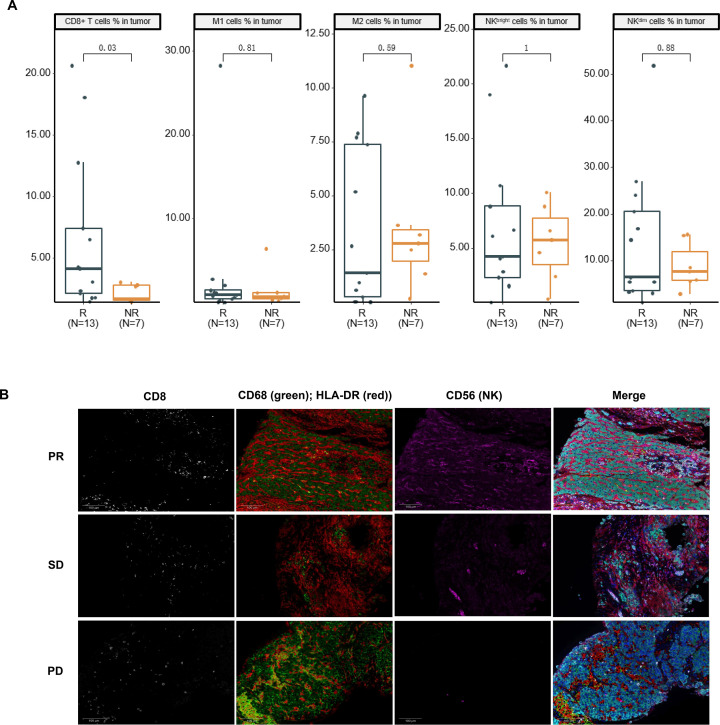
Tumor immune microenvironment analysis was performed using mIF on pretreatment tumor samples from 20 patients. The response group presented a significantly high percentage of CD8+ T cells (p=0.03) versus the non-response group (figure 4A). No significant differences were observed between the two groups for M1 macrophages, M2 macrophages, CD56dim natural killer (NK) cells, and CD56bright NK cells (figure 4A). Representative mIF images of tumor-infiltrating immune cells were displayed in figure 4B.
Figure 4.
Association between immune cell infiltration and response to lenvatinib plus PD-1 inhibitors in 20 patients with HCC. Response was assessed by IIR per mRECIST. (A) The differences between the response and non-response groups for CD8+ T cells, M1 macrophages, M2 macrophages, CD56dim NK cells, and CD56bright NK cells. (B) The representative images of immunohistochemical staining of tumor-infiltrating immune cells. Cells with only CD68 expression were considered M2 macrophages, and dual-labeled cells for CD68 and HLA-DR were considered M1 macrophages. HCC, hepatocellular carcinoma; IIR, independent imaging review; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NK, natural killer; NR, non-response; PD, progressive disease; PD-1, programmed cell death protein-1; PR, partial response; R, response; SD, stable disease; HLA-DR, human leukocyte antigen DR.
Discussion
This phase II study demonstrated that the combination of lenvatinib and PD-1 blockade was well tolerated and effective in converting patients with unresectable intermediate-advanced HCC to become resectable. Pre-existing CD8+ T cells in the tumor were identified as a promising biomarker for responding to this regimen.
Conversion therapy offers the potential for tumor-free survival and increased OS for patients with advanced cancer. However, it is a relatively nascent field with ongoing debates concerning its target population, treatment scheme, and postoperative management. Despite preliminary studies into HCC conversion therapy, the lack of high-grade evidence and generally accepted standard therapy limit its widespread application. Our principle was to select a therapeutic regimen with the highest ORR to achieve optimal chances of downstaging or downsizing HCC and conversion to resection. Among systemic treatment regimens, lenvatinib plus PD-1 inhibitor is associated with the highest ORR (36.0–54.2%) by far, along with a low progressive disease rate,7 12 13 and has been evaluated in several studies with conversion success rates between 15.9% and 30.8%.14–17 While these data provide evidence that this strategy can be effective, the limitations of the small sample size or retrospective nature temper the conclusions drawn from those studies. We thus designed a prospective phase II study to assess the conversion success rate of lenvatinib plus PD-1 inhibitors in 56 patients with unresectable intermediate-advanced HCC and prespecified the criteria for unresectable HCC and resection.
Here, ORR by lenvatinib plus anti-PD-1 therapy was 53.6% (30/56) per mRECIST and 44.6% (25/56) per RECIST 1.1, higher than 40.8% per mRECIST and 26.1% per RECIST 1.1 reported in the LEAP-002 trial, potentially due to the different proportions of HBV-associated HCC. Most patients in our study suffered from HBV-related HCC (87.5%), whereas only 48.6% of participants in the LEAP-002 trial had HBV-associated HCC.10 Lenvatinib has been reported to have greater activity in HBV-related HCC.18 Moreover, a recent meta-analysis revealed that patients with HBV-related or HCV-related HCC benefited more from checkpoint inhibition than those with non-viral HCC.19
In this study, the conversion success rate was 55.4% (31/56), and the conversion resection rate was 37.5% (21/56), with an R0-resection rate of 32.1% (18/56) and a pCR rate of 14.3% (8/56) in the intention-to-treat population. These results were promising, which were better than the data from a retrospective study regarding TKI plus anti-PD-1 therapy in a similar population, where a conversion success rate of 19.1%, an actual surgery rate of 15.9%, an R0 resection rate of 15.9%, and a pCR rate of 9.5% were reported.15 This could potentially be explained by the difference in the criteria for surgical resection. Our results also outperformed the conversion success rate of 30.8% (8/26) presented at the 2022 American Society of Clinical Oncology (ASCO) annual meeting, emanating from a phase II study exploring sintilimab plus lenvatinib as conversion therapy.18 This study consisted of 26 patients with unresectable intermediate-advanced HCC, none of whom had extrahepatic metastasis. In contrast, our study included 16 patients with extrahepatic metastasis. Despite their challenging condition, their conversion success rate and postoperative outcomes paralleled those patients without extrahepatic metastasis (online supplemental figure 4), indicating the potential for conversion therapy even in this more complex patient subset. Notably, our conversion success rate and conversion resection rate obtained by systemic treatment alone are comparable to the recently reported results of local therapy plus systemic therapy.20 21 Nevertheless, the addition of local therapy inevitably increases cost, trauma, and side effects and complicates the evaluation of drug therapy effectiveness.
The timing of surgery, the interval between lenvatinib withdrawal and surgery, and the postoperative treatment protocol were determined based on the literature and our previous work.9 16 22 23 In comparison with previous studies,9 the survival benefit from this conversion regimen in the whole cohort was desirable, with a 12-month PFS rate of 46.2% (95% CI 25.7% to 66.7%) and a 12-month OS rate of 72.8% (95% CI 61.9% to 85.5%). The patients undergoing conversion resection showed encouraging outcomes with a median RFS of 11.6 months and a median postoperative OS of 31.4 months. However, for patient #025 who underwent surgery but did not convert successfully, even if R0 resection was achieved, the prognosis was poor, with a postoperative OS of 3.7 months. Together, these data demonstrate the effectiveness of this conversion regimen and the crucial role of successful conversion for surgical benefit.
It should be mentioned that patient #015, who met the criteria for surgical resection at 75 weeks and was assigned to the conversion failure group, obtained a good postoperative prognosis with an ongoing RFS of 8.3 months and an OS of 27.3 months. This finding might suggest that even if the conversion is unsuccessful within 48 weeks, patients can continue with the regimen as long as they do not progress and still have the possibility to undergo radical resection. Notably, a significantly poor RFS and postoperative OS were observed in the patients with high post-conversion AFP versus those with low AFP, suggesting that AFP levels might guide the timing of surgery following successful conversion. Patients with high AFP could consider extending conversion therapy duration to reduce AFP levels, thereby potentially improving postoperative survival.
The conversion regimen used in this trial was generally well-tolerated, and adverse events were manageable, with no new or unexpected toxic effects noted. Both the rate of any grade and grade 3 or higher toxicity were lower than that of the LEAP-002 trial (any grade, 89.3% vs 96.5%; grade≥3, 42.9% vs 62.5%), possibly due to the relatively young age of our patients (median age, 54.5 vs 66.0 years).10 Severe postoperative complications (Clavien-Dindo grade 3–5) occurred in two patients (9.5%), an incidence comparable to the previously reported 7.1%.24
To gain better insight into drivers of response or resistance and identify possible biomarkers predictive of response to lenvatinib plus anti-PD-1 antibodies, we performed multiplex immunohistochemistry on pretreatment tumor samples. CD8+ T cells were significantly more abundant in the responding tumors, aligning with previous studies suggesting that the presence of these cells is necessary for anti-PD-1/L1 therapy to be effective and could serve as an indicator of the antitumor immune response across multiple tumor types.25–27
There are some limitations to this study. First, successful conversion is a somewhat subjective endpoint. Although the criteria for successful conversion are clearly defined with reference to Chinese expert consensus on conversion therapy for HCC,28 these definitions and related decisions are biased by Chinese institutional practices and may not be representative of institutional practices in other countries. Nevertheless, this study illustrates the possible outcomes of combining systemic therapy and surgery. Given the single-arm study design, it is unclear whether surgery brings additional benefits to the patients who underwent successful conversion, but the rate of long-term tumor-free survival is an undisputable result that infrequently happens with systemic therapy alone. A multicenter, large-sample, multiarm, prospective study is warranted for selecting post-conversion success treatment strategies.
In conclusion, this is the first prospective study demonstrating that lenvatinib combined with PD-1 inhibitors is a safe and effective conversion therapy for patients with unresectable intermediate-advanced HCC. Tumors with a higher percentage of CD8+ T cells might respond better to this regimen. These findings provide some references for optimizing the management of patients with HCC.
Acknowledgments
The authors would like to acknowledge the patients who participated in this study.
Footnotes
WZ, ST and BH contributed equally.
Contributors: Conception and design: SL. Development of methodology: SL, WZ, and YB. Acquisition of data: WZ, ST, BH, TW, HT, TJ, JL, ZZ, HY, ZW, SC, YW, XL, FW, JC, LT, and XZ. Analysis and interpretation of data: FZ, WZ, ST, TJ, JL, and ZZ. Drafting of the manuscript: JC, WZ, and ST. Review and/or revision of the manuscript: WZ, YB, and SL. Administrative, technical, or material support: MC, HW, SC, and MH. Study supervision: SL. Final approval of manuscript: All authors. SL accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ST, FZ, JC, SC, and XZ are employees of 3D Medicines Inc. Other authors declare no potential conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol and any amendments were approved by the Medical Ethics Committee of PLA General Hospital (approval ID: S2018-111-03). Participants gave informed consent to participate in the study before taking part.
References
- 1.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589–604. 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin 2020;70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–1314. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 4.Zhu X-D, Sun H-C. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol 2019;12:1–10. 10.1186/s13045-019-0794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song T, Lang M, Ren S, et al. The past, present and future of conversion therapy for liver cancer. Am J Cancer Res 2021;11:4711–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The lancet oncology 2009;10:25–34. 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Ikeda M, Motomura K, et al. A phase IB study of Lenvatinib (Len) plus Nivolumab (Niv) in patients (Pts) with Unresectable hepatocellular carcinoma (Uhcc): study 117. JCO 2020;38(4_suppl):513. 10.1200/JCO.2020.38.4_suppl.513 [DOI] [Google Scholar]
- 8.Finn RS, Qin S, Ikeda M, et al. Imbrave150: updated overall survival (os) data from a global, randomized, open-label phase III study of Atezolizumab (Atezo) + Bevacizumab (BEV) versus sorafenib (Sor) in patients (Pts) with Unresectable hepatocellular carcinoma (HCC). JCO 2021;39(3_suppl):267. 10.1200/JCO.2021.39.3_suppl.267 [DOI] [Google Scholar]
- 9.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. Journal of Clinical Oncology 2020;38:2960–2970. 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RS, Kudo M, Merle P, et al. Lba34 primary results from the phase III LEAP-002 study: Lenvatinib plus Pembrolizumab versus Lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Annals of Oncology 2022;33:S1401. 10.1016/j.annonc.2022.08.031 [DOI] [Google Scholar]
- 11.Chong JU, Choi GH, Han DH, et al. Downstaging with localized concurrent Chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2018;25:3308–15. 10.1245/s10434-018-6653-9 [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Zhang Y, Guo Y, et al. 987P A phase IB study of the PD-1 antagonist Cs1003 plus Lenvatinib (LEN) in Chinese patients (Pts) with the first-line (1L) Unresectable hepatocellular carcinoma (uHCC).Annals of oncology. Annals of Oncology 2020;31:S690–1. 10.1016/j.annonc.2020.08.1103 [DOI] [Google Scholar]
- 13.Bai L, Sun M, Xu A, et al. Phase 2 study of Ak104 (Pd-1/Ctla-4 Bispecific antibody) plus Lenvatinib as first-line treatment of Unresectable hepatocellular carcinoma. JCO 2021;39(15_suppl):4101. 10.1200/JCO.2021.39.15_suppl.4101 [DOI] [Google Scholar]
- 14.Zhu X-D, Huang C, Shen Y-H, et al. Downstaging and resection of initially Unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer 2021;10:320–9. 10.1159/000514313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun H-C, Zhu X-D, Huang C, et al. Initially Unresectable hepatocellular carcinoma treated by combination therapy of tyrosine kinase inhibitor and anti-Pd-1 antibody followed by resection. JCO 2020;38(15_suppl):e16690. 10.1200/JCO.2020.38.15_suppl.e16690 [DOI] [Google Scholar]
- 16.Zhang W, Hu B, Han J, et al. Surgery after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol 2021;11. 10.3389/fonc.2021.747950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Wang H, Cui Y, et al. Sintilimab plus Lenvatinib as conversion therapy in patients with Unresectable locally Intermediate to advanced hepatocellular carcinoma: a single-arm, single-center, open-label, phase 2 study. JCO 2022;40(4_suppl):449. 10.1200/JCO.2022.40.4_suppl.449 [DOI] [Google Scholar]
- 18.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet 2018;391:1163-1173. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 19.Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021;592:450–456. 10.1038/s41586-021-03362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, Ming S, Lai Z, et al. A phase II trial of Lenvatinib plus Toripalimab and hepatic arterial infusion chemotherapy as a first-line treatment for advanced hepatocellular carcinoma [LTHAIC study]. JCO 2021;39(15_suppl):4083. 10.1200/JCO.2021.39.15_suppl.4083 [DOI] [Google Scholar]
- 21.Zhang X, Zhu X, Liu C, et al. The safety and efficacy of Transarterial Chemoembolization (Tace) + Lenvatinib + programmed cell death protein 1 (Pd-1) antibody of advanced Unresectable hepatocellular carcinoma. JCO 2022;40(4_suppl):453. 10.1200/JCO.2022.40.4_suppl.453 [DOI] [Google Scholar]
- 22.Ikeda M, Sung MW, Kudo M, et al. A phase 1B trial of Lenvatinib (Len) plus Pembrolizumab (Pem) in patients (Pts) with Unresectable hepatocellular carcinoma (Uhcc). JCO 2018;36(15_suppl):4076. 10.1200/JCO.2018.36.15_suppl.4076 [DOI] [Google Scholar]
- 23.Llovet J, Shepard KV, Finn RS, et al. A phase IB trial of Lenvatinib (LEN) plus Pembrolizumab (PEMBRO) in Unresectable hepatocellular carcinoma (uHCC): updated results. Annals of Oncology 2019;30:v286–7. 10.1093/annonc/mdz247.073 [DOI] [Google Scholar]
- 24.Giani A, Cipriani F, Famularo S, et al. Performance of comprehensive complication index and Clavien-Dindo complication scoring system in liver surgery for hepatocellular carcinoma. Cancers (Basel) 2020;12:3868. 10.3390/cancers12123868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun R, Limkin EJ, Vakalopoulou M, et al. A Radiomics approach to assess tumour-infiltrating Cd8 cells and response to anti-PD-1 or anti-PD-L1 Immunotherapy: an imaging biomarker, retrospective Multicohort study. Lancet Oncol 2018;19:1180–91. 10.1016/S1470-2045(18)30413-3 [DOI] [PubMed] [Google Scholar]
- 26.Durgeau A, Virk Y, Corgnac S, et al. Recent advances in targeting Cd8 T-cell immunity for more effective cancer Immunotherapy. Front Immunol 2018;9:14. 10.3389/fimmu.2018.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclerc M, Voilin E, Gros G, et al. Regulation of Antitumour Cd8 T-cell immunity and Checkpoint blockade Immunotherapy by Neuropilin-1. Nat Commun 2019;10:3345. 10.1038/s41467-019-11280-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, Zhang W, Zhao H-T, et al. Chinese expert consensus on conversion therapy of immune Checkpoint inhibitors combined Antiangiogenic targeted drugs for advanced hepatocellular carcinoma (2021 edition). Eletronic Journal of Liver Tumor 2021;8:9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007366supp001.pdf (6.3MB, pdf)
jitc-2023-007366supp002.pdf (1.8MB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.