Abstract
The irrational and prolonged use of antibiotics in orthopaedic infections poses a major threat to the development of antimicrobial resistance. To combat antimicrobial resistance, researchers have implemented various novel and innovative modalities to curb infections. Nanotechnology involves doping ions/metals onto the scaffolds to reach the target site to eradicate the infective foci. In this connotation, we reviewed silver nanoparticle technology in terms of mechanism of action, clinical applications, toxicity, and regulatory guidelines to treat orthopaedic infections.
Keywords: Antimicrobial resistance, Biofilms, Silver, Nanoparticles, Orthopaedics
Core Tip: To overcome antimicrobial resistance, researchers explored the alternate technology to curb infections in musculoskeletal disorders. Nanotechnology forms an eye opener in the usage of silver nanoparticles to eradicate infections in osteoarticular system.
INTRODUCTION
Globally, Orthopaedic infections pose a great challenge among orthopaedic surgeons and orthopaedic researchers. In the literature, the incidence of infection after orthopaedic surgeries was 1% to 2% in closed fractures and approximately 30% in open fractures[1]. Universally, there is no accepted classification for infection after orthopaedic surgeries. To overcome these infections, antibiotics were used irrationally which led to antimicrobial resistance (AMR) due to the development of biofilm by the micro-organisms[2,3]. Jefferson reported that biofilm formation is due to (1) protection from host defense; (2) colonization; (3) local environment benefits; and (4) planktonic cultures as in vitro artifacts[4]. Aparna et al[5] gave 5 stages of the growth cycle of a biofilm namely stage 1 – attachment phase, stage 2 – irreversible binding phase, stage 3 – maturation 1 phase, stage 4 – maturation 2 phase, and stage 5 – cellular dispersion phase.
In humans, 80% of microbial infections are due to non-healing chronic wounds, osteomyelitis, prosthesis- and implant-related infections, endocarditis, rhinosinusitis, and cystic fibrosis[6-9]. Literature states that both gram-positive and gram-negative bacteria form biofilms on the surface of medical devices which are E. faecalis, S. aureus, S. epidermidis, S. viridans, E. coli, K. pneumoniae, P. mirabilis, and P. aeruginosa[10]. Out of all these organisms, S. aureus and coagulase-negative staphylococci pose a greater risk of forming biofilms in orthopaedic implants[11,12], whereas P. aeruginosa sustain and survive in harsh environments and forms a resistant biofilm[13].
With the rise of AMR, antibiotic prophylaxis has become ineffective in curbing infections. Novel techniques such as nanomedicine, bacteriophage therapy, antimicrobial peptides, sonic therapies, scaffold-loaded nanoparticles, antimicrobial adjuvants in the form of silver, electrical and electromagnetic methods, bioacoustic effects, surface modification of biomaterials, antimicrobial photodynamic therapies, biosensors, hyperbaric oxygen, and fecal microbiota transplantation have been introduced to combat AMR[10,14,15]. With the profound literature evidence of the antimicrobial property of silver (Ag), nanotechnology experts doped nanoparticles (NP) with Ag for targeted drug delivery and enhanced interaction with the surrounding environment to curb biofilm-producing organisms[16]. In this article, the usage of AgNP in curbing orthopaedic infections is narrated.
AGNP TECHNOLOGY
In literature, Ag has been identified to have antimicrobial properties[17-19], but in recent years clinicians have demonstrated this property in various clinical conditions. The oligodynamic action of Ag refers to the toxic nature of metal ions on microbes by integrating with microbial deoxyribonucleic acid (DNA)[20-22]. Few studies have emphasized the precipitation of DNA within the microbial cell[23]. Ag exerts antibacterial action by inhibiting cell wall synthesis[24,25].
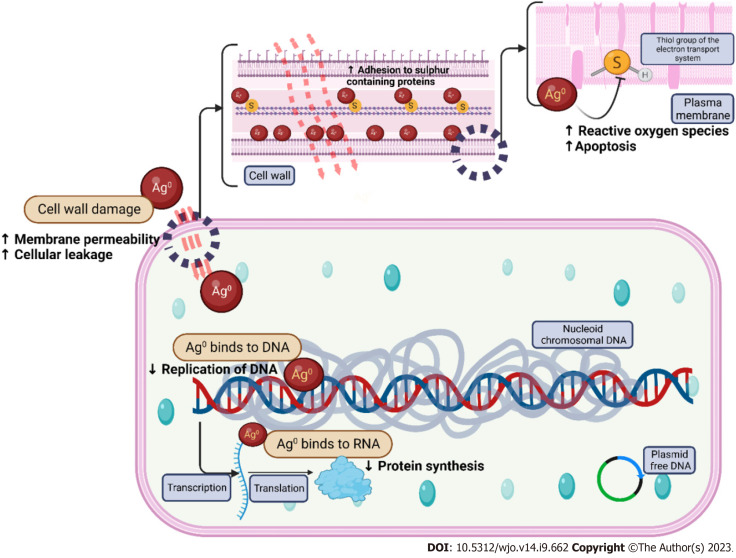
Recent technologies use NP to load Ag ions which may be used as an antimicrobial agent in a target-specific manner[23]. AgNP permeates into cells and interferes with the enzymes of bacterial respiratory chains to inhibit ATP production and growth of the bacteria[16,26]. The particle size of AgNP determines the bactericidal activity. 10-nm Ag demonstrates the complete bacterial interaction, henceforth AgNP exerts a higher bactericidal effect[27]. The mechanism of action of AgNP in infections is as follows (as depicted in Figure 1).
Figure 1.
Mechanism of action of silver nanoparticles. DNA: Deoxyribonucleic acid.
Direct contact with microbes through leakage of cellular contents and bacterial death due to the damage of cell membrane and higher production of reactive oxygen species and free radical species, release of Ag+ ions through interaction with sulfhydryl groups (cysteine) in cell wall proteins and enzymes, induction of bacterial death when Ag+ ions in AgNPs by bombarding the electron transport chain in bacterial mitochondria. Entering of Ag+ ions into periplasmic space which leads to the separation of the cytosol from the cell membrane, occurrence of cellular pits after the exposure of Ag+ ions, inhibition of ribosomal functions leading to enhanced ROS production, malformation of proteins resulting in improper DNA function, antibiofilm activity of AgNPs, and dose-dependent cytotoxic and genotoxic effects of AgNP on osteoblasts and impaired cellular viability of AgNPs at 10 µg/g.
To impart biocompatibility of AgNP in musculoskeletal tissues: (1) Biosynthesis process from bacteria, yeast, and fungi; (2) physical property adjustment; and (3) biomolecule combinations can be tried.
AGNP IN ORTHOPAEDIC INFECTIONS
Recently, the modification of orthopaedic implants with the application of AgNPs on the surface leads to the prevention of implant-associated infections. With the availability of AgNP-coated external fixators, mega prostheses, and AgNP-coated bone cement, orthopaedic infections are showing a downtrend. Since AgNP-coated orthopaedic implants demonstrate antimicrobial activity, the specific molecular mechanism of osteogenic-related cells warrants an understanding. Due to dose-dependent cytotoxicity, the effect of AgNP on osteoblast and osteoclast is controversial. Aurore et al[28] demonstrated the bactericidal effect of AgNP against non-virulent E.coli and virulent MRSA at non-toxic concentrations. Macrophage polarization towards the M1 phenotype enables the cells to kill the engulfed microorganisms. Elevated reactive oxygen species (ROS) responses were found in AgNP-treated osteoclasts. The modification of orthopaedic implants by using AgNPs enhances antimicrobial effects through plasma immersion ion implantation (PIII), magnetron sputtering, plasma electrolytic oxidation, and 3DP-Ag-containing scaffolds[16].
Tumor prosthesis: In orthopaedic oncology, peri-prosthetic infection rates from 9% to 29%. Due to the immunosuppressive environment, this group of patients is more prone to infection than arthroplasty patients. Gosheger et al[29] demonstrated a superior antimicrobial effect with a silver-coated mega prosthesis (7% infection rate) than titanium prosthesis (47% infection rate) in a rabbit model. Ag coated group showed fewer signs of inflammation as measured by ESR, CRP, and neutrophil count. In sarcoma patients, Hardes et al[30] observed 17.6% of infection in the non-silver coated mega prosthesis group than 5.6% of infection in silver coated mega prosthesis group. About 38.5% of cases of amputation in the non-silver-coated mega prosthesis group were due to deep infections whereas no case of amputation was reported in the silver-coated mega prosthesis group. Hence, silver-coated mega prosthesis reduces the risk of further infection in oncology cases as they are already in an immunocompromised state. However, large-scale blinded controlled trials have to prove the safety and efficacy of infection prevention with the silver-coated prosthesis in orthopaedic oncological cases.
External fixator pins: Pin tract infections mount for 42% of orthopaedic infections which results in loosening of the implant, fracture non-union, and osteomyelitis. An in-vitro study demonstrated a 3-log step reduction of S. epidermidis biofilm producers when incubating the stainless steel pins coated with AgNP compared with titanium and copper pins for 20 h (9). Wassall et al[31] revealed the antimicrobial effects of Ag in Ag-coated pins with the significant reduction of adhesion of E. coli, P. aeruginosa, and S. aureus when compared with normal stainless steel pins. Loosening of external fixator pins was less frequently found with Ag-coated pins. Hence silver inhibits microbial adhesion by inhibiting the formation of the glycocalyx on the surface of the pins.
Osteomyelitis and infected non-union: Ag ionophoresis act as an adjunct treatment option for osteomyelitis and infection in non-union of fractures. The continuous inflow of electrically driven Ag into the infective foci curbs the infection and promotes the environment for tissue regeneration. At the end of 3 mo follow-up, radiographic and histological analysis revealed neo-osteogenesis in a 6 mm critical bone defect in the femoral diaphysis of rats managed with bone graft with BMP-2 coupled with AgNP and poly lactic-co-glycolic acid (PLGA) scaffold injected with 10 CFUs of vancomycin-resistant MRSA[32]. Microbial elimination with 2% Ag NP coupled with composite bioscaffold resulted in fracture union.
Bone cement: Alt et al[33] proved that nanosilver-coated cement produced a high index of antimicrobial activity against S. epidermidis, MRSE, and MRSA. Tiopronin, a stabilizing agent, coupled with AgNP in bone cement expressed a good antimicrobial efficacy without displaying any cement-related cytotoxicity[34]. AgNP coated bone cement resulted in antibacterial activity against MRSA and decrease the formation of polymer debris in joint replacement[35].
TOXICITY OF AGNP
Though nanotechnology poses a greater advantage in clinical applications, a considerable note of precautions and toxic effects are observed with AgNP. The interaction of AgNP with biological media leads to Ag agglomeration and dissolution. Doping of Ag into NP results in the induction of toxic responses. The failure of protective coatings on NPs to prevent aggregation in biological fluids leads to AgNP instability. In vitro and in vivo AgNP studies demonstrated the induction of lung fibroblasts, genotoxicity, chromosomal aberrations, DNA damage, and apoptosis of cells. There is only limited evidence for carcinogenicity in any biological tissues.
The interaction of human alveolar basal epithelial cells with AgNP results in the generation of reactive oxygen species, reduction of mitochondrial membrane potential and cellular viability, and enhance cellular apoptosis. Exposure to higher concentrations of AgNP in human cell lines induces not only cellular apoptosis but also induces cellular morphology and genetic mutations. The toxicity of AgNP has been demonstrated in microbes ranging from bacteria, viruses, fungi, and algae. AgNP molecules penetrate the skin and blood tube of zebrafish larvae in aggregate form whereas it induces heat shock, oxidate stress, and DNA damage in Drosophila melanogaster.
AgNP technology is associated with developmental abnormalities in zebrafish embryos, cytotoxic, and genotoxic effects with systemic immunosuppression. AgNP-mediated cytotoxicity with 10 μg/g is observed on primary human MSCs and osteoblast cells[36]. In zebrafish, AgNP induced neurotoxicity and persistent abnormal behavior[36]. Cytotoxicity of AgNP depends on the particle size of Ag. Literature has documented the cytotoxic effects of Ag of 24 nm causing an intrinsic pro-inflammatory response and apoptosis of surrounding cells[36].
Drake et al[37] and Lansdown[38] have done an extensive review of the exposure-related health effects of silver and its related compounds in health. The evidence of toxic effects and complications of AgNP in human studies is limited. Silver represents occupational health hazards like argyria in long-term exposure[37]. The critical oral dosage of silver varies for every individual. The accumulation of silver and nanosilver particles occur in the liver, spleen, kidney, nails, and mucous membranes[39]. With the topical application of Ag, the risk of percutaneous absorption is very low as the epidermis is a relatively impenetrable barrier[40]. Munger et al[41] performed a cross-over time exposure study with oral AgNP (5-10 mm) and demonstrated no change in metabolic, hematologic, physical, or morphological findings. However, the toxicity of AgNP in humans is understudied.
Due to the increase of AgNP usage in the market, governmental regulations in the United States [Environment Protection Agency on the nanomaterials regulation], European Union [European Strategy for Nanotechnology], and Canada [Health Canada and Environment Canada] have been implemented[42]. These agencies mentioned that the size of AgNP must be ranging from 1–100 nm in at least one spatial dimension[42]. No specific occupational exposure limits have been laid by these governmental agencies on AgNPs. Global organizations focus on the safety and sustainability of AgNPs in the market for optimal benefits in the community.
RECENT ADVANCES IN AGNP TECHNOLOGY
With the introduction of new fabricating methods, the toxic effects of AgNP are minimized in the microenvironment[43]. Doping of copolymers and growth factors with AgNPs is more effective in hastening wound healing. Green-synthesized AgNPs are cheaper and eco-friendly for the desired environment. The phytochemicals to be doped with AgNPs have to be characterized and seek appropriate regulatory approvals before commercializing the product for preclinical and clinical studies[44,45]. The addition of electrospun nanofibers along with AgNP offers a great advantage in curbing the infection along with debridement and antibiotics. Once the wound is healed, such electrospun nanofibers provide a naïve extracellular matrix in the newly regenerated tissues[46].
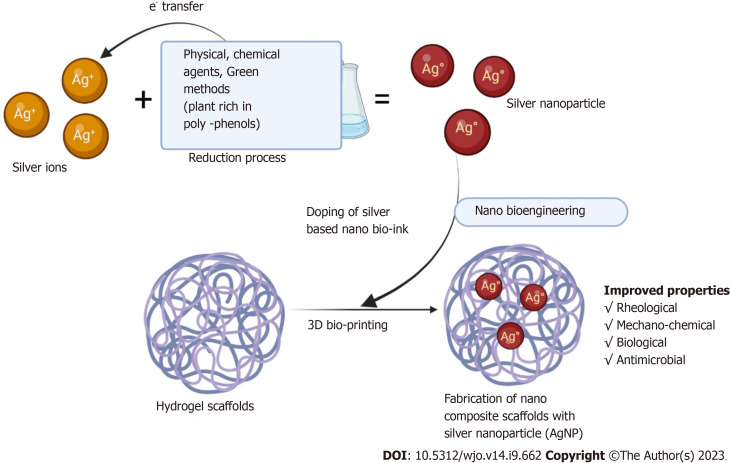
With the evolution of 3-D printing technology, the fabrication of scaffolds with Ag coating prevents infections in complex orthopaedic cases[47,48]. 3D scaffolds with porous structures are ideal for loading biomolecules and ions for targeting the desired site. Doping of AgNP into scaffolds with antimicrobial activity and biocompatibility properties in musculoskeletal tissues aids in curbing infection and promoting tissue regeneration as depicted in Figure 2.
Figure 2.
Doping of silver nanoparticles in scaffolds.
3D bioprinting technology dispenses “bio-inks” which contain cells with regenerative potential, scaffolds doped with metal/ion nanoparticles, and biomicromolecules in a temporospatial controlled fashion[49,50]. Such bio-inks with antimicrobial properties aim at uprooting the infection and facilitating the regeneration of tissues. Damle et al[51] proved the proliferation and differentiation of mesenchymal stromal cells when doped with AgNPs which gave further insights in osseous tissue engineering. The concept of “Smart Coating” depends on light responsiveness, temperature responsiveness, pH responsiveness, and piezo responsiveness for improving osseous integration, inhibting biofilm formation, and preventing post-operative complications associated with orthopaedic infections[52].
CONCLUSION
In orthopaedics, AgNP technology has the potential to reduce implant-related orthopaedic infections. Doping AgNP with scaffolds and bio-inks must adhere to the regulatory guidelines to avoid toxicity in clinical applications. With the evidence of preclinical studies, large-scale blinded controlled trials on AgNP in orthopaedic infections have to be assessed for further validation.
Footnotes
Conflict-of-interest statement: All authors declare no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 18, 2023
First decision: July 18, 2023
Article in press: September 4, 2023
Specialty type: Nanoscience and nanotechnology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hadianamrei R, United Kingdom; Koubaa M, Tunisia; Soriano-Ursúa MA, Mexico S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Madhan Jeyaraman, Department of Orthopaedics, ACS Medical College and Hospital, Dr MGR Educational and Research Institute, Chennai 600077, Tamil Nadu, India; Department of Orthopaedics, South Texas Orthopaedic Research Institute, Laredo, TX 78045, United States. madhanjeyaraman@gmail.com.
Naveen Jeyaraman, Department of Orthopaedics, ACS Medical College and Hospital, Dr MGR Educational and Research Institute, Chennai 600077, Tamil Nadu, India.
Arulkumar Nallakumarasamy, Department of Orthopaedics, ACS Medical College and Hospital, Dr MGR Educational and Research Institute, Chennai 600077, Tamil Nadu, India.
Karthikeyan P Iyengar, Department of Trauma and Orthopaedics, Southport & Ormskirk Hosp NHS Trust Southport, Richmond PR8 6PN, Southport, United Kingdom.
Vijay Kumar Jain, Department of Orthopaedics, Atal Bihari Vajpayee Institute of Medical Sciences, Dr Ram Manohar Lohia Hospital, Delhi 110001, New Delhi, India.
Anish G Potty, Department of Orthopaedics, South Texas Orthopaedic Research Institute, Laredo, TX 78045, United States.
Ashim Gupta, Department of Orthopaedics, South Texas Orthopaedic Research Institute, Laredo, TX 78045, United States; Department of Regenerative Medicine, Regenerative Orthopaedics, Noida 201301, Uttar Pradesh, India; Department of Regenerative Medicine, Future Biologics, Lawrenceville, GA 30043, United States; Department of Regenerative Medicine, BioIntegarte, Lawrenceville, GA 30043, United States.
References
- 1.Steinmetz S, Wernly D, Moerenhout K, Trampuz A, Borens O. Infection after fracture fixation. EFORT Open Rev. 2019;4:468–475. doi: 10.1302/2058-5241.4.180093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat MKF, Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett. 2004;236:163–173. doi: 10.1016/j.femsle.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Aparna MS, Yadav S. Biofilms: microbes and disease. Braz J Infect Dis. 2008;12:526–530. doi: 10.1590/s1413-86702008000600016. [DOI] [PubMed] [Google Scholar]
- 6.Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 9.Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev. 2009;73:310–347. doi: 10.1128/MMBR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2:176–194. doi: 10.4161/biom.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 13.Chang CY. Surface Sensing for Biofilm Formation in Pseudomonas aeruginosa. Front Microbiol. 2017;8:2671. doi: 10.3389/fmicb.2017.02671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramires LC, Santos GS, Ramires RP, da Fonseca LF, Jeyaraman M, Muthu S, Lana AV, Azzini G, Smith CS, Lana JF. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? Int J Mol Sci. 2022;23 doi: 10.3390/ijms23031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeyaraman M, Sami A, Nallakumarasamy A, Jeyaraman N, Jain VK. Hyperbaric Oxygen Therapy in Orthopaedics: An Adjunct Therapy with an Emerging Role. Indian J Orthop. 2023;57:748–761. doi: 10.1007/s43465-023-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qing Y, Cheng L, Li R, Liu G, Zhang Y, Tang X, Wang J, Liu H, Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomedicine. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int J Nanomedicine. 2020;15:2555–2562. doi: 10.2147/IJN.S246764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver Nanoparticles and Their Antibacterial Applications. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalwar K, Shan D. Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism - a mini review. Micro & Nano Letters. 2018;13:277–280. [Google Scholar]
- 20.Zhang E, Zhao X, Hu J, Wang R, Fu S, Qin G. Antibacterial metals and alloys for potential biomedical implants. Bioact Mater. 2021;6:2569–2612. doi: 10.1016/j.bioactmat.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules. 2016;21 doi: 10.3390/molecules21070836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans A, Kavanagh KA. Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens. J Med Microbiol. 2021;70 doi: 10.1099/jmm.0.001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rtimi S, Dionysiou DD, Pillai SC, Kiwi J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Applied Catalysis B: Environmental 2019; 240: 291-318. [Google Scholar]
- 24.Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Liao C, Li Y, Tjong SC. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anees Ahmad S, Sachi Das S, Khatoon A, Tahir Ansari M, Afzal Mohd, Saquib Hasnain M, Kumar Nayak A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater Sci Energy Technol. 2020;3:756–769. [Google Scholar]
- 28.Aurore V, Caldana F, Blanchard M, Kharoubi Hess S, Lannes N, Mantel PY, Filgueira L, Walch M. Silver-nanoparticles increase bactericidal activity and radical oxygen responses against bacterial pathogens in human osteoclasts. Nanomedicine. 2018;14:601–607. doi: 10.1016/j.nano.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, Gunsel A, Kemper FH, Winkelmann W, Von Eiff C. Silver-coated megaendoprostheses in a rabbit model--an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25:5547–5556. doi: 10.1016/j.biomaterials.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, Hauschild G, Ahrens H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 31.Wassall MA, Santin M, Isalberti C, Cannas M, Denyer SP. Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pins. J Biomed Mater Res. 1997;36:325–330. doi: 10.1002/(sici)1097-4636(19970905)36:3<325::aid-jbm7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Z, Yin W, Zara JN, Li W, Kwak J, Mamidi R, Lee M, Siu RK, Ngo R, Wang J, Carpenter D, Zhang X, Wu B, Ting K, Soo C. The use of BMP-2 coupled - Nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials. 2010;31:9293–9300. doi: 10.1016/j.biomaterials.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 34.Prokopovich P, Leech R, Carmalt CJ, Parkin IP, Perni S. A novel bone cement impregnated with silver-tiopronin nanoparticles: its antimicrobial, cytotoxic, and mechanical properties. Int J Nanomedicine. 2013;8:2227–2237. doi: 10.2147/IJN.S42822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakthi Devi R, Girigoswami A, Siddharth M, Girigoswami K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl Biochem Biotechnol. 2022;194:4187–4219. doi: 10.1007/s12010-022-03963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brennan SA, Ní Fhoghlú C, Devitt BM, O'Mahony FJ, Brabazon D, Walsh A. Silver nanoparticles and their orthopaedic applications. Bone Joint J. 2015;97-B:582–589. doi: 10.1302/0301-620X.97B5.33336. [DOI] [PubMed] [Google Scholar]
- 37.Drake PL, Hazelwood KJ. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005;49:575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 38.Lansdown AB. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharmacol Sci. 2010;2010:910686. doi: 10.1155/2010/910686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferdous Z, Nemmar A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- 41.Munger MA, Radwanski P, Hadlock GC, Stoddard G, Shaaban A, Falconer J, Grainger DW, Deering-Rice CE. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomedicine. 2014;10:1–9. doi: 10.1016/j.nano.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonilla-Gameros L, Chevallier P, Sarkissian A, Mantovani D. Silver-based antibacterial strategies for healthcare-associated infections: Processes, challenges, and regulations. An integrated review. Nanomedicine. 2020;24:102142. doi: 10.1016/j.nano.2019.102142. [DOI] [PubMed] [Google Scholar]
- 43.Nqakala ZB, Sibuyi NRS, Fadaka AO, Meyer M, Onani MO, Madiehe AM. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aboyewa JA, Sibuyi NRS, Meyer M, Oguntibeju OO. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants (Basel) 2021;10 doi: 10.3390/plants10091929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon S, Sibuyi NRS, Fadaka AO, Meyer M, Madiehe AM, du Preez MG. The antimicrobial activity of biogenic silver nanoparticles synthesized from extracts of Red and Green European pear cultivars. Artif Cells Nanomed Biotechnol. 2021;49:614–625. doi: 10.1080/21691401.2021.1980884. [DOI] [PubMed] [Google Scholar]
- 46.Stojanov S, Berlec A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front Bioeng Biotechnol. 2020;8:130. doi: 10.3389/fbioe.2020.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Do AV, Khorsand B, Geary SM, Salem AK. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv Healthc Mater. 2015;4:1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Baik JM, Yu YS, Kim JH, Ahn CB, Son KH, Choi ES, Lee JW. Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci Rep. 2020;10:7554. doi: 10.1038/s41598-020-64573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty J, Mu X, Pramanick A, Kaplan DL, Ghosh S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials. 2022;287:121672. doi: 10.1016/j.biomaterials.2022.121672. [DOI] [PubMed] [Google Scholar]
- 50.Veiga A, Silva IV, Duarte MM, Oliveira AL. Current Trends on Protein Driven Bioinks for 3D Printing. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13091444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damle A, Sundaresan R, Rajwade JM, Srivastava P, Naik A. A concise review on implications of silver nanoparticles in bone tissue engineering. Biomater Adv. 2022;141:213099. doi: 10.1016/j.bioadv.2022.213099. [DOI] [PubMed] [Google Scholar]
- 52.Joshi MU, Kulkarni SP, Choppadandi M, Keerthana M, Kapusetti G. Current state of art smart coatings for orthopedic implants: A comprehensive review. Smart Mater Med. 2023;4:661–679. [Google Scholar]