Abstract
BACKGROUND
The prevalence of osteoporosis and low bone mass is steadily rising each year. Low body weight is commonly linked to diminished bone mass and serves as a robust predictor of osteoporosis. Nonetheless, the connection between body mass index (BMI), bone mineral density, and lipid profiles among the elderly remains elusive.
AIM
To examine the association between BMI and bone mass, explore the correlation between lipid profiles and bone mass, and delve into the interplay between lipid metabolism and bone health.
METHODS
The study included 520 patients aged ≥ 65 years (178 men and 342 women). Age, sex, weight, and height were recorded. Femoral neck bone mineral density and T scores were determined using a dual-energy X-ray absorptiometry scanner. Blood calcium (Ca), phosphorus (P), albumin (ALB), alkaline phosphatase (ALP), aspartate aminotransferase, alanine aminotransferase, triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were measured. Patients were classified by sex (male and female), age (65-79 years and ≥ 80 years), and T score (normal bone mineral density, osteopenia and osteoporosis).
RESULTS
Age, sex, BMI, and ALP and TG levels were independent risk factors for osteoporosis. For the 65-79- and ≥ 80-year-old groups, females presented lower T scores than males. Ca, P, ALB, ALP, TC, HDL and LDL levels were significantly different between men and women in the 65-79-year-old group. In addition, BMI and TG levels were significantly decreased in osteoporotic patients compared with patients with normal bone mass. TC levels declined in 65- to 79-year-old male and female osteoporosis patients. In the group of women aged ≥ 80 years, osteoporotic patients showed significantly increased ALP levels. Furthermore, we found positive correlations between BMI and TG levels in the male and female patient groups. However, we found no significant differences in ALB, Ca, P, HDL and LDL levels in osteoporotic patients compared to patients with normal bone mass.
CONCLUSION
Osteoporotic patients showed significantly decreased BMI and TG levels compared with those with normal bone mass. BMI showed positive correlations with TG levels in male and female patients. These results indicate correlations between BMI and bone mass and between lipid profiles and bone mass.
Keywords: Osteoporosis, Weight loss, Elderly patients, Body mass index, Lipid profiles
Core Tip: Older age, female gender, low body mass index (BMI), and low triglycerides (TG) were identified as overall independent factors for osteoporosis. Furthermore, low total cholesterol represented a gender-unspecific risk factor for osteoporosis in elderly patients aged 65-79 years, and high alkaline phosphatase represented a specific risk factor for osteoporosis in elderly male patients aged 80+ years. In addition, positive correlations were found between BMI and serum TG levels, suggesting an interaction between bone and fat metabolism that may have an impact on the development of osteoporosis and would provide a new strategy for the treatment of osteoporosis.
INTRODUCTION
The incidence of osteoporosis and low bone mass is progressively rising on an annual basis. Osteoporosis affects over 33% of individuals aged 50 years or older in China[1]. Osteoporosis is marked by diminished bone mineral density (BMD) and compromised bone microarchitecture, resulting in reduced bone mass and heightened bone fragility, thereby elevating the susceptibility to fractures[2]. Numerous factors contribute to osteoporosis, encompassing age, sex, lifestyle, and various medical conditions[3]. Alongside the T score, dual-energy X-ray absorptiometry (DXA) is deemed a pivotal and extensively employed technique for osteoporosis diagnosis[4]. Typically, osteoporosis is classified based on the T score, following the World Health Organization (WHO) guidelines: Normal bone density (≥ -1.0), osteopenia (-1.0 to -2.5), and osteoporosis (≤ -2.5)[5,6].
Recently, numerous studies have examined osteoporosis risk factors, including body mass index (BMI), serum lipid profiles, serum calcium (Ca) and phosphorus (P), serum albumin (ALB), alkaline phosphatase (ALP), and serum alanine transaminase (ALT) and aspartate aminotransferase (AST)[7-11].
Previous research has reported a link between obesity and osteoporosis, suggesting that obesity might act as a protective factor against osteoporosis[12,13]. Obese individuals subject their bones to greater mechanical loads, which can be advantageous for bone mass[14,15]. Furthermore, weight loss detrimentally impacts musculoskeletal health[16], and it has been linked to bone loss and identified as a potent predictor of osteoporosis[17]. Elderly women who experienced weight loss demonstrated heightened bone loss in the hip region[18]. Another investigation noted a relationship between weight loss and hip-bone loss among elderly men and women[17]. These findings imply that elderly individuals with lower body weight face greater risks of both osteoporosis and fractures than their counterparts with higher body weight. Additionally, plasma lipid profiles have been demonstrated to undergo alterations in response to weight changes[19]. Assessing BMI provides a straightforward approach to categorizing an individual's weight status and is linked to the proportion of body fat[20].
Nevertheless, the relationship among BMI, bone mass, and lipid profiles remains unexplored in populations with osteoporosis and fragility fractures. Fragility fractures frequently occur in elderly patients with severe osteoporosis, exacerbating the prognosis[21]. The impact of weight gain on enhancing the lipid profile and subsequently mitigating the prevalence of osteoporosis and fragility fractures remains uncertain. This study aimed to examine the interaction between BMI and bone mass, explore the correlation between lipid profiles and bone mass, and further analyze the interrelationship between lipid metabolism and bone health in individuals experiencing fragility fractures.
MATERIALS AND METHODS
Study participants
This retrospective study was conducted at a singular orthopaedic trauma centre during the time span of January 2017 to December 2020. The study meticulously applied specific inclusion and exclusion criteria as delineated below:
Inclusion criteria were as follows: (1) Age exceeding 65 years at the moment of injury; (2) confirmed diagnosis of fractures in the hip, vertebral region, distal radius, or proximal humerus; and (3) hospitalization at our centre.
Exclusion criteria were as follows: (1) Patients afflicted with cancer, thyroid disorders, hypopituitarism, rheumatoid arthritis, chronic renal failure, or renal insufficiency; (2) patients undergoing lipid-lowering, synthetic thyroid, or hormone replacement therapies; or (3) presence of incomplete data.
Our study managed to acquire comprehensive clinical data suitable for analysis. Participants were categorized based on gender (male and female), age groups (65-79 years and ≥ 80 years), BMI ranges (< 18.5, 18.5-24, > 24 kg/m2), and T scores [normal BMD (≥ -1.0), osteopenia (-1.0 to -2.5), and osteoporosis (≤ -2.5)]. All laboratory data used in this study were obtained from blood samples collected during the initial admission.
Data collection
Age, sex, height, and weight were recorded from patient documents. BMI was calculated using weight and height (kg/m2). Femoral neck BMD and T scores were determined using a DXA scanner (Hologic Discovery Wi with software version 13.2). According to the WHO classification, the T score was used to define the BMD categories. Serum samples were collected immediately after the patients were admitted, and serum Ca, P, ALB, AST, ALT, ALP, triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were analyzed.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). IBM SPSS statistics software (version 26.0, SPSS Inc., Chicago, United States) and GraphPad Prism software (version 8.4.0, GraphPad Software, Boston, United States) were used to analyze the data. After analysing the normality using the Shapiro-Wilk test, data between two groups were evaluated by the t test and nonparametric test. For three groups, data were evaluated by one-way ANOVA. Multivariate analysis was performed by multiple linear regression. The Pearson correlation test was used to analyse the association between BMI and TG levels. aP < 0.05, bP < 0.01, cP < 0.001 and dP < 0.0001 indicated significant differences.
RESULTS
In total, 520 patients aged ≥ 65 years were included in our study. A total of 178 male patients were enrolled, including 48 normal, 81 osteopenic and 49 osteoporotic patients. A total of 342 female patients were included in our study, including 37 normal, 103 osteopenic, and 202 osteoporotic patients (Table 1). Age, sex, BMI and ALP and TG concentrations were significantly different among the groups of normal, osteopenic and osteoporotic patients. Moreover, age, sex, BMI and ALP and TG concentrations were independent risk factors for osteoporosis (Table 1).
Table 1.
Univariate and multivariate analysis of related factors for osteoporosis
|
|
Univariate analysis
|
Multivariate analysis
|
|||||
|
Normal (n = 85)
|
Osteopenia (n = 184)
|
Oeteoporosis (n = 251)
|
P value
|
OR
|
95%CI
|
P value
|
|
| Age (year old) | 75.54 ± 8.22 | 76.31 ± 8.00 | 79.72 ± 7.62 | < 0.001 | -0.04 | 0.016-0.064 | 0.001 |
| Gender (n) | < 0.001 | 1.34 | 0.968-1.720 | < 0.001 | |||
| Male | 48 | 81 | 49 | ||||
| Female | 37 | 103 | 202 | ||||
| BMI (kg/m2) | 25.46 ± 3.32 | 23.43 ± 3.32 | 21.83 ± 3.10 | < 0.001 | -0.018 | -0.373 | < 0.001 |
| ≤ 18.5 (n) | 1 (1.18) | 9 (4.89) | 38 (15.14) | < 0.001 | |||
| 18.5-24 (n) | 27 (31.76) | 92 (50.00) | 148 (58.96) | ||||
| ≥ 24 (n) | 57 (67.06) | 83 (45.11) | 65 (25.90) | ||||
| Ca (mmol/L) | 2.21 ± 0.16 | 2.20 ± 0.15 | 2.22 ± 0.15 | 0.69 | |||
| P (mmol/L) | 1.05 ± 0.29 | 1.02 ± 0.19 | 1.04 ± 0.20 | 0.56 | |||
| ALB (g/L) | 38.21 ± 4.72 | 39.27 ± 4.26 | 38.49 ± 4.36 | 0.09 | |||
| ALP (U/L) | 64.02 ± 41.37 | 59.40 ± 38.83 | 70.89 ± 39.63 | 0.01 | 0.01 | 0.006-0.015 | < 0.001 |
| AST (U/L) | 26.19 ± 14.73 | 27.64 ± 15.36 | 24.83 ± 16.01 | 0.19 | |||
| ALT (U/L) | 43.89 ± 30.07 | 48.50 ± 31.54 | 42.16 ± 31.87 | 0.11 | |||
| TG (mmol/L) | 1.62 ± 0.83 | 1.35 ± 0.87 | 1.09 ± 0.47 | < 0.001 | -0.56 | -1.132 | < 0.001 |
| TC (mmol/L) | 4.48 ± 1.15 | 4.27 ± 1.06 | 4.22 ± 1.01 | 0.16 | |||
| HDL (mmol/L) | 1.16 ± 0.31 | 1.21 ± 0.28 | 1.28 ± 0.44 | 0.1 | |||
| LDL (mmol/L) | 2.67 ± 0.93 | 2.48 ± 0.84 | 2.40 ± 0.80 | 0.12 | |||
| CHD (n) | 7 | 14 | 27 | 0.52 | |||
BMI: Body mass index; Ca: Calcium; P: Phosphorus; ALB: Albumin; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine transaminase; TG: Triglyceride; TC: Total cholesterol; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; CHD: Coronary heart diseases.
Patients were divided into two groups according to age (65-79 years and ≥ 80 years). To analyze sex differences, a total of 106 men and 188 women were included in the 65- to 79-year age group (Table 2). As shown in Table 2, there were significant differences in the T score and Ca, P, ALB, ALP, TC, HDL and LDL concentrations between male and female patients. However, male patients showed no difference in age, BMI, or AST, ALT and TG concentrations in comparison with female patients.
Table 2.
Male and female patients aged between 65 to 79 years old in related factors for osteoporosis
|
|
Male (n = 106)
|
Female (n = 188)
|
P value
|
| Age (year) | 71.25 ± 4.39 | 72.18 ± 4.52 | 0.095 |
| T-value | -1.51 ± 0.94 | -2.5 ± 1.03 | < 0.0001 |
| BMI (kg/m2) | 24.33 ± 3.52 | 23.63 ± 3.37 | 0.134 |
| ≤ 18.5 (n) | 3 (2.83) | 13 (6.91) | 0.316 |
| 18.5-24(n) | 48 (45.28) | 85 (45.21) | |
| ≥ 24 (n) | 55 (51.89) | 90 (47.88) | |
| Ca (mmol/L) | 2.21 ± 0.13 | 2.25 ± 0.14 | 0.027 |
| P (mmol/L) | 1.00 ± 0.19 | 1.06 ± 0.14 | 0.01 |
| ALB (g/L) | 38.40 ± 4.11 | 39.67 ± 4.21 | 0.013 |
| ALP (U/L) | 69.29 ± 39.15 | 77.79 ± 35.53 | 0.032 |
| AST (U/L) | 26.86 ± 18.85 | 27.30 ± 19.34 | 0.258 |
| ALT (U/L) | 40.06 ± 27.97 | 38.94 ± 28.56 | 0.953 |
| TG (mmol/L) | 1.39 ± 0.73 | 1.45 ± 0.91 | 0.727 |
| TC (mmol/L) | 4.15 ± 0.95 | 4.52 ± 1.11 | 0.005 |
| HDL (mmol/L) | 1.13 ± 0.26 | 1.33 ± 0.45 | < 0.001 |
| LDL (mmol/L) | 2.42 ± 0.83 | 2.68 ± 0.86 | 0.048 |
BMI: Body mass index; Ca: Calcium; P: Phosphorus; ALB: Albumin; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine transaminase; TG: Triglyceride; TC: Total cholesterol; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
A total of 72 men and 154 women aged ≥ 80 years were examined in our study. The T score was significantly different in male patients compared to female patients. There were no significant differences in BMI or Ca, P, ALB, ALP, AST, ALT, TG, TC, HDL and LDL concentrations between male and female patients (Table 3).
Table 3.
Male and female patients aged ≥ 80 years old in related factors for osteoporosis
|
|
Male (n = 72)
|
Female (n = 154)
|
P value
|
| Age (year) | 85.60 ± 4.08 | 85.65 ± 33.89 | 0.839 |
| T-value | -2.06 ± 1.21 | -2.94 ± 1.29 | < 0.0001 |
| BMI (kg/m2) | 22.34 ± 3.26 | 21.68 ± 3.12 | 0.15 |
| ≤ 18.5 (n) | 10 (13.89) | 22 (14.29) | 0.829 |
| 18.5-24 (n) | 41 (56.94) | 93 (60.39) | |
| ≥ 24 (n) | 21 (29.17) | 39 (25.32) | |
| Ca (mmol/L) | 2.20 ± 0.17 | 2.21 ± 0.19 | 0.234 |
| P (mmol/L) | 1.01 ± 0.28 | 1.09 ± 0.58 | 0.092 |
| ALB (g/L) | 38.19 ± 4.34 | 38.04 ± 4.68 | 0.823 |
| ALP (U/L) | 56.21 ± 38.95 | 52.95 ± 40.44 | 0.455 |
| AST (U/L) | 23.61 ± 9.93 | 25.08 ± 10.77 | 0.238 |
| ALT (U/L) | 45.61 ± 28.78 | 54.45 ± 36.12 | 0.06 |
| TG (mmol/L) | 1.07 ± 0.44 | 1.08 ± 0.49 | 0.857 |
| TC (mmol/L) | 4.03 ± 0.99 | 4.20 ± 1.03 | 0.171 |
| HDL (mmol/L) | 1.17 ± 0.30 | 1.21 ± 0.32 | 0.425 |
| LDL (mmol/L) | 2.31 ± 0.78 | 2.29 ± 0.78 | 0.926 |
BMI: Body mass index; Ca: Calcium; P: Phosphorus; ALB: Albumin; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine transaminase; TG: Triglyceride; TC: Total cholesterol; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
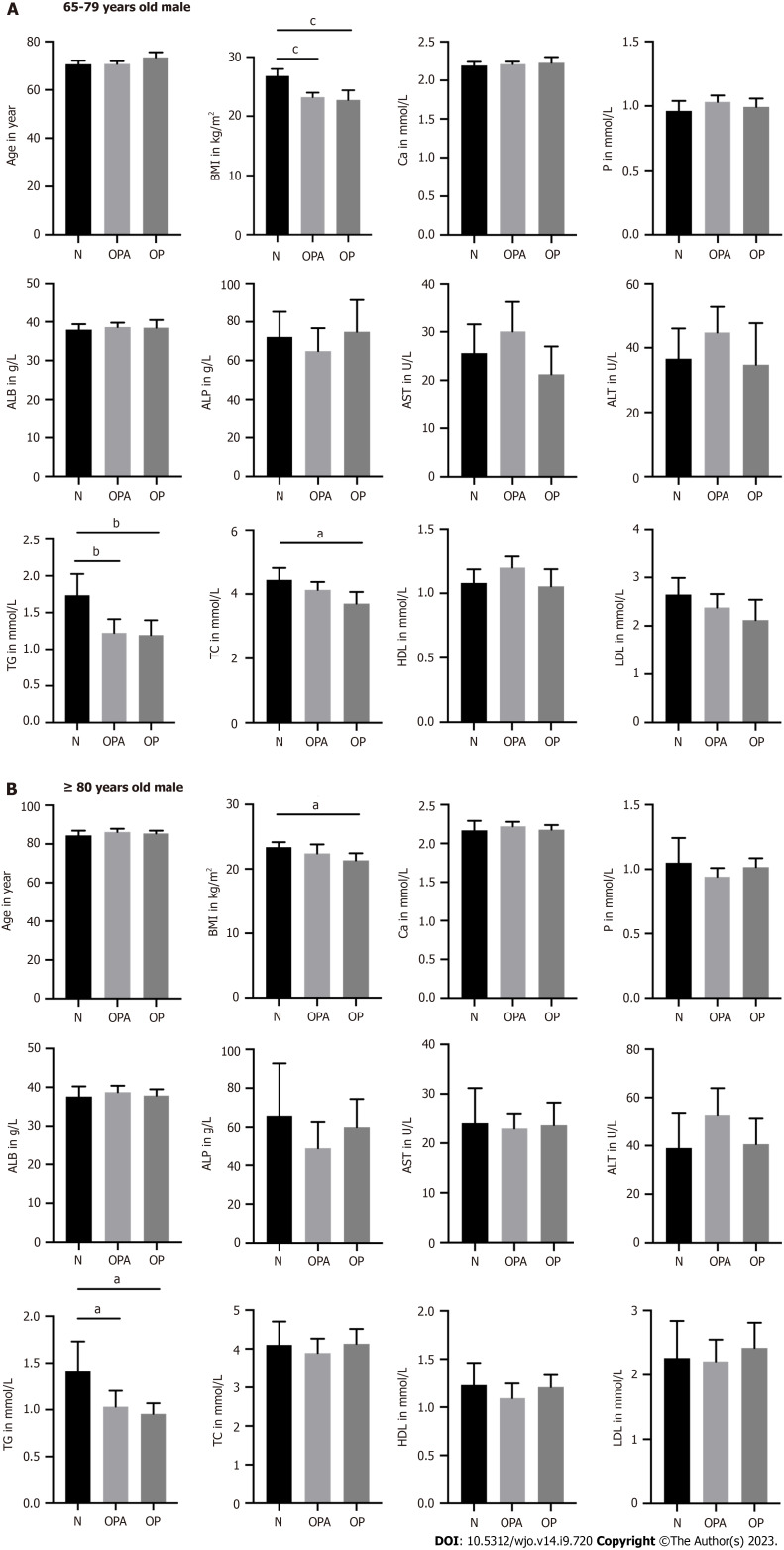
In the group of 65- to 79-year-old male patients, BMI was lower in osteoporotic patients than in patients with normal bone mass. Furthermore, we found a positive correlation between BMI and T-value in the groups of male patients (Supplementary Figure 1). Moreover, TG and TC levels were also lower in patients with osteoporosis compared to those with normal bone mass. Osteoporotic patients showed no significant differences in Ca, P, ALB, ALP, AST, ALT, HDL and LDL levels compared to patients with normal bone mass. In men aged ≥ 80 years, BMI was significantly lower in osteoporotic patients than in patients with normal bone mass. We also observed decreased TG levels in osteoporotic patients compared to those in patients with normal bone mass. Ca, P, ALB, ALP, AST, ALT, TC, HDL and LDL levels were not significantly different between patients with and without osteoporosis (Figure 1).
Figure 1.
The differences of age, body mass index, calcium, phosphorus, albumin, alkaline phosphatase, aspartate aminotransferase, alanine transaminase, triglyceride, total cholesterol, high-density lipoprotein, and low-density lipoprotein in male patients. A: Presented the group of 65-79 years old man; N(N) = 35, N(OPA) = 50, N(OP) = 21; B: Presented the group of ≥ 80 years old man; N(N) = 13, N(OPA) = 31, N(OP) = 28. aP < 0.05, bP < 0.01 and cP < 0.001 as different significance levels. BMI: Body mass index; Ca: Calcium; P: Phosphorus; ALB: Albumin; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine transaminase; TG: Triglyceride; TC: Total cholesterol; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; N: Normal bone mass; OPA: Osteopenia; OP: Osteoporosis.
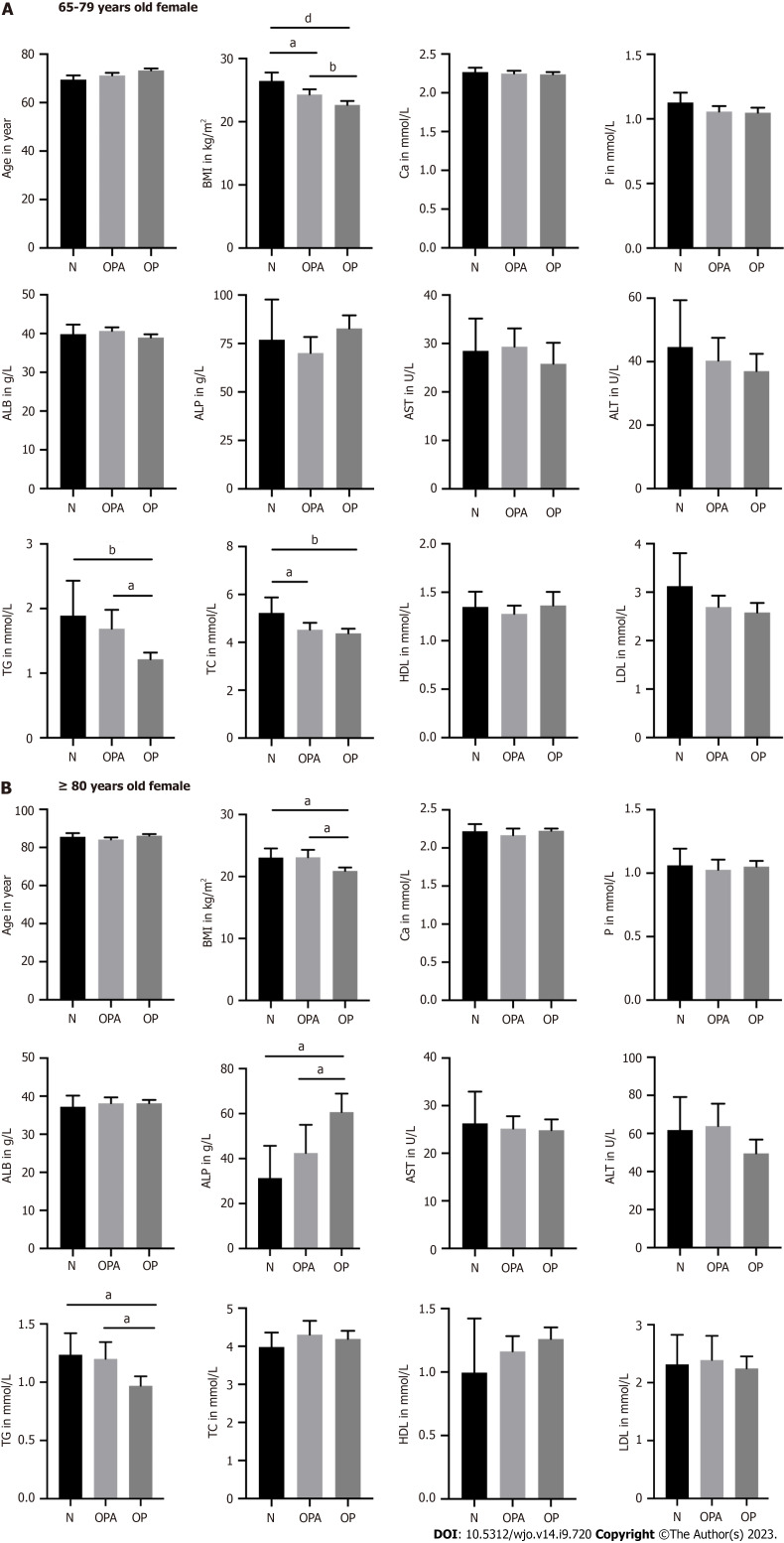
In the group of 65- to 79-year-old females, BMI and TG and TC levels were significantly lower in osteoporotic patients than patients with normal bone mass. We also found a positive correlation between BMI and T-value in the groups of female patients (Supplementary Figure 1). Osteoporotic patients also showed no significant differences in Ca, P, ALB, ALP, AST, ALT, HDL and LDL levels compared to patients with normal bone mass. In the group of women aged ≥ 80 years, osteoporotic patients showed significantly decreased BMI and TG levels as well as increased ALP levels. We also did not find significant differences in the Ca, P, ALB, AST, ALT, TC, HDL and LDL levels in osteoporotic patients compared with patients with normal bone mass (Figure 2).
Figure 2.
The differences of age, body mass index, calcium, phosphorus, albumin, alkaline phosphatase, aspartate aminotransferase, alanine transaminase, triglyceride, total cholesterol, high-density lipoprotein, and low-density lipoprotein in female patients. A: Presented the group of 65-79 years old female; N(N) = 19, N(OPA) = 65, N(OP) = 104; B: Presented the group of ≥ 80 years old female; N(N) = 17, N(OPA) = 38, N(OP) = 99. aP < 0.05, bP < 0.01 and dP < 0.0001 as different significance levels. BMI: Body mass index; Ca: Calcium; P: Phosphorus; ALB: Albumin; ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine transaminase; TG: Triglyceride; TC: Total cholesterol; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; N: Normal bone mass; OPA: Osteopenia; OP: Osteoporosis.
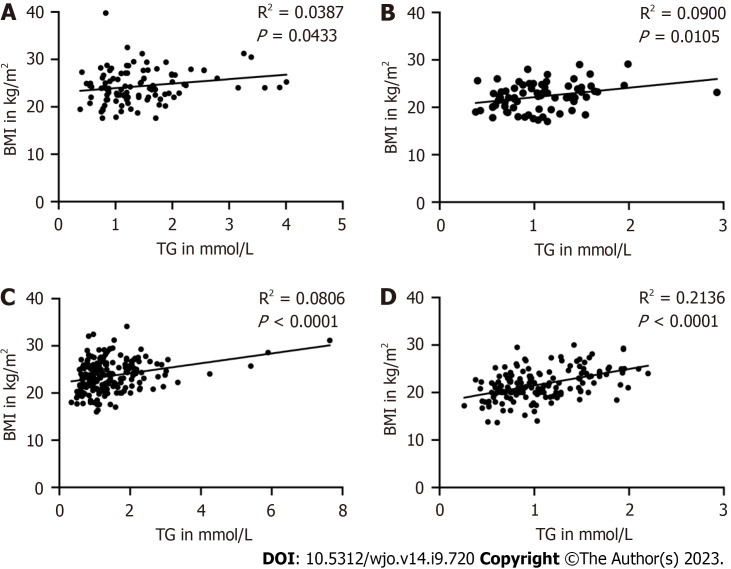
Furthermore, we found a positive correlation between BMI and TG levels in the groups of male patients aged 65-79 and ≥ 80 years. A positive correlation was also found between BMI and TG levels in the groups of 65-79- and ≥ 80-year-old female patients (Figure 3).
Figure 3.
The correlation between body mass index and triglyceride in male and female patients. A: Presented the group of 65-79 years old male patients; n = 106; B: Presented the group of ≥ 80 years old male patients; n = 72; C: Presented the group of 65-79 years old female patients; n = 188; D: Presented the group of ≥ 80 years old female patients; n = 154. P < 0.05 as a different significance level. BMI: Body mass index; TG: Triglyceride.
DISCUSSION
In our study, we analyzed the clinical data of 520 patients aged ≥ 65 years. We found that osteoporotic patients showed significantly decreased BMI and TG levels in comparison with patients with normal bone mass. The number of patients diagnosed with osteoporosis is increasing annually, and previous studies have shown that many factors contribute to osteoporosis, including age, sex, BMI, and Ca, P, ALB, ALP, AST, ALT, TG, TC, HDL and LDL levels.
Previous studies have reported that many factors contribute to osteoporosis[3,22]. In our study, we found that age, sex, BMI, and ALP and TG levels were independent risk factors for osteoporosis. With increasing age, a series of factors are altered, such as a lack of sex steroids, declining levels of growth factors and changes in food intake, exercise and mechanical loading, leading to bone loss. Based on this, we classified the groups according to age. Regarding the relationship between sex and bone mass, previous studies have demonstrated that men tend to have a higher BMD at a later age and a lower rate of bone mass loss than women[23,24], suggesting that bone mass loss differs by sex. In our study, female patients in the 65-79- and ≥ 80-year-old groups presented lower T scores than male patients. The reason for the differences in bone mass between sexes might be that hormones differ in males and females[23]. Moreover, significant differences were observed in the Ca, P, ALB, ALP, TC, HDL and LDL levels between men and women in the 65- to 79-year-old group. These results indicate that men and women present differences in many factors for osteoporosis. Cui et al[7] supported our results in their analysis of 1035 men and 3953 women, where they also showed significant differences in BMI and T scores, as well as in TG, TC, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels, between men and women. According to their and our results, we classified the groups by sex and age (65-79 and ≥ 80 years old).
Previous studies have revealed the correlation among lipid profiles, BMI, and BMD[7,25,26]. In our study, we found that male and female osteoporotic patients showed significantly decreased BMI in the groups aged 65-79 and ≥ 80 years. Supporting our findings, many studies have reported an association between BMI and BMD. Alay et al[27] investigated 452 postmenopausal women in an outpatient clinic between 2012 and 2015 and observed that a decreased BMI was related to a lower BMD. In addition, Fawzy et al[28] analyzed 101 men and women and found that a decline in BMI was associated with a lower BMD. The possible mechanisms for the association between BMI and BMD might be that weight gain places greater static mechanical loads on the bones and increases various hormone levels, which are beneficial for bone mass and bone remodelling[26,29-31].
Moreover, our study demonstrated that male and female osteoporotic patients presented obviously decreased TG levels in the groups aged 65-79 years and ≥ 80 years. In addition, male and female osteoporotic patients also showed obviously decreased TC levels in the group aged 65-79 years. However, we did not find any significant differences in the HDL and LDL levels between the two groups in our study. Although previous studies have reported the association between serum lipid profiles and BMD, the literature is conflicting. Our study findings were in agreement with those of Cui et al[7], who showed that BMI and TG levels were significantly lower in patients with osteoporosis than in those with normal BMD. However, there was no significant difference in TC levels. In a meta-analysis, Chen et al[32] selected ten publications and investigated the relationship between lipid profiles (HDL, LDL, TG, and TC levels) and osteoporosis in postmenopausal women, finding significantly higher HDL and TC levels in the postmenopausal osteoporosis group than in the normal BMD group. Although the difference was not significant, postmenopausal osteoporosis patients presented with a lower TG level. Alfahal et al[33] also reported a lower TG level in postmenopausal individuals with osteoporosis compared with individuals with normal bone mass individuals. However, Bijelic et al[34] evaluated lipid profiles and BMI in 100 postmenopausal osteoporotic patients and patients with normal BMD. They proved that in postmenopausal patients, osteoporosis was associated with LDL, TC, and TG levels. Contrary to our results, they showed increased TG levels in osteoporotic patients compared with those with a normal BMD. The reason might be that, in their study, there were 72 overweight patients in the osteoporosis group and 54 overweight patients in the normal BMD group. However, Alay et al[27] did not observe significant differences in HDL, LDL, TG, and TC levels between groups with normal bone mass and osteoporosis. BMI and TG levels were independent risk factors for osteoporosis in our study. Moreover, we also observed a positive correlation between BMI and TG levels in the groups of male and female patients aged 65-79 and ≥ 80 years. These results suggest a positive relationship among BMI, TG levels and BMD.
It has been reported that lipids may be a predisposing factor for osteoporosis and are associated with bone fragility[11], and TG metabolism is considered to be correlated with bone metabolism. Dragojevič et al[35] performed a gene expression study by analysing bone tissue from 50 patients with osteoporosis and 62 controls. They reported that osteoporosis patients had decreased osteoblastogenesis, increased osteoclastogenesis, and lower TG metabolism than controls. TGs are considered an important form for the storage of fatty acids. Fatty acids are essential components of all lipids and are involved in producing energy in all vertebrates; fatty acid metabolism plays essential roles in osteoblast and osteoclast function and activities as well as in bone remodelling[36-38]. This could be one explanation for the relationship between serum TG levels and bone remodelling. However, the underlying mechanism between TG metabolism and bone metabolism remains unclear, and more studies should be performed in the future.
In addition, we did not observe significant differences in other factors for osteoporosis, such as Ca, P, AST, ALT and ALB levels, between the osteoporosis and control groups in our study. Regarding the association between serum Ca and P levels and osteoporosis, many studies have shown different results. Our study reported that serum Ca and P levels were normal in postmenopausal women with or without osteoporosis. This may be because the levels of Ca and P in serum may not reflect their storage in bones. Serum Ca and P levels are regulated, and homeostasis is maintained in serum[39,40]. In previous studies, nutrition was reported to be related to osteoporosis, and serum ALB levels were lower in patients with osteoporosis[41,42]. By analysing the serum ALB levels of 15539 individuals, hypoalbuminemia (serum ALB less than 3.5 g/dL) was found to be associated with osteoporosis[42]. However, the mean serum ALB levels were more than 35 g/L in patients with normal bone mass and osteoporotic patients and did not show differences between groups in our study. This might be the reason why serum ALB was not a factor for diagnosing osteoporosis in our study. AST and ALT are liver enzymes, and they were also reported to be associated with osteoporosis. In agreement with our study, Do et al[9] selected 7160 subjects to analyse the association between liver enzymes and BMD in Koreans. They also did not show differences in the association of AST and ALT with femur BMD. We discovered that ALP was an independent factor for osteoporosis, and osteoporotic female patients aged ≥ 80 years showed significantly increased ALP levels. Supporting our results, increased serum ALP levels in postmenopausal women indicate high bone turnover and are helpful for diagnosing osteoporosis[43-45].
This study investigated the interplay between BMI, lipid profile, and bone mass within a population vulnerable to brittle fractures. Our research has several strengths. Firstly, our study utilized blood samples collected from fragility fracture patients upon admission to assess pertinent lipid metabolism markers. Secondly, individuals with fragility fractures frequently experience bone loss or osteoporosis. Our findings introduce a novel perspective for future endeavours in osteoporosis and fragility fracture prevention. Furthermore, our hierarchical analysis provides preliminary results, enhancing the generalizability of our research outcomes.
Nonetheless, our research bears certain limitations. Firstly, being a single-centre study, not all patients had lipid metabolism indicator data available for analysis due to admission discrepancies among fragility fracture patients, which resulted in a significant reduction in sample size. Among patients undergoing DXA assessments, the T-value of the proximal femur was the only site measurement employed for standardization, with lumbar and radius measurements being omitted. Furthermore, follow-up data for the study cohort is absent, impeding outcome comparison. Moving forward, we aspire to extend this discourse through a multicentre, large-sample study. Additionally, we intend to investigate the nexus between BMI, lipid profile, and bone mass via prospective studies involving nutritional interventions in patients.
CONCLUSION
In conclusion, osteoporotic patients showed significantly decreased BMI and TG levels in comparison with patients with normal bone mass in our study. These results indicate an association between TG metabolism and bone metabolism and provide a new method for the treatment of osteoporosis.
ARTICLE HIGHLIGHTS
Research background
The increasing incidence of osteoporosis and low bone mass, affecting a significant portion of individuals aged 50 years or older in China, underscores the urgent need to address this public health concern. While obesity's potential protective role and the complex interplay between body mass index (BMI), lipid profiles, and bone health are subjects of recent investigation, their specific impact on populations with osteoporosis and fragility fractures remains relatively unexplored.
Research motivation
The relationship between BMI, bone mass, and lipid profiles in populations with osteoporosis and fragility fractures remains understudied.
Research objectives
This study aims to shed light on the potential impact of weight gain and lipid profiles on bone health in individuals with fragility fractures which may provide a new method for the treatment of osteoporosis.
Research methods
This retrospective study conducted at a single orthopaedic trauma center between January 2017 and December 2020 included participants aged 65 years and above with diagnosed fractures in specific region. Participants' comprehensive clinical data, including gender, age groups, BMI ranges, DXA scores, and laboratory measurements, were collected and analyzed using statistical software. Data were analyzed using IBM SPSS and GraphPad Prism software, employing t-tests, nonparametric tests, one-way ANOVA, multiple linear regression, and Pearson correlation tests (aP < 0.05, bP < 0.01, cP < 0.001, dP < 0.0001 denoting significance) after assessing normality with the Shapiro-Wilk test.
Research results
In this study involving 520 participants aged ≥ 65 years, distinct gender and age-related disparities were observed in osteoporosis prevalence and associated factors. While a significant divergence in age, sex, BMI, alkaline phosphatase (ALP), and triglyceride (TG) concentrations was noted among normal, osteopenic, and osteoporotic groups, multivariate analysis revealed age, sex, BMI, ALP, and TG concentrations as independent risk factors for osteoporosis. Differential correlations between BMI and bone health parameters, along with lipid profiles, were elucidated across age and gender cohorts. Notably, these findings underscore the intricate interplay between metabolic and skeletal factors in the context of osteoporosis.
Research conclusions
In conclusion, osteoporotic patients showed significantly decreased BMI and TG levels in comparison with patients with normal bone mass in our study.
Research perspectives
These results indicate an association between TG metabolism and bone metabolism and provide a new method for the treatment of osteoporosis.
Footnotes
Institutional review board statement: The study was reviewed and approved by the IEC for Clinical Research of Zhongda Hospital, Affiliated to Southeast University [Approval No.2022ZDSYLL183-P01].
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no competing financial interests or conflicts of interest that could have influenced the design, data collection, analysis, interpretation, or publication of this study.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 6, 2023
First decision: August 4, 2023
Article in press: August 29, 2023
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bondarenko S, Ukraine; Roomi AB, Iraq S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
Contributor Information
Xiang-Xu Chen, Department of Orthopaedics, Trauma Center, Southeast University, Nanjing 210009, Jiangsu Province, China.
Chu-Wei Tian, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Li-Yong Bai, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Ya-Kuan Zhao, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Cheng Zhang, Department of Orthopaedics, Trauma Center, Southeast University, Nanjing 210009, Jiangsu Province, China.
Liu Shi, Department of Orthopaedics, Trauma Center, Southeast University, Nanjing 210009, Jiangsu Province, China.
Yuan-Wei Zhang, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Wen-Jun Xie, Department of Orthopaedics, Trauma Center, Southeast University, Nanjing 210009, Jiangsu Province, China.
Huan-Yi Zhu, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Hui Chen, Department of Orthopaedics, Trauma Center, Southeast University, Nanjing 210009, Jiangsu Province, China.
Yun-Feng Rui, Department of Orthopaedics, Trauma Center, Southeast University, Nanjing 210009, Jiangsu Province, China. ruiyunfeng@126.com.
Data sharing statement
The data presented in this study are available upon reasonable request to qualified researchers for the purpose of academic and scientific collaboration. Requests for data access should be directed to the corresponding author at ruiyunfeng@126.com.
References
- 1.Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. 2016;16:1039. doi: 10.1186/s12889-016-3712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinese Orthopaedic Association. Diagnosis and treatment of osteoporotic fractures. Orthop Surg. 2009;1:251–257. doi: 10.1111/j.1757-7861.2009.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayak S, Olkin I, Liu H, Grabe M, Gould MK, Allen IE, Owens DK, Bravata DM. Meta-analysis: accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med. 2006;144:832–841. doi: 10.7326/0003-4819-144-11-200606060-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 7.Cui R, Zhou L, Li Z, Li Q, Qi Z, Zhang J. Assessment risk of osteoporosis in Chinese people: relationship among body mass index, serum lipid profiles, blood glucose, and bone mineral density. Clin Interv Aging. 2016;11:887–895. doi: 10.2147/CIA.S103845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Kumar D, Lal AK. Serum Osteocalcin as a Diagnostic Biomarker for Primary Osteoporosis in Women. J Clin Diagn Res. 2015;9:RC04–RC07. doi: 10.7860/JCDR/2015/14857.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do HJ, Shin JS, Lee J, Lee YJ, Kim MR, Nam D, Kim EJ, Park Y, Suhr K, Ha IH. Association between liver enzymes and bone mineral density in Koreans: a cross-sectional study. BMC Musculoskelet Disord. 2018;19:410. doi: 10.1186/s12891-018-2322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan B, Zhao Q, Wang L, Xue S, Cai H, Yang S. Association between lipid biomarkers and osteoporosis: a cross-sectional study. BMC Musculoskelet Disord. 2021;22:759. doi: 10.1186/s12891-021-04643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Tian H, Pan J, Qiao D, Dong X, Li R, Wang Y, Tu R, Abdulai T, Liu X, Hou J, Zhang G, Wang C. Adiposity reduces the risk of osteoporosis in Chinese rural population: the Henan rural cohort study. BMC Public Health. 2020;20:285. doi: 10.1186/s12889-020-8379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bierhals IO, Dos Santos Vaz J, Bielemann RM, de Mola CL, Barros FC, Gonçalves H, Wehrmeister FC, Assunção MCF. Associations between body mass index, body composition and bone density in young adults: findings from a southern Brazilian cohort. BMC Musculoskelet Disord. 2019;20:322. doi: 10.1186/s12891-019-2656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep. 2005;3:46–51. doi: 10.1007/s11914-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 16.Papageorgiou M, Kerschan-Schindl K, Sathyapalan T, Pietschmann P. Is Weight Loss Harmful for Skeletal Health in Obese Older Adults? Gerontology. 2020;66:2–14. doi: 10.1159/000500779. [DOI] [PubMed] [Google Scholar]
- 17.Knoke JD, Barrett-Connor E. Weight loss: a determinant of hip bone loss in older men and women. The Rancho Bernardo Study. Am J Epidemiol. 2003;158:1132–1138. doi: 10.1093/aje/kwg265. [DOI] [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR Study of Osteoporotic Fractures Research Group. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 19.Noakes M, Clifton PM. Weight loss and plasma lipids. Curr Opin Lipidol. 2000;11:65–70. doi: 10.1097/00041433-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Kontogianni MD, Panagiotakos DB, Skopouli FN. Does body mass index reflect adequately the body fat content in perimenopausal women? Maturitas. 2005;51:307–313. doi: 10.1016/j.maturitas.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Migliorini F, Giorgino R, Hildebrand F, Spiezia F, Peretti GM, Alessandri-Bonetti M, Eschweiler J, Maffulli N. Fragility Fractures: Risk Factors and Management in the Elderly. Medicina (Kaunas) 2021;57 doi: 10.3390/medicina57101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14:2029–2049. doi: 10.2147/TCRM.S138000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alswat KA. Gender Disparities in Osteoporosis. J Clin Med Res. 2017;9:382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compston J. Clinical and therapeutic aspects of osteoporosis. Eur J Radiol. 2009;71:388–391. doi: 10.1016/j.ejrad.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 25.Morin S, Leslie WD Manitoba Bone Density Program. High bone mineral density is associated with high body mass index. Osteoporos Int. 2009;20:1267–1271. doi: 10.1007/s00198-008-0797-6. [DOI] [PubMed] [Google Scholar]
- 26.Ma M, Feng Z, Liu X, Jia G, Geng B, Xia Y. The Saturation Effect of Body Mass Index on Bone Mineral Density for People Over 50 Years Old: A Cross-Sectional Study of the US Population. Front Nutr. 2021;8:763677. doi: 10.3389/fnut.2021.763677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alay I, Kaya C, Cengiz H, Yildiz S, Ekin M, Yasar L. The relation of body mass index, menopausal symptoms, and lipid profile with bone mineral density in postmenopausal women. Taiwan J Obstet Gynecol. 2020;59:61–66. doi: 10.1016/j.tjog.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Fawzy T, Muttappallymyalil J, Sreedharan J, Ahmed A, Alshamsi SO, Al Ali MS, Al Balsooshi KA. Association between Body Mass Index and Bone Mineral Density in Patients Referred for Dual-Energy X-Ray Absorptiometry Scan in Ajman, UAE. J Osteoporos. 2011;2011:876309. doi: 10.4061/2011/876309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanyon LE. Control of bone architecture by functional load bearing. J Bone Miner Res. 1992;7 Suppl 2:S369–S375. doi: 10.1002/jbmr.5650071403. [DOI] [PubMed] [Google Scholar]
- 30.Movérare-Skrtic S, Wu J, Henning P, Gustafsson KL, Sjögren K, Windahl SH, Koskela A, Tuukkanen J, Börjesson AE, Lagerquist MK, Lerner UH, Zhang FP, Gustafsson JÅ, Poutanen M, Ohlsson C. The bone-sparing effects of estrogen and WNT16 are independent of each other. Proc Natl Acad Sci U S A. 2015;112:14972–14977. doi: 10.1073/pnas.1520408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232:R99–R113. doi: 10.1530/JOE-16-0405. [DOI] [PubMed] [Google Scholar]
- 32.Chen YY, Wang WW, Yang L, Chen WW, Zhang HX. Association between lipid profiles and osteoporosis in postmenopausal women: a meta-analysis. Eur Rev Med Pharmacol Sci. 2018;22:1–9. doi: 10.26355/eurrev_201801_14093. [DOI] [PubMed] [Google Scholar]
- 33.Alfahal AO, Ali AE, Modawe GO, Doush WM. Association between serum lipid profile, body mass index and osteoporosis in postmenopausal Sudanese women. Afr Health Sci. 2022;22:399–406. doi: 10.4314/ahs.v22i3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bijelic R, Balaban J, Milicevic S. Correlation of the Lipid Profile, BMI and Bone Mineral Density in Postmenopausal Women. Mater Sociomed. 2016;28:412–415. doi: 10.5455/msm.2016.28.412-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragojevič J, Zupan J, Haring G, Herman S, Komadina R, Marc J. Triglyceride metabolism in bone tissue is associated with osteoblast and osteoclast differentiation: a gene expression study. J Bone Miner Metab. 2013;31:512–519. doi: 10.1007/s00774-013-0445-x. [DOI] [PubMed] [Google Scholar]
- 36.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Kushwaha P, Wolfgang MJ, Riddle RC. Fatty acid metabolism by the osteoblast. Bone. 2018;115:8–14. doi: 10.1016/j.bone.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Oh B, Park-Min KH. Regulation of Osteoclast Differentiation and Activity by Lipid Metabolism. Cells. 2021;10 doi: 10.3390/cells10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali NK. Estimation of Some Mineral (Calcium, Phosphorous, Vitamin 25(OH) D, And Alkaline Phosphatase) in Osteoporosis Patients In Kirkuk City. J Osteopor Phys Act. 2018;6:215. [Google Scholar]
- 40.Jayaram N, Bijoor AR, Rajagopalan N, Venkatesh T. The value of serum and urinary n-telopeptide in the diagnosis of osteoporosis. Indian J Orthop. 2002 [Google Scholar]
- 41.Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients. 2020;12 doi: 10.3390/nu12071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afshinnia F, Pennathur S. Association of Hypoalbuminemia With Osteoporosis: Analysis of the National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2016;101:2468–2474. doi: 10.1210/jc.2016-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5:18. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fink HA, Litwack-Harrison S, Taylor BC, Bauer DC, Orwoll ES, Lee CG, Barrett-Connor E, Schousboe JT, Kado DM, Garimella PS, Ensrud KE Osteoporotic Fractures in Men (MrOS) Study Group. Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int. 2016;27:331–338. doi: 10.1007/s00198-015-3356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res. 2015;27:413–418. doi: 10.1007/s40520-014-0296-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon reasonable request to qualified researchers for the purpose of academic and scientific collaboration. Requests for data access should be directed to the corresponding author at ruiyunfeng@126.com.