Abstract
Background
Pulse oximetry has been used in medical care for decades. Its use quickly became standard of care in high resource settings, with delayed widespread availability and use in lower resource settings. Pulse oximetry training initiatives have been ongoing for years, but a map of the literature describing such initiatives among health care workers in low- and middle-income countries (LMICs) has not previously been conducted. Additionally, the coronavirus disease 2019 (COVID-19) pandemic further highlighted the inequitable distribution of pulse oximetry use and training. We aimed to characterise the landscape of pulse oximetry training for health care workers in LMICs prior to the COVID-19 pandemic as described in the literature.
Methods
We systematically searched six databases to identify studies reporting pulse oximetry training among health care workers, broadly defined, in LMICs prior to the COVID-19 pandemic. Two reviewers independently assessed titles and abstracts and relevant full texts for eligibility. Data were charted by one author and reviewed for accuracy by a second. We synthesised the results using a narrative synthesis.
Results
A total of 7423 studies were identified and 182 screened in full. A total of 55 training initiatives in 42 countries met inclusion criteria, as described in 66 studies since some included studies reported on different aspects of the same training initiative. Five overarching reasons for conducting pulse oximetry training were identified: 1) anaesthesia and perioperative care, 2) respiratory support programme expansion, 3) perinatal assessment and monitoring, 4) assessment and monitoring of children and 5) assessment and monitoring of adults. Educational programmes varied in their purpose with respect to the types of patients being targeted, the health care workers being instructed, and the depth of pulse oximetry specific training.
Conclusions
Pulse oximetry training initiatives have been ongoing for decades for a variety of purposes, utilising a multitude of approaches to equip health care workers with tools to improve patient care. It is important that these initiatives continue as pulse oximetry availability and knowledge gaps remain. Neither pulse oximetry provision nor training alone is enough to bolster patient care, but sustainable solutions for both must be considered to meet the needs of both health care workers and patients.
Pulse oximetry has been used in medical care since the 1970s; a decade later its use became standard of care in high resource settings, first in the perioperative space and subsequently for use in routine vital sign monitoring [1-3]. Numerous multilateral organisations and global public health initiatives associated with them recommend increased pulse oximetry training and use, from surgical and anaesthesia initiatives to child health and welfare programmes [4-7]. Despite this, pulse oximetry is not universally available, and the COVID-19 pandemic has further highlighted the inequitable distribution of pulse oximetry use and training, among other key health care capacity measures [8,9].
In the perioperative setting, pulse oximetry use in low-resource settings has lagged behind use in high-resource settings due, in part, to decreased oximetry availability and non-universal training initiatives for all health care workers [10]. Studies have demonstrated a need for continued strengthening of pulse oximetry use and training given identified capacity and knowledge gaps across multiple countries and additional practice areas, including trauma, obstetrics, paediatrics and neonatology [11-16].
Pulse oximetry training initiatives have been ongoing for years, but a map of the literature describing such initiatives among health care workers in low- and middle-income countries (LMICs) has not previously been conducted. To continue making progress in strengthening health care delivery, it is important to understand what pulse oximetry training initiatives have been done, the settings in which they have been conducted, and the health care workers who have been prioritised for training. We aimed to characterise the landscape of pulse oximetry training for health care workers in LMICs prior to the COVID-19 pandemic as described in the literature. To do this, we aim to address the following evaluation questions as part of our scoping review: 1) who (i.e. what type of health care workers) are being trained to use pulse oximetry in LMICs?; 2) what resources are used and how is training structured?; 3) when have they been trained?; 4) where are they being trained?; 5) why are they being trained (i.e. for what application of pulse oximetry)?; 6) how effective has the training been?
METHODS
Protocol
We developed a protocol a priori using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P). Given the aim to map literature related to pulse oximetry training among health care workers in LMICs prior to the COVID-19 pandemic, a scoping review was deemed the most appropriate methodology [17]. This scoping review is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist presented in Table S1 in the Online Supplementary Document [18].
Search strategy and sources of evidence
We systematically searched 6 databases (PubMed, Embase, CINAHL, Cochrane Library, Scopus, and World Health Organization (WHO) Global Index Medicus) to identify studies reporting pulse oximetry training among health care workers, broadly defined, in LMICs prior to the COVID-19 pandemic. Search terms and MeSH headings related to pulse oximetry, LMIC countries, and education initiatives were developed in PubMed and adapted for other databases in collaboration with a specialist librarian presented in Table S2 in the Online Supplementary Document. We searched databases from their inception to 14 May 2021 and made no language exclusions. No exclusions were made based on study design and letters to the editor and editorials were also assessed for inclusion criteria if they were identified in the database searches. References of identified studies and relevant reviews and editorials were also reviewed. We attempted to locate full texts of studies for relevant conference abstracts and study protocols through hand searching. If no full study could be identified, protocols were excluded and abstracts were included if there was enough information for data charting; otherwise, they were also excluded.
Assessment of eligibility
We imported all references to Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org), where duplicates were removed. Two reviewers (MEP and SD or DRRB) independently assessed titles and abstracts and relevant full texts for eligibility. Studies were eligible if they 1) described training health care workers from an LMIC as defined by The World Bank in calendar year 2020 [19], 2) described educational interventions surrounding the use of pulse oximeters and 3) the educational initiative occurred prior to 2020. Of note, pulse oximetry did not need to be the primary focus of the educational initiative. Studies were excluded if they 1) did not conduct pulse oximetry training in an LMIC, 2) only described the introduction of pulse oximeters without mention of any training received, such as capacity or knowledge assessments without an education or training component, or 3) pulse oximetry training was not conducted for health care workers.
Evidence synthesis
Data were charted using forms created in Covidence by one author (MEP, DRRB, JA or SA) and reviewed for accuracy by a second (MEP, DRRB, JA or SA), with a singular author (MEP) completing one of these steps for all included studies. Discrepancies were discussed between the two authors and a final decision made. Items charted from each study included: 1) training setting, 2) year of training, 3) population being trained, 4) reason for training, 5) training structure (pulse oximetry training materials used and length of training, when reported) and 6) pulse oximetry specific outcomes of training, when reported. If pulse oximetry specific outcomes were not reported, an alternative relevant study outcome was charted. We synthesised the results using a narrative synthesis.
RESULTS
A total of 7423 studies were identified and 182 screened in full. Of these, 66 met our inclusion criteria (Figure 1). A total of 55 training initiatives were identified in 42 countries, with some included studies reporting on different aspects of the same training initiative. Five overarching reasons for conducting pulse oximetry training were identified: 1) anaesthesia and perioperative care, 2) respiratory support programme expansion, 3) perinatal assessment and monitoring, 4) assessment and monitoring of children and 5) assessment and monitoring of adults.
Figure 1.
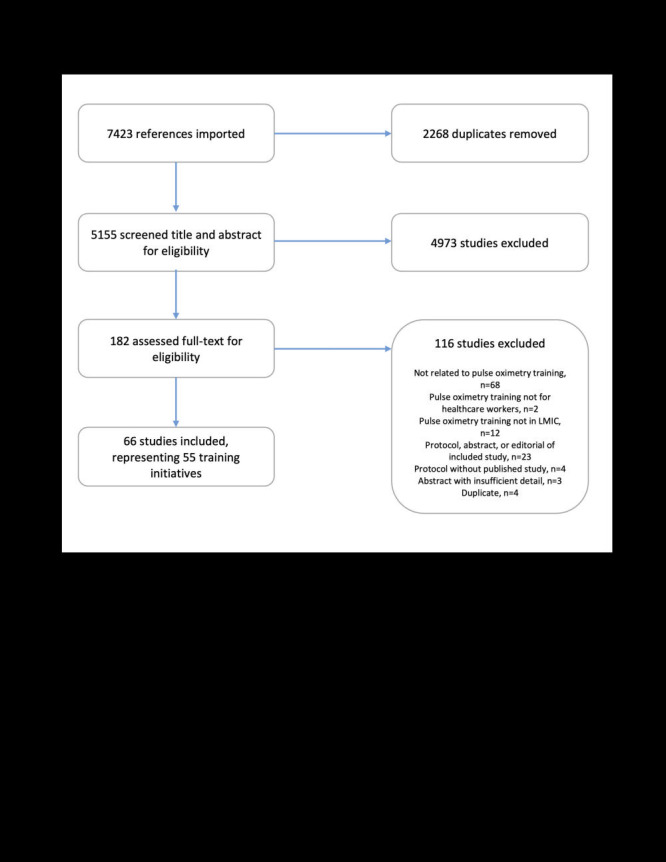
PRISMA flow diagram.
Training initiatives utilised a variety of structures, teaching styles and methods of assessment. They ranged from solely focused on pulse oximetry to being broader in their focus with pulse oximetry training being a small component of the overall educational agenda. Similarly, training ranged from a brief one-hour lecture to multiday workshops with practical skills assessments participants were required to pass. Some initiatives were longitudinal in nature and followed up with trainees overtime to assess continued pulse oximetry competency and use, while others studies’ only touchpoint with trainees was at the time of the initial training session. Such differences are further described in detail below in each respective section and results table.
Anaesthesia and perioperative care
Twenty-four studies described conducting pulse oximetry training focused on perioperative teams in 21 countries globally, including 15 in the African Region, one in the European Region, three in the South-East Asia Region, and two in the Western Pacific Region (Table 1). Of these, nine had low-Income designation, nine lower-middle income and three upper-middle income. The primary foci of these efforts involved anaesthesia capacity-building and establishing the use of the World Health Organization Surgical Safety Checklist (WHO SSC). Training focused on anaesthesia providers, surgeons and nurses. Although training structure, length, and evaluation methods differed, many studies reported an increased use of pulse oximetry after training. Often, pulse oximetry capabilities were limited by availability of pulse oximeters and, secondarily, staff trained in its use. Multiple studies report increased intraoperative monitoring as well as the establishment of postoperative monitoring capabilities after training and pulse oximetry provision.
Table 1.
Studies describing pulse oximetry training initiatives: Anaesthesia and perioperative care*
| WHERE: country (LMIC designation), sites | WHEN: year of training | WHO: population trained | WHY: purpose of training | WHAT: structure of training | HOW: pulse oximetry related outcomes |
|---|---|---|---|---|---|
|
African Region
| |||||
| Benin (UMIC), 36 hospitals nationwide [20] |
2016-2017 |
Surgeons, anaesthesia providers, nurses, and other perioperative staff |
SSC implementation |
3-d Mercy Ships led workshop |
Always using a pulse oximeter increased from 86.6 to 97.0% 12-18 mo after training among 17 hospitals selected for follow-up. |
| Burkina Faso (LIC), 57 hospitals nationwide [21] |
2013 |
Anaesthetists |
To improve the practice of pulse oximetry and SCC implementation |
Lifebox workshop |
Systematic use of pulse oximetry during anaesthesia increased from 73% to 100% of hospitals. Prior to training, 17% of hospitals had a PACU and used the pulse oximeter from theatre to monitor patients post-operatively. After training, 94% of hospitals used post-operative pulse oximetry monitoring. |
| Cameroon (LMIC), 25 hospitals nationwide [22] |
2017-2018 |
Operating room staff |
SSC implementation |
3-d multidisciplinary training course developed by Mercy Ships |
“Always” or “often” intraoperative pulse oximetry use increased from 74% before training to 93% 4 mo after training. |
| Republic of Congo (LMIC), 1 hospital in Dolisie [23] |
2014 |
Operating team personnel |
SSC implementation |
4-d pilot SSC training course developed by Mercy Ships |
Intraoperative pulse oximetry “always” use increased from 0% before training to 86% 15 mo after training. |
| Ethiopia (LIC), 1 hospital in Addis Ababa [24] |
2011-2012 |
Plastic and reconstructive surgery surgeons, anaesthetists, nurses, and other perioperative staff |
Implement anaesthetic pre-assessment, SSC, continuous pulse oximetry monitoring in recovery areas, improved observation protocols in recovery areas, and the development of an HDU |
Teaching sessions and simulation workshops |
Pulse oximetry used intraoperatively in 98% of cases during the 8 mo after training. Continuous pulse oximetry available for all recovery beds after training. |
| Ethiopia (LIC), 1 hospital in Addis Ababa (primarily), 9 hospitals in southwestern Ethiopia (pulse oximeters only) [25] |
2012-2018 |
Anaesthesia providers |
Improve morale and retention, establish postgraduate physician training, SSC implementation, develop PACU |
Lifebox workshop |
Pulse oximeters: 6-mo follow-up showed retained pulse oximetry knowledge and use. SSC: >90% use of SSC. PACU: Patients now monitored postoperatively in PACU. |
| Ghana (LMIC), 1 nurse anaesthetist school in Kumasi [26] |
Since 1987 |
Nurse anaesthetists |
To improve anaesthetic patient care and safety |
18-mo training programme in collaboration with University of Utah |
In 2000, pulse oximetry was not used in the affiliate hospital. In 2009, 70% of the district and regional hospitals use pulse oximetry, including in PACUs. |
| Ghana (LMIC), 1 nurse anaesthetist school in Accra [27] |
Since 2009 |
Nurse anaesthetists |
To increase the number of anaesthesia providers |
18-mo training programme |
95% of graduates (representing 39 hospitals across 7 of the 10 regions) surveyed had access to pulse oximetry at their hospital. |
| Guinea (LIC), 6 hospitals nationwide [28] |
2012-2013 |
Surgeons, anaesthetists, and nurses |
To evaluate three different methods of SSC implementation |
Training delivered by Mercy Ships: 1) team training in operating room and classroom (surgeon AND anaesthesia provider or nurse); 2) individual training in operating room and classroom (surgeon or anaesthesia provider); 3) individual training in the classroom only (anaesthesia provider) |
4 of 6 hospitals had no pulse oximetry. Pulse oximetry was occasionally available in the other 2. No pulse oximeters were provided as part of the study. However, participants agreed that it would be a valuable addition in the OR and recovery wards. Multidisciplinary courses more impactful than single discipline at 3-6 mo. |
| Kenya (LMIC), 3 sub-district hospitals in Western Kenya [29] |
2013-2014 |
Non-anaesthetist clinicians (nurses, clinical officers, medical officers, nurse aid) |
Safer surgical care when no anaesthetist is available |
5-d Every Second Matters-Ketamine (ESM-Ketamine) training course |
Surgeries able to be performed when ESM-Ketamine protocol enacted, pulse oximeters alerted desaturation events. |
| Liberia (LIC), 2 hospitals in Monrovia [30] |
2008-2009 |
Surgical team |
SSC implementation |
2-week training programme |
Pulse oximetry use increased from 34.9% to 88.2% in Hospital 1 and 75.7% to 88.5% in Hospital 2 after training. |
| Madagascar (LIC), 21 hospitals nationwide [31-33] |
2015-2016 |
Operating room staff |
SSC implementation |
3-d multidisciplinary course |
Prior to training, no hospital routinely used pulse oximetry due to lack of supply. 3-4 mo after training, 63% of participants surveyed reported “Always” using pulse oximetry in theatre and 11% using it “most of the time.” 12-18 mo after training, 88% of participants surveyed reported “Always” using pulse oximetry in theatre and 9% using it “most of the time.” |
| Malawi (LIC), 27 hospitals [34] |
2014 |
Anaesthesia providers |
Perioperative monitoring |
1-d Lifebox workshop |
Improved pulse oximetry knowledge via MCQs immediately after training that was maintained after 8 mo. 82% of donated pulse oximeters were located at follow up. 97% of located pulse oximeters were in regular use at follow up. 8% relative reduction in the odds of desaturation event for every 10 cases during first 100 cases after training. |
| Niger (LIC), 40 public hospitals nationwide [35] |
2014 |
Anaesthesiologists and surgeons |
Pulse oximetry use, hypoxia management, implementation of SSC |
Lifebox workshop |
Average number of pulse oximeters in each hospital increased from 1 to 8. Logbook for notification and management of hypoxia introduced. |
| Togo (LIC), providers nationwide [36] |
2012 |
Anaesthesia providers |
To improve surgical and anaesthesia safety |
Lifebox workshop |
An audit of a maternity unit in 2014 demonstrated all patients receiving anaesthesia were monitored with pulse oximetry perioperatively and pulse oximetry training and provision enabled early hypoxia detection and interventions. |
| Uganda (LIC): 12 hospitals nationwide [37] |
2007 |
Anaesthesia providers |
Identify pulse oximetry gaps and training needs for perioperative monitoring |
2 half-day Global Oximetry project workshop with refresher 1 y later |
Test scores improved for all but two participants after training. All participants were able to demonstrate basic oximetry use after training. Demonstrating a change of practice. |
| Uganda (LIC), providers nationwide [38] |
2011 |
Non-physician anaesthetists |
Oximetry and hypoxia management |
2.5 d Lifebox training course |
Pulse oximetry knowledge improved from a median score of 36 / 50 to 41 / 50 (P < 0.0001) immediately after course. 3-5 mo later, the median score was 41 / 50 (P = 0.001 compared with immediate post-training test scores), and 95% of oximeters were in routine clinical use. Participants felt oximeters improved patient safety. |
| Zambia (LMIC), no site specified [39] |
No year noted. Abstract presented in 2016. |
Physicians and clinical officers throughout Zambia |
Improve anaesthesia capacity, SSC, and pulse oximetry monitoring to reduce maternal mortality |
1-d Lifebox workshop and 3-d Safe Anesthesia From Education (SAFE) obstetric anaesthesia courses |
Lifebox MCQ, SAFE MCQ, and SAFE skills scores all improved after the training course. |
|
European Region
| |||||
| Moldova (UMIC), 1 hospital in Chisinau [40] |
2010 |
Operating Room Staff |
SSC implementation |
Train-the-trainer approach with months long progressive rollout using course materials developed by WHO, Harvard School of Public Health, the World Federation of Societies of Anaesthesiologists, and the Association of Anaesthetists of Great Britain and Ireland, and intraoperative teaching |
Pulse oximeters in operating stations increased from 14 to 100%. Pulse oximetry use in cases increased from 16 to 99.6%. Hypoxemic episodes lasting 2 min or longer per 100 h of oximetry decreased from 11.5 to 6.4 (P < 0.002). |
|
South-East Asia Region
| |||||
| India (LMIC), 4 hospitals in 1 state [41] |
2007 |
Anaesthetists |
To increase oximetry provision and perioperative monitoring |
Training manual designed for Global Oximetry (GO) subproject initially used in Uganda |
10 mo after training, 11 / 12 pulse oximeters were still regularly used. Anaesthetists report early detection of hypoxia, improved perioperative monitoring, and enhanced team communication. |
| Nepal (LMIC), 12 districts nationwide [42] |
2014-2015 |
Anaesthesia assistants |
To provide anaesthesia assistant continuing professional development |
A refresher course of 5 d, 1 y with tablet-based self-learning modules and clinical case logs, regular educational mentor communication, a midcourse 2-week contact time at an anaesthesia assistant training site, regular text messaging, and clinical and MCQ examinations |
Pulse oximetry was used in 98% of cases. |
| Thailand (UMIC), 1 hospital in Bangkok and 1 hospital in Pitsanulok [43] |
Ongoing. Anaesthesia training for physicians began in 1951 and anaesthetic nurses in 1965. |
Anaesthesia residents, anaesthesia fellows, and anaesthetic nurses from Thailand and nearby countries |
Increasing anaesthesia workforce in the region |
3-y residency for physicians, 1-y programme for nurse anaesthetists |
In 2016, 25 new anaesthesiologists and 40 anaesthesia nurses trained each year. Pulse oximetry monitoring now standard. |
|
Western Pacific Region
| |||||
| The Philippines (LMIC): 16 hospitals in Cebu province [37] |
2007 |
Acute care doctors and nurses |
Identify pulse oximetry gaps and training needs for perioperative monitoring |
1 d training course (for doctors and nurses) in the use of oximeters |
Use of the oximeters throughout the project, demonstrating a change of practice. |
| Vietnam (LMIC): 15 hospitals in Binh Dinh province [37] | 2007 | Anaesthesia providers | Identify pulse oximetry gaps and training needs for perioperative monitoring | 1 d Global Oximetry project workshop with refreshers 6 mo and 1 y later | All participants were able demonstrate basic oximetry use after training. |
LMIC – lower middle-income country, UMIC – upper middle-income country, SSC – World Health Organization’s (WHO) Safe Surgical Checklist, d – days, mo – months, LIC – low-income country, HDU – high dependency unit, PACU – post-anaesthesia care unit, y – year, MCQ – multiple choice question
*Stratified by WHO Regions.
Respiratory support programme expansion
Fourteen studies in six countries representing the African Region (n = 4), South-East Asia Region (n = 1), and Western Pacific Region (n = 1) described pulse oximetry training prior to oxygen programme implementation, improved oxygen use, or expansion of bubble continuous positive airway pressure (bCPAP) or ventilatory support programmes (Table 2). One country was low-income and five were lower-middle income. Training was focused on doctors and nurses and ranged from 45-minute lectures to 3-day workshops. Studies found increased use of ventilatory and bCPAP support after training and increased use of pulse oximetry monitoring in children with pneumonia on oxygen support. One study found decreased mortality after pulse oximetry monitoring training and oxygen introduction [55-57].
Table 2.
Studies describing pulse oximetry training initiatives: Respiratory support programme expansion*
| WHERE: country (LMIC designation), sites | WHEN: year of training | WHO: population trained | WHY: purpose of training | WHAT: structure of training | HOW: pulse oximetry related outcomes |
|---|---|---|---|---|---|
|
African Region
| |||||
| Ghana (LMIC), 1 hospital in the Sissala East District [44] |
2011-2012 |
Medical officer and nurse anaesthetists |
Introduction of non-invasive positive pressure ventilators (NIPPV) |
3-d workshop |
NIPPV was successfully used in 130 children and 11 adults |
| Kenya (LMIC), 1 hospital in Nakuru [45] |
2018 |
Nurses and physicians |
Increasing use of bCPAP for neonates |
8-h course covering assessment of neonatal respiratory distress, bCPAP eligibility, and bCPAP use. Refresher training 2 and 9 mo later. |
Use of bCPAP for neonates in respiratory distress increased from 2% pre-initiative to 17.6% post-initiative. |
| Nigeria (LMIC), 29 secondary and tertiary hospitals in Kaduna, Kano, and Niger states [46] |
2017-2018 |
Doctors, nurses, pharmacists, and community health extension workers |
Oxygen programme implementation |
Train-the-trainer approach with 5-d training for trainers and 2-d training for trainees. Used curriculum based on WHO guidelines and modified from Graham et al.'s studies in Southwestern Nigeria. |
Among all sites, use of pulse oximetry for pneumonia patients rose from 13.7 to 82.4% after the intervention. |
| Nigeria (LMIC), 12 secondary-level hospitals in 7 urban areas of Southwest Nigeria [47,48] |
2014-2017 |
Nursing and medical staff |
Implementation of oxygen system and pulse oximeters |
1-h initial training session based on WHO guidelines with quarterly support visits for 18 mo |
Use of pulse oximetry knowledge and practice increased after initial training but further increased and was more sustained after complete oxygen system training and implementation. Pulse oximetry introduction may have reduced the risk of death from pneumonia by ~ 50%. |
| Rwanda (LIC), 1 hospital in Kigali [49] |
No year provided. Study was published in 2018. |
Nurses, resident physicians, general practice physicians |
Improving oxygen use in the emergency department |
45-min training and MCQ assessment. Nurses received more specific pulse oximetry training. |
Examination score increased from 60% average prior to the training to 80% average after the training. Proportion of patients with target SpO2 increased from 18.7% at baseline to 38.5% at 4 weeks and 42.0% at 12 weeks. |
|
South-East Asia Region
| |||||
| India (LMIC), 4 hospitals in Maharashtra [50,51] |
2017-2018 |
Providers (unspecified) |
Introducing bCPAP |
Every Second Matters-Newborn and Infant Respiratory Bundle |
Decreased Respiratory Severity Score on average by 1.31 with bCPAP use |
|
Western Pacific Region
| |||||
| Papua New Guinea (LMIC), 5 hospitals nationwide [52-54] |
2004 |
Doctors and nurses |
Determining the incidence of hypoxemia and improving hypoxemia detection in admitted children prior to oxygen programme implementation |
Practical teaching and provision of protocol for pulse oximetry use |
Prior to training, pulse oximetry was not used. After training, 98.9% of admitted children were evaluated with pulse oximetry. Pulse oximetry more reliably detected hypoxemia compared to clinical signs alone, which missed 29% of children with hypoxemia. Pulse oximetry was underutilised at 14-mo follow-up. |
| Papua New Guinea (LMIC), 39 remote health centres and district hospitals nationwide [55-57] | 2016-2018 | Health care workers (cadre not specified) | Implementing oxygen system | Annual training using WHO guidelines for the Clinical Use of Oxygen in Children and WHO Hospital Care for Children | Pneumonia cases and deaths, as well as referrals to escalate care, decreased after the intervention. Pulse oximetry allowed for hypoxemia monitoring and solar-powered oxygen concentrators effectively decreased morbidity and mortality in children. |
LIC – low-income country, d – days, LMIC – lower middle-income country, bCPAP – bubble continuous positive airway pressure, h – hours, WHO – World Health Organization, mo – months, min – minutes, MCQ – multiple choice question
*Stratified by WHO Regions.
Perinatal assessment and monitoring
Eight studies representing 16 countries focused on pulse oximetry training of health care workers in the perinatal period, 12 of which were in the Region of the Americas and one representing the African, Eastern Mediterranean, South-East Asia, and Western Pacific Regions (Table 3). Five countries were lower-middle income and 11 were upper-middle income. One study focused on perinatal monitoring of mothers, one focused on maternal and neonatal assessment and six focused on neonatal assessment and monitoring. Among those focusing on neonates, training aimed to instruct health care workers in the use of pulse oximetry to decrease the burden of retinopathy of prematurity (ROP) and as a screening tool for critical congenital heart disease (CCHD), sepsis and pneumonia. Educational efforts most often involved neonatal nurses, obstetricians and neonatologists, although one study included community health workers and another traditional birth attendants [60,64]. Commonly cited limitations in expanding oximetry included the lack of pulse oximeters, lack of trained staff, or limited staff availability. Reported study outcomes varied but many reported increased pulse oximetry knowledge or improved neonatal screening rates after training.
Table 3.
Studies describing pulse oximetry training initiatives: perinatal assessment and monitoring*
| WHERE: country (LMIC designation), sites | WHEN: year of training | WHO: population trained | WHY: purpose of training | WHAT: structure of training | HOW: pulse oximetry related outcomes |
|---|---|---|---|---|---|
|
Region of the Americas
| |||||
| Argentina (UMIC): 1 hospital in San Luis, 1 hospital in Rosario [58] |
San Luis – 2019; Rosario – 2017-2018 |
Neonatal nurses and neonatologists |
CCHD screening |
SIBEN Clinical Consensus |
Increased screening after training with plans for universal screening |
| Bolivia (LMIC): 1 hospital in La Paz, 1 hospital in Sucre [58] |
2018 |
Staff |
CCHD screening |
SIBEN Clinical Consensus |
Unable to routinely screen due to lack of equipment and staff |
| Brazil (UMIC), 5 NICUs in Rio de Janeiro [59] |
2009 |
Neonatal nurses and nurse assistants |
Improving nursing care to decrease mortality and incidence of ROP |
3-mo self-administered education package |
78% of qualified nurses and 82% of nurse assistants were trained and knowledge improved after training. No significant impact on survival or ROP was found. |
| Colombia (UMIC): 1 hospital in Barranquilla [58] |
2014 |
Neonatal group |
CCHD screening |
SIBEN Clinical Consensus |
Increased screening after training and screening became mandatory in 2018 |
| Costa Rica (UMIC): Nationwide [58] |
2016 |
Not clearly stated, screening by respiratory therapists, nurses |
CCHD screening |
SIBEN Clinical Consensus |
Screening nationwide after training |
| Cuba (UMIC): not stated [58] |
Cuba: not provided |
Not stated, screening in some neonatal centres |
CCHD screening |
SIBEN Clinical Consensus |
Limited screening due to pulse oximetry shortage |
| Dominican Republic (UMIC): Nationwide [58] |
2019 |
Neonatologists, neonatal nurses, neonatology residents |
CCHD screening |
SIBEN Clinical Consensus |
Screening not yet started |
| El Salvador (LMIC): 1 hospital in San Salvador [58] |
Not provided |
Not stated |
CCHD screening |
SIBEN Clinical Consensus |
Screening not yet started |
| Guatemala (UMIC), Tecpán municipality [60] |
Year not provided. Study published in 2018. |
Traditional birth attendants |
Improve detection of maternal and perinatal complications and facility referral |
4-d training on perinatal complications, indications for referral, and use of the mHealth platform and device (which included a pulse oximeter) |
Rate of emergency referrals to facilities increased with the mHealth intervention and training. |
| Honduras (LMIC): Nationwide [58] |
Not provided |
Staff providing neonatal care |
CCHD screening |
SIBEN Clinical Consensus |
Increased screening after training |
| Paraguay (UMIC): 1 hospital in Asunción [58] |
2013 |
Nurses |
CCHD screening |
SIBEN Clinical Consensus |
Increased screening after training |
| Peru (UMIC): 1 hospital in Lima. [58] |
2018 |
Not clearly stated, screening by nurses |
CCHD screening |
SIBEN Clinical Consensus |
Increased screening after training, pulse oximeter provision, and hiring dedicated staff for screening |
|
African Region
| |||||
| South Africa (UMIC), 1 hospital in Port Elizabeth [61] |
2012-2014 |
Paediatricians and NICU nurses |
ROP prevention |
Paediatric academic programme for paediatricians and educational sessions for nursing staff |
More infants being screened for ROP after developing an ROP screening clinic. Training and provision of pulse oximeters and oxygen blenders made targeted oxygen supplementation possible. |
|
Eastern Mediterranean Region
| |||||
| Iran (LMIC), Sistan and Baluchestan provinces [62] |
2014 |
Midwives and general practitioners |
Improve management of postpartum haemorrhage |
2-d training based on WHO guidelines |
Pulse oximetry use increased from 0 to 81.5% after the workshop. |
|
South-East Asia Region
| |||||
| India (LMIC), Centres with high incidence of aggressive posterior ROP in North Karnataka [63] |
2011-2015 |
NICU nurses and paramedical staff, paediatricians |
Attempt to reduce severe ROP |
ROP prevention guidelines and targeted training |
More infants being are being screened for ROP but no mention of improved pulse oximetry use |
|
Western Pacific Region
| |||||
| China (UMIC), Rural areas [64] |
2015 |
Public health stakeholders, clinicians, rural health workers |
CCHD, pneumonia, and sepsis detection |
Train the trainer model |
More than 52 000 newborns have been screened |
| China (UMIC), Rural county hospitals in Yunnan Province [65] | 2015-2016 | Obstetricians and obstetric nurses | CCHD screening training | 1-d hands on training | Improved knowledge via MCQs immediately after training and at 3 mo follow up 61.6% of nurses reported frequent pulse oximeter and 23.2% reported sometimes using pulse oximeters at follow up. Newborn screening rates were 90.6%-98.0% |
LMIC – lower middle-income country, UMIC – upper middle-income country, CCHD – critical congenital heart defects, SIBEN – Ibero American Society of Neonatology, NICU – neonatal intensive care unit, ROP – retinopathy of prematurity, m – months, d – days, MCQ – multiple choice question
*Stratified by WHO Regions.
Paediatric assessment and monitoring
Seven studies from four countries in the African Region, three of which were low-income countries and one was lower-middle income, focused on training health care workers caring for non-neonatal paediatric populations, especially those responsible for assessing children for pneumonia (Table 4). Most studies focused on training community health workers and nurses to regularly and routinely conduct vital signs assessments that included measurement of oxygen saturation. Training ranged from one-hour pulse oximetry training supplemented with additional clinical management training to a four-day emergency triaging workshop. After training, studies found increased rates of oxygen saturation measurements with several studies reporting improved morbidity and mortality among the studied population.
Table 4.
Studies describing pulse oximetry training initiatives: paediatric assessment and monitoring*
| WHERE: country (LMIC designation), sites | WHEN: year of training | WHO: population trained | WHY: purpose of training | WHAT: structure of training | HOW: pulse oximetry related outcomes |
|---|---|---|---|---|---|
|
African Region
| |||||
| Ethiopia (LIC), first-level health facilities and in the community in Sodo Zuria, Damote Sore, and Damote Gale districts [66] |
2018 |
Health extension workers and first-level health facility workers |
Assessment of children with respiratory illness |
2-d iCCM/IMNCI and pulse oximetry training |
83.9% of pneumonia consultations were assessed using pulse oximetry in the 2 mo following training |
| Malawi (LIC), Health centres in Lilongwe and Mchinji districts [67,68] |
2011 |
Rural practitioners and community health workers |
Improve identification of children with severe respiratory illness |
1-d training with continued monthly mentorship visits |
94.1% of children had oxygen saturation measured during the 3 y after training. Moderate agreement was found between expert oxygen saturation measurements and newly trained providers. Pulse oximetry identified fatal episodes of childhood pneumonia that did not have identified clinical signs. |
| Malawi (LIC), 1 hospital in Lilongwe [69] |
2011 |
Nurses, clinicians, and vital sign assistants (VSAs) |
Improve inpatient paediatric surveillance |
Half-day training for nurses, 2-d training for VSAs based on PEWS concepts |
Patients' vital signs, including oxygen saturation, were monitored more frequently and accurately than before the training when VSAs were included in the health care team. |
| Malawi (LIC), 2 clinics in the Mulanje district [70] |
2019 |
Clinical officers and nurses |
Assessing whether pulse oximeter screening in addition to IMCI education improves respiratory illness diagnosis and decreases antibiotic prescriptions in children |
1-h long session using WHO pulse oximetry manual supplemented with additional recommendations |
30% of children were evaluated with pulse oximeters at clinics with this capability. Clinic sites with pulse oximeters improved illness classification (diagnosed severe respiratory illnesses less frequently in children with normal oxygen saturation and more frequently in children with low oxygen saturation) and prescribed antibiotics less frequently. Pulse oximeters improved provider confidence. |
| Sierra Leone (LIC), 1 hospital in Freetown [71] |
2009 |
Nurses |
Improve paediatric hospital emergency medical care |
4-d adapted WHO Emergency Triage Assessment and Treatment course |
Decreased mortality rate immediately and 4 mo after interventions |
| Zambia (LMIC), 1 hospital in Lusaka [72] | 2013 | Nurses | Implementation of a clinical guidance tool, which included prompts for regular vital sign checks (including oxygen saturation), to improve the care of children hospitalised with pneumonia | Modified WHO recommendations for the management of acute respiratory illness were used to make the tool | Nurses believed the clinical guidance tool led to improved care through closer and more consistent monitoring and rapid identification of problems. Increase of the proportion of children with oxygen saturation ≤92% receiving oxygen on admission (83.3% vs. 93.8%) and at 48 h follow-up (76.4% vs. 95.3%) |
LMIC – lower middle-income country, LIC – low-income country, d – days, iCCM/IMNCI – integrated community case management/integrated management of newborn and childhood illnesses, mo – months, PEWS – paediatric early warning score, IMCI – integrated management of childhood illness, h – hours, WHO – World Health Organization
*Stratified by WHO Regions.
Adult assessment and monitoring
Ten studies were conducted in six countries globally, representing the Region of the Americas (n = 3), African Region (n = 2) and the Eastern Mediterranean Region (n = 1) (Table 5). One country was low-income, three were lower-middle income and two were upper-middle income. Studies focus on training health care workers in the assessment and monitoring of adults. Six of these studies primarily focused on improving patient monitoring and the early identification of complications by increasing the frequency of vital signs measurement. As such, these studies primarily focused their training on nurses. Three studies focused on improving trauma and emergency services in LMICs. In these studies, pulse oximetry training was not the primary focus but was included as part of comprehensive training programmes that included vital sign assessments and monitoring. Training often focused on non-physician health care workers who either triage emergency and trauma patients or provide bedside care. One study focused on training primary care providers to use pulse oximetry as an adjunct in pulmonary disease management. Training ranged from 30-minute lectures to a 7-day long workshop. Pulse oximetry use and vital sign monitoring increased after these sessions, but studies reported that additional improvements could still be made, such as further increasing oximetry use and vital sign monitoring, as well as reacting to abnormal oximetry values.
Table 5.
Studies describing pulse oximetry training initiatives: adult assessment and monitoring
| WHERE: country (LMIC designation), sites | WHEN: year of training | WHO: population trained | WHY: purpose of training | WHAT: structure of training | HOW: pulse oximetry related outcomes |
|---|---|---|---|---|---|
|
Region of the Americas
| |||||
| Ecuador (UMIC), 1 pre-hospital training site in Cuenca [73,74] |
No year provided. Abstracts presented in 2017. |
Pre-hospital personnel (doctors, firefighters, paramedics, ambulance operators, medical dispatchers, medical auditors, and Ministry of Health administrators) |
To improve prehospital to hospital communication for injured patients |
1-h long communication course and introduction of a communication checklist |
Communication of pulse oximetry values increased from 37.5 to 72% after training in observed scenarios. |
| Haiti (LMIC), 1 hospital in Port-au-Prince [75] |
2013-2014 |
Physicians and nurses |
Implementation of an adapted WHO severe sepsis protocol and vital signs monitoring |
31-h lectures on severe sepsis management, protocol implementation (including new triage and vital sign forms), and clinical data recording |
After protocol implementation, 83.6% of patients had their vital signs taken a second time compared to 78.8% before the protocol implementation.
Patients were more likely to have their second vital signs taken sooner after protocol implementation compared to before (140 min vs. 240 min, respectively). |
| Nicaragua (LMIC), Managua [76] |
Year not provided. Article published in 1997. |
Nurses and physicians |
Critical care training, including pulse oximetry and oxyhaemoglobin dissociation curve |
5-d course |
No outcomes provided. |
|
African Region
| |||||
| South Africa (UMIC), 1 hospital in Cape Town [77] |
2010 |
Nurses on postoperative wards |
MEWS system implementation |
2-h Cape Town MEWS training programme |
Nurses who underwent training recorded oxygen saturation in 12.3% of patients compared to the control group, which only had 3.5% of patients with oxygen saturation recorded. |
| South Africa (UMIC), 6 government-sector adult hospitals in the Western Cape [78] |
2014 |
Nurses in medical and surgical wards |
MEWS system implementation |
8-h MEWS and SBAR training |
Nurses who underwent the training recorded pulse oximetry in 54.0% of patients compared to nurses in the control group who only recorded oxygen saturation in 17.6% of patients. Abnormal oxygen saturation values in the intervention group did not trigger assistance as they should have. |
| Uganda (LIC), 4 health facilities in Western Uganda [79] |
2015 |
Health facility staff |
Improved vital sign monitoring and recognition of severe illness |
7 d WHO Quick Check + training programme |
Pulse oximetry monitoring increased from 0.2% of patients to 19% |
| Uganda (LIC), 1 emergency centre in Kampala [80] |
2016 |
Emergency centre nurses |
Traumatic brain injury nursing care, vital sign monitoring, and charting |
30-40-min lecture |
Nurses felt that the vital signs chart improved monitoring and was useful for tracking patient progress and guiding management but felt routine vital sign monitoring may be difficult to integrate into their practice. |
| Uganda (LIC), Multiple regions nationwide [81] |
No year provided. Abstract presented in 2019. |
Primary care clinicians (doctors, clinical officers, nurses, midwives) |
Improve assessment and management of pulmonary diseases in primary care, including use of pulse oximetry |
iBreath |
Participants report increased knowledge, skills, and improved attitudes after the course |
|
Eastern Mediterranean Region
| |||||
| Iran (LMIC), 1 prehospital emergency centre in Tabriz [82] | 2016-2018 | Prehospital emergency staff | Improve prehospital trauma care | 12 session Prehospital Trauma Life Support course | Vital signs control and pulse oximetry measurement increased from 95.7 to 100% after the course |
LMIC – lower middle-income country, UMIC – upper middle-income country, h – hours, WHO – World Health Organization, min – minutes, d – days, MEWS – modified early warning score, SBAR – situation, background, assessment, recommendation, LIC – low-income country.
*Stratified by WHO Regions.
DISCUSSION
Pulse oximetry education initiatives among health care workers in LMICs focused primarily on monitoring and evaluation of patients during times of potential deterioration – such as during the perioperative period or respiratory compromise – or as a means of routine assessment or screening – such as during vital signs measurement. Training and educational programmes varied in their purpose with respect to the types of patients being targeted, the types of providers being instructed, and the depth of pulse oximetry-specific training. These initiatives included training anaesthetists or perioperative care staff, ensuring appropriate and adequate oxygen use, pulse oximetry screening for congenital diseases and infectious diseases and improving vital signs assessment for patients of all ages. Most studies focused on training doctors and nurses working in hospitals, with fewer focused on pre-hospital and community-based health care workers. Identified studies reported heterogeneous training structures for pulse oximetry, even among studies with the same goals of teaching pulse oximetry use. As such, no determination could be made about if a certain training style or method was more, or less, effective compared to another style. While these findings do not encompass all possible reasons pulse oximetry training could be conducted, they provide an overview of the training landscape as described in the literature prior to the COVID-19 pandemic.
Prior studies demonstrate that availability and use of pulse oximetry does not guarantee a proper understanding of oximetry practices, interpretation, or indications for intervention [15,83,84]. Additionally, pulse oximetry may not have been covered in primary qualification training for some health care workers [85]. Finally, knowledge without access cannot lead to improvements in patient care, just as access without proper understanding also does not lead to improvements in care. Thus, it is important for educational initiatives to occur in tandem with provision of devices, as one is not successful without the other.
In addition to addressing improved pulse oximetry availability and education, other barriers to its use also need to be assessed. Such barriers will vary based on context-specific concerns. An appropriate, thorough, and regionally specific understanding of why pulse oximetry is not utilised in particular areas where it may be beneficial should be explored, including cost, staffing shortages and power outages. This should be enabled by engagement with local health care workers to address the most relevant and pressing concerns. Future research focusing on these issues would be beneficial to inform pulse oximetry implementation and policy reforms, with a focus on local health care workers’ needs, workflows, and behaviours. Furthermore, implementation science approaches and methodologies would help standardise the way impact of such initiatives is assessed.
Globally, the COVID-19 pandemic demonstrated the need for widespread pulse oximetry availability and training for early detection of hypoxemia [9]. It laid bare the inequitable distribution of pulse oximeters and the need for increased access to pulse oximeters and health provider training in LMICs. Thousands of pulse oximeters have been distributed in LMICs throughout the pandemic by multilateral organisations and non-governmental organisations (NGOs), such as UNICEF and Lifebox [86,87]. The required rapid up scaling of pulse oximetry training and the increased availability of devices highlight a health systems area in need of strengthening. It showed that while pulse oximetry training has been conducted for many years across settings described in this review, it could benefit from additional investment. Improvements achieved since the start of the pandemic represent a momentary success, but for sustainability, widespread pulse oximetry introduction and appropriate training will need to continue [8].
This scoping review is not without limitations. This review focused on data gathered from the published literature, which we acknowledge fails to capture non-published pulse oximetry education initiatives. There may be a publication bias as to which initiatives are published and which are not. Additionally, if within a particular setting pulse oximetry is widely available and is a standard topic covered in health care schooling, educational efforts may be less likely published given its ubiquitous nature. Limitations notwithstanding, we believe this review will be beneficial to researchers, educators, and policy makers to provide a baseline understanding of many pulse oximetry education foci in LMICs prior to the COVID-19 pandemic.
CONCLUSIONS
Pulse oximetry training initiatives have been ongoing for decades for a variety of purposes, utilising a multitude of approaches to equip various health care workers with tools to improve patient care. It is important that these initiatives continue as pulse oximetry availability and knowledge gaps remain. Neither pulse oximetry provision nor training alone is enough to bolster patient care, but sustainable solutions for both must be considered in order to meet the needs of both health care workers and patients.
Additional material
Acknowledgements
We would like to thank the specialist librarians who assisted with drafting our database search strategy.
Footnotes
Funding: Meagan E Peterson received funding from the Stanford Medical Scholars Research Program for this work. The funding body played no role in the design of the study; collection, analysis, and interpretation of data; or in writing the manuscript. No other author received funding for this work.
Authorship contributions: MEP helped with study design, data collection, data interpretation, and drafted the manuscript. SD helped with data collection, data interpretation, and critically revised the manuscript. DRRB helped with data collection, data interpretation, and critically revised the manuscript. JA helped with data collection, data interpretation, and critically revised the manuscript. SA helped with data collection, data interpretation, and critically revised the manuscript. TGW helped with study design, data interpretation, and critically revised the manuscript.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) declare the following activities and relationships: TGW is a member of The Lifebox Global Governance Council. SA is a joint fellow of Lifebox and Operation Smile. Lifebox and Operation Smile as organisations did not play a role in the design of the study; collection, analysis, and interpretation of data; or in writing the manuscript. All other authors (MEP, SD, DRRB, JA) declare that they have no competing interests to report.
REFERENCES
- 1.Eichhorn JH.Standards for Patient Monitoring During Anesthesia at Harvard Medical School. JAMA. 1986;256:1017. 10.1001/jama.1986.03380080063029 [DOI] [PubMed] [Google Scholar]
- 2.Van Meter A, Williams U, Zavala A, Kee J, Rebello E, Tsai J, et al. Beat to Beat: A Measured Look at the History of Pulse Oximetry. J Anesth Hist. 2017;3:24-6. 10.1016/j.janh.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Kelleher JF.Pulse oximetry. J Clin Monit. 1989;5:37-62. 10.1007/BF01618369 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Guidelines for Safe Surgery 2009. 2009. [PubMed]
- 5.The Lancet Commission on Global Surgery. Global Surgery 2030: Evidence and Solutions for Achieving Health, Welfare, and Economic Development. 2015. [DOI] [PubMed]
- 6.World Health Organization. Integrated Management of Childhood Illness: Chart Booklet. 2014.
- 7.World Health Organization. Exploratory meeting to review new evidence for Integrated Management of Childhood Illness danger signs. 2018.
- 8.Starr N, Rebollo D, Asemu YM, Akalu L, Mohammed HA, Menchamo MW, et al. Pulse oximetry in low-resource settings during the COVID-19 pandemic. Lancet Glob Health. 2020;8:e1121-2. 10.1016/S2214-109X(20)30287-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobin MJ.Basing Respiratory Management of COVID-19 on Physiological Principles. Am J Respir Crit Care Med. 2020;201:1319-20. 10.1164/rccm.202004-1076ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk LM, Weiser TG, Berry WR, Lipsitz SR, Merry AF, Enright AC, et al. Global operating theatre distribution and pulse oximetry supply: an estimation from reported data. Lancet. 2010;376:1055-61. 10.1016/S0140-6736(10)60392-3 [DOI] [PubMed] [Google Scholar]
- 11.Rishipathak P, Sengupta N, Hinduja A.To Assess the Awareness, Beliefs and Practice Patterns Regarding Oxygen Therapy in Prehospital Management of Respiratory Emergencies amongst EMS Professionals in Pune, India. Indian J Forensic Med Toxicol. 2020;14:3682–3687. [Google Scholar]
- 12.Mugyenyi GR, Ngonzi J, Wylie BJ, Haberer JE, Boatin AA.Quality of vital sign monitoring during obstetric hospitalizations at a regional referral and teaching hospital in Uganda: an opportunity for improvement. Pan Afr Med J. 2021;38:252. 10.11604/pamj.2021.38.252.21749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabwire J, Namasopo S, Hawkes M.Oxygen Availability and Nursing Capacity for Oxygen Therapy in Ugandan Paediatric Wards. J Trop Pediatr. 2018;64:97-103. 10.1093/tropej/fmx033 [DOI] [PubMed] [Google Scholar]
- 14.Hadler RA, Chawla S, Stewart BT, McCunn MC, Kushner AL.Anesthesia Care Capacity at Health Facilities in 22 Low- and Middle-Income Countries. World J Surg. 2016;40:1025-33. 10.1007/s00268-016-3430-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabherwal S, Gilbert C, Foster A, Kumar P.Status of oxygen monitoring in four selected special care newborn units in India. Indian Pediatr. 2020;57:317-20. 10.1007/s13312-020-1783-0 [DOI] [PubMed] [Google Scholar]
- 16.Sawe HR, Reynolds TA, Weber EJ, Mfinanga JA, Coats TJ, Wallis LA.Trauma care and capture rate of variables of World Health Organisation data set for injury at regional hospitals in Tanzania: first steps to a national trauma registry. BMC Emerg Med. 2020;20:29. 10.1186/s12873-020-00325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E.Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 19.World Bank Group. World Bank Country and Lending Groups. 2022. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed: 26 September 2022.
- 20.White MC, Randall K, Capo-Chichi N, Sodogas F, Quenum S, Wright K, et al. Implementation and evaluation of nationwide scale-up of the Surgical Safety Checklist. Br J Surg. 2019;106:e91-102. 10.1002/bjs.11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bougouma CT, Ouédraogo N, Kaboré RAF, Ki KB, Traoré IA, Ouattara SA, et al. Évaluation de l’impact du projet Lifebox au Burkina Faso sur la pratique de l’oxymétrie pulsée et la check-list de l’organisation mondiale de la santé pour la sécurité du patient au bloc opératoire. Can J Anaesth. 2019;66:232-3. 10.1007/s12630-018-1263-3 [DOI] [PubMed] [Google Scholar]
- 22.White MC, Daya L, Brice Karel FK, White G, Abid S, Fitzgerald A, et al. Using the knowledge to action framework to describe a nationwide implementation of the WHO surgical safety checklist in Cameroon. Anesth Analg. 2020;130:1425-34. 10.1213/ANE.0000000000004586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White MC, Peterschmidt J, Callahan J, Fitzgerald JE, Close KL.Interval follow up of a 4-day pilot program to implement the WHO surgical safety checklist at a Congolese hospital. Global Health. 2017;13:42. 10.1186/s12992-017-0266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashford T. Improving anaesthetic safety in an Ethiopian Specialised Hospital: A pathway approach. Conference: Anaesthesia Special Issue: Abstracts of the AAGBI Annual Congress; 19-21 Sep 2012; Bournemouth UK;67: p. 24-85. [Google Scholar]
- 25.Burton ZA, Ayele Y, McDonald P.Establishing a sustainable anaesthetic education programme at Jimma University Medical Centre, Ethiopia. Anaesth Intensive Care. 2019;47:334-42. 10.1177/0310057X19860984 [DOI] [PubMed] [Google Scholar]
- 26.Peters JL, Boakye G, Harris M, Nsiah-Asare A, Antwi-Kusi A, Jabir AR, et al. Anesthesia teaching in Ghana: A 10-year experience. Int Anesthesiol Clin. 2010;48:23-37. 10.1097/AIA.0b013e3181cd1603 [DOI] [PubMed] [Google Scholar]
- 27.Potisek MG, Hatch DM, Atito-Narh E, Agudogo J, Olufolabi AJ, Rieker M, et al. Where Are They Now? Evolution of a Nurse Anesthesia Training School in Ghana and a Survey of Graduates. Front Public Health. 2017;5:78. 10.3389/fpubh.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White M, Millimouno F. Observational study of three different methods of implementing the WHO surgical safety checklist in Guinea. Glob Anesth Periop Med. 2016. [Google Scholar]
- 29.Burke T, Manglani Y, Altawil Z, Dickson A, Clark R, Okelo S, et al. A Safe-Anesthesia Innovation for Emergency and Life-Improving Surgeries When no Anesthetist is Available: A Descriptive Review of 193 Consecutive Surgeries. World J Surg. 2015;39:2147-52. 10.1007/s00268-015-3118-1 [DOI] [PubMed] [Google Scholar]
- 30.Yuan CT, Walsh D, Tomarken JL, Alpern R, Shakpeh J, Bradley EH.Incorporating the World Health Organization surgical safety checklist into practice at two hospitals in Liberia. Jt Comm J Qual Patient Saf. 2012;38:254-60. 10.1016/S1553-7250(12)38032-X [DOI] [PubMed] [Google Scholar]
- 31.White MC, Randall K, Ravelojaona VA, Andriamanjato HH, Andean V, Callahan J, et al. Sustainability of using the WHO surgical safety checklist: a mixed-methods longitudinal evaluation following a nationwide blended educational implementation strategy in Madagascar. BMJ Glob Health. 2018;3:e001104. 10.1136/bmjgh-2018-001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Close KL, Baxter LS, Ravelojaona VA, Rakotoarison HN, Bruno E, Herbert A, et al. Overcoming challenges in implementing the WHO Surgical Safety Checklist: lessons learnt from using a checklist training course to facilitate rapid scale up in Madagascar. BMJ Glob Health. 2017;2:e000430. 10.1136/bmjgh-2017-000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White MC, Baxter LS, Close KL, Ravelojaona VA, Rakotoarison HN, Bruno E, et al. Evaluation of a countrywide implementation of the world health organisation surgical safety checklist in Madagascar. PLoS One. 2018;13:e0191849. 10.1371/journal.pone.0191849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert V, Mndolo S, Harrison EM, O’Sullivan E, Wilson IH, Walker IA.Lifebox pulse oximeter implementation in Malawi: evaluation of educational outcomes and impact on oxygen desaturation episodes during anaesthesia. Anaesthesia. 2017;72:686-93. 10.1111/anae.13838 [DOI] [PubMed] [Google Scholar]
- 35.Maman Sani C, Kader A, Adamou F, Angela E, Remy T, Hadjara D.Lifebox project on pulse oximetry in Niger Republic. Anesth Analg. 2016;123:752. 10.1213/01.ane.0000492971.15752.72 [DOI] [Google Scholar]
- 36.Sama HD, Maman AF, Walker IA.Incidence of hypoxia and related events detected by pulse oximeters provided by the Lifebox Foundation in the maternity unit at Sylvanus Olympio University Teaching Hospital, Togo. J Anesth. 2015;29:971-3. 10.1007/s00540-015-2048-2 [DOI] [PubMed] [Google Scholar]
- 37.Walker IA, Merry AF, Wilson IH, McHugh GA, O’Sullivan E, Thoms GM, et al. Global oximetry: an international anaesthesia quality improvement project. Anaesthesia. 2009;64:1051-60. 10.1111/j.1365-2044.2009.06067.x [DOI] [PubMed] [Google Scholar]
- 38.Finch LC, Kim RY, Ttendo S, Kiwanuka JK, Walker IA, Wilson IH, et al. Evaluation of a large-scale donation of Lifebox pulse oximeters to non-physician anaesthetists in Uganda. Anaesthesia. 2014;69:445-51. 10.1111/anae.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coley EL, Colhoun R, Bonnett P.SAFE obstetrics course: Education aimed at reducing maternal mortality in Zambia [abstract]. Int J Obstet Anesth. 2016;26:S35. [Google Scholar]
- 40.Kwok AC, Funk LM, Baltaga R, Lipsitz SR, Merry AF, Dziekan G, et al. Implementation of the World Health Organization surgical safety checklist, including introduction of pulse oximetry, in a resource-limited setting. Ann Surg. 2013;257:633-9. 10.1097/SLA.0b013e3182777fa4 [DOI] [PubMed] [Google Scholar]
- 41.McHugh GA, Pollard BJ, Hooda S, Thoms GM.The impact of increasing oximetry usage in India: A pilot study. Indian J Anaesth. 2011;55:235-41. 10.4103/0019-5049.82662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah S, Ross O, Pickering S, Knoble S, Rai I.Tablet e-Logbooks: Four Thousand Clinical Cases and Complications e-Logged by 14 Nondoctor Anesthesia Providers in Nepal. Anesth Analg. 2017;125:1337-41. 10.1213/ANE.0000000000002094 [DOI] [PubMed] [Google Scholar]
- 43.Lertakyamanee J.50th year anniversary of department of anesthesiology, faculty of medicine Siriraj Hospital, Mahidol University. J Med Assoc Thai. 2016;99:618-21. [PubMed] [Google Scholar]
- 44.Balfour-Lynn RE, Marsh G, Gorayi D, Elahi E, LaRovere J.Non-invasive ventilation for children with acute respiratory failure in the developing world: Literature review and an implementation example. Paediatr Respir Rev. 2014;15:181-7. 10.1016/j.prrv.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 45.Switchenko N, Kibaru E, Tsimbiri P, Grubb P, Anderson Berry A, Fassl B.Implementation of a Bubble CPAP Treatment Program for Sick Newborns in Nakuru, Kenya: A Quality Improvement Initiative. Glob Pediatr Health. 2020;7:X20939756. 10.1177/2333794X20939756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fashanu C, Mekonnen T, Amedu J, Onwundiwe N, Adebiyi A, Omokere O, et al. Improved oxygen systems at hospitals in three Nigerian states: An implementation research study. Pediatr Pulmonol. 2020;55:S65-77. 10.1002/ppul.24694 [DOI] [PubMed] [Google Scholar]
- 47.Graham HR, Bakare AA, Ayede AI, Gray AZ, McPake B, Peel D, et al. Oxygen systems to improve clinical care and outcomes for children and neonates: a stepped-wedge cluster-randomised trial in Nigeria. PLoS Med. 2019;16:e1002951. 10.1371/journal.pmed.1002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham HR, Bakare AA, Gray A, Ayede AI, Qazi S, McPake B, et al. Adoption of paediatric and neonatal pulse oximetry by 12 hospitals in Nigeria: a mixed-methods realist evaluation. BMJ Glob Health. 2018;3:e000812. 10.1136/bmjgh-2018-000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutherland T, Moriau V, Niyonzima JM, Mueller A, Kabeja L, Twagirumugabe T, et al. The “Just Right” Amount of Oxygen. Improving Oxygen Use in a Rwandan Emergency Department. Ann Am Thorac Soc. 2019;16:1138-42. 10.1513/AnnalsATS.201811-763QI [DOI] [PubMed] [Google Scholar]
- 50.Ravi R, Bhagat S, Kurundwadkar M, Won A, Mollazadeh-Moghaddam K, Garg L, et al. Feasibility of an innovative, ultra-low-cost bubble cpap device for Newborns in India [abstract]. Int J Gynaecol Obstet. 2018;143:668. [Google Scholar]
- 51.Ravi R, Burke T, Bhagat S, Kurundwadkar M, Won A, Moghaddam K, et al. Outcomes of newborns and infants associated with use of an innovative, ultra-low-cost, bubble continuous positive airway pressure (CPAP) package, with a novel blender and pulse oximeter, in Mahaarashtra, India. Pediatrics. 2019;144:561. 10.1542/peds.144.2MA6.561 [DOI] [Google Scholar]
- 52.Matai S, Peel D, Wandi F, Jonathan M, Subhi R, Duke T.Implementing an oxygen programme in hospitals in Papua New Guinea. Ann Trop Paediatr. 2008;28:71-8. 10.1179/146532808X270716 [DOI] [PubMed] [Google Scholar]
- 53.Wandi F, Peel D, Duke T.Hypoxaemia among children in rural hospitals in Papua New Guinea: epidemiology and resource availability—a study to support a national oxygen programme. Ann Trop Paediatr. 2006;26:277-84. 10.1179/146532806X152791 [DOI] [PubMed] [Google Scholar]
- 54.Duke T, Wandi F, Jonathan M, Matai S, Kaupa M, Saavu M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328-33. 10.1016/S0140-6736(08)61164-2 [DOI] [PubMed] [Google Scholar]
- 55.Duke T, Hwaihwanje I, Kaupa M, Karubi J, Panauwe D, Sa’avu M, et al. Solar powered oxygen systems in remote health centers in Papua New Guinea: a large scale implementation effectiveness trial. J Glob Health. 2017;7:010411. 10.7189/jogh.07.010411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duke T, Pulsan F, Panauwe D, Hwaihwanje I, Sa’Avu M, Kaupa M, et al. Solar-powered oxygen, quality improvement and child pneumonia deaths: A large-scale effectiveness study. Arch Dis Child. 2021;106:224-30. 10.1136/archdischild-2020-320107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pulsan F, Duke T.Response to oxygen therapy using oxygen concentrators run off solar power in children with respiratory distress in remote primary health facilities in Papua New Guinea. Trop Doct. 2021;51:15-9. 10.1177/0049475520947886 [DOI] [PubMed] [Google Scholar]
- 58.Sola A, Rodríguez S, Young A, Varela LL, Villamayor RM, Cardetti M, et al. CCHD screening implementation efforts in Latin American countries by the Ibero American Society of Neonatology (SIBEN). Int J Neonatal Screen. 2020;6:21. 10.3390/ijns6010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert C, Darlow B, Zin A, Sivasubramaniam S, Shah S, Gianini N, et al. Educating neonatal nurses in Brazil: A before-and-after study with interrupted time series analysis. Neonatology. 2014;106:201-8. 10.1159/000362532 [DOI] [PubMed] [Google Scholar]
- 60.Martinez B, Ixen EC, Hall-Clifford R, Juarez M, Miller AC, Francis A, et al. mHealth intervention to improve the continuum of maternal and perinatal care in rural Guatemala: a pragmatic, randomized controlled feasibility trial. Reprod Health. 2018;15:120. 10.1186/s12978-018-0554-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacoby MR, Du Toit L.Screening for retinopathy of prematurity in a provincial hospital in Port Elizabeth, South Africa. S Afr Med J. 2016;106. 10.7196/SAMJ.2016.v106i6.10663 [DOI] [PubMed] [Google Scholar]
- 62.Moudi Z, Tabatabaei SM, Sargazi-Moakhar Z, Mollashahi S, Zaboli M, Boroojeny SB.Empowering midwives to manage postpartum haemorrhage in rural areas of islamic republic of iran: Lessons learnt from cases of maternal death. East Mediterr Health J. 2019;25:637-46. 10.26719/emhj.19.008 [DOI] [PubMed] [Google Scholar]
- 63.Vinekar A, Jayadev C, Kumar S, Mangalesh S, Dogra MR, Bauer NJ, et al. Impact of improved neonatal care on the profile of retinopathy of prematurity in rural neonatal centers in India over a 4-year period. Eye Brain. 2016;8:45-53. 10.2147/EB.S98715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saarinen A, Kochilas L.Can a little red beam of light save newborn lives? J Perinat Med. 2015;43. [Google Scholar]
- 65.Guo F, Tang S, Li Y, Loh C, Guo T, Bartell S, et al. The implementation of newborn cardiac screening in developing regions: Evaluating a training program in rural China. J Neonatal Nurs. 2019;25:16-9. 10.1016/j.jnn.2018.09.004 [DOI] [Google Scholar]
- 66.Baker K, Ward C, Maurel A, de Cola MA, Smith H, Getachew D, et al. Usability and acceptability of a multimodal respiratory rate and pulse oximeter device in case management of children with symptoms of pneumonia: A cross-sectional study in Ethiopia. Acta Paediatr. 2021;110:1620-32. 10.1111/apa.15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colbourn T, King C, Beard J, Phiri T, Mdala M, Zadutsa B, et al. Predictive value of pulse oximetry for mortality in infants and children presenting to primary care with clinical pneumonia in rural Malawi: A data linkage study. PLoS Med. 2020;17:e1003300. 10.1371/journal.pmed.1003300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCollum ED, King C, Deula R, Zadutsa B, Mankhambo L, Nambiar B, et al. Pulse oximetry for children with pneumonia treated as outpatients in rural Malawi. Bull World Health Organ. 2016;94:893-902. 10.2471/BLT.16.173401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson D, Preidis GA, Milazi R, Spinler JK, Lufesi N, Mwansambo C, et al. Task shifting an inpatient triage, assessment and treatment programme improves the quality of care for hospitalised Malawian children. Trop Med Int Health. 2013;18:879-86. 10.1111/tmi.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sylvies F, Nyirenda L, Blair A, Baltzell K.The impact of pulse oximetry and Integrated Management of Childhood Illness (IMCI) training on antibiotic prescribing practices in rural Malawi: A mixed-methods study. PLoS One. 2020;15:e0242440. 10.1371/journal.pone.0242440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark M, Spry E, Daoh K, Baion D, Skordis-Worrall J.Reductions in Inpatient Mortality following Interventions to Improve Emergency Hospital Care in Freetown, Sierra Leone. PLoS One. 2012;7:e41458. 10.1371/journal.pone.0041458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutcliffe CG, Thea DM, Seidenberg P, Chipeta J, Mwyanayanda L, Somwe SW, et al. A clinical guidance tool to improve the care of children hospitalized with severe pneumonia in Lusaka, Zambia. BMC Pediatr. 2016;16:136. 10.1186/s12887-016-0665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter J, Hopkins M, Trieu E, Morocho E, Mosquera C, Prieto J, et al. Feasibility of Standardizing Prehospital Communication in Cuenca, Ecuador. Ann Glob Health. 2017;83:28. 10.1016/j.aogh.2017.03.060 [DOI] [Google Scholar]
- 74.Trieu E, Hopkins M, Carter J, Morocho E, Prieto JL, Mosquera C, et al. Improving the quality of prehospital to hospital communication in cuenca, ecuador using a standardized course. Ann Glob Health. 2017;83:145. 10.1016/j.aogh.2017.03.323 [DOI] [Google Scholar]
- 75.Papali A, Eoin West T, Verceles AC, Augustin ME, Nathalie Colas L, Jean-Francois CH, et al. Treatment outcomes after implementation of an adapted WHO protocol for severe sepsis and septic shock in Haiti. J Crit Care. 2017;41:222-8. 10.1016/j.jcrc.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 76.Goodfellow LM.Application of pulse oximetry and the oxyhemoglobin dissociation curve in respiratory management. Crit Care Nurs Q. 1997;20:22-7. 10.1097/00002727-199708000-00006 [DOI] [PubMed] [Google Scholar]
- 77.Kyriacos U, Jelsma J, James M, Jordan S.Early warning scoring systems versus standard observations charts for wards in South Africa: a cluster randomized controlled trial. Trials. 2015;16:103. 10.1186/s13063-015-0624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kyriacos U, Burger D, Jordan S.Testing effectiveness of the revised Cape Town modified early warning and SBAR systems: a pilot pragmatic parallel group randomised controlled trial. Trials. 2019;20:809. 10.1186/s13063-019-3916-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cummings MJ, Goldberg E, Mwaka S, Kabajaasi O, Vittinghoff E, Cattamanchi A, et al. A complex intervention to improve implementation of World Health Organization guidelines for diagnosis of severe illness in low-income settings: a quasi-experimental study from Uganda. Implement Sci. 2017;12:126. 10.1186/s13012-017-0654-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wynveen L, Gamble M, Nabulime J, Luggya T, Kalanzi JK, Mowafi H.A qualitative study exploring nurses’ attitudes, confidence, and perceived barriers to implementing a traumatic brain injury nursing chart in Uganda. Afr J Emerg Med. 2018;8:64-8. 10.1016/j.afjem.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kayingo G. iBreath: An education intervention to improve lung health in Uganda...American Academy of Physician Assistants (AAPA) Conference; 2019 May 18-20; Denver, Colorado. JAAPA: Journal of the American Academy of Physician Assistants (Lippincott Williams & Wilkins). 2019. [Google Scholar]
- 82.Dehghannezhad J, Rahmani F, Ghafouri R, Hassankhani H, Dadashzadeh A, Damanabad ZH.Promotion of knowledge, skill, and performance of emergency medical technicians in prehospital care of traumatic patients: An action-research study. Arch Trauma Res. 2020;9:81-6. 10.4103/atr.atr_112_19 [DOI] [Google Scholar]
- 83.Nantanda R, Kayingo G, Jones R, van Gemert F, Kirenga BJ.Training needs for Ugandan primary care health workers in management of respiratory diseases: a cross sectional survey. BMC Health Serv Res. 2020;20:402. 10.1186/s12913-020-05135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milutinović D, Repić G, Aranđelović B.Clinical nurses’ knowledge level on pulse oximetry: A descriptive multi-centre study. Intensive Crit Care Nurs. 2016;37:19-26. 10.1016/j.iccn.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 85.Dauncey JW, Olupot-Olupot P, Maitland K.Healthcare-provider perceptions of barriers to oxygen therapy for paediatric patients in three government-funded eastern Ugandan hospitals; a qualitative study. BMC Health Serv Res. 2019;19:335. 10.1186/s12913-019-4129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lifebox Foundation Lifebox. 2018. Available: https://www.lifebox.org/. Accessed: 26 September 2022.
- 87.UNICEF. Oxygen and COVID-19: UNICEF’s global, rapid and multi-faceted approach. 2020. Available: https://www.unicef.org/supply/stories/oxygen-covid-19-unicefs-global-rapid-and-multi-faceted-approach. Accessed: 26 September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


