Abstract
Cowden syndrome (CS) or multiple hamartoma syndrome (MIM 158350) is an autosomal dominant disorder with an increased risk for breast and thyroid carcinoma. The diagnosis of CS, as operationally defined by the International Cowden Consortium, is made when a patient, or family, has a combination of pathognomonic major and/or minor criteria. The CS gene has recently been identified as PTEN, which maps at 10q23.3 and encodes a dual specificity phosphatase. PTEN appears to function as a tumour suppressor in CS, with between 13-80% of CS families harbouring germline nonsense, missense, and frameshift mutations predicted to disrupt normal PTEN function. To date, only a small number of tumour suppressor genes, including BRCA1, BRCA2, and p53, have been associated with familial breast or breast/ovarian cancer families. Given the involvement of PTEN in CS, we postulated that PTEN was a likely candidate to play a role in families with a "CS-like" phenotype, but not classical CS. To answer these questions, we gathered a series of patients from families who had features reminiscent of CS but did not meet the Consortium Criteria. Using a combination of denaturing gradient gel electrophoresis (DGGE), temporal temperature gel electrophoresis (TTGE), and sequence analysis, we screened 64 unrelated CS-like subjects for germline mutations in PTEN. A single male with follicular thyroid carcinoma from one of these 64 (2%) CS-like families harboured a germline point mutation, c.209T-->C. This mutation occurred at the last nucleotide of exon 3 and within a region homologous to the cytoskeletal proteins tensin and auxilin. We conclude that germline PTEN mutations play a relatively minor role in CS-like families. In addition, our data would suggest that, for the most part, the strict International Cowden Consortium operational diagnostic criteria for CS are quite robust and should remain in place.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairns P., Okami K., Halachmi S., Halachmi N., Esteller M., Herman J. G., Jen J., Isaacs W. B., Bova G. S., Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997 Nov 15;57(22):4997–5000. [PubMed] [Google Scholar]
- Chiariello E., Roz L., Albarosa R., Magnani I., Finocchiaro G. PTEN/MMAC1 mutations in primary glioblastomas and short-term cultures of malignant gliomas. Oncogene. 1998 Jan 29;16(4):541–545. doi: 10.1038/sj.onc.1201689. [DOI] [PubMed] [Google Scholar]
- Dahia P. L., Marsh D. J., Zheng Z., Zedenius J., Komminoth P., Frisk T., Wallin G., Parsons R., Longy M., Larsson C. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 1997 Nov 1;57(21):4710–4713. [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Eng C. From bench to bedside... but when? Genome Res. 1997 Jul;7(7):669–672. doi: 10.1101/gr.7.7.669. [DOI] [PubMed] [Google Scholar]
- Eng C., Vijg J. Genetic testing: the problems and the promise. Nat Biotechnol. 1997 May;15(5):422–426. doi: 10.1038/nbt0597-422. [DOI] [PubMed] [Google Scholar]
- Furnari F. B., Lin H., Huang H. S., Cavenee W. K. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg P., thor Straten P., Birck A., Ahrenkiel V., Kirkin A. F., Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997 Sep 1;57(17):3660–3663. [PubMed] [Google Scholar]
- Jacoby R. F., Schlack S., Cole C. E., Skarbek M., Harris C., Meisner L. F. A juvenile polyposis tumor suppressor locus at 10q22 is deleted from nonepithelial cells in the lamina propria. Gastroenterology. 1997 Apr;112(4):1398–1403. doi: 10.1016/s0016-5085(97)70156-2. [DOI] [PubMed] [Google Scholar]
- Li D. M., Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997 Jun 1;57(11):2124–2129. [PubMed] [Google Scholar]
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997 Mar 28;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liaw D., Marsh D. J., Li J., Dahia P. L., Wang S. I., Zheng Z., Bose S., Call K. M., Tsou H. C., Peacocke M. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997 May;16(1):64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Liu W., James C. D., Frederick L., Alderete B. E., Jenkins R. B. PTEN/MMAC1 mutations and EGFR amplification in glioblastomas. Cancer Res. 1997 Dec 1;57(23):5254–5257. [PubMed] [Google Scholar]
- Lynch E. D., Ostermeyer E. A., Lee M. K., Arena J. F., Ji H., Dann J., Swisshelm K., Suchard D., MacLeod P. M., Kvinnsland S. Inherited mutations in PTEN that are associated with breast cancer, cowden disease, and juvenile polyposis. Am J Hum Genet. 1997 Dec;61(6):1254–1260. doi: 10.1086/301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
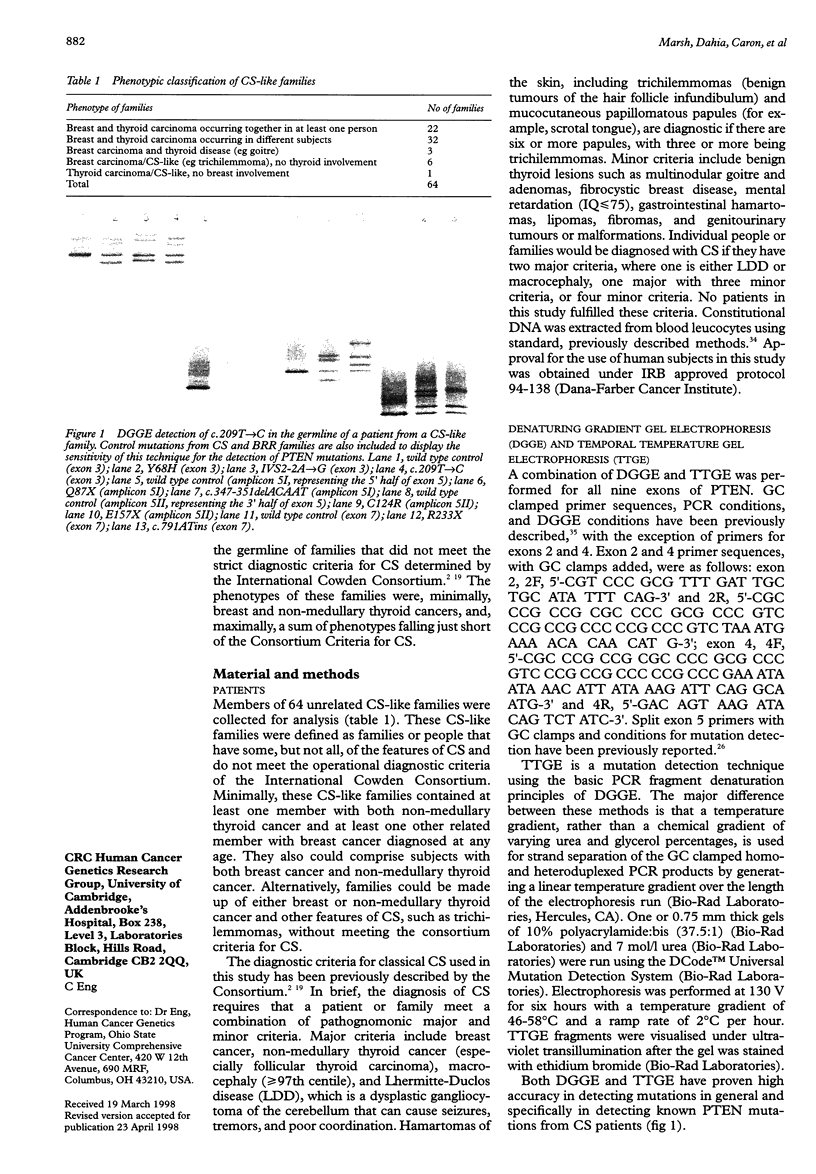
- Marsh D. J., Coulon V., Lunetta K. L., Rocca-Serra P., Dahia P. L., Zheng Z., Liaw D., Caron S., Duboué B., Lin A. Y. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998 Mar;7(3):507–515. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Dahia P. L., Zheng Z., Liaw D., Parsons R., Gorlin R. J., Eng C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997 Aug;16(4):333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Roth S., Lunetta K. L., Hemminki A., Dahia P. L., Sistonen P., Zheng Z., Caron S., van Orsouw N. J., Bodmer W. F. Exclusion of PTEN and 10q22-24 as the susceptibility locus for juvenile polyposis syndrome. Cancer Res. 1997 Nov 15;57(22):5017–5021. [PubMed] [Google Scholar]
- Mathew C. G., Smith B. A., Thorpe K., Wong Z., Royle N. J., Jeffreys A. J., Ponder B. A. Deletion of genes on chromosome 1 in endocrine neoplasia. Nature. 1987 Aug 6;328(6130):524–526. doi: 10.1038/328524a0. [DOI] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Myers M. P., Stolarov J. P., Eng C., Li J., Wang S. I., Wigler M. H., Parsons R., Tonks N. K. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelen M. R., Padberg G. W., Peeters E. A., Lin A. Y., van den Helm B., Frants R. R., Coulon V., Goldstein A. M., van Reen M. M., Easton D. F. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996 May;13(1):114–116. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- Nelen M. R., van Staveren W. C., Peeters E. A., Hassel M. B., Gorlin R. J., Hamm H., Lindboe C. F., Fryns J. P., Sijmons R. H., Woods D. G. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997 Aug;6(8):1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- Olschwang S., Serova-Sinilnikova O. M., Lenoir G. M., Thomas G. PTEN germ-line mutations in juvenile polyposis coli. Nat Genet. 1998 Jan;18(1):12–14. doi: 10.1038/ng0198-12. [DOI] [PubMed] [Google Scholar]
- Rasheed B. K., Stenzel T. T., McLendon R. E., Parsons R., Friedman A. H., Friedman H. S., Bigner D. D., Bigner S. H. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res. 1997 Oct 1;57(19):4187–4190. [PubMed] [Google Scholar]
- Rhei E., Kang L., Bogomolniy F., Federici M. G., Borgen P. I., Boyd J. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res. 1997 Sep 1;57(17):3657–3659. [PubMed] [Google Scholar]
- Risinger J. I., Hayes A. K., Berchuck A., Barrett J. C. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997 Nov 1;57(21):4736–4738. [PubMed] [Google Scholar]
- Sakurada A., Suzuki A., Sato M., Yamakawa H., Orikasa K., Uyeno S., Ono T., Ohuchi N., Fujimura S., Horii A. Infrequent genetic alterations of the PTEN/MMAC1 gene in Japanese patients with primary cancers of the breast, lung, pancreas, kidney, and ovary. Jpn J Cancer Res. 1997 Nov;88(11):1025–1028. doi: 10.1111/j.1349-7006.1997.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Starink T. M., van der Veen J. P., Arwert F., de Waal L. P., de Lange G. G., Gille J. J., Eriksson A. W. The Cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet. 1986 Mar;29(3):222–233. doi: 10.1111/j.1399-0004.1986.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Steck P. A., Pershouse M. A., Jasser S. A., Yung W. K., Lin H., Ligon A. H., Langford L. A., Baumgard M. L., Hattier T., Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997 Apr;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Tashiro H., Blazes M. S., Wu R., Cho K. R., Bose S., Wang S. I., Li J., Parsons R., Ellenson L. H. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997 Sep 15;57(18):3935–3940. [PubMed] [Google Scholar]
- Teng D. H., Hu R., Lin H., Davis T., Iliev D., Frye C., Swedlund B., Hansen K. L., Vinson V. L., Gumpper K. L. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997 Dec 1;57(23):5221–5225. [PubMed] [Google Scholar]
- Tsuchiya K. D., Wiesner G., Cassidy S. B., Limwongse C., Boyle J. T., Schwartz S. Deletion 10q23.2-q23.33 in a patient with gastrointestinal juvenile polyposis and other features of a Cowden-like syndrome. Genes Chromosomes Cancer. 1998 Feb;21(2):113–118. doi: 10.1002/(sici)1098-2264(199802)21:2<113::aid-gcc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ueda K., Nishijima M., Inui H., Watatani M., Yayoi E., Okamura J., Yasutomi M., Nakamura Y., Miyoshi Y. Infrequent mutations in the PTEN/MMAC1 gene among primary breast cancers. Jpn J Cancer Res. 1998 Jan;89(1):17–21. doi: 10.1111/j.1349-7006.1998.tb00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley J. M., McGown G., Thorncroft M., Santibanez-Koref M. F., Kelsey A. M., Tricker K. J., Evans D. G., Birch J. M. Germ-line mutations of TP53 in Li-Fraumeni families: an extended study of 39 families. Cancer Res. 1997 Aug 1;57(15):3245–3252. [PubMed] [Google Scholar]
- Wang S. I., Puc J., Li J., Bruce J. N., Cairns P., Sidransky D., Parsons R. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997 Oct 1;57(19):4183–4186. [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]