Abstract
Mexico virus (MXV) is a genogroup II human calicivirus (HuCV). We conducted an epidemiological study to determine the prevalence of MXV infection in infants and adults in Japan and Southeast Asia by enzyme-linked immunosorbent assays (ELISAs) developed by using baculovirus-expressed recombinant MXV (rMXV) capsids. Of 155 stool specimens obtained from children younger than 10 years old with acute clinical gastroenteritis (diarrhea and vomiting) associated with small, round-structured viruses in Japan from 1987 to 1989, only 2 were positive for MXV antigen. In 42 outbreaks of acute gastroenteritis in Japan from 1986 to 1994, 1 in an infant home and 1 among adults were positive for MXV antigen. The pattern of acquisition of antibody to rMXV was different from that of acquisition of antibody to group A rotavirus, the prototype HuCV Sapporo virus, and Norwalk virus. The prevalence of antibody to rMXV remained low for the first 3 years of life, showed a steep rise during nursery school age, reaching a prevalence of 50%, and another steep rise during adolescence, reaching 80%; and steadily increased thereafter. A high prevalence of antibody (82 to 88%) was observed in adult populations in Japan and Southeast Asia, suggesting that MXV infection is common in these areas. The discrepancy between the high prevalence of antibody to MXV and a low rate of detection of MXV antigen may be explained by a high specificity of the antigen ELISA for the prototype and closely related MXV strains while serological responses can detect responses to a broader group of viruses.
Based on sequence difference of the RNA-dependent RNA polymerase and capsid regions of genomes, human caliciviruses (HuCVs) have been divided into at least three genogroups, the Norwalk virus (NV)-like, Snow Mountain agent-like, and Sapporo virus-like genogroups, which are also referred to as genogroups I, II, and III, respectively (2, 5, 16, 21). The Mexico virus (MXV) belongs to genogroup II. Genogroup II viruses are proposed to be further divided into two subgenogroups. Subgenogroup 1 is represented by the prototype Snow Mountain agent, and subgenogroup 2 is represented by MXV based on the antigenic relationships between the two subgenogroups determined by the recombinant MXV (rMXV) antibody and antigen enzyme-linked immunosorbent assays (ELISAs) (11).
As for NV, the recent success of MXV gene cloning and the production of the rMXV capsid protein by using the baculovirus expression system have resulted in the availability of an unlimited amount of rMXV antigen and high-titered immune sera to rMXV which can enable large-scale epidemiological studies (4, 23). Epidemiological studies using these ELISAs and other genomic methods indicate that viruses in genogroup II, including MXV, are the predominant viruses detected currently in many countries, including Mexico, the United Kingdom, the United States, South Africa, Canada, Japan, and Spain. Genogroup II viruses have been reported to cause mainly sporadic cases of gastroenteritis in infants and outbreaks of gastroenteritis among school-aged children and adults (1, 6, 8, 9, 13–15, 17, 21, 24).
In this study, we conducted epidemiological surveys by using antigen and antibody ELISAs for rMXV to determine the prevalence of MXV infection in infants and adults in Japan and Southeast Asia and demonstrated that MXV infection is common in these areas.
MATERIALS AND METHODS
Stool samples.
Four hundred twenty stool samples were tested by the antigen ELISA for MXV. Of these stool specimens, 155 were collected from children younger than 10 years of age with acute clinical gastroenteritis (diarrhea and vomiting) who had visited 10 pediatric outpatient clinics in Sapporo, Japan, from 1987 to 1989 and 1 outpatient clinic in Ehime prefecture, Japan, from 1984 to 1988. These samples were positive for small, round-structured viruses (SRSV) by electron microscopy and had been examined for NV (22) and HuCV Sapporo (HuCV/Sa/82/J) by ELISAs (12). Two hundred forty-five stool samples were obtained from patients involved in 42 outbreaks of acute gastroenteritis in Japan from 1986 to 1994. One outbreak occurred in a pediatric ward of the Hakodate city hospital in 1982, 18 outbreaks occurred among school children and adults in Aichi prefecture from 1987 to 1991, and 23 outbreaks occurred in an infant home in Sapporo from 1986 to 1994. Ten samples collected from patients with group A rotavirus gastroenteritis in Sapporo, 10 specimens obtained from patients with gastroenteritis due to enteric adenovirus type 40 or 41 (22), and one sample positive for group C rotavirus were tested as negative controls. Stool samples were prepared as a 10% (wt/vol) suspension in 10 mM phosphate-buffered saline (PBS; pH 7.4) and clarified by centrifugation at 3,000 × g for 20 min. The supernatant was extracted with an equal volume of Difulon solvent (trichlorotrifluoroethane; Daikin Kogyo Ltd., Tokyo, Japan) and clarified by centrifugation at 7,000 × g for 20 min. The aqueous phase was stored at 4°C until testing.
Serum samples.
Six hundred eighty-four serum samples collected from healthy adults or ill children without gastroenteritis were tested by ELISA for antibody to rMXV. One hundred eighty serum samples were collected from children (1 month to 19 years of age) without gastroenteritis who were outpatients or inpatients at the Sapporo Medical University hospital from 1986 to 1991. They were divided into six age groups (0 to 3 months, 4 to 11 months, 1 to 2 years, 3 to 6 years, 7 to 11 years, and 12 to 19 years), and 30 samples from each age group were tested. Thirty cord blood samples were obtained by the Sapporo Medical University Hospital in 1991. One hundred thirty-four serum samples were collected from healthy adults (over 20 years of age) in 1992 in Hokkaido, Japan, and kindly supplied by S. Sekiguchi (Japan Red Cross Society, Hokkaido, Japan). They were divided into four age groups (20 to 29, 30 to 39, 40 to 49, and over 50 years), and 30, 30, 30, and 44 samples from each age group were tested. The other 190 serum samples from healthy adults aged 20 to 49 years were collected from four prefectures in Japan in 1978, of which 40 were from Miyagi-ken, 50 were from Saitama-ken, 50 were from Kyoto-fu, and 50 were from Fukuoka-ken. Of these four prefectures, Miyagi-ken is located in the northern part of Japan and Fukuoka-ken is in the southern part of Japan. These samples were kindly provided by S. Yamazaki (National Institute of Health, Tokyo, Japan). One hundred fifty serum samples were obtained from healthy adults in three countries in Southeast Asia and were kindly supplied by S. Yamazaki. Fifty of these were obtained from adults aged 20 to 37 years in Singapore in 1975, 50 were obtained from adults aged 20 to 29 years in Indonesia in 1975 and 1976, and 50 were obtained from adults aged 20 to 44 years in Papua, New Guinea in 1979. All serum samples were stored at −20°C and tested under code.
Baculovirus-expressed rMXV capsid protein and preimmune and hyperimmune antisera.
rMXV capsid protein and guinea pig and rabbit preimmune and hyperimmune sera against rMXV were prepared as described previously (10, 11).
ELISA for antigen detection.
ELISAs for MXV antigen were performed in 96-well polyvinyl chloride flat-bottom microtiter plates (Dynatech Laboratories, Inc., Chantilly, Va.) as described previously, with minor modifications (11, 20). PBS (pH 7.4) containing 10% fetal calf serum and 1% bovine serum albumin was used as the diluent for stool samples, blocking serum, and detector antibody. Peroxidase-conjugated goat antibody to rabbit immunoglobulin G (Seikagaku Kogyo Co., Ltd., Tokyo, Japan) was diluted in PBS containing 5% normal guinea pig serum and 1% bovine serum albumin. o-Phenylenediamine dihydrochloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in citric acid buffer (pH 4.0) containing 0.4 μl of 30% H2O2 per ml was used as the substrate. The reaction was stopped by adding 100 μl of 1 M H2SO4 into each well, and the reactions in the plates were measured by determining the A492 with an ELISA reader (Easy Reader EAR400; SLT-Labinstruments). Each sample giving an A492 of >0.1 and a P/N ratio of >1.8 (mean plus 3.0 standard deviations of 20 control stool samples) was considered to be positive. All tests were performed in duplicate, and the results were averaged.
ELISA-BL.
Antibodies to rMXV were measured by an ELISA-blocking test (ELISA-BL) previously used in the laboratory to measure antibodies to a variety of enteric viruses (20, 22). In each microtiter plate, an optimal dilution of hyperimmune serum, which produced >90% reduction in the A492, served as the positive control; diluent alone served as the negative control. A result of >50% inhibition of the A492 produced by the serum samples compared with that of the buffer control was considered to be positive for antibody to rMXV. All tests were performed in duplicate, and the results were averaged.
RESULTS
Specificity and sensitivity of ELISA for detection of MXV.
The specificity of the guinea pig and rabbit hyperimmune sera for the rMXV and rMXV protein used in the ELISAs done in this study was established in previous experiments (10, 11) and was further confirmed in this study by testing stool specimens and cell culture fluids containing different enteric viruses (12). None of the stool samples obtained from patients with group A rotavirus, enteric adenovirus, and group C rotavirus were positive in the antigen ELISA for rMXV. Five cell culture fluids containing group A rotavirus, enteric adenovirus, poliovirus, echovirus, and coxsackievirus also were negative. The sensitivity of the ELISA for detection of MXV was determined by testing serial dilutions of rMXV protein. An end point of 1.5-ng/ml rMXV protein was detected by this ELISA (data not shown).
Specificity and sensitivity of ELISA-BL for detection of antibody to MXV.
The specificity and sensitivity of the ELISA-BL for detection of antibody to MXV were evaluated by using hyperimmune and preimmune sera against different enteric viruses from laboratory animals. Guinea pig and rabbit hyperimmune sera to rMXV were used as positive controls and found to have antibody titers of 1:50,000 and 1:20,000, respectively. Three guinea pig hyperimmune sera to group A, B, and C rotaviruses; two sets of guinea pig and rabbit hyperimmune sera to rNV (22) and HuCV/Sa/82/J (20); and two preimmune sera were all negative in the ELISA-BL for detection of antibody to MXV (<1:50).
Detection of MXV antigen in sporadic and outbreak cases of gastroenteritis.
A total of 155 samples previously confirmed to be positive for SRSVs by electron microscopy had previously been tested for NV and HuCV/Sa/82/J by ELISA (12, 22) or dot blot assay (12). One of 105 samples from Sapporo and 1 of 50 samples from Ehime prefecture were positive for MXV antigen but negative for NV and HuCV/Sa/82/J (Table 1). Of 245 stool samples obtained from 42 outbreaks, stool samples positive for MXV came from one outbreak among adults in Aichi prefecture and from another outbreak in an infant home (Table 2).
TABLE 1.
Detection of MXV antigen in stool of children with sporadic SRSV gastroenteritis by rMXV ELISA
TABLE 2.
Detection of MXV antigen in stool of infants or adults involved in outbreaks of acute gastroenteritis by rMXV ELISA
| Geographic origin | No. (%) positive for rMXV by ELISA | No. (%) positive for rNV by ELISAa | No. (%) positive for Sapporo virus by ELISAb | No. tested |
|---|---|---|---|---|
| Hakodate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| Aichi | 1 (5.6) | 3 (16.7) | 0 (0.0) | 18 |
| Otaru | 1 (4.3) | 0 (0.0) | 0 (0.0) | 23 |
| Total | 2 (4.8) | 3 (7.1) | 0 (0.0) | 42 |
From Numata et al. (22).
Unpublished data.
Age-related prevalence of antibody to MXV in Hokkaido, Japan.
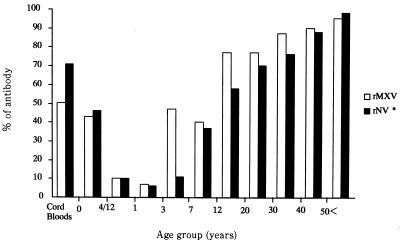
Age-related prevalence of antibody to MXV in Hokkaido, Japan, is shown in Fig. 1. The samples tested included 30 cord blood samples, from 10 age groups ranging from birth to >60 years, that had previously been tested for antibodies to NV (22). Sixteen (53.3%) of the 30 cord blood samples had a titer of antibody to rMXV which was lower than that of the 20 (76.7%)- and 30 (86.7%)-year-old age groups (Table 1). The prevalence of antibody was 45% at 0 to 3 months of age, decreased thereafter, and reached a minimum of 6.7% at 1 to 2 years of age. The positivity rate showed a steep rise during nursery school age and adolescence, reaching about 80% by the age of 12. Antibody prevalence continued to rise throughout adulthood, reaching almost 100% by the age of 60.
FIG. 1.
Age-related prevalence of serum antibody to rMXV from children and adults in Hokkaido, Japan. ∗, from Numata et al. (22).
Prevalence of MXV infection in Japan and Southeast Asia.
The prevalence of antibody to rMXV by age and sex in five districts of Japan is shown in Table 3. No significant difference of antibody prevalence was observed among these areas, whereas the positivity rate gradually increased with age. The total proportion of seropositivity to rMXV in Japan was 86.7% (281 of 324 samples). A similar high prevalence of MXV infection (82 to 88%, with an average of 85.3%) was observed in healthy adults in three Southeast Asian countries (Table 4). No significant difference was observed among the three countries.
TABLE 3.
Prevalence of serum antibody to rMXV by age and sex in healthy adults from five districts in Japan
| District | No. positivea/no. tested (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 20–29 yr old | 30–39 yr old | 40–49 yr old | 50–59 yr old | >60 | Males | Females | Total | |
| Hokkaido | 23/30 (77) | 26/30 (87) | 27/30 (90) | 28/30 (93) | 14/14 (100) | 79/90 (88) | 39/44 (89) | 118/134 (88) |
| Miyagi-ken | 25/30 (83) | 10/10 (100) | — | — | — | 14/15 (93) | 21/25 (84) | 35/40 (88) |
| Saitama-ken | 35/39 (89) | 8/10 (80) | 1/1 (100) | — | — | 1/1 (100) | 43/49 (88) | 44/50 (88) |
| Kyoto-fu | 33/40 (83) | 9/10 (90) | — | — | — | 15/15 (100) | 27/35 (77) | 42/50 (84) |
| Fukuoka-ken | 30/38 (79) | 10/10 (100) | 2/2 (100) | — | — | 1/1 (100) | 41/49 (84) | 42/50 (84) |
| Total | 146/177 (82) | 63/70 (90) | 30/33 (91) | 28/30 (93) | 14/14 (100) | 110/122 (90) | 171/202 (85) | 281/324 (87) |
Serum samples with >50% inhibition of the A492 in the rMXV ELISA were considered positive for antibody to MXV.—, no sera available.
TABLE 4.
Prevalence of serum antibody to rMXV by age and sex in healthy adults from three Southeast Asian countries
| Country | No. positivea/no. tested (%)
|
|||||
|---|---|---|---|---|---|---|
| 20–29 yr old | 30–39 yr old | 40–49 yr old | Males | Females | Total | |
| Singapore | 22/30 (73) | 19/20 (95) | — | 41/50 (82) | — | 41/50 (82) |
| Indonesia | 43/49 (88) | 1/1 (100) | — | 21/25 (84) | 23/25 (92) | 44/50 (88) |
| Papua New Guinea | 24/27 (89) | 17/19 (89) | 2/4 (50) | 13/18 (72) | 30/32 (94) | 43/50 (86) |
| Total | 89/106 (84) | 37/40 (93) | 2/4 (50) | 75/93 (81) | 53/57 (93) | 128/150 (85) |
Serum samples with >50% inhibition of the A492 in the rMXV ELISA were considered positive for antibody to MXV. —, no sera available.
DISCUSSION
This is the first report of a study using rMXV ELISAs to describe HuCV infection in Japan and Southeast Asia. In 155 stool samples obtained from children with sporadic SRSV gastroenteritis in Japan, we found only 2 MXV antigen-positive samples. The low rate of MXV antigen detection in this study was similar to the results found in London, where only 1 of 206 samples was positive for MXV (3). Several explanations may account for the low virus detection rate. (i) The ELISA for MXV antigen may be quite specific for prototype MXV and detect only a few strains of genogroup II HuCVs which are antigenically close to the prototype virus. (ii) The stool samples in our study were collected mainly from infants younger than 3 years of age, while the MXV infections in Sapporo may not be common until nursery school age based on our serological data. (iii) MXV-associated gastroenteritis may be mild and not require visiting an outpatient clinic. (iv) The rMXV ELISA does not detect subgenogroup 1 HuCVs, which could be more prevalent during the study period than subgenogroup 2 within genogroup II of HuCV (11). Use of antigen ELISAs for subgenogroup 1 viruses of genogroup II HuCV (7) or genetic analysis of SRSV-positive samples may be required to distinguish between these possibilities.
Although genogroup II HuCVs are considered to be the predominant viruses currently detected in the world (Mexico, the United Kingdom, the United States, South Africa, Canada, Japan, and Spain) (1, 6, 9, 13–15, 17, 24), outbreaks of acute gastroenteritis due to genogroup II HuCVs have been reported only from the United Kingdom (3, 8), the United States (11, 21), and Japan (25). Those outbreaks occurred mainly in geriatric wards and nursing homes for the elderly. Our case is the second report of an outbreak affecting infants (unpublished data) in addition to a Toronto virus-associated outbreak (13). From our serological data obtained in Sapporo, Japan, MXV infection is not common in children younger than 3 years of age. Children under this age are considered to be susceptible to MXV, and outbreaks are likely to occur once enteric viruses, including MXV, are introduced into the community, e.g., in infant homes with particularly crowded conditions.
The serosurveillance results presented in this report indicate that MXV infection is common in Japan and Southeast Asian countries, confirming the previous report by Yamazaki et al. (25) in that many outbreaks of acute gastroenteritis in adults that occurred between 1985 and 1995 in eight prefectures in Japan were associated with genogroup II HuCVs. However, the detection of antigen in stool specimens by rMXV ELISA remains low based on our study. Several possibilities may explain the high prevalence of antibody to MXV but the low level of MXV antigen detection in stools. First, repeated asymptomatic MXV infections may boost the antibody titer to this virus, as found in HuCV/Sa/82/J infection (18). Second, the high prevalence of antibody in the adult population may reflect separate infections with viruses antigenically related to MXV but distinct from MXV by the rMXV antigen ELISA (11). Finally, MXV may cause diseases other than acute gastroenteritis.
The age-related prevalence of antibody to rMXV in Hokkaido was strikingly different from the prevalence of antibody to group A rotavirus and HuCV/Sa/82/J and slightly different from that of antibody to NV. In our study, acquisition of antibody to rMXV remained at a low level for the first 3 years and then showed a steep rise during the nursery school ages and the adolescence, whereas antibodies to group A rotavirus or HuCV/Sa/82/J were acquired in early childhood (20). In contrast, antibody to NV was first acquired by school age children and in early adulthood (22). Thus, MXV infection in Hokkaido occurs in older children when they start to attend kindergarten, indicating that infection with MXV may require a high population density.
The higher prevalence of antibody detected in cord blood and serum samples obtained from infants aged 0 to 3 months, which declined to 6.7 to 10.0% in infants aged 4 months to 3 years, suggests that antibody detected in early infancy was probably passively acquired from mothers. The lower reduction rates of the ELISA-BL found in the 0- to 3-month age group compared with the higher reduction rates found in the childhood and older age groups support the above possibility (data not shown). There is no clear explanation for the discrepancy between the prevalence of antibody to rMXV in cord blood samples and that in serum samples obtained from the maternal age group (20 to 39 years old). One possibility might be the difference in the periods when the serum samples were collected.
Further epidemiological studies using the rMXV ELISA are required to clarify the significance of MXV infection in the world. New ELISA methods for a broader range of HuCVs should be useful for antigenic and serologic studies on the relationships among several HuCVs.
ACKNOWLEDGMENTS
This study was supported in part by grant 044543878 from the Ministry of Education, Science, and Culture of Japan, by grants from the U.S. Public Health Service (HD-13021 and AI 28544), and by the Jeffress Research Grant Foundation (J-303).
We thank Mary K. Estes (Baylor College of Medicine, Houston, Tex.) for suggestions and criticism.
REFERENCES
- 1.Ando T, Monroe S S, Gentsch J R, Jin Q, Lewis D C, Glass R I. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J Clin Microbiol. 1995;33:64–71. doi: 10.1128/jcm.33.1.64-71.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berke T, Golding B, Jiang X, Cubitt W D, Wolfaardt M, Smith A W, Matson D O. Phylogenetic analysis of the caliciviruses. J Med Virol. 1997;52:419–424. doi: 10.1002/(sici)1096-9071(199708)52:4<419::aid-jmv13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Cubitt W D, Jiang X, Wang J, Estes M K. Sequence similarity of human caliciviruses and small round structured viruses. J Med Virol. 1994;43:252–258. doi: 10.1002/jmv.1890430311. [DOI] [PubMed] [Google Scholar]
- 4.Cubitt W D, Jiang X. Study on occurrence of human calicivirus (Mexico strain) as cause of sporadic cases and outbreaks of calicivirus-associated diarrhoea in the United Kingdom, 1983–1995. J Med Virol. 1996;48:273–277. doi: 10.1002/(SICI)1096-9071(199603)48:3<273::AID-JMV10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Estes M K, Hardy M E. Norwalk virus and other enteric caliciviruses. In: Blaser M J, et al., editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 1009–1034. [Google Scholar]
- 6.Green J, Norcott J P, Lewis D, Arnold C, Brown D W G. Norwalk-like viruses: demonstration of genomic diversity by polymerase chain reaction. J Clin Microbiol. 1993;31:3007–3012. doi: 10.1128/jcm.31.11.3007-3012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green K Y, Kapikian A Z, Valdesuso J, Sosnovtsev S, Treanor J J, Lew J F. Expression and self-assembly of recombinant capsid protein from the antigenically distinct Hawaii human calicivirus. J Clin Microbiol. 1997;35:1909–1914. doi: 10.1128/jcm.35.7.1909-1914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green S M, Lambden P R, Caul E O, Clarke I N. Capsid sequence diversity in small round structured viruses from recent UK outbreaks of gastroenteritis. J Med Virol. 1997;52:14–19. [PubMed] [Google Scholar]
- 9.Jiang X, Wang J, Estes M K. Characterization of SRSVs using RT-PCR and a new antigen ELISA. Arch Virol. 1995;140:363–374. doi: 10.1007/BF01309870. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Matson D O, Ruiz-Palacios G M, Hu J, Treanor J, Pickering L K. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J Clin Microbiol. 1995;33:1452–1455. doi: 10.1128/jcm.33.6.1452-1455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Cubitt D, Hu J, Dai X, Treanor J, Matson D O, Pickering L K. Development of an ELISA to detect MX virus, a human calicivirus in the Snow Mountain agent genogroup. J Gen Virol. 1995;76:2739–2747. doi: 10.1099/0022-1317-76-11-2739. [DOI] [PubMed] [Google Scholar]
- 12.Kogawa K, Nakata S, Ukae N, Adachi N, Numata K, Matson D O, Estes M K, Chiba S. Dot blot hybridization with a cDNA probe derived from the human calicivirus Sapporo 1982 strain. Arch Virol. 1996;141:1949–1959. doi: 10.1007/BF01718206. [DOI] [PubMed] [Google Scholar]
- 13.Lew J F, Petric M, Kapikian A Z, Jiang X, Estes M K, Green K Y. Identification of minireovirus as a Norwalk-like virus in pediatric patients with gastroenteritis. J Virol. 1994;68:3391–3396. doi: 10.1128/jvi.68.5.3391-3396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lew J F, Valdesuso J, Vesikari T, Kapikian A Z, Jiang X, Estes M K, Green K Y. Detection of Norwalk virus or Norwalk-like virus infections in Finnish infants and young children. J Infect Dis. 1994;169:1364–1367. doi: 10.1093/infdis/169.6.1364. [DOI] [PubMed] [Google Scholar]
- 15.Lew J F, Kapikian A Z, Valdesuso J, Green K Y. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J Infect Dis. 1994;170:535–542. doi: 10.1093/infdis/170.3.535. [DOI] [PubMed] [Google Scholar]
- 16.Matson D O, Zhong W M, Nakata S, Numata K, Jiang X, Pickering L K, Chiba S, Estes M K. Molecular characterization of a human calicivirus with sequence relationships closer to animal caliciviruses than other known human caliciviruses. J Med Virol. 1995;45:215–222. doi: 10.1002/jmv.1890450218. [DOI] [PubMed] [Google Scholar]
- 17.Moe C L, Gentsch J, Ando T, Grohmann G, Monroe S S, Jiang X, Wang J, Estes M K, Seto Y, Humphrey C. Application of PCR to detect Norwalk virus in fecal specimens from outbreaks of gastroenteritis. J Clin Microbiol. 1994;32:642–648. doi: 10.1128/jcm.32.3.642-648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakata S, Chiba S, Terashima H, Yokoyama T, Nakao T. Humoral immunity in infants with gastroenteritis caused by human calicivirus. J Infect Dis. 1985;152:274–279. doi: 10.1093/infdis/152.2.274. [DOI] [PubMed] [Google Scholar]
- 19.Nakata S, Chiba S, Terashima T, Nakao H. Prevalence of antibody to human calicivirus in Japan and Southeast Asia determined by radioimmunoassay. J Clin Microbiol. 1985;22:519–521. doi: 10.1128/jcm.22.4.519-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakata S, Estes M K, Chiba S. Detection of human calicivirus antigen and antibody by enzyme-linked immunosorbent assays. J Clin Microbiol. 1988;26:2001–2005. doi: 10.1128/jcm.26.10.2001-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noel J S, Ando T, Leite J P, Green K Y, Dingle K E, Estes M K, Seto Y, Monroe S S, Glass R I. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J Med Virol. 1997;53:372–383. doi: 10.1002/(sici)1096-9071(199712)53:4<372::aid-jmv10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Numata K, Nakata S, Jiang X, Estes M K, Chiba S. Epidemiological study of Norwalk virus infections in Japan and Southeast Asia by enzyme-linked immunosorbent assays with Norwalk virus capsid protein produced by the baculovirus expression system. J Clin Microbiol. 1994;32:121–126. doi: 10.1128/jcm.32.1.121-126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker S P, Cubitt W D, Jiang X. Enzyme immunoassay using baculovirus-expressed human calicivirus (Mexico) for the measurement of IgG responses and determining its seroprevalence in London, UK. J Med Virol. 1995;46:194–200. doi: 10.1002/jmv.1890460305. [DOI] [PubMed] [Google Scholar]
- 24.Wolfaardt M, Taylor M B, Grabow W, Cubitt W D, Jiang X. Molecular characterization of small round structured viruses associated with gastroenteritis in South Africa. J Med Virol. 1995;47:386–391. doi: 10.1002/jmv.1890470415. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki K, Oseto M, Seto Y, Utagawa E, Kimoto T, Minekawa Y, Inoue S, Yamazaki S, Okuno Y, Oishi I. Reverse transcription-polymerase chain reaction detection and sequence analysis of small round-structured viruses in Japan. Arch Virol. 1996;12:271–276. doi: 10.1007/978-3-7091-6553-9_29. [DOI] [PubMed] [Google Scholar]