Abstract
Development of novel adsorbents often neglect the competitive adsorption between co-occurring oxo-anions, over-estimating realistic pollutant removal potentials, and overlook the need to improve selectivity of materials. This critical review focuses on adsorptive competition between commonly co-occurring oxo-anions in water and mechanistic approaches for the design and development of selective adsorbents. Five “target” metal(loid) oxo-anion pollutants (arsenate, arsenite, selenate, selenite, and chromate) were selected for study. Five “competing” co-occurring oxo-anions (phosphate, sulfate, bicarbonate, silicate, and nitrate) were selected due to their potential to compete with target oxo-anions for sorption sites resulting in decreased removal of the target oxo-anions. First, a comprehensive review of competition between target and competitor oxo-anions to sorb on commonly used, non-selective, metal hydr(oxide) materials is presented and the strength of competition between each target and competitive oxo-anion pair is classified. This is followed by a critical discussion of the different equations and models used to quantify selectivity. Next, five mechanisms that have been successfully utilized in the development of selective adsorbents are reviewed: variation in surface complexation, Lewis acid/base hardness, steric hindrance, electrostatic interactions, and hydrophobic interactions. For each mechanism, the oxo-anions, both target and competitors, are ranked in terms of adsorptive attraction and technologies that exploit this mechanism are reviewed. Third, given the significant effort to evaluate these systems empirically, the potential to use computational quantum techniques, such as density functional theory (DFT), for modeling and prediction is explored. Finally, areas within the field of selective adsorption requiring further research are detailed with guidance on priorities for screening and defining selective adsorbents.
1. Introduction
Exposure to toxic metal(loid) oxo-anions, such as arsenic, selenium, and chromium, through contaminated groundwater-sourced drinking water poses a threat to human and environmental health.1 The scope of this challenge is large with approximately 40% of the United States population served by groundwater-sourced drinking water, through public supply or self-supplied (primarily from private domestic wells) systems.2,3 Globally, arsenic contamination of drinking water has been reported in more than 70 countries, mainly in South and Southeastern Asia.4
Of the traditional water treatment technologies to remove oxo-anions, adsorbent processes are an attractive option for smaller-sized communities, which face high rates of water quality violations,5 while for larger-sized communities it is often more cost-effective to use chemical coagulants (e.g., iron, aluminum) that form floc and adsorb pollutants but form sludges that must be dewatered prior to landfill disposal. Advantages of adsorbents include their generally high removal efficiencies, feasible logistic use in decentralized field conditions, and regeneration potential.1,5–9 However the low specificity of traditional non-selective adsorbent materials can lead to less effective removal of the target contaminants, contributing to failure in achieving regulatory limits.10 As many oxo-anions share similar chemical structure and behavior, their adsorptive mechanisms also tend to be alike, thus promoting competition for adsorption sites.11 Additionally, lower concentrations of contaminants such as arsenic, selenium, and chromium, compared to background ions that compete for adsorption binding sites can result in decreased removal efficiency due to adsorption sites becoming saturated with competitive ions rather than target contaminants.1,11
Selective adsorption, or the favorable removal of a target contaminant over competing and co-occurring background ions, overcomes competition by promoting target contaminant adsorption and/or hindering background oxo-anion adsorption.12 Selective adsorbent material properties (surface area, pore size, surface charge, energy density of binding sites) can be developed by exploiting chemical behavioral differences, such as complexation strength, Lewis acid/base hardness, hydrophobicity, polarizability, size, and shape between target contaminants and competitive ions.11 Depending the strength of competition, which is typically controlled by the degree of similarity in binding mechanisms and chemical structure,13–16 multiple mechanisms may be required to realize selectivity.17 Likewise, competitive adsorption between bulk natural organic matter and target synthetic organic pollutants on activated carbon or other porous adsorbents has been well studied and numerous mechanistic and empirical models developed to aid in predictive pollutant removals or improvements in material designs.18–21 However, there has been less focus on selectivity and competitive adsorption for co-occurring oxo-anions on metal oxide adsorbents, despite their widespread use and development for removing metallic oxo-anions from drinking waters.1,22,23
Interference from competing ions greatly reduces removal effectiveness of the target contaminant increasing the requirements for sorbent material, and subsequently costs; as such, a tailored approach to water treatment that considers selectivity can increase material and resource efficiency.24,25 Selectivity also results in a waste stream of reduced complexity due to the singular removal of a contamiant.26 This could, in turn, decrease the energy and costs required for proper disposal.
In this critical review, we will classify the strength of competition between target/competitive oxo-anion pairs, review equations used to quantify selectivity, and discuss four mechanisms that have been successfully utilized in the development of selective adsorbents: variation in surface complexation, Lewis acid/base hardness, steric hindrance, and electrostatic interactions (Figure 1). For each mechanism, the oxo-anions are ranked in terms of adsorptive attraction and technologies that exploit this mechanism are discussed. Next, the potential to use computational quantum techniques to model and predict selective adsorption is explored. Finally, areas within the field of selective adsorption that require further research are detailed with guidance on priorities for designing, screening, and assessing selective adsorbents.
Figure 1.
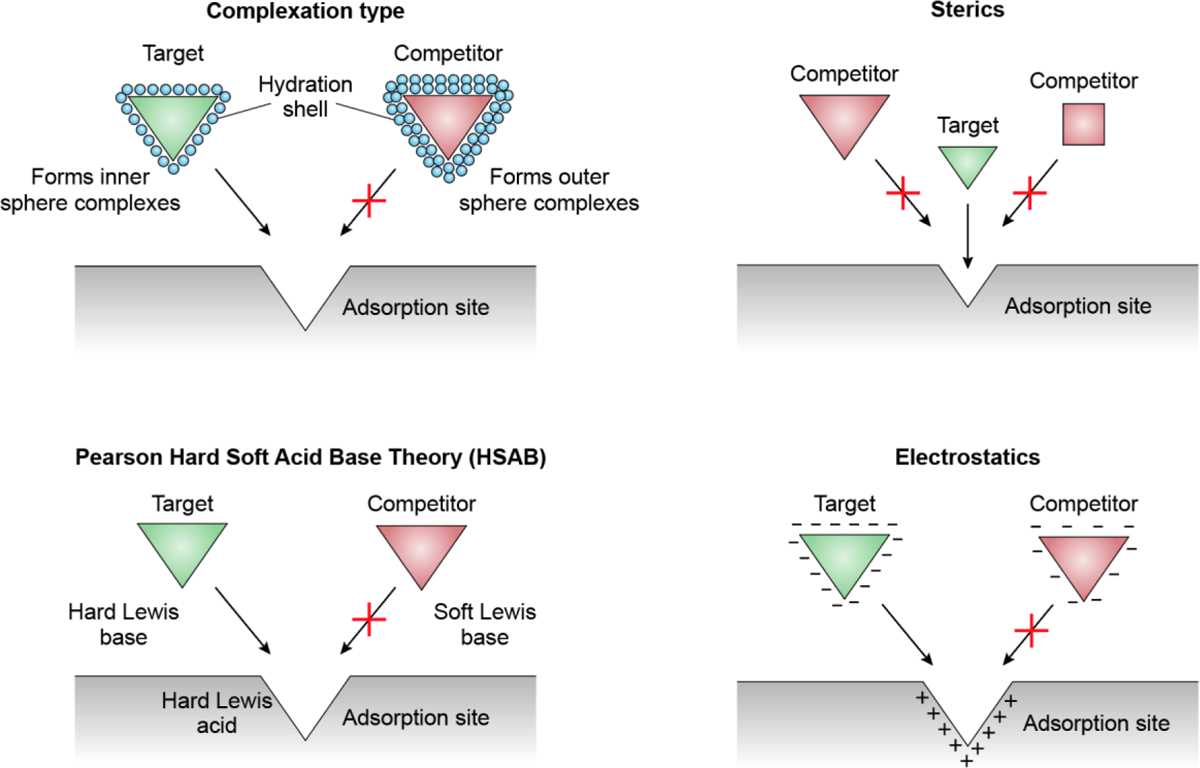
Mechanisms for the selective adsorption of target oxo-anions over competitor oxo-anions. Green indicates target oxo-anion and red the competitor oxo-anion(s).
2. Target Oxo-anions and Competitors
This review will focus on three target drinking water contaminants of current interest to human health and the environment – arsenic, selenium, and chromium (Table 1) – and their most competitive oxo-anions – phosphate, sulfate, silicate, bicarbonate and nitrate (Table 1) under typical environmental conditions (pH ~6). This review will not focus on OH−, or pH, as a competitive anion since this circumstance can be adjusted during treatment conditions. Rather, this review is aimed at the inherent properties of sorbent materials that can be intentionally designed. It is important to note that the classifications of “target” and “competitor” are flexible as some of the competitor oxo-anions could also be considered “targets”. For example, phosphate and nitrate are also pollutants of environmental concern due to their role in eutrophication.27,28 Additionally, this review will focus solely on targeting toxic metal(loid) oxo-anions. There are other contaminants of concern, such as uranyl and perchlorate, which warrant development of selective adsorption technologies; however, since uranyl is an oxo-cation and perchlorate is neither a metal nor a metalloid oxo-anion, these two contaminants fall outside the scope of this review.
Table 1.
Target and Competitor Oxo-anion pKa’s, Geometry, and Common Occurrence Range
| Oxo-anion | pKa’s | Protonated Structure at pH 6 | Oxo-anion Geometry | Common Occurrence Concentration Range in Groundwater | Refs. |
|---|---|---|---|---|---|
| Target Oxo-anions | |||||
| Arsenate As(V) H2AsO4− |
pKa1 = 2.2 pKa2 = 7.0 pKa3 = 11.6 |
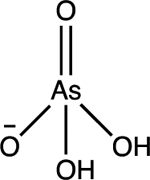
|
Tetrahedral | Background: < 0.01 mg/L Affected waters: 0.08 to 5 mg/ L |
29–31 |
| Arsenite As(III) H3AsO3 |
pKa1 = 9.2 |
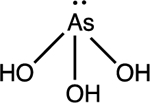
|
Trigonal pyramid | Background: < 0.01 mg/L Affected waters: 0.01 to 5 mg/ L |
30,32 |
| Selenate Se(VI) SeO42- |
pKa1 = −3.0 pKa2 = 1.7 |
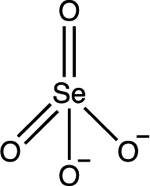
|
Tetrahedral | Background: < 2×10−5 mg/L Affected waters: 0.14 to 1.4 mg/L |
33–35 |
| Selenite Se(VI) HSeO3− |
pKa1 = 2.6 pKa2 = 8.2 |
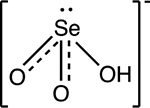
|
Trigonal pyramid | Background: < 2×10−5 mg/L Affected waters: 0.14 to 1.4 mg/L |
33–35 |
| Chromate Cr(VI) HCrO4− |
pKa1 = −0.98 pKa2 = 6.5 |
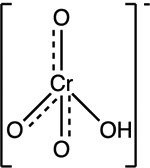
|
Tetrahedral | Background: 0.002 to 0.014 mg/L Affected waters: 0.05 to 0.43 mg/L |
36,37 |
| Competitor Oxo-anions | |||||
| Phosphate H2PO4− |
pKa1 = 2.1 pKa2 = 7.2 pKa3 = 12.7 |
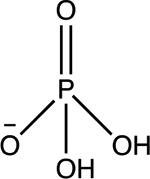
|
Tetrahedral | Background (total phosphorus): 0.05 to 2 mg/L | 29,38–40 |
| Sulfate SO42- |
pKa1 = −3.0 pKa2 = 2.0 |
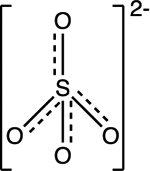
|
Tetrahedral | Background: 1 to 250 mg/L | 41–43 |
| Silicate H4SiO4 |
pKa1 = 9.8 |
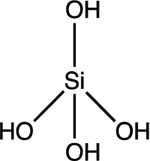
|
Tetrahedral | Background: 1 to 30 mg/L |
42
44 |
| Bicarbonate HCO3− |
pKa1 = 6 pKa2 = 10.3 |
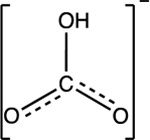
|
Trigonal planar | Background: 150 to 450 mg/L | 42,45 |
| Nitrate NO3− |
pKa1 = −1.3 |
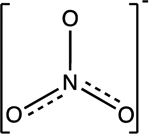
|
Trigonal planar | Background: 0.2 to 10 mg/L | 39,41,46 |
Arsenic, selenium, and chromium represent typical oxo-anion contaminants of concern with their multiple relevant redox states and known toxicity concerns. Arsenic frequently occurs as an oxo-anion in either +III (arsenite) or +V (arsenate) oxidation states, depending upon the redox conditions and dissolved oxygen concentration of a water.1 Arsenite is more difficult to adsorb than arsenate as a result of its neutral charge in environmental conditions.1,47,48 The WHO regulatory guideline for total arsenic concentration in drinking water is 10 ppb.31 Selenium exists in the environment predominantly as selenite (Se (IV)) and selenate (Se (VI)). Selenate is more difficult to remove due to its high solubility and weaker complexation with adsorbents.49–52 While selenium is an essential nutrient at low doses, it is a toxin and potentially carcinogenic at higher doses.53 The regulatory drinking water guideline set by the WHO for total selenium is 40 ppb, but because selenium also impacts aquatic ecosystems – its removal is often required from a diverse array of water sources.31 Chromium is predominantly found in the environment as Cr(III) (Cr2O3) or Cr(VI) (HCrO4−). While Cr(III) has relatively low toxicity and is insoluble in most environmentally relevant conditions, Cr(VI) is extremely toxic and highly mobile.54,55 The WHO regulatory guideline for the total level of chromium (Cr(III) and Cr(VI)) allowed in drinking water is 50 ppb.31
While reductions in removal efficiency of target oxo-anions in the presence of competitive ions is well-known and varies by adsorbent material (Table 2), studies do not consistently report on strong competition pairs or effectively assess selectivity. For example, phosphate and silicate are generally strong competitors and should be considered priorities in designs of selective adsorbent. Bicarbonate competes moderately to weakly, never decreasing removal by more than 50%. The strength of competition by sulfate varies as it weakly competes with arsenate, arsenite, and selenite, but is a strong to moderate competitor for selenate and chromate. Nitrate is generally a weak competitor. Further, most studies examine the individual effects between target and competitive oxo-anions, but do not systematically consider the most relevant competition pairs. Additionally, under environmental conditions, multiple competitive oxo-anions will frequently be present, and the presence of more than one competitive oxo-anion tends to further decrease removal efficiency compared to singular competitors.56
Table 2.
Strength of competitive adsorption on traditional adsorptive media by oxo-anions
| Target | Competitor | Similar traits | Dissimilar traits | Adsorptive Media | Approximate Reduction in Removal Efficiency |
|---|---|---|---|---|---|
| Arsenate H2AsO4− |
Phosphate H2PO4− |
pKa,57,58LBH,59,60 geometry14,58 | Size*,11,61,62 CD,11,57,63 hydration11,64,65 | Fe oxide, hematite | 50–70%66,67 |
| Ferrihydrite | 90%68 | ||||
| Goethite | 32%69 | ||||
| Fe-Mn oxide | 53%70 | ||||
| Fe-Ce oxide | 47%71 | ||||
| Fe-Ni oxide | 40%72 | ||||
| Zr-Fe hydroxide | 57%73 | ||||
| Al oxide | 80–83%14,74 | ||||
| Al-Mn oxide | 44%75 | ||||
| Nano-Ti oxide | 10%76 | ||||
| Cu oxide | 51%77 | ||||
| Sulfate SO42- |
Geometry78 | LBH,24,60,79 pKa, size*,11,61,80 CD, hydration | Fe (hydr)oxide, hematite | 2–14%14,66,81 | |
| Ferrihydrite | 0%68 | ||||
| Goethite | 2%69 | ||||
| Fe-Ni oxide | 7%72 | ||||
| Zr-Fe hydroxide | 7%73 | ||||
| Al oxide | 15–30%14,74 | ||||
| Al-Mn oxide | 46%75 | ||||
| Cu oxide | 0%77 | ||||
| Bicarbonate HCO3− |
pKa, LBH59,60 | Geometry,78 size*,11,61,62 CD, hydration | Fe oxide | 13–64%66,67 | |
| Fe-Mn oxide | 12%70 | ||||
| Fe-Ni oxide | 20%72 | ||||
| Zr-Fe hydroxide | 33%73 | ||||
| Al oxide | 50%74 | ||||
| Al-Mn oxide | 31%75 | ||||
| Silicate H4SiO4 |
Geometry78 | pKa, LBH,60 size*,11,61 CD, hydration | Fe oxide | 77–83%14,66 | |
| Ferrihydrite | 93%82 | ||||
| Goethite | 35%69 | ||||
| Fe-Mn oxide | 48%70 | ||||
| Fe-Ni oxide | 30%72 | ||||
| Zr-Fe hydroxide | 40%73 | ||||
| Al oxide | 63–86%14,74 | ||||
| Al-Mn oxide | 27%75 | ||||
| Cu oxide | 1%77 | ||||
| Nitrate NO3− |
pKa, LBH,60 CD,63 geometry,78 size*,11,61,62 hydration | Fe oxide | 2%14 | ||
| Zr-Fe hydroxide | 0%73 | ||||
| Al oxide | 0%14 | ||||
| Arsenite H3AsO3 |
Phosphate H2PO4− |
Size* | pKa,83 LBH,60,84–86 geometry,78 CD, hydration | Fe hydroxide | 71%56 |
| Ferrihydrite | 62–75%68,87 | ||||
| Goethite | 70%69 | ||||
| Fe-Mn oxide | 48%−70%75,88 | ||||
| Fe-Ni oxide | 56%72 | ||||
| Zr-Fe hydroxide | 50%73 | ||||
| Al-Mn oxide | 76%75 | ||||
| Cu oxide | 44%77 | ||||
| Sulfate SO42- |
Size* | pKa, hydration,65,89 LBH,59,60,85,86 geometry,78 CD | Fe hyroxide | 50%81 | |
| Ferrihydrite | 7–15%68,87 | ||||
| Goethite | 30%69 | ||||
| Fe-Mn oxide | 2%88 | ||||
| Fe-Ni oxide | 5%72 | ||||
| Zr-Fe hydroxide | 10%73 | ||||
| Al-Mn oxide | 16%75 | ||||
| Cu oxide | 7%77 | ||||
| Bicarbonate HCO3− |
pKa, LBH,59,60,85,86 geometry, size*,62 CD, hydration | Fe-Mn oxide | 3–23%70,88 | ||
| Fe-Ni oxide | 11%72 | ||||
| Zr-Fe hydroxide | 18%73 | ||||
| Al-Mn oxide | 24%75 | ||||
| Silicate H4SiO4 |
pKa, LBH,59,60 geometry, size*62 | Goethite | 25%69 | ||
| Ferrihydrite | 90%82 | ||||
| Fe-Mn oxide | 38%70 | ||||
| Fe-Ni oxide | 22%72 | ||||
| Zr-Fe hydroxide | 26%73 | ||||
| Al-Mn oxide | 34%75 | ||||
| Cu oxide | 6%77 | ||||
| Nitrate NO3− |
pKa, LBH,59,60,85,86 size*,62 geometry, CD, hydration | Zr-Fe hydroxide | 0%73 | ||
| Selenite HSeO3− |
Phosphate H2PO4− |
pKa, LBH,59 Size*, CD, hydration | Geometry | Nano-hematite | 82%90 |
| Fe oxyhydroxide | 83%91 | ||||
| Fe-Mn (hydr)oxide | 20–39%92,93 | ||||
| Nano-Mn oxide | 63%94 | ||||
| Sulfate SO42- |
LBH,59,95 size*62,80 | pKa, CD, hydration,65,89, geometry | Fe oxide | 6%90 | |
| Fe oxyhydroxide | 1%91 | ||||
| Fe-Mn (hydr)oxide | 0–3%92,93 | ||||
| Nano-Mn oxide | 30%94 | ||||
| Bicarbonate HCO3− |
pKa, LBH,59,95 geometry | Size*,62,80 CD, hydration | Nano-hematite | 20%90 | |
| Fe-Mn hydroxide | 2%92 | ||||
| Silicate H4SiO4 |
pKa, geometry, size*,62 CD, hydration | Nano-hematite | 71%90 | ||
| Fe oxyhydroxide | 39%91 | ||||
| Fe-Mn hydroxide | 28%92 | ||||
| Nitrate NO3− |
LBH,59,95 molecular geometry | pKa, size*62, CD, hydration | Nano-hematite | 1%90 | |
| Fe-Mn oxide | 0%93 | ||||
| Nano-Mn oxide | 8%94 | ||||
| Selenate SeO42- |
Phosphate H2PO4− |
LBH,59 geometry | pKa, size*,11,62 CD, hydration | Fe-Mn oxide | 85%93 |
| Nano-Mn oxide | 84%94 | ||||
| Nano-Mg oxide | 20%96 | ||||
| Sulfate SO42- |
pKa, geometry97,98 | LBH,16,60,99,100 size*,62,80 CD, hydration | Goethite | 50%101 | |
| Schwertmannite | 70%102 | ||||
| Zero valent Fe | 63%93 | ||||
| Basaluminite | 52%102 | ||||
| Nano-Mn oxide | 84%94 | ||||
| Nano-Mg oxide | 10%96 | ||||
| Nitrate NO3− |
pKa | Geometry, size*,62 CD, hydration | Fe-Mn oxide | 0%93 | |
| Nano-Mn oxide | 60%94 | ||||
| Chromate HCrO4− |
Phosphate H2PO4− |
pKa, hydration,65,103 size*,11,61,62,103 CD, geometry | LBH60 | Fe-Ni oxide | 30%104 |
| Al hydroxide | 57%105 | ||||
| Mn oxide | 64%106 | ||||
| Fe-Al hydroxide | 80%107 | ||||
| Sulfate SO42- |
LBH,60,108 geometry, size*61,62,80 | pKa,109 CD, hydration65,110 | Fe-Ni oxide | 2%104 | |
| Fe-Mn oxide | 8%111 | ||||
| Al oxide | 75%109 | ||||
| Mn oxide | 30%106 | ||||
| Bicarbonate HCO3− |
pKa | LBH,60 geometry, size*,61,62 CD, hydration | Mn oxide | 42%106 | |
| Silicate H4SiO4 |
Geometry | pKa, LBH,60 size,61,62 CD, hydration | Fe-Mn oxide | 27%111 | |
| Nitrate NO3− |
pKa, LBH,60 geometry, size*,61,62 CD, hydration | Fe-Mn oxide | 7%111 | ||
| Mn oxide | 0%106 |
3. Quantification of Selectivity
The need for a standardized quantitative definition of selective adsorption is critical in order to appropriately compare functional performance of commercially available or novel adsorbents. An effective selectivity metric should capture the antagonistic competition between the target and competitor oxo-anions as well as overall performance removal. This requires quantifying the adsorption affinity and removal efficiency for both target and competitor oxo-anions. The metric should also have a clear numerical threshold for selectivity. However, none of the selectivity metrics most commonly reported in the literature (Table 3) comprehensively capture all of these sorbent attributes.
Table 3.
Commonly Used Metrics to Quantify Selectivity
| Eqn # | Relationship | Definition of Terms | Ref(s) |
|---|---|---|---|
| 1 | %R is percent removal, C0 is the initial concentration of the adsorbate (ppm) Ce is the equilibrium concentration of the adsorbate (ppm) |
15,112 | |
| 2 | is the equilibrium adsorption capacity for a target contaminant (mg/g), is the volume of adsorbate solution (mL), and is the mass of adsorbent (mg) | 110 | |
| 3 | %I is percent interference, qt is the adsorption capacity for the target contaminant and qt+c is the adsorption capacity for the target contaminant with a competitive oxo-anion present. | 113 | |
| 4 | 114 | ||
| 5 | is the binary separation factor for the adsorption of target oxoanion, t, in the presence of competitive oxo-anion, c, is the equilibrium concentration of the competitive oxoanion in the mixed ion system, and is the equilibrium concentration of the target oxoanion in the mixed ion system. | 11,24,29,79,110,114–119 | |
| 6 | Kd is the distribution coefficient, C0 the initial concentration of the target oxo-anion, and Ce the equilibrium concentration | 120–123 | |
| 7 | is the selectivity coefficient for the target anion, the distribution coefficient for the target ion with a competitor present, and is the distribution coefficient for the competitor with the target ion present | 124–127 |
The most commonly used metric for selectivity assesses changes in removal performance of the target contaminant when a competitive oxo-anion is introduced, defined as % removal (%R) (Table 3, Eqn.1). By comparing %R of the target contaminant with/without a competitive ion, if and how much a competitor decreases adsorptive performance for the target contaminant can be quantified. Some studies have defined a “tolerance” limit on an acceptable change in %R as a result of introduction of a competitive ion (e.g., the highest concentration of a competitive ion that can be introduced to the system while still allowing >90% recovery of the target oxo-anion).128–130
In this review, %R was chosen as the metric for quantifying the competitive effect of oxo-anions (Table 2) as it was the most widely reported in the literature or could be readily calculated from the reported data. However, %R does not adequately quantify several key aspects for developing an effective selective adsorbent. Critically, %R does not evaluate how efficient the adsorbent is at removing the competitive oxo-anion. In order for an adsorbent to be truly selective, it must not only be effective in removing the target contaminant, but it also must be ineffective in removing competitive ions. This metric also does not consider the mass of adsorbent required to achieve selective removal, a key aspect of assessing the efficiency of an adsorbent, or have a threshold limit for selectivity.
Another commonly used metric is adsorption capacity, q. (Table 3, Eqn. 2). Similar to %R, strength of competition between target and competitive ions can be quantified by assessing adsorption capacity for the target ion with/without a competitive oxo-anion present, referred to as % interference (%I) (Table 3, Eqn. 3).113,131–133 Li et al. quantified the change in q as an adsorption capacity ratio (Table 3, Eqn. 4).114 An alternative is to model competitive adsorption using Ideal Adsorption Isotherm Theory (IAST). This approach, which takes parameters derived from two sets of Freundlich isotherm models, is currently more widely used for sorption of organics to porous adsorbents such as granular activated carbon.134,135 However, IAST could be easily applied to selective and competitive oxo-anion sorption.136
Measuring q, is slightly more robust than %R given that it does assess the mass of adsorbent required to remove the target contaminant, and thus the efficiency of the material. However, this metric is not capable of evaluating the strength of adsorption towards both target and competitor simultaneously, and thus, is inadequate in effectively characterizing selectivity. Both %I and the adsorption capacity ratio are variations on using q as a selectivity metric that attempt to mathematically define the strength of competition between the target and competitor. None of these selectivity metrics (e.g., %I, the adsorption capacity ratio, %R and q) provide a defined threshold value above which selectivity is demonstrated For example, an adsorbent could report >90% removal of a contaminant, but fail to decrease contaminant concentrations below MCLs depending on the starting concentration or mass of sorbent material.
An alternative metric that has emerged is the binary separation factor, sometimes referred to as the selectivity factor for target (t) and competitor (c) sorbates, (Table 3, Eqn. 5).11,24,29,79,110,114–119 A indicates that the target contaminant is preferred and the adsorbent is selective. is a particularly robust selectivity metric as it compares adsorption affinity towards both the target and competitive oxo-anions within the same system while also measuring efficiency of the material. However, does not effectively capture the ability of a sorbent to realize compliance with regulatory limits.
Another option is the distribution coefficient, Kd (Table 3, Eqn. 6).120–123 The decrease in Kd of the target anion upon introduction of a competitive ion can be used to quantify selectivity towards the target anion.120–123 However, similar to most of the above metrics (all except for ), Kd does not define a threshold limit above or below which selectivity has been demonstrated and it also alone does not evaluate removal of target and contaminant oxo-anions within the same system. Therefore, Kd alone cannot be used to effectively assess selectivity.
Some studies will also analyze Kd of the competitive ion with/without the target anion present. They will then compare the ratio of Kd of the target and competitor ions in the mixed oxo-anion system, defined as the selectivity coefficient, (Table 3, Eqn. 7).124–127 is an effective selectivity metric as it quantifies the adsorption affinity for both oxo-anions, not just the target contaminant and additionally has a clear definition of selectivity where values above 1 demonstrate selectivity towards the target competitor. However, is mathematically equivalent to , which was not previously recognized in the literature, and needs to be clarified in order to avoid confusion.
In order to compare the metrics listed in Table 3, a test system (Table S1) was evaluated and selectivity quantified through each of the equations discussed above. The results of this are reported in Table S2. Overall, and were found to be the most effective metrics to quantify selectivity due to their clear threshold limit for selectivity and that they evaluate adsorption affinity towards both the target and competitive oxo-anion.
While using or is a much more robust metric than %R or q, additional challenges remain. For example, there not yet a clear definition for the systems conditions to quantify or . Initial concentrations of both the target and competitor oxo-anion must be agreed upon in addition to other critical considerations such as pH or presence of additional background electrolytes. To enable rigorous comparisons across sorbent materials, selectivity should be evaluated in standardized testing waters (e.g., NSF 53 Challenge Water).137,138
An alternative approach to quantify selectivity is equilibrium surface complexation models (SCMs) that consider competition for binding sites in multi-ion systems such as the diffuse layer model (DLM) and charge distribution multi-site complexation (CD-MUSIC).136,139–141 Once parameterized with a subset of data, SCMs are able to predict the nature of competition or selectivity between multiple oxo-anions in a system in a wide range of conditions beyond those studied in limited isotherm tests.69,136 However, SCMs require more skill to parameterize and information than more traditional quantitative methods such as those reported in Table 3 (e.g., binding site density, strength of binding sites, zero point of charge for adsorbent, etc.),136 they lack a clear quantitative definition for selectivity, but they also are mechanistic in nature and could compliment techniques such as DFT.
Also lacking from the above metrics is consideration of adsorptive kinetics which can have a strong influence on competitive adsorption.142,143 For example, silicate adsorbs more slowly than As(V) due to its surface polymerization.142 Thus, in a competitive system containing As(V) and silicate, the slower kinetics of silicate can lead to a decrease in both the kinetics of As(V) adsorption, as well as the quantity of arsenic adsorbed.142 Models such as the homogeneous diffusion model (HDSM) can be used to better understand differences in oxo-anion diffusion rates on various adsorptive materials by taking into account the concentration of solute in the bulk phase and the adsorption density gradient within individual adsorbent particles.136,144 This knowledge could then be exploited in the design of selective adsorbents that promote faster diffusion of target oxo-anions over competitive oxo-anions, or that minimize the detrimental effects of competitive oxo-anions with slower kinetics.
4. Selectivity due to Differences in Surface Complexation
4.1. Surface Complexation Geometries
Metal (hydr)oxides form both inner-sphere and outer-sphere complexes with oxo-anions.145,146 Outer-sphere complexes form between the fully coordinated shells of oxo-anions and metal (hydr)oxides.147,148 The weak electrostatic attraction between the protonated surface hydroxyl groups (OH2+) and the negatively charged oxo-anions is often facilitated through extensive hydrogen bond networks (Figure 2).149 In comparison, the much stronger inner-sphere complexes form as a result of direct covalent bonding between the adsorbed oxo-anion and the sorbate.147,148 The oxo-anion and sorbate first form a weak outer-sphere complex, then a ligand exchange between the surface hydroxyl groups and oxo-anions occurs, forming a stronger inner-sphere complex.149–151, Inner-sphere complexes are most commonly monodentate (one bond between oxo-anion and the sorbate surface) or bidentate (two bonds between oxo-anion and sorbate) (Figure 2). Bidentate complexes can be further categorized as either mononuclear or binuclear, corresponding to the formation of edge-sharing complexes with a single surface atom, or corner-sharing complexes with two adjacent terminal atoms, respectively.
Figure 2.
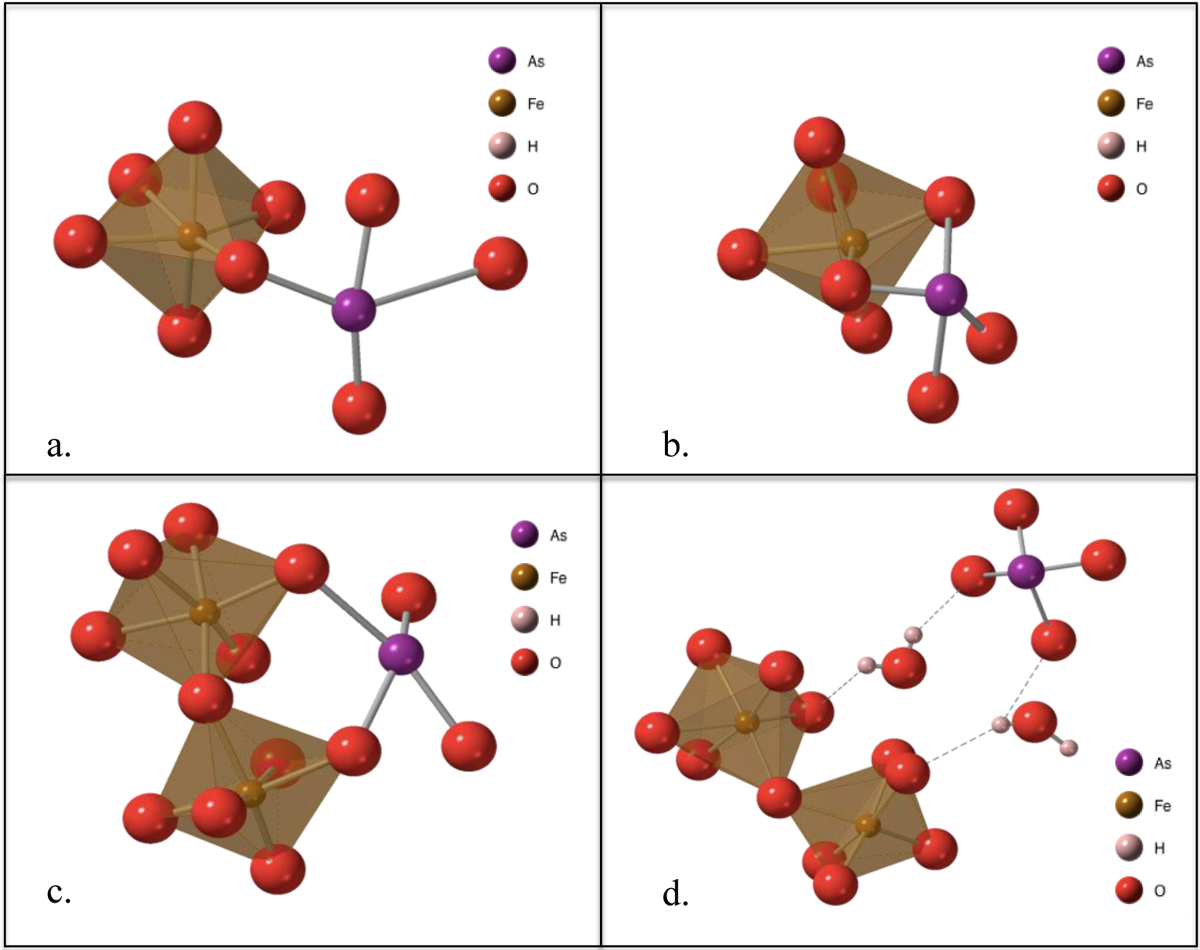
Common coordination complex geometries between metal (hydr)oxides and oxo-anions: (a.) monodentate mononuclear complex; (b.) bidentate mononuclear edge-sharing complex; (c.) bidentate binuclear corner-sharing complex; (d.) outer-sphere complex.
The most commonly studied metal (hydr)oxides are hematite (α-Fe2O3), TiO2, Al2O3, and goethite (α-FeOOH). Iron oxides (hematite and goethite) have shown greater adsorption strength than their aluminum counterparts.13,14,152 The increased reactivity of iron oxides, especially hematite, is theorized to be due to the presence of reactive and highly exchangeable hydroxyl groups on the surface.153,154 Titanium dioxide performs comparably to iron oxides in the ability to sorb oxo-anions.
Strong inner-sphere complexes typically occur with metal oxides that readily exchange surface hydroxyl groups (e.g. α-Fe2O3), and particularly with oxo-anions that have a strong affinity for the metal (hydr)oxide surface (e.g. HSeO3− H2PO4−, H2AsO4).155 These high-affinity oxo-anions often form strong bidentate complexes regardless of the metal (hydr)oxide composition.156–160 In comparison, “intermediate” oxo-anions (e.g. SO42-, HCO3−, SeO42-, H3AsO3, HCrO4−) have a lower affinity for oxide surfaces and can participate in both inner and outer-sphere complexation depending on the metal (hydr)oxide surface.145,155,156,158,161–163 Lastly, weakly adsorbing oxo-anions (e.g. NO3−) have a low affinity for the surface of metal oxides and thus primarily form outer-sphere complexes.14,164
Exploiting the surface chemistry of metal (hydr)oxides and other sorbents to target contaminants based on their mechanism of complexation will make it possible to design efficient, and possibly selective, sorption systems to remove target oxo-anion contaminants by maximizing their interaction with metal (hydr)oxide surfaces.
4.2. Facet-Dependent Adsorption Mechanisms
Current research has begun to correlate surface chemistry to adsorptive function by studying the role of different metal (hydr)oxides crystal facets on sorption mechanisms and capacity.157,165,166 Facets are classified by their Miller indices {hkl}, where the curly brackets indicate an equivalent family of planes. This notation derives from the coordinates of the plane’s interception on the x, y, and z axes.
Recent research has suggested that adsorption capacity can be improved by synthesizing metal (hydr)oxides with more reactive exposed facets, which could be beneficial in sorbing contaminants with high and intermediate affinities. Additionally, exposing facets that promote certain complexation types (e.g. monodentate, bidentate, outer-sphere) may make it possible to selectively sorb contaminants with matching preferred geometries (Table 4).
Table 4.
Facet Preference for Target and Competitive Oxo-anions
| Oxo-anion | Adsorbent Material | High-Affinity Facet(s) | Complexation Type | Low-Affinity Facet(s) | Complexation Type | Solution Chemistry | Reference(s) |
|---|---|---|---|---|---|---|---|
| Target | |||||||
| H2AsO4− | TiO2 Anatase | {001} | Not Studied | {101} | Not studied | DFT calculation | 166 |
| α-MnO2 | {100} | Bidentate/monodentate, more stable as bidentate | {110} | Bidentate/ monodentate, more stable as bidentate | DFT calculation | 169 | |
| H3AsO3 | TiO2 Anatase | {001} | Not Studied | {101} | Not studied | DFT calculation | 166 |
| α-MnO2 | {100} | Bidentate/ monodentate, more stable as bidentate | {110} | Bidentate/ monodentate, more stable as bidentate | DFT calculation | 169 | |
| HSeO3− | Nano-hematite (α-Fe2O3) | {012} | Bidentate mononuclear | {110} | Bidentate binuclear | 0.01 M KCl pH = 3.5 | 162 |
| HCrO4− | Nano-hematite (α-Fe2O3) | {110} | Bidentate binuclear | {001} | Monodentate mononuclear | 0.01 M NaCl pH = 2 | 168 |
| Competitor | |||||||
| SO42- | Hematite | {012} | Bidentate | {001} | Monodentate | pH = 1 | 170 |
The majority of research on facet-dependent adsorption mechanisms focuses on hematite (α-Fe2O3) because it is a strong sorbent, ubiquitous in the natural environment, and thermodynamically stable. Given that different crystal facets have different surface oxygen configurations and availabilities, synthesizing hematite to expose crystal facets with a high density of singly coordinated oxygens could promote strong sorption of intermediate and high affinity oxo-anions.167 For example, Catalano et al. showed that selenite and arsenic form strong, bidentate inner-sphere complexes preferentially at the singly-coordinated surface oxygen despite the availability of doubly-coordinated oxygen sites.157
This variation in surface chemistry can therefore promote different sorption mechanisms on different facets. For example, it has been shown that that both selenite and chromate form binuclear, bidentate complexes on the {110} facet of hematite. Further, selenite forms mononuclear, bidentate complex on the {012} facet162 while chromate sorbs via monodentate, mononuclear geometry on the {001} facet.167,168 Notably, the {012} facet showed a higher adsorption capacity than the {110} facet for selenite while the {110} facet had higher adsorption capacity for chromate than the {001} facet.162,167,168 The enhanced reactivity on the singly-coordinated oxygen to form inner-sphere bidentate geometries indicates potential for utilizing controlled-synthesis methods to make iron oxide particles with highly reactive, and potentially highly selective, facets. This may promote the formation of strong bidentate geometries for high- and intermediate-affinity oxo-anions and thus resulting in increased sorption capacity.
These results broadly suggest that the mechanism and strength of sorption is dependent on the crystal facet, and thus surface functional groups, of the adsorbent material. With limited experimental and computational studies analyzing this effect on a variety of metal oxides, facet-dependent sorption is an understudied area. This is an important gap in the pursuit of developing selective and efficient metal (hydr)oxide sorbents. With recent research suggesting that different exposed facets promote different sorption mechanisms, a robust understanding of preferred complexation geometry for different oxo-anions could make it possible to selectively promote adsorption of target oxo-anions over competitors (Table 4). Future work establishing a comprehensive correlation between the crystallographic structure and sorptive function of metal (hydr)oxides, will elucidate favorable sorbent qualities and aid in the design of selective sorbent materials.
5. Selectivity due to Steric Interactions
Steric hindrance as it relates to adsorption can be described as interference in adsorption due to the molecular structure (size and molecular geometry) of either the adsorbate or the adsorption site. In reviewing the literature there are numerous reports of oxo-anion size reported as ionic radius, hydrated ionic radius, and molecular volume.11,61,62,80,171 However, many of these values differ significantly between sources, or are not calculated at the correct degree of protonation for environmental conditions. Therefore, in order to directly compare between target and competitive oxo-anions, we calculated the molecular volume of both target and competitor oxo-anions for their degree of protonation in environmental conditions (Table 5, SI). These calculations resulted in the following ranking of oxo-anions listed in order of decreasing molecular volume: H2AsO4−~SeO42->H4SiO4>HCrO4−>H2PO4−>SO42->H3AsO3>HSeO3−>HCO3−>NO3−. The molecular volume of silicate was calculated assuming a single tetrahedral molecule; however, it is known to polymerize frequently in the environment, so may be present at much higher molecular volumes. Comparing molecular geometry, arsenate, selenate, chromate, phosphate, sulfate, and silicate all have tetrahedral structure, while arsenite and selenite have trigonal pyramidal structure, and nitrate and bicarbonate a trigonal planar structure (Tables 1 and 2). These differences in molecular volume and geometry can potentially be exploited in the development of sterically selective adsorbents.
Table 5.
Molecular volume and Laplacian bond order (LBO) of target and competitor oxo-anions
| Oxo-anion | Volume | LBO | ||||
|---|---|---|---|---|---|---|
| Bohr3 | Å3 | X-O1 | X-O2 | X-O3 | X-O4 | |
| Target | ||||||
| H2AsO4− | 690.9 | 102.4 | 0.338 | 0.338 | 0.232* | 0.233* |
| H3AsO3 | 602.6 | 89.3 | - | 0.228* | 0.242* | 0.231* |
| HSeO3− | 598.7 | 88.7 | 0.341 | 0.206* | 0.357 | - |
| SeO42- | 690.9 | 102.4 | 0.324 | 0.324 | 0.324 | 0.324 |
| HCrO4− | 656 | 97.2 | 0.836 | 0.845 | 0.829 | 0.384* |
| Competitor | ||||||
| H2PO4− | 635.3 | 94.1 | 0.751 | 0.774 | 0.415* | 0.415* |
| SO42- | 623 | 92.3 | 0.860 | 0.860 | 0.860 | 0.860 |
| NO3− | 424 | 62.8 | 0.863 | 0.865 | 0.866 | - |
| H4SiO4 | 658.8 | 97.6 | 0.379* | 0.379* | 0.379* | 0.379* |
| HCO3− | 474.6 | 70.3 | 1.078 | 1.033 | 0.447* | - |
Note:
values for the O atoms additionally bonded to H atoms
Several efforts aimed at developing a sterically selective adsorbent based on materials that contain an interlayer space where larger or more geometrically bulky molecules may be excluded, such as layered double hydroxides (LDH) and functionalized clay.172 However, multiple studies based on this approach found that selectivity for target oxo-anions is not reliably developed. For example, Mg-Al LDHs selected (measured as %I) the following: H2PO4−>H2AsO4−>HCO3−~SO42->NO3−.173,174 Similarly, using Fe-functionalized Akadama clay, phosphate and sulfate were preferred (measured via Kd) over chromate.120 These materials may not reliably develop steric selectivity as the adsorption sites are not arranged to specifically prefer a given molecular geometry or size, and the interlayer space can shrink and contract.62
An alternative approach is through cationic MOFs (Table 6) where by controlling the pore size and rigidity of the MOF, steric selectivity can be developed.17,98,175 The rigidity of the MOF is critical in avoiding free rearrangement to accommodate anions of various size and molecular geometry, rather than sterically exclude them.17,98 This type of steric selectivity is particularly strong as it does not singularly favor smaller anions, instead taking into account both size and molecular geometry to develop a high degree of anion shape recognition.17,98 Cai et al. developed a Fe(III)-MOF which selects for As(V) over sulfate, nitrate, and bicarbonate (measured as %R), but did not test against phosphate.175 Selectivity for chromate over nitrate, sulfate, and bicarbonate has also been developed using Ag(I)-,176 Co(II)-,177 Cd(II)-,178 Zn(II)-,177 and Zr(VI)-112,122 cationic MOFs (Table 6). MOFs that select for As(III), Se(IV) or Se(VI) over other oxo-anions have not yet been developed.
Table 6.
Adsorbents selective for the removal of target oxo-anions
| Target Oxo-anion | Technology Type | Adsorption Technology | Observed Selectivity | Selectivity Metric(s) | Mechanism(s) of Selectivity | References | |
|---|---|---|---|---|---|---|---|
| H2AsO4− | PLE | Cu(II)-PLE | As(V) ~ P > C > S | Electrostatic, HSAB | 24,79,117,179 | ||
| Zr(VI)-PLE | As(V) ~ P > C ~ S | %R | Electrostatic, HSAB | 16,99 | |||
| ZV-PLE | As(V) > S > N > P | %R | Hydrophobicity, sterics | 180 | |||
| Cu(II)-chitosan | As(V) > P | , %R | Electrostatic, HSAB | 11,133,181 | |||
| AE | WB-AE | S > As(V) > N | %I | Electrostatic, HSAB | 25 | ||
| WB-AE | P > As(V) > S > N | %I | Electrostatic, hydrophobicity | 57 | |||
| MOF | Fe(III)-MOF | As(V) > S ~ N > C | %R | Sterics | 175 | ||
| SM-Zr(VI)-MOF | P ~ As(V) > Si > S > N | TL | Electrostatic | 182 | |||
| LDH | Mg-Al LDH | P > As(V) > C ~ S > N | %I | Electrostatic, sterics | 173,174 | ||
| H3AsO3 | MIP | MIP | As(III) > P > S > N | Kd, | Electrostatic, sterics | 126 | |
| MIP | S > As(III) > Si ~ P > N | Kd, | Electrostatic, sterics | 183 | |||
| HSeO3− | PLE | Cu(II)-PLE | Se(IV) > S | Electrostatic, HSAB | 117 | ||
| Cu(II)-chitosan | Se(IV) > P | , %R | Electrostatics, sterics | 11 | |||
| MMSi | MMSi | Se(IV) > S ~ N > P | %R | Electrostatics | 184 | ||
| SeO42- | HBC | Mg(II)-HBC | S > Se(VI) > C | Hydrophobicity, sterics, electrostatic | 98 | ||
| HCrO4− | PLE | ZVE-PLE | Cr(VI) > S > N > P | %R | Hydrophobicity, sterics | 180 | |
| AE | SB-AE | Cr(VI) > S > N | Hydrophobicity, sterics, electrostatic | 110 | |||
| MOF | Zr(VI)-MOF | Cr(VI) > S > N | K d , %R | Electrostatic, HSAB, sterics | 112,122 | ||
| Co(II)-MOF | Cr(VI) > S ~ N | K d | Electrostatic, sterics | 177 | |||
| Zn(II)-MOF | Cr(VI) > S ~ N | K d | Electrostatic, sterics | 177 | |||
| Cd(II)-MOF | Cr(VI) > S > N | %R | Electrostatic, sterics | 178 | |||
| Ag(I)-MOF | Cr(VI) > N > C | %R | Electrostatic, sterics | 176 | |||
| SM-clay | SM-clay | Cr(VI) > N > S − | qr | Sterics | 172 | ||
| MIP | MIP | Cr(VI) > S ~ N | Kd, | Sterics | 127 | ||
| MIP | Cr(VI) > S > N | Kd, | Sterics | 185 | |||
| MIP | Cr(VI) > S > P > N | Kd, | Sterics | 186,187 | |||
| NMO-MIP | Cr(VI) > N > S > P | TL | Electrostatic, sterics | 188 | |||
| SM-MCWNT | SM-MCWNT | Cr(VI) > C ~ P ~ N | %R | Electrostatic | 189 | ||
| SM-activated C | SM-activated C | Cr(VI) > P > S > N | %I | Sterics | 190 | ||
Note: PLE is polymeric ligand exchanger, HSAB is hard-soft acid base principle, ZV is zero valent iron, SB-AE is a strong base anion exchanger, WB-AE is weak base anion exchanger, MOF is metal organic framework, LDH is layered double hydroxide, SM is surfactant modified, and MIP is molecularly imprinted polymer, MMSi is modified mesoporous silica, HBC is hydrogen bonding capsule, MCWNT is multi-walled carbon nanotube. is binary separation factor, %R is % removal, %I is % interference, TL is tolerance limit, Kd is distribution coefficient, is the selectivity coefficient, is energy of crystallization, qr is ratio of q the adsorption capacity.
Molecularly imprinted polymers (MIP)s are also effective at developing steric selectivity (Table 6). Similar to MOFs, MIPs contain a pore structure that can recognize potentially and select for the target oxo-anion through the macrocyclic affect.126 Specific recognition cavities are developed by adsorbing the target oxo-anion, then adding a crosslinking agent to rigidly set the MIP structure to be complementary to the target oxo-anion.127,191 The target oxo-anion is then desorbed, leaving pores within the MIP with functional groups rigidly set complementary to the target oxo-anion shape, size and coordination geometry.127,191 Fang et al. successfully developed a MIP which selects for (measured as Kd and ) As(III) over phosphate, sulfate, and nitrate.126 This is a particularly compelling technology as phosphate is a strong competitor with arsenic (Table 2). However, this technology has not yet been evaluated for competition between As(V) and phosphate. MIPs have also been used to selectively adsorb (measured as Kd and ) Cr(VI) over phosphate, nitrate, and sulfate.127,185–187,190 Qi et al. combined nanometal oxides with MIPs to design a hybrid MIP-functionalized-nanoparticle that selects for (measured as TL) HCrO4−>NO3−>SO42->H2PO4−.188 Sterically selective MIPs have not yet been developed that target As(V) and selenium.
Of the three technologies reviewed that utilize steric hindrance to develop selectivity, MIPs are particularly promising since they successfully removed As(III) and Cr(VI) in the presence of phosphate, a strong adsorptive competitor (Table 2).126,185–188,190 MOFs are highly selective towards arsenate and chromate, but studies have not yet tested their selectivity in the presence of strong competitors such as silicate and phosphate.
6. Selectivity driven by the Pearson Hard Soft Acid Base Principle (HSAB)
According to the Pearson Hard Soft Acid Base Principle (HSAB), Lewis bases (electron donors), and Lewis acids (electron acceptors) can be divided into three categories: hard, intermediate, and soft.59,192 A hard Lewis base is an electron donor with low polarizability, high pKa, high negative charge density, and inaccessible high energy empty orbitals.192,193 A hard Lewis acid is an electron acceptor with low polarizability, small size, high positive charge density, and no easily excited outer electrons; soft acids/bases have inverse traits, and borderline have intermediate traits.192 More stable complexes are formed between acids/bases of similar hardness than between acids/bases of dissimilar hardness.59
Applying the HSAB principle to oxo-anion remediation, both target and competitive oxo-anions are Lewis bases, and metal sorption sites are Lewis acids. Target oxo-anions arsenate, selenate, selenite, and chromate are all hard Lewis bases, while arsenite is a borderline Lewis base.59,194 The competitive oxanions phosphate, silicate, sulfate, nitrate, and bicarbonate are also hard Lewis bases.59,60,194 Trivalent metals Al3+ and Fe3+ are hard Lewis acids, and divalent metals Cu2+, Zn2+, Ni2+, and Fe2+ are borderline Lewis acids.59 While most target and competitive oxo-anions are classified as hard Lewis bases, the relative hardness varies within this general classification. There is not yet a comprehensive classification of oxo-anions ranked according to their relative Lewis base hardness; however synthesis of the available studies suggests the following ranking: silicate > phosphate ~ arsenate > bicarbonate > sulfate ~ chromate > nitrate > arsenite.24,60,79
Differences in Lewis acid/base hardness were utilized in the design selective polymeric ligand exchangers (PLE)29 and MOFs (Table 6). Henry et al. developed a Cu(II)-PLE that selected (measured by ) for arsenate and phosphate over sulfate;179 however, it failed to separate the most difficult competitive pair, arsenate and phosphate. Similar Cu(II)-PLEs were selective (measured by ) for arsenate and selenite over weakly competing bicarbonate and sulfate; however, strongly competitive phosphate was not evaluated.24,79,117,195 Zr(IV)-PLEs selected for (measured by %R) arsenate and phosphate over bicarbonate and sulfate.16,99 MOFs are less studied than PLEs for HSAB driven selective adsorption. However, Rapti et al. found that a Zr(IV) MOF selected (measured by Kd) for chromate in the presence of only weakly competitive sulfate and nitrate.122
Overall, PLEs and MOFs were effective in their selectivity for arsenate, selenate, and chromate over only relatively weak competitors: bicarbonate, sulfate, and nitrate (Table 6). As silicate is a harder Lewis base than most target oxo-anions,60 theoretically, HSAB-selectivity could be developed using a weaker Lewis acid adsorption site. Overall though, the HSAB principle must be combined with other mechanisms of selective sorption such as electrostatic preference or steric hinderance in order to separate strong sorbing competitors such as arsenate and phosphate, due to their similar Lewis base hardness.11,17,64,133
7. Electrostatic Selectivity
Electrostatic intermolecular interactions are mediated by forces that can be broadly categorized into van der Waals forces, ion-dipole forces, and hydrogen bonding. Van der Waals forces can be further separated into dipole-dipole, dipole-induced dipole, and London dispersion forces.196 The type of intermolecular interactions that occur are dependent on the polarity of both adsorbent and adsorbate. If both the adsorbent and adsorbate are polar molecules, they can participate in dipole-dipole interactions.196 For example, at environmental pH, As(III) is neutrally charged as H3AsO3, and has a permanent dipole moment due to its trigonal pyramid structure, so can participate in dipole-dipole interactions with polar adsorbents. As most target contaminants are negatively charged at environmental pH, they will participate in ion-dipole forces where anions and polar adsorbents are electrostatically attracted to each other. In ion-dipole interactions, the smaller the oxo-anion, the higher the charge density (ratio of charge/volume) and the stronger the interaction with the dipole.98,110,196 Therefore, the following charge density ranking is expected (from highest to lowest) based on the molecular volumes calculated in Table 5 and the protonation expected in environmental conditions: SO42->SeO42->NO3−>HCO3−>HSeO3−>H2PO4−>HCrO4−>H2AsO4−>H3AsO3>H4SiO4.
In order to systematically probe electrostatic differences between oxo-anions, we calculated the Laplacian bond order (LBO) values (Table 5) and Electron Localization Functions (ELF) for each of the target and competitive oxo-anions (Fig.S1–S4). LBO values measure the local charge concentration or depletion within an oxo-anion,197 with positive LBO values corresponding to locally depleted electron density. This parameter correlates directly with the bond dissociation energy, bond polarity and bond vibrational energy. LBO varies greatly between all the oxo-anions analyzed, with the largest LBO values, and strongest bonds observed for HCO3− (Table 5). Generally, C-O, Cr-O, S-O and N-O bonds among the strongest, P-O bonds are moderately strong, and As-O and Se-O are weakest amongst the oxo-anions. These results enable development of electrostatically selective adsorbents as oxo-anions with higher LBO values are less polarizable.11,133,181
Polarizability differences can be exploited in the development of selective adsorbents, particularly for targeting oxo-anions with relatively low charge density and high polarizability such as arsenate and selenite. For example, Yamani et al. used a Cu(II)-chitosan adsorbent to electrostatically select for (measured as ) arsenate and selenite in the presence of phosphate.11 As Cu(II) binds to the amine groups of chitosan, the Cu(II)-chitosan complex gains cationic character that electrostatically attracts oxo-anions.11,24,29,79,133,181 Chitosan is a large polymeric molecule with many electrons, so would expected to be highly polarizable.196 Therefore, arsenate and selenite, both of which are more polarizable than phosphate,11,57,133,181 will be more attracted to Cu(II)-chitosan bidentate complex due to their similar diffusion of charge.11,133,181 Pincus et al. further developed this adsorbent, optimizing the loading of Cu(II) and n-TiO2 to selectively adsorb (measured as %R) As(III) and As(V) over phosphate.133,181 These adsorbents were notable as they are able to select for arsenate over its strongest adsorptive competitor, phosphate, by taking advantage of some of their few dissimilar traits, charge density and polarizability (Table 2).11,57,63,133,181
Other studies have focused on developing adsorbents that select for oxo-anions of higher charge density. Many of these technologies use amine,198 amide,198 urea,98 and pyridinium98 groups among others to promote electrostatic interactions and hydrogen bonding between oxo-anions of higher charge density and the adsorbent. Hydrogen bonding is more directional than Coulombic and van der Waals forces, meaning it can potentially be better exploited for selective adsorption.17,64 Generally, smaller anions interact more strongly as hydrogen bond acceptors due to their relatively higher charge density.17 For example, Hu et al. found that for strong base anion exchangers, development of selectivity is dependent on both the polymer composition and the functional groups on the polymer.110 Strong base anion exchangers with smaller spacing between functional groups electrostatically prefer divalent ions with higher charge density compared to anion exchangers with wider spaced functional groups.110 Their study took place at pH >8 where chromate is divalent, and were successfully able to select for (measured by ) chromate over sulfate and nitrate using a hydrophobic strong base anion exchange resin with small spacing between amine functional groups.
An additional approach is developing 3D hydrogen bonding networks through ring structures in hydrogen bonding capsules, MOFs, and MIPs. For example, Custelcean et al. developed hydrogen-bonding capsules lined with urea and pyridinium binding groups and found that using six urea groups to develop twelve hydrogen bonds was most complementary to tetrahedral sulfate and selenate.98 Fang et al. developed a MIP with imidazole functional groups that formed four hydrogen bonds with As(III) had the following selectivity (measured as Kd and ): As(III)>P>S>N.
These electrostatically selective adsorbents have shown great promise in their ability to separate between the strongest competitive adsorptive pairs, such as arsenate and phosphate. However, one important note regarding this mechanism is that its success is highly pH dependent. Therefore, pH must be carefully monitored and controlled in order to optimize performance.
A further subset of electrostatic interactions is hydrophobic effects. As dictated by the Hofmeister Bias, oxo-anions with a lower degree of hydration are more strongly attracted to hydrophobic surfaces, and conversely, more hydrated oxo-anions are more attracted to hydrophilic surfaces.17,98,103,199 Therefore, by controlling the hydrophobicity/hydrophilicity of the adsorbent material, more or less hydrated oxo-anions can be rejected or selected.
The degree of hydration of an oxo-anion is largely controlled by the ratio of charge/volume.17,103 For ions with the same charge, the larger the radius, the lower the charge density and hydration of the ion, and therefore the more hydrophobic the ion.110 In reviewing the literature, there is not yet a comprehensive comparison of degree of hydration between various oxo-anions. Based on our calculations of molecular volume and assuming protonation in environmental conditions, we can rank the oxo-anions in order of decreasing hydration energy: SO42->SeO42->NO3−>HCO3−>HSeO3−>H2PO4−>HCrO4−>H2AsO4−>H3AsO3>H4SiO4.65,200,201 Generally, monovalent oxo-anions have a lower hydration energy, and therefore are more strongly attracted to hydrophobic adsorbents, than divalent oxo-anions.17,64,199
Several technologies have utilized differences in hydrophobicity to develop selectivity. For example, it was found that hydrophobic activated carbon preferred (measured as %I) HCrO4− over H2PO4−, NO3− and SO42-.103 Luo et al. used surfactant modified clays and found that generally, monovalent ions were preferred for adsorption within the hydrophobic, surfactant-coated interlayer space over divalent ions due to their lower hydration energies.199 They observed the following selectivity sequence (measured as %I): NO3− >HCrO4− > SeO42-~SO42- > HSeO3−>H2AsO4−.
Another hydrophobic material type frequently utilized in selective adsorbents is polymers. Resin hydrophobicity can be varied by changing the composition of the polymer and the chain length of functional groups on the polymer.110 Polymers have been widely used in a variety of adsorbents including MOFs64 and anion exchange resins29,57,110,202 to enable selective adsorption of oxo-anions (Table 6). Custelcean and Moyer found that by including higher charged metals such as Fe(III) and Zr(VI) in their MOF framework, they selected for smaller and more hydrophobic oxo-anions.64 Further research is needed to fully explore the potential of MOFs to hydrophobically select for target oxo-anions.
Many anion exchange resins are made up of cross-linked polymers with functional groups such as amine groups that serve as binding sites for oxo-anions.202 Conventional anion exchange resins, due to their hydrophobic nature, follow the Hofmeister bias and prefer less hydrated, more hydrophobic anions.57,202 When targeting HCrO4−, a more hydrophobic ion than most of its strongest competitors, a more hydrophobic adsorbent is more effective in developing selectivity. Hydrophobicity of the adsorbent can be influenced by polymer composition, and the chain length of the functional groups.110 For example, in a bicarbonate-form strong base anion exchanger, by selecting a more hydrophobic resin they were able to successfully select for (measured as ) HCrO4− over weakly competing SO42- and NO3−, but did not evaluate stronger competitive oxo-anions such as phosphate.110
Several studies used of weak-base anion exchange resins and found that they typically exhibit non-Hofmeister selectivity.57,202 For example, Awual et al. modified cross-linked polystyrene through the addition of primary amino groups, resulting in a more hydrophilic weak-base anion exchange resin that exhibited the following selectivity (measured as %R): H2PO4−>H2AsO4−>SO42->NO3−.57 While this weak-base anion exchange resin was successful at reversing the Hofmeister bias, it was not able to select for As(V) over phosphate. Some studies also examined the potential of MOFs and hydrogen bonding capsules to exhibit non-Hofmeister selectivity.17,64,98 They found that through combinations of rigid hydrogen bonding networks and metal coordination, anti-Hofmeister selectivity can be developed. For example, Custelcean et al. observed the following selectivity (measured via differences in Gibbs free energy of crystallization) with a Mg(II)-hydrogen bond capsule: SO42->SeO42->HCO3−.98
8. Use of Quantum Simulation to Understand and Predict Oxo-anion Sorption
Over the years, density functional theory (DFT) and wave-function methods (Hartree-Fock, MP2, couple cluster) have found important application in understanding oxo-anion adsorption on the metal oxide surfaces.203–209 Specifically, DFT methods provide two tools to complement and propel experimental approaches in development of selective adsorbents: 1) the ability to isolate individual cause/effects by focusing on processes at the site of interaction of the studied system; and 2) the use of rapid screening approaches to identify novel sorbent materials, predicting the most promising candidates that could then be validated experimentally.
Due to limitations dictated by system size (<500–1000 atoms) and simulation time scale (either thermodynamic states or a few femto-seconds), the DFT calculation of competitive adsorption energies and Gibbs free energies210 must be completed on a specific material surface facet, with a specific pH, ionic strength, etc.168,211 Competing effects, such as presence of multiple facets, defect sites, and sample analysis treatments are generally avoided allowing the direct delineation of the effect of changing variables. This is commonly done by calculating oxo-anion-surface complexation energies;212–214 with DFT calculated Ultra Violet, Infrared and EXAFS spectra of adsorbed oxo-anions aiding in experimental interpretation and cross-method validation.214,215 In addition, DFT calculations enabled direct elucidation of the nature of the chemical and physical bonds between adsorbate and surface168,209,210,216 becomes useful in identifying material characteristics required for higher adsorption selectivity.217 The predictive and design power of DFT methods have been underutilized in selective sorbent design. In general, DFT based design of novel materials follows two routes: 1) brute force materials screening or 2) descriptor identification and materials selection.
Effective screening requires rapid calculation of competitive oxo-anion adsorption on hundreds to thousands of candidate material compositions and morphologies which is complicated and time-consuming process. Only limited number of studies going beyond simple comparisons of two or three materials have been reported to date.210,217–219 Several recent studies of Cr(VI) adsorption on hematite have suggested outer-sphere, monodentate, bidentate mononuclear, and bidentate binuclear inner-sphere complexes as being favorable configurations.209,215,220,221 However, the specific hematite facet was not considered, even though it was later found to be critical factor in determining the most energetically stable (i.e. lowest energy) configuration.168,215,220,221 Therefore, screening studies require physically representative model systems, where the material composition, surface characteristics, and facet; pH dependent effects; as well as surface and oxo-anion solvation effects, are necessary.168,213,216,221,222 Additional effects such as oxo-anion/oxo-anion interactions,212,223 and pH / presence of other ions in the solution should also be included in the screening models.
The development of adsorption descriptors which use simple formulas or material characteristics to predict adsorptivity mostly rely on adsorption energy and selectivity derived from the DFT calculations and identification of materials characteristics that correlate with desired adsorptive performance. For example, Corum and Mason found that strong adsorption of As oxo-anions on Keggin Al-nanoclusters was correlated with large local shape and electronic potential gradients.211 With a suitable and clearly defined descriptor, materials could be screened without detailed DFT calculation for interaction of oxo-anions and each sorbent material, and only candidates with most promising properties would be subjected to full DFT calculations (i.e. adsorption energies, Gibbs free energies, spectra calculations, etc.). This approach has become standard in other fields (e.g., use of Volcano plots in catalysis224). One of the difficulties in effective application becomes establishing an adequate database of selective or non-selective sorbents that would be used for defining the most appropriate descriptor(s).
DFT has a great potential to aid in the discovery of selective oxo-anion adsorbents. The key areas that limit its implementation are the size of model system needed to adequately describe the complex sorbent/sorbate environment, the development of efficient computational materials screening tools, and sufficient high-quality experimental validation data. Use of novel methods, based on linear scaling methods,225 artificial intelligence/machine learning methods,226 or combined quantum mechanics/molecular model systems227 can help with development of complex model systems and identifying features that categorize behavior while decreasing computational time.
9. Future Directions and Opportunities
Reviewing the literature, it is clear that variation in surface complexation, HSAB interactions, sterics, and electrostatics, can be exploited for the development of selective adsorbents. Rarely do these mechanisms occur singularly, often acting synergistically to strengthen the specificity of a selective adsorbent (Table 6). The strength of these mechanisms varies, with those that involve inner-sphere complexation and steric interactions exhibiting stronger selective adsorptive performance compared to weaker outer-sphere or electrostatic interactions in antagonistic situations. Further research is needed to elucidate these synergistic and antagonistic relationships between selectivity mechanisms. The most widely used mechanisms to develop selectivity were electrostatics and sterics, with HSAB and crystal facet engineering requiring more study. However, very few of the relevant studies contained discussions of adsorption mechanisms, and even fewer critically examined and proved the adsorption and selectivity mechanisms. In addition, due to a lack of a standardized definition for quantification of selectivity, it is difficult to quantify the reliability of removing target contaminants to beneath regulatory limits in competitive systems. Kinetics also warrant more consideration since differences in diffusion rates between oxo-anions can have a large influence on selectivity in competitive systems.142 This is particularly important since steric selectivity mechanisms tend to more strongly control selectivity, leading to a need to more carefully consider the influence tortuosity and pore size on diffusion rates in these competitive systems.
Great opportunities exist to develop selective adsorbents for arsenite, selenite, and selenate as very few studies have targeted these oxo-anions (Table 6). The largest number of selective sorbents have been developed for chromate and arsenate. However, critically, very few of these studies have successfully developed selectivity for these target contaminants over their strongest competitors (e.g., phosphate and silicate) or if silicate is evaluated, its surface polymerization is rarely considered. Studies of singular target/contaminant pairs are important to truly understand fundamental competitive relationships, more complex multicomponent waters which more accurately reflect environmental conditions should also be tested. Use of SCMs derived from this data could enable easier prediction of systems conditions in which selectivity will be achieved. Additionally, the potential trade-off between highly efficient selective removal and decreased regeneration potential also needs to be evaluated.228
The majority of adsorbent materials reviewed that successfully developed selectivity were organometallics including MOFs, PLEs, AEs, and MIPs; however, these were also the mostly studied materials (Table 6), so there is room for innovation to other sorbent systems. Further systematic empirical studies, iterated with computational techniques, are needed to more accurately and efficiently screen materials and develop selective adsorbents. For example, DFT modeling could be used determine which crystal facets of NMOs show high binding energy for targets and low binding energy for competitive oxo-anions. Sustainability considerations should also be considered during this screening process, including questions of materials criticality, energy consumption for production and regeneration, and environmental and human health impacts from any potential waste streams across the life cycle of these systems. Additionally, any opportunities to develop multifunctionality such as simultaneous reduction/oxidation of a contaminant into a more easily adsorbed form and sensing/quantification of contaminants upon adsorption are worthy of exploration and development.
Supplementary Material
Acknowledgements
This work was supported by the NSF Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (ERC-1449500).
References
- (1).Mohan D; Pittman CU Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater 2007, 142 (1), 1–53. 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- (2).Ryker SJ Arsenic in Ground Water Used for Drinking Water in the United States. In Arsenic in Ground Water: Geochemistry and Occurrence; Welch AH, Stollenwerk KG, Eds.; Springer US: Boston, MA, 2003; pp 165–178. 10.1007/0-306-47956-7_6. [DOI] [Google Scholar]
- (3).Solley WB; Pierce RR; Perlman HA Estimated Use of Water in the United States in 1995; U.S. Government Printing Office, 1998. [Google Scholar]
- (4).Ravenscroft P; Brammer H; Richards K Arsenic Pollution: A Global Synthesis; John Wiley & Sons, 2011. [Google Scholar]
- (5).Allaire M; Wu H; Lall U National Trends in Drinking Water Quality Violations. Proc. Natl. Acad. Sci 2018, 115 (9), 2078–2083. 10.1073/pnas.1719805115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ahmaruzzaman M; Gupta VK Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res 2011, 50 (24), 13589–13613. 10.1021/ie201477c. [DOI] [Google Scholar]
- (7).Lata S; Singh P; Samadder S Regeneration of Adsorbents and Recovery of Heavy Metals: A Review. Int. J. Environ. Sci. Technol. IJEST 2015, 12 (4), 1461–1478. 10.1007/s13762-014-0714-9. [DOI] [Google Scholar]
- (8).Ranjan D; Talat M; Hasan SH Biosorption of Arsenic from Aqueous Solution Using Agricultural Residue ‘Rice Polish.’ J. Hazard. Mater 2009, 166 (2–3), 1050–1059. 10.1016/j.jhazmat.2008.12.013. [DOI] [PubMed] [Google Scholar]
- (9).Wu Y; Wen Y; Zhou J; Cao J; Jin Y; Wu Y Comparative and Competitive Adsorption of Cr(VI), As(III), and Ni(II) onto Coconut Charcoal. Environ. Sci. Pollut. Res 2013, 20 (4), 2210–2219. 10.1007/s11356-012-1066-y. [DOI] [PubMed] [Google Scholar]
- (10).Sarkar S; Greenleaf JE; Gupta A; Uy D; Sengupta AK Sustainable Engineered Processes to Mitigate the Global Arsenic Crisis in Drinking Water: Challenges and Progress. Annu. Rev. Chem. Biomol. Eng 2012, 3, 497–517. 10.1146/annurev-chembioeng-062011-081101. [DOI] [PubMed] [Google Scholar]
- (11).Yamani JS; Lounsbury AW; Zimmerman JB Towards a Selective Adsorbent for Arsenate and Selenite in the Presence of Phosphate: Assessment of Adsorption Efficiency, Mechanism, and Binary Separation Factors of the Chitosan-Copper Complex. Water Res. 2016, 88, 889–896. 10.1016/j.watres.2015.11.017. [DOI] [PubMed] [Google Scholar]
- (12).Pincus LN; Melnikov F; Yamani JS; Zimmerman JB Multifunctional Photoactive and Selective Adsorbent for Arsenite and Arsenate: Evaluation of Nano Titanium Dioxide-Enabled Chitosan Cross-Linked with Copper. J. Hazard. Mater 2018, 358, 145–154. 10.1016/j.jhazmat.2018.06.033. [DOI] [PubMed] [Google Scholar]
- (13).Wijnja H; Schulthess CP Vibrational Spectroscopy Study of Selenate and Sulfate Adsorption Mechanisms on Fe and Al (Hydr)Oxide Surfaces. J. Colloid Interface Sci 2000, 229 (1), 286–297. 10.1006/jcis.2000.6960. [DOI] [PubMed] [Google Scholar]
- (14).Youngran J; FAN M; Van Leeuwen J; Belczyk JF Effect of Competing Solutes on Arsenic(V) Adsorption Using Iron and Aluminum Oxides. J. Environ. Sci 2007, 19 (8), 910–919. 10.1016/S1001-0742(07)60151-X. [DOI] [PubMed] [Google Scholar]
- (15).Goh K-H; Lim T-T Geochemistry of Inorganic Arsenic and Selenium in a Tropical Soil: Effect of Reaction Time, PH, and Competitive Anions on Arsenic and Selenium Adsorption. Chemosphere 2004, 55 (6), 849–859. 10.1016/j.chemosphere.2003.11.041. [DOI] [PubMed] [Google Scholar]
- (16).Awual Md. R.; El-Safty SA; Jyo A Removal of Trace Arsenic(V) and Phosphate from Water by a Highly Selective Ligand Exchange Adsorbent. J. Environ. Sci 2011, 23 (12), 1947–1954. 10.1016/S1001-0742(10)60645-6. [DOI] [PubMed] [Google Scholar]
- (17).Custelcean R Anions in Crystal Engineering. Chem. Soc. Rev 2010, 39 (10), 3675–3685. 10.1039/B926221K. [DOI] [PubMed] [Google Scholar]
- (18).Quinlivan PA; Li L; Knappe DRU Effects of Activated Carbon Characteristics on the Simultaneous Adsorption of Aqueous Organic Micropollutants and Natural Organic Matter. Water Res. 2005, 39 (8), 1663–1673. 10.1016/j.watres.2005.01.029. [DOI] [PubMed] [Google Scholar]
- (19).Crittenden John C; Speth Thomas F; Hand David W; Luft Paul J; Lykins Ben. Evaluating Multicomponent Competitive Adsorption in Fixed Beds. J. Environ. Eng 1987, 113 (6), 1363–1375. 10.1061/(ASCE)0733-9372(1987)113:6(1363). [DOI] [Google Scholar]
- (20).Matsui Y; Knappe DRU; Takagi R Pesticide Adsorption by Granular Activated Carbon Adsorbers. 1. Effect of Natural Organic Matter Preloading on Removal Rates and Model Simplification. Environ. Sci. Technol 2002, 36 (15), 3426–3431. 10.1021/es0113652. [DOI] [PubMed] [Google Scholar]
- (21).Newcombe G; Morrison J; Hepplewhite C; Knappe DRU Simultaneous Adsorption of MIB and NOM onto Activated Carbon: II. Competitive Effects. Carbon 2002, 40 (12), 2147–2156. 10.1016/S0008-6223(02)00098-2. [DOI] [Google Scholar]
- (22).Cumbal L; SenGupta AK Arsenic Removal Using Polymer-Supported Hydrated Iron(III) Oxide Nanoparticles: Role of Donnan Membrane Effect. Environ. Sci. Technol 2005, 39 (17), 6508–6515. 10.1021/es050175e. [DOI] [PubMed] [Google Scholar]
- (23).Westerhoff P; De H; Martindale A; Badruzzaman M Arsenic Adsorptive Media Technology Selection Strategies. Water Qual. Res. J. Can 2006, 41 (2), 171–184. [Google Scholar]
- (24).An B; Steinwinder TR; Zhao D Selective Removal of Arsenate from Drinking Water Using a Polymeric Ligand Exchanger. Water Res. 2005, 39 (20), 4993–5004. 10.1016/j.watres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- (25).Awual MR; Hossain MA; Shenashen MA; Yaita T; Suzuki S; Jyo A Evaluating of Arsenic(V) Removal from Water by Weak-Base Anion Exchange Adsorbents. Environ. Sci. Pollut. Res. Int 2013, 20 (1), 421–430. 10.1007/s11356-012-0936-7. [DOI] [PubMed] [Google Scholar]
- (26).Sarkar S; Blaney LM; Gupta A; Ghosh D; SenGupta AK Arsenic Removal from Groundwater and Its Safe Containment in a Rural Environment: Validation of a Sustainable Approach. Environ. Sci. Technol 2008, 42 (12), 4268–4273. 10.1021/es702556t. [DOI] [PubMed] [Google Scholar]
- (27).Bhatnagar A; Sillanpää M A Review of Emerging Adsorbents for Nitrate Removal from Water. Chem. Eng. J 2011, 168 (2), 493–504. 10.1016/j.cej.2011.01.103. [DOI] [Google Scholar]
- (28).An B; Jung K-Y; Lee S-H; Lee S; Choi J-W Effective Phosphate Removal from Synthesized Wastewater Using Copper–Chitosan Bead: Batch and Fixed-Bed Column Studies. Water. Air. Soil Pollut 2014, 225 (8), 1–12. 10.1007/s11270-014-2050-6. [DOI] [Google Scholar]
- (29).SenGupta AK Ion Exchange in Environmental Processes, 1st ed.; John Wiley & Sons, Ltd, 2017. 10.1002/9781119421252. [DOI] [Google Scholar]
- (30).Smedley PL; Kinniburgh DG A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem 2002, 17 (5), 517–568. 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- (31).World Health Organization. Guidelines for Drinking-Water Quality; 2017.
- (32).Sillén LG; Martell AE; Bjerrum J Stability Constants of Metal-Ion Complexes; Chemical Society: London, 1964. [Google Scholar]
- (33).Ramana Anuradha; Sengupta Arup K. Removing Selenium(IV) and Arsenic(V) Oxyanions with Tailored Chelating Polymers. J. Environ. Eng 1992, 118 (5), 755–775. 10.1061/(ASCE)0733-9372(1992)118:5(755). [DOI] [Google Scholar]
- (34).Fernández-Martínez A; Charlet L Selenium Environmental Cycling and Bioavailability: A Structural Chemist Point of View. Rev. Environ. Sci. Biotechnol 2009, 8 (1), 81–110. 10.1007/s11157-009-9145-3. [DOI] [Google Scholar]
- (35).Santos S; Ungureanu G; Boaventura R; Botelho C Selenium Contaminated Waters: An Overview of Analytical Methods, Treatment Options and Recent Advances in Sorption Methods. Sci. Total Environ 2015, 521–522, 246–260. 10.1016/j.scitotenv.2015.03.107. [DOI] [PubMed] [Google Scholar]
- (36).Bailey N; Carrington A; Lott KAK; Symons MCR Structure and Reactivity of the Oxyanions of Transition Metals. Part VIII. Acidities and Spectra of Protonated Oxyanions. J. Chem. Soc. Resumed 1960, 290–297. [Google Scholar]
- (37).McNeill LS; McLean JE; Parks JL; Edwards MA Hexavalent Chromium Review, Part 2: Chemistry, Occurrence, and Treatment. J. Am. Water Works Assoc 2012, 104 (7), E395–E405. [Google Scholar]
- (38).Wu B; Wan J; Zhang Y; Pan B; Lo IMC Selective Phosphate Removal from Water and Wastewater Using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol 2019, acs.est.9b05569. 10.1021/acs.est.9b05569. [DOI] [PubMed] [Google Scholar]
- (39).Mueller DK; Hamilton PA; Helsel DR; Hitt KJ; Ruddy BC Nutrients in Ground Water and Surface Water of the United States; an Analysis of Data through 1992; Water-Resources Investigations Report; USGS Numbered Series 95–4031; U.S. Geological Survey : Earth Science Information Center, Open-File Reports Section [distributor], 1995. [Google Scholar]
- (40).Domagalski JL; Johnson H Phosphorus and Groundwater: Establishing Links Between Agricultural Use and Transport to Streams: U.S. Geological Survey Fact Sheet 2012–3004; U.S. Geological Survey, 2012; p 4. [Google Scholar]
- (41).Kolthoff IM; Elving PJ Treatise on Analytical Chemistry.; Interscience Encyclopedia: New York, 1959. [Google Scholar]
- (43).World Health Organization. Sulfate in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; 2004.
- (44).Khan A; Umar R; Khan HH Significance of Silica in Identifying the Processes Affecting Groundwater Chemistry in Parts of Kali Watershed, Central Ganga Plain, India. Appl. Water Sci 2015, 5 (1), 65–72. 10.1007/s13201-014-0164-z. [DOI] [Google Scholar]
- (45).Trainer FW; Heath RC Bicarbonate Content of Groundwater in Carbonate Rock in Eastern North America. J. Hydrol 1976, 31 (1), 37–55. 10.1016/0022-1694(76)90019-6. [DOI] [Google Scholar]
- (46).Nolan BT; Ruddy BC; Hitt KJ; Helsel DR A National Look at Nitrate Contamination of Ground Water. Water Cond. Purif 1998, 39 (12), 7679. [Google Scholar]
- (47).Yamani JS; Miller SM; Spaulding ML; Zimmerman JB Enhanced Arsenic Removal Using Mixed Metal Oxide Impregnated Chitosan Beads. Water Res. 2012, 46 (14), 4427–4434. 10.1016/j.watres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- (48).Zavareh S; Zarei M; Darvishi F; Azizi H As(III) Adsorption and Antimicrobial Properties of Cu–Chitosan/Alumina Nanocomposite. Chem. Eng. J 2015, 273, 610–621. 10.1016/j.cej.2015.03.112. [DOI] [Google Scholar]
- (49).Mondal K; Jegadeesan G; Lalvani SB Removal of Selenate by Fe and NiFe Nanosized Particles. Ind. Eng. Chem. Res 2004, 43 (16), 4922–4934. 10.1021/ie030715l. [DOI] [Google Scholar]
- (50).Plant JA; Kinniburgh DG; Smedley PL; Fordyce FM; Klinck BA 9.02 - Arsenic and Selenium. In Treatise on Geochemistry; Holland HD, Turekian KK, Eds.; Pergamon: Oxford, 2003; pp 17–66. 10.1016/B0-08-043751-6/09047-2. [DOI] [Google Scholar]
- (51).Sheoran AS; Sheoran V Heavy Metal Removal Mechanism of Acid Mine Drainage in Wetlands: A Critical Review. Miner. Eng 2006, 19 (2), 105–116. 10.1016/j.mineng.2005.08.006. [DOI] [Google Scholar]
- (52).Yamani JS; Lounsbury AW; Zimmerman JB Adsorption of Selenite and Selenate by Nanocrystalline Aluminum Oxide, Neat and Impregnated in Chitosan Beads. Water Res. 2014, 50, 373–381. 10.1016/j.watres.2013.10.054. [DOI] [PubMed] [Google Scholar]
- (53).Goldhaber SB Trace Element Risk Assessment: Essentiality vs. Toxicity. Regul. Toxicol. Pharmacol 2003, 38 (2), 232–242. 10.1016/S0273-2300(02)00020-X. [DOI] [PubMed] [Google Scholar]
- (54).Ernst WG Overview of Naturally Occurring Earth Materials and Human Health Concerns. J. Asian Earth Sci 2012, 59, 108–126. 10.1016/j.jseaes.2012.05.030. [DOI] [Google Scholar]
- (55).Mohan D; Pittman CU Activated Carbons and Low Cost Adsorbents for Remediation of Tri- and Hexavalent Chromium from Water. J. Hazard. Mater 2006, 137 (2), 762–811. 10.1016/j.jhazmat.2006.06.060. [DOI] [PubMed] [Google Scholar]
- (56).Meng X; Korfiatis GP; Bang S; Bang KW Combined Effects of Anions on Arsenic Removal by Iron Hydroxides. Toxicol. Lett 2002, 133 (1), 103–111. 10.1016/S0378-4274(02)00080-2. [DOI] [PubMed] [Google Scholar]
- (57).Awual Md. R.; Jyo A; El-Safty SA; Tamada M; Seko N A Weak-Base Fibrous Anion Exchanger Effective for Rapid Phosphate Removal from Water. J. Hazard. Mater 2011, 188 (1), 164–171. 10.1016/j.jhazmat.2011.01.092. [DOI] [PubMed] [Google Scholar]
- (58).Yazdani M. (Roza); Bhatnagar A; Vahala R Synthesis, Characterization and Exploitation of Nano-TiO2/Feldspar-Embedded Chitosan Beads towards UV-Assisted Adsorptive Abatement of Aqueous Arsenic (As). Chem. Eng. J 2017, 316, 370–382. 10.1016/j.cej.2017.01.121. [DOI] [Google Scholar]
- (59).Pearson RG Hard and Soft Acids and Bases, HSAB, Part 1: Fundamental Principles. J. Chem. Educ 1968, 45 (9), 581. 10.1021/ed045p581. [DOI] [Google Scholar]
- (60).Hawthorne FC A Bond-Topological Approach to Theoretical Mineralogy: Crystal Structure, Chemical Composition and Chemical Reactions. Phys. Chem. Miner 2012, 39 (10), 841–874. 10.1007/s00269-012-0538-4. [DOI] [Google Scholar]
- (61).Regenspurg S; Peiffer S Arsenate and Chromate Incorporation in Schwertmannite. Appl. Geochem 2005, 20 (6), 1226–1239. 10.1016/j.apgeochem.2004.12.002. [DOI] [Google Scholar]
- (62).Goh K-H; Lim T-T; Dong Z Application of Layered Double Hydroxides for Removal of Oxyanions: A Review. Water Res. 2008, 42 (6–7), 1343–1368. 10.1016/j.watres.2007.10.043. [DOI] [PubMed] [Google Scholar]
- (63).Paikaray S; Hendry MJ; Essilfie-Dughan J Controls on Arsenate, Molybdate, and Selenate Uptake by Hydrotalcite-like Layered Double Hydroxides. Chem. Geol 2013, 345, 130–138. 10.1016/j.chemgeo.2013.02.015. [DOI] [Google Scholar]
- (64).Custelcean R; Moyer BA Anion Separation with Metal–Organic Frameworks. Eur. J. Inorg. Chem 2007, 2007 (10), 1321–1340. 10.1002/ejic.200700018. [DOI] [PubMed] [Google Scholar]
- (65).Marcus Y Ions in Solution and Their Solvation; John Wiley & Sons, Incorporated: New York, UNITED STATES, 2015. [Google Scholar]
- (66).Hsu J-C; Lin C-J; Liao C-H; Chen S-T Evaluation of the Multiple-Ion Competition in the Adsorption of As(V) onto Reclaimed Iron-Oxide Coated Sands by Fractional Factorial Design. Chemosphere 2008, 72 (7), 1049–1055. 10.1016/j.chemosphere.2008.03.060. [DOI] [PubMed] [Google Scholar]
- (67).Yang G; Liu Y; Song S Competitive Adsorption of As(V) with Co-Existing Ions on Porous Hematite in Aqueous Solutions. J. Environ. Chem. Eng 2015, 3 (3), 1497–1503. 10.1016/j.jece.2015.05.011. [DOI] [Google Scholar]
- (68).Jain A; Loeppert RH Effect of Competing Anions on the Adsorption of Arsenate and Arsenite by Ferrihydrite. J. Environ. Qual. Madison 2000, 29 (5), 1422. [Google Scholar]
- (69).Kanematsu M; Young TM; Fukushi K; Green PG; Darby JL Arsenic(III, V) Adsorption on a Goethite-Based Adsorbent in the Presence of Major Co-Existing Ions: Modeling Competitive Adsorption Consistent with Spectroscopic and Molecular Evidence. Geochim. Cosmochim. Acta 2013, 106, 404–428. 10.1016/j.gca.2012.09.055. [DOI] [Google Scholar]
- (70).Wu K; Liu R; Liu H; Zhao X; Qu J Arsenic(III,V) Adsorption on Iron-Oxide-Coated Manganese Sand and Quartz Sand: Comparison of Different Carriers and Adsorption Capacities. Environ. Eng. Sci 2011, 28 (9), 643–651. 10.1089/ees.2010.0307. [DOI] [Google Scholar]
- (71).Zhang Y; Dou X-M; Yang M; He H; Jing C-Y; Wu Z-Y Removal of Arsenate from Water by Using an Fe–Ce Oxide Adsorbent: Effects of Coexistent Fluoride and Phosphate. J. Hazard. Mater 2010, 179 (1–3), 208–214. 10.1016/j.jhazmat.2010.02.081. [DOI] [PubMed] [Google Scholar]
- (72).Liu S; Kang S; Wang G; Zhao H; Cai W Micro/Nanostructured Porous Fe–Ni Binary Oxide and Its Enhanced Arsenic Adsorption Performances. J. Colloid Interface Sci 2015, 458, 94–102. 10.1016/j.jcis.2015.07.038. [DOI] [PubMed] [Google Scholar]
- (73).Sun X; Hu C; Qu J Preparation and Evaluation of Zr-β-FeOOH for Efficient Arsenic Removal. J. Environ. Sci 2013, 25 (4), 815–822. 10.1016/S1001-0742(12)60085-0. [DOI] [PubMed] [Google Scholar]
- (74).Tchieda VK; D’Amato E; Chiavola A; Parisi M; Chianese A; Amamra M; Kanaev A Removal of Arsenic by Alumina: Effects of Material Size, Additives, and Water Contaminants. CLEAN – Soil Air Water 2016, 44 (5), 496–505. 10.1002/clen.201400599. [DOI] [Google Scholar]
- (75).Wu K; Zhang J; Chang B; Liu T; Zhang F; Jin P; Wang W; Wang X Removal of Arsenic(III,V) by a Granular Mn-Oxide-Doped Al Oxide Adsorbent: Surface Characterization and Performance. Environ. Sci. Pollut. Res. Int. Heidelb 2017, 24 (22), 18505–18519. 10.1007/s11356-017-9465-8. [DOI] [PubMed] [Google Scholar]
- (76).Ma L; Tu SX Removal of Arsenic from Aqueous Solution by Two Types of Nano TiO2 Crystals. Environ. Chem. Lett 2011, 9 (4), 465–472. 10.1007/s10311-010-0303-1. [DOI] [Google Scholar]
- (77).Martinson CA; Reddy KJ Adsorption of Arsenic(III) and Arsenic(V) by Cupric Oxide Nanoparticles. J. Colloid Interface Sci 2009, 336 (2), 406–411. 10.1016/j.jcis.2009.04.075. [DOI] [PubMed] [Google Scholar]
- (78).Kim Y; Kim C; Choi I; Rengaraj S; Yi J Arsenic Removal Using Mesoporous Alumina Prepared via a Templating Method. Environ. Sci. Technol 2004, 38 (3), 924–931. 10.1021/es0346431. [DOI] [PubMed] [Google Scholar]
- (79).An B; Fu Z; Xiong Z; Zhao D; SenGupta AK Synthesis and Characterization of a New Class of Polymeric Ligand Exchangers for Selective Removal of Arsenate from Drinking Water. React. Funct. Polym 2010, 70 (8), 497–507. 10.1016/j.reactfunctpolym.2010.01.006. [DOI] [Google Scholar]
- (80).Marcus Y Ionic Radii in Aqueous Solutions. Chem. Rev 1988, 88 (8), 1475–1498. 10.1021/cr00090a003. [DOI] [Google Scholar]
- (81).Wilkie JA; Hering JG Adsorption of Arsenic onto Ferric Hydroxide: Effects of Adsorbate/Adsorbent Ratios and Co‐Occurring Solutes. Colloids Surf. Physicochem. Eng. Asp 1996, 107, 97–110. [Google Scholar]
- (82).Gao X; Root RA; Farrell J; Ela W; Chorover J Effect of Silicic Acid on Arsenate and Arsenite Retention Mechanisms on 6-L Ferrihydrite: A Spectroscopic and Batch Adsorption Approach. Appl. Geochem 2013, 38, 110–120. 10.1016/j.apgeochem.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Lu P; Zhu C Arsenic Eh–PH Diagrams at 25°C and 1 Bar. Environ. Earth Sci 2011, 62 (8), 1673–1683. 10.1007/s12665-010-0652-x. [DOI] [Google Scholar]
- (84).Kitchin KT; Wallace K; Andrewes P Chapter 26 - Some Chemical Properties Underlying Arsenic’s Biological Activity*. In Arsenic Exposure and Health Effects V; Chappell WR, Abernathy CO, Calderon RL, Thomas DJ, Eds.; Elsevier Science B.V.: Amsterdam, 2003; pp 345–354. 10.1016/B978-044451441-7/50027-0. [DOI] [Google Scholar]
- (85).Prabhu SM; Koilraj P; Sasaki K Synthesis of Sucrose-Derived Porous Carbon-Doped ZrxLa1-XOOH Materials and Their Superior Performance for the Simultaneous Immobilization of Arsenite and Fluoride from Binary Systems. Chem. Eng. J 2017, 325, 1–13. 10.1016/j.cej.2017.05.052. [DOI] [Google Scholar]
- (86).Martin M; Violante A; Ajmone-Marsan F; Barberis E Surface Interactions of Arsenite and Arsenate on Soil Colloids. Soil Sci. Soc. Am. J. Madison 2014, 78 (1), 157–170. [Google Scholar]
- (87).Zhu J; Pigna M; Cozzolino V; Caporale AG; Violante A Sorption of Arsenite and Arsenate on Ferrihydrite: Effect of Organic and Inorganic Ligands. J. Hazard. Mater 2011, 189 (1), 564–571. 10.1016/j.jhazmat.2011.02.071. [DOI] [PubMed] [Google Scholar]
- (88).Zhang G; Qu J; Liu H; Liu R; Wu R Preparation and Evaluation of a Novel Fe–Mn Binary Oxide Adsorbent for Effective Arsenite Removal. Water Res. 2007, 41 (9), 1921–1928. 10.1016/j.watres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- (89).Fowler CJ; Haverlock TJ; Moyer BA; Shriver JA; Gross DE; Marquez M; Sessler JL; Hossain, Md. A.; Bowman-James, K. Enhanced Anion Exchange for Selective Sulfate Extraction: Overcoming the Hofmeister Bias. J. Am. Chem. Soc 2008, 130 (44), 14386–14387. 10.1021/ja806511b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Ma Z; Shan C; Liang J; Tong M Efficient Adsorption of Selenium(IV) from Water by Hematite Modified Magnetic Nanoparticles. Chemosphere 2018, 193, 134–141. 10.1016/j.chemosphere.2017.11.005. [DOI] [PubMed] [Google Scholar]
- (91).Balistrieri LS; Chao TT Adsorption of Selenium by Amorphous Iron Oxyhydroxide and Manganese Dioxide. Geochim. Cosmochim. Acta 1990, 54 (3), 739–751. 10.1016/0016-7037(90)90369-V. [DOI] [Google Scholar]
- (92).Szlachta M; Chubar N The Application of Fe–Mn Hydrous Oxides Based Adsorbent for Removing Selenium Species from Water. Chem. Eng. J 2013, 217, 159–168. 10.1016/j.cej.2012.11.100. [DOI] [Google Scholar]
- (93).Gonzalez CM; Hernandez J; Parsons JG; Gardea-Torresdey JL A Study of the Removal of Selenite and Selenate from Aqueous Solutions Using a Magnetic Iron/Manganese Oxide Nanomaterial and ICP-MS. Microchem. J 2010, 96 (2), 324–329. 10.1016/j.microc.2010.05.005. [DOI] [Google Scholar]
- (94).Gonzalez CM; Hernandez J; Parsons JG; Gardea-Torresdey JL Adsorption of Selenite and Selenate by a High- and Low-Pressure Aged Manganese Oxide Nanomaterial. Instrum. Sci. Technol 2011, 39 (1), 1–19. 10.1080/10739149.2010.537721. [DOI] [Google Scholar]
- (95).Amin N; Afkhami A; Madrakian T Construction of a Novel “Off-On” Fluorescence Sensor for Highly Selective Sensing of Selenite Based on Europium Ions Induced Crosslinking of Nitrogen-Doped Carbon Dots. J. Lumin 2018, 194, 768–777. 10.1016/j.jlumin.2017.09.048. [DOI] [Google Scholar]
- (96).Cui W; Li P; Wang Z; Zheng S; Zhang Y Adsorption Study of Selenium Ions from Aqueous Solutions Using MgO Nanosheets Synthesized by Ultrasonic Method. J. Hazard. Mater 2018, 341, 268–276. 10.1016/j.jhazmat.2017.07.073. [DOI] [PubMed] [Google Scholar]
- (97).Shan C; Chen J; Yang Z; Jia H; Guan X; Zhang W; Pan B Enhanced Removal of Se(VI) from Water via Pre-Corrosion of Zero-Valent Iron Using H2O2/HCl: Effect of Solution Chemistry and Mechanism Investigation. Water Res. 2018, 133, 173–181. 10.1016/j.watres.2018.01.038. [DOI] [PubMed] [Google Scholar]
- (98).Custelcean R; Bock A; Moyer BA Selectivity Principles in Anion Separation by Crystallization of Hydrogen-Bonding Capsules. J. Am. Chem. Soc 2010, 132 (20), 7177–7185. 10.1021/ja101354r. [DOI] [PubMed] [Google Scholar]
- (99).Awual Md. R.; Shenashen MA; Yaita; Shiwaku H; Jyo A Efficient Arsenic(V) Removal from Water by Ligand Exchange Fibrous Adsorbent. Water Res. 2012, 46 (17), 5541–5550. 10.1016/j.watres.2012.07.038. [DOI] [PubMed] [Google Scholar]
- (100).Sengupta S; Pandit A Selective Removal of Phosphorus from Wastewater Combined with Its Recovery as a Solid-Phase Fertilizer. Water Res. 2011, 45 (11), 3318–3330. 10.1016/j.watres.2011.03.044. [DOI] [PubMed] [Google Scholar]
- (101).Wijnja H; Schulthess CP Effect of Carbonate on the Adsorption of Selenate and Sulfate on Goethite. Soil Sci. Soc. Am. J 2002, 66 (4), 1190–1197. 10.2136/sssaj2002.1190. [DOI] [Google Scholar]
- (102).Carrero S; Fernandez-Martinez A; Pérez-López R; Poulain A; Salas-Colera E; Nieto JM Arsenate and Selenate Scavenging by Basaluminite: Insights into the Reactivity of Aluminum Phases in Acid Mine Drainage. Environ. Sci. Technol 2017, 51 (1), 28–37. 10.1021/acs.est.6b03315. [DOI] [PubMed] [Google Scholar]
- (103).Duranoğlu D; Trochimczuk AW; Beker U Kinetics and Thermodynamics of Hexavalent Chromium Adsorption onto Activated Carbon Derived from Acrylonitrile-Divinylbenzene Copolymer. Chem. Eng. J 2012, 187, 193–202. 10.1016/j.cej.2012.01.120. [DOI] [Google Scholar]
- (104).Wei L; Yang G; Wang R; Ma W Selective Adsorption and Separation of Chromium (VI) on the Magnetic Iron–Nickel Oxide from Waste Nickel Liquid. J. Hazard. Mater 2009, 164 (2), 1159–1163. 10.1016/j.jhazmat.2008.09.016. [DOI] [PubMed] [Google Scholar]
- (105).Aide MT; Cummings MF The Influence of PH AND Phosphorus on the Adsorption of Chromium (VI) on Boehmite. Soil Sci 1997, 162 (8), 599. [Google Scholar]
- (106).Gheju M; Balcu I; Mosoarca G Removal of Cr(VI) from Aqueous Solutions by Adsorption on MnO2. J. Hazard. Mater 2016, 310, 270–277. 10.1016/j.jhazmat.2016.02.042. [DOI] [PubMed] [Google Scholar]
- (107).Wang X-H; Liu F-F; Lu L; Yang S; Zhao Y; Sun L-B; Wang S-G Individual and Competitive Adsorption of Cr(VI) and Phosphate onto Synthetic Fe–Al Hydroxides. Colloids Surf. Physicochem. Eng. Asp 2013, 423, 42–49. 10.1016/j.colsurfa.2013.01.026. [DOI] [Google Scholar]
- (108).Suh YJ; Chae JW; Jang HD; Cho K Role of Chemical Hardness in the Adsorption of Hexavalent Chromium Species onto Metal Oxide Nanoparticles. Chem. Eng. J 2015, 273, 401–405. 10.1016/j.cej.2015.03.095. [DOI] [Google Scholar]
- (109).Álvarez-Ayuso E; García-Sánchez A; Querol X Adsorption of Cr(VI) from Synthetic Solutions and Electroplating Wastewaters on Amorphous Aluminium Oxide. J. Hazard. Mater 2007, 142 (1), 191–198. 10.1016/j.jhazmat.2006.08.004. [DOI] [PubMed] [Google Scholar]
- (110).Hu Y; Foster J; Boyer TH Selectivity of Bicarbonate-Form Anion Exchange for Drinking Water Contaminants: Influence of Resin Properties. Sep. Purif. Technol 2016, 163, 128–139. 10.1016/j.seppur.2016.02.030. [DOI] [Google Scholar]
- (111).Du X; Yu Z; Zhu Y Cr(VI) Adsorption from Aqueous Solution and Its Reactions Behavior on the Surfaces of Granular Fe–Mn Binary Oxides. Environ. Prog. Sustain. Energy 2019. 10.1002/ep.12968. [DOI] [Google Scholar]
- (112).Lin Z-J; Zheng H-Q; Zheng H-Y; Lin L-P; Xin Q; Cao R Efficient Capture and Effective Sensing of Cr2O72–from Water Using a Zirconium Metal–Organic Framework. Inorg. Chem 2017, 56 (22), 14178–14188. 10.1021/acs.inorgchem.7b02327. [DOI] [PubMed] [Google Scholar]
- (113).Davis CC; Knocke WR; Edwards M Implications of Aqueous Silica Sorption to Iron Hydroxide: Mobilization of Iron Colloids and Interference with Sorption of Arsenate and Humic Substances. Environ. Sci. Technol 2001, 35 (15), 3158–3162. 10.1021/es0018421. [DOI] [PubMed] [Google Scholar]
- (114).Li K; Li P; Cai J; Xiao S; Yang H; Li A Efficient Adsorption of Both Methyl Orange and Chromium from Their Aqueous Mixtures Using a Quaternary Ammonium Salt Modified Chitosan Magnetic Composite Adsorbent. Chemosphere 2016, 154, 310–318. 10.1016/j.chemosphere.2016.03.100. [DOI] [PubMed] [Google Scholar]
- (115).Zhang H; Selim HM COMPETITIVE SORPTION-DESORPTION KINETICS OF ARSENATE AND PHOSPHATE IN SOILS: Soil Sci. 2008, 173 (1), 3–12. 10.1097/ss.0b013e31815ce750. [DOI] [Google Scholar]
- (116).An B; Jung K-Y; Zhao D; Lee S-H; Choi J-W Preparation and Characterization of Polymeric Ligand Exchanger Based on Chitosan Hydrogel for Selective Removal of Phosphate. React. Funct. Polym 2014, 85, 45–53. 10.1016/j.reactfunctpolym.2014.10.003. [DOI] [Google Scholar]
- (117).Ramana A; Sengupta AK Removing Selenium(IV) and Arsenic(V) Oxyanions with Tailored Chelating Polymers. J. Environ. Eng 1992, 118 (5), 755–775. 10.1061/(ASCE)0733-9372(1992)118:5(755). [DOI] [Google Scholar]
- (118).Zhao D; Sengupta AK Ultimate Removal of Phosphate from Wastewater Using a New Class of Polymeric Ion Exchangers. Water Res. 1998, 32 (5), 1613–1625. 10.1016/S0043-1354(97)00371-0. [DOI] [Google Scholar]
- (119).Zhao D; Sengupta AK Selective Removal and Recovery of Phosphate in a Novel Fixed-Bed Process. Water Sci. Technol 1996, 33 (10), 139–147. 10.1016/0273-1223(96)00415-5. [DOI] [Google Scholar]
- (120).Ji M; Su X; Zhao Y; Qi W; Wang Y; Chen G; Zhang Z Effective Adsorption of Cr(VI) on Mesoporous Fe-Functionalized Akadama Clay: Optimization, Selectivity, and Mechanism. Appl. Surf. Sci 2015, 344, 128–136. 10.1016/j.apsusc.2015.03.006. [DOI] [Google Scholar]
- (121).Muller J; Abdelouas A; Ribet S; Grambow B Sorption of Selenite in a Multi-Component System Using the “Dialysis Membrane” Method. Appl. Geochem 2012, 27 (12), 2524–2532. 10.1016/j.apgeochem.2012.07.023. [DOI] [Google Scholar]
- (122).Rapti S; Pournara A; Sarma D; Papadas IT; Armatas GS; Tsipis AC; Lazarides T; Kanatzidis MG; Manos MJ Selective Capture of Hexavalent Chromium from an Anion-Exchange Column of Metal Organic Resin–Alginic Acid Composite. Chem. Sci 2016, 7 (3), 2427–2436. 10.1039/c5sc03732h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Dhillon SK; Dhillon KS Selenium Adsorption in Soils as Influenced by Different Anions. J. Plant Nutr. Soil Sci 2000, 163 (6), 577–582. . [DOI] [Google Scholar]
- (124).Liu B; Wang D; Li H; Xu Y; Zhang L As(III) Removal from Aqueous Solution Using α-Fe2O3 Impregnated Chitosan Beads with As(III) as Imprinted Ions. Desalination 2011, 272 (1–3), 286–292. 10.1016/j.desal.2011.01.034. [DOI] [Google Scholar]
- (125).Etemadi M; Samadi S; Yazd SS; Jafari P; Yousefi N; Aliabadi M Selective Adsorption of Cr(VI) Ions from Aqueous Solutions Using Cr6+-Imprinted Pebax/Chitosan/GO/APTES Nanofibrous Adsorbent. Int. J. Biol. Macromol 2017, 95, 725–733. 10.1016/j.ijbiomac.2016.11.117. [DOI] [PubMed] [Google Scholar]
- (126).Fang L; Min X; Kang R; Yu H; Pavlostathis SG; Luo X Development of an Anion Imprinted Polymer for High and Selective Removal of Arsenite from Wastewater. Sci. Total Environ 2018, 639, 110–117. 10.1016/j.scitotenv.2018.05.103. [DOI] [PubMed] [Google Scholar]
- (127).Hassanpour S; Taghizadeh M; Yamini Y Magnetic Cr(VI) Ion Imprinted Polymer for the Fast Selective Adsorption of Cr(VI) from Aqueous Solution. J. Polym. Environ 2018, 26 (1), 101–115. 10.1007/s10924-016-0929-6. [DOI] [PubMed] [Google Scholar]
- (128).Qiu H; Yang L; Liu F; Zhao Y; Liu L; Zhu J; Song M Highly Selective Capture of Phosphate Ions from Water by a Water Stable Metal-Organic Framework Modified with Polyethyleneimine. Environ. Sci. Pollut. Res 2017, 24 (30), 23694–23703. 10.1007/s11356-017-9946-9. [DOI] [PubMed] [Google Scholar]
- (129).Marwani HM; Albishri HM; Soliman EM; Jalal TA Selective Adsorption and Determination of Hexavalent Chromium in Water Samples by Chemically Modified Activated Carbon with Tris(Hydroxymethyl)Aminomethane. J. Dispers. Sci. Technol 2012, 33 (4), 549–555. 10.1080/01932691.2011.574941. [DOI] [Google Scholar]
- (130).Shahat A; Hassan HMA; Azzazy HME; Hosni M; Awual Md. R. Novel Nano-Conjugate Materials for Effective Arsenic(V) and Phosphate Capturing in Aqueous Media. Chem. Eng. J 2018, 331, 54–63. 10.1016/j.cej.2017.08.037. [DOI] [Google Scholar]
- (131).Liu B; Jian M; Liu R; Yao J; Zhang X Highly Efficient Removal of Arsenic(III) from Aqueous Solution by Zeolitic Imidazolate Frameworks with Different Morphology. Colloids Surf. Physicochem. Eng. Asp 2015, 481, 358–366. 10.1016/j.colsurfa.2015.06.009. [DOI] [Google Scholar]
- (132).Zhang B; Chen N; Feng C; Zhang Z Adsorption for Phosphate by Crosslinked/Non-Crosslinked-Chitosan-Fe(III) Complex Sorbents: Characteristic and Mechanism. Chem. Eng. J 2018, 353, 361–372. 10.1016/j.cej.2018.07.092. [DOI] [Google Scholar]
- (133).Pincus LN; Melnikov F; Yamani JS; Zimmerman JB Multifunctional Photoactive and Selective Adsorbent for Arsenite and Arsenate: Evaluation of Nano Titanium Dioxide-Enabled Chitosan Cross-Linked with Copper. J. Hazard. Mater 2018, 358, 145–154. 10.1016/j.jhazmat.2018.06.033. [DOI] [PubMed] [Google Scholar]
- (134).Benjamin MM New Conceptualization and Solution Approach for the Ideal Adsorbed Solution Theory (IAST). Environ. Sci. Technol 2009, 43 (7), 2530–2536. 10.1021/es803652r. [DOI] [PubMed] [Google Scholar]
- (135).Wigton A; Kilduff JE Modeling Trichloroethylene Adsorption by Activated Carbon Preloaded with Natural Dissolved Organic Matter Using a Modified IAST Approach. Environ. Sci. Technol 2004, 38 (22), 5825–5833. 10.1021/es049676a. [DOI] [PubMed] [Google Scholar]
- (136).Speitel GE Jr.; Katz LE; Chen C-C; Stokes S; Westerhoff P; Shafieian P Surface Complexation and Dynamic Transport Modeling of Arsenic Removal on Adsorptive Media; Water Research Foundation and Arsenic Water Technology Partnership: U.S.A., 2010; p 166. [Google Scholar]
- (137).Lakshmanan D; Clifford DA; Samanta G Comparative Study of Arsenic Removal by Iron Using Electrocoagulation and Chemical Coagulation. Water Res. 2010, 44 (19), 5641–5652. 10.1016/j.watres.2010.06.018. [DOI] [PubMed] [Google Scholar]
- (138).Möller T; Sylvester P Effect of Silica and PH on Arsenic Uptake by Resin/Iron Oxide Hybrid Media. Water Res. 2008, 42 (6), 1760–1766. 10.1016/j.watres.2007.10.044. [DOI] [PubMed] [Google Scholar]
- (139).Yu Y; Zhou Z; Ding Z; Zuo M; Cheng J; Jing C Simultaneous Arsenic and Fluoride Removal Using {201}TiO2–ZrO2: Fabrication, Characterization, and Mechanism. J. Hazard. Mater 2019, 377, 267–273. 10.1016/j.jhazmat.2019.05.060. [DOI] [PubMed] [Google Scholar]
- (140).Komárek M; Koretsky CM; Stephen KJ; Alessi DS; Chrastný V Competitive Adsorption of Cd(II), Cr(VI), and Pb(II) onto Nanomaghemite: A Spectroscopic and Modeling Approach. Environ. Sci. Technol 2015, 49 (21), 12851–12859. 10.1021/acs.est.5b03063. [DOI] [PubMed] [Google Scholar]
- (141).Zeng H; Fisher B; Giammar DE Individual and Competitive Adsorption of Arsenate and Phosphate To a High-Surface-Area Iron Oxide-Based Sorbent. Environ. Sci. Technol 2008, 42 (1), 147–152. 10.1021/es071553d. [DOI] [PubMed] [Google Scholar]
- (142).Hu S; Yan W; Duan J Polymerization of Silicate on TiO2 and Its Influence on Arsenate Adsorption: An ATR-FTIR Study. Colloids Surf. Physicochem. Eng. Asp 2015, 469, 180–186. 10.1016/j.colsurfa.2015.01.021. [DOI] [Google Scholar]
- (143).Price HL; Teasdale PR; Jolley DF An Evaluation of Ferrihydrite- and MetsorbTM-DGT Techniques for Measuring Oxyanion Species (As, Se, V, P): Effective Capacity, Competition and Diffusion Coefficients. Anal. Chim. Acta 2013, 803, 56–65. 10.1016/j.aca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- (144).Badruzzaman M; Westerhoff P; Knappe DRU Intraparticle Diffusion and Adsorption of Arsenate onto Granular Ferric Hydroxide (GFH). Water Res. 2004, 38 (18), 4002–4012. 10.1016/j.watres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- (145).Peak D; Sparks DL Mechanisms of Selenate Adsorption on Iron Oxides and Hydroxides. Environ. Sci. Technol 2002, 36 (7), 1460–1466. 10.1021/es0156643. [DOI] [PubMed] [Google Scholar]
- (146).Brechbühl Y; Christl I; Elzinga EJ; Kretzschmar R Competitive Sorption of Carbonate and Arsenic to Hematite: Combined ATR-FTIR and Batch Experiments. J. Colloid Interface Sci 2012, 377 (1), 313–321. 10.1016/j.jcis.2012.03.025. [DOI] [PubMed] [Google Scholar]
- (147).Taube Henry. Rates and Mechanisms of Substitution in Inorganic Complexes in Solution. Chem. Rev 1952, 50 (1), 69–126. 10.1021/cr60155a003. [DOI] [Google Scholar]
- (148).Taube H; Myers H Evidence for a Bridged Activated Complex for Electron Transfer Reactions. J. Am. Chem. Soc 1954, 76 (8), 2103–2111. 10.1021/ja01637a020. [DOI] [Google Scholar]
- (149).Sposito G The Surface Chemistry of Soils.; Oxford University Press: New York, USA, 1984. [Google Scholar]
- (150).Casey WH; Swaddle TW Why Small? The Use of Small Inorganic Clusters to Understand Mineral Surface and Dissolution Reactions in Geochemistry. Rev. Geophys 2003, 41 (2). 10.1029/2002RG000118. [DOI] [Google Scholar]
- (151).Cornell RM; Schwertmann U The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons, 2003. [Google Scholar]
- (152).Hristovski K; Baumgardner A; Westerhoff P Selecting Metal Oxide Nanomaterials for Arsenic Removal in Fixed Bed Columns: From Nanopowders to Aggregated Nanoparticle Media. J. Hazard. Mater 2007, 147 (1–2), 265–274. 10.1016/j.jhazmat.2007.01.017. [DOI] [PubMed] [Google Scholar]
- (153).Trainor TP; Chaka AM; Eng PJ; Newville M; Waychunas GA; Catalano JG; Brown GE Structure and Reactivity of the Hydrated Hematite (0001) Surface. Surf. Sci 2004, 573 (2), 204–224. 10.1016/j.susc.2004.09.040. [DOI] [Google Scholar]
- (154).Mason SE; Iceman CR; Tanwar KS; Trainor TP; Chaka AM Pb(II) Adsorption on Isostructural Hydrated Alumina and Hematite (0001) Surfaces: A DFT Study. J. Phys. Chem. C 2009, 113 (6), 2159–2170. 10.1021/jp807321e. [DOI] [Google Scholar]
- (155).Manceau A; Charlet L The Mechanism of Selenate Adsorption on Goethite and Hydrous Ferric Oxide. J. Colloid Interface Sci 1994, 168 (1), 87–93. 10.1006/jcis.1994.1396. [DOI] [Google Scholar]
- (156).Catalano JG; Park C; Fenter P; Zhang Z Simultaneous Inner- and Outer-Sphere Arsenate Adsorption on Corundum and Hematite. Geochim. Cosmochim. Acta 2008, 72 (8), 1986–2004. 10.1016/j.gca.2008.02.013. [DOI] [Google Scholar]
- (157).Catalano JG; Zhang Z; Fenter P; Bedzyk MJ Inner-Sphere Adsorption Geometry of Se(IV) at the Hematite (100)–Water Interface. J. Colloid Interface Sci 2006, 297 (2), 665–671. 10.1016/j.jcis.2005.11.026. [DOI] [PubMed] [Google Scholar]
- (158).Parfitt RL; Smart RSC The Mechanism of Sulfate Adsorption on Iron Oxides 1. Soil Sci. Soc. Am. J 1978, 42 (1), 48–50. 10.2136/sssaj1978.03615995004200010011x. [DOI] [Google Scholar]
- (159).Pena ME; Korfiatis GP; Patel M; Lippincott L; Meng X Adsorption of As(V) and As(III) by Nanocrystalline Titanium Dioxide. Water Res. 2005, 39 (11), 2327–2337. 10.1016/j.watres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- (160).Svecova L; Dossot M; Cremel S; Simonnot M-O; Sardin M; Humbert B; Den Auwer C; Michot LJ Sorption of Selenium Oxyanions on TiO2 (Rutile) Studied by Batch or Column Experiments and Spectroscopic Methods. J. Hazard. Mater 2011, 189 (3), 764–772. 10.1016/j.jhazmat.2011.02.090. [DOI] [PubMed] [Google Scholar]
- (161).Hayes KF; Roe AL; Brown GE; Hodgson KO; Leckie JO; Parks GA In Situ X-Ray Adsorption Study of Surface Complexes: Selenium Oxyanions on a-FeOOH. Science 1987, 238 (4828), 783–786. [DOI] [PubMed] [Google Scholar]
- (162).Lounsbury AW; Wang R; Plata DL; Billmyer N; Muhich C; Kanie K; Sugimoto T; Peak D; Zimmerman JB Preferential Adsorption of Selenium Oxyanions onto {1 1 0} and {0 1 2} Nano-Hematite Facets. J. Colloid Interface Sci 2019, 537, 465–474. 10.1016/j.jcis.2018.11.018. [DOI] [PubMed] [Google Scholar]
- (163).Rahnemaie R; Hiemstra T; van Riemsdijk WH Carbonate Adsorption on Goethite in Competition with Phosphate. J. Colloid Interface Sci 2007, 315 (2), 415–425. 10.1016/j.jcis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- (164).Yates DE; Healy TW Mechanism of Anion Adsorption at the Ferric and Chromic Oxide/Water Interfaces. J. Colloid Interface Sci 1975, 52 (2), 222–228. 10.1016/0021-9797(75)90192-7. [DOI] [Google Scholar]
- (165).Tamura H; Tanaka A; Mita K; Furuichi R Surface Hydroxyl Site Densities on Metal Oxides as a Measure for the Ion-Exchange Capacity. J. Colloid Interface Sci 1999, 209 (1), 225–231. 10.1006/jcis.1998.5877. [DOI] [PubMed] [Google Scholar]
- (166).Yan L; Du J; Jing C How TiO2 Facets Determine Arsenic Adsorption and Photooxidation: Spectroscopic and DFT Studies. Catal. Sci. Technol 2016, 6 (7), 2419–2426. 10.1039/C5CY01679G. [DOI] [Google Scholar]
- (167).Barrón V; Torrent J Surface Hydroxyl Configuration of Various Crystal Faces of Hematite and Goethite. J. Colloid Interface Sci 1996, 177 (2), 407–410. 10.1006/jcis.1996.0051. [DOI] [Google Scholar]
- (168).Huang X; Hou X; Song F; Zhao J; Zhang L Facet-Dependent Cr(VI) Adsorption of Hematite Nanocrystals. Environ. Sci. Technol 2016, 50 (4), 1964–1972. 10.1021/acs.est.5b05111. [DOI] [PubMed] [Google Scholar]
- (169).Luo J; Meng X; Crittenden J; Qu J; Hu C; Liu H; Peng P Arsenic Adsorption on α-MnO2 Nanofibers and the Significance of (100) Facet as Compared with (110). Chem. Eng. J 2018, 331, 492–500. 10.1016/j.cej.2017.08.123. [DOI] [Google Scholar]
- (170).Sugimoto T; Wang Y Mechanism of the Shape and Structure Control of Monodispersed α-Fe2O3Particles by Sulfate Ions. J. Colloid Interface Sci 1998, 207 (1), 137–149. 10.1006/jcis.1998.5741. [DOI] [PubMed] [Google Scholar]
- (171).Constantino LV; Quirino JN; Monteiro AM; Abrão T; Parreira PS; Urbano A; Santos MJ Sorption-Desorption of Selenite and Selenate on Mg-Al Layered Double Hydroxide in Competition with Nitrate, Sulfate and Phosphate. Chemosphere 2017, 181, 627–634. 10.1016/j.chemosphere.2017.04.071. [DOI] [PubMed] [Google Scholar]
- (172).Dultz S; An J-H; Riebe B Organic Cation Exchanged Montmorillonite and Vermiculite as Adsorbents for Cr(VI): Effect of Layer Charge on Adsorption Properties. Appl. Clay Sci 2012, 67–68, 125–133. 10.1016/j.clay.2012.05.004. [DOI] [Google Scholar]
- (173).Violante A; Pucci M; Cozzolino V; Zhu J; Pigna M Sorption/Desorption of Arsenate on/from Mg–Al Layered Double Hydroxides: Influence of Phosphate. J. Colloid Interface Sci 2009, 333 (1), 63–70. 10.1016/j.jcis.2009.01.004. [DOI] [PubMed] [Google Scholar]
- (174).Wang S-L; Liu CH; Wang MK; Chuang YH; Chiang PN Arsenate Adsorption by Mg/Al–NO3 Layered Double Hydroxides with Varying the Mg/Al Ratio. Appl. Clay Sci 2009, 43 (1), 79–85. 10.1016/j.clay.2008.07.005. [DOI] [Google Scholar]
- (175).Cai J; Wang X; Zhou Y; Jiang L; Wang C Selective Adsorption of Arsenate and the Reversible Structure Transformation of the Mesoporous Metal–Organic Framework MIL-100(Fe). Phys. Chem. Chem. Phys 2016, 18 (16), 10864–10867. 10.1039/C6CP00249H. [DOI] [PubMed] [Google Scholar]
- (176).Fei H; Bresler MR; Oliver SRJ A New Paradigm for Anion Trapping in High Capacity and Selectivity: Crystal-to-Crystal Transformation of Cationic Materials. J. Am. Chem. Soc 2011, 133 (29), 11110–11113. 10.1021/ja204577p. [DOI] [PubMed] [Google Scholar]
- (177).Fei H; Han CS; Robins JC; Oliver SRJ A Cationic Metal–Organic Solid Solution Based on Co(II) and Zn(II) for Chromate Trapping. Chem. Mater 2013, 25 (5), 647–652. 10.1021/cm302585r. [DOI] [Google Scholar]
- (178).Guo M; Guo H; Liu S; Sun Y; Guo X A Microporous Cationic Metal–Organic Framework for the Efficient Removal of Dichromate and the Selective Adsorption of Dyes from Water. RSC Adv. 2017, 7 (80), 51021–51026. 10.1039/C7RA09429A. [DOI] [Google Scholar]
- (179).Henry WD; Zhao D; SenGupta AK; Lange C Preparation and Characterization of a New Class of Polymeric Ligand Exchangers for Selective Removal of Trace Contaminants from Water. React. Funct. Polym 2004, 60, 109–120. 10.1016/j.reactfunctpolym.2004.02.016. [DOI] [Google Scholar]
- (180).Azzam AM; Shenashen MA; Selim MM; Yamaguchi H; El-Sewify IM; Kawada S; Alhamid AA; El-Safty SA Nanospherical Inorganic α-Fe Core-Organic Shell Necklaces for the Removal of Arsenic(V) and Chromium(VI) from Aqueous Solution. J. Phys. Chem. Solids 2017, 109, 78–88. 10.1016/j.jpcs.2017.05.017. [DOI] [Google Scholar]
- (181).Pincus LN; Lounsbury AW; Zimmerman JB Toward Realizing Multifunctionality: Photoactive and Selective Adsorbents for the Removal of Inorganics in Water Treatment. Acc. Chem. Res 2019. 10.1021/acs.accounts.8b00668. [DOI] [PubMed] [Google Scholar]
- (182).Shahat A; Hassan HMA; Azzazy HME; Hosni M; Awual Md. R. Novel Nano-Conjugate Materials for Effective Arsenic(V) and Phosphate Capturing in Aqueous Media. Chem. Eng. J 2018, 331, 54–63. 10.1016/j.cej.2017.08.037. [DOI] [Google Scholar]
- (183).Song Z; Feng L; Zhang T Synthesis and Performance Evaluation of As (III) -Ion-Imprinted Polymer. Chin. J. Environ. Eng 2014, 8 (5), 2141–2145. [Google Scholar]
- (184).Awual Md. R.; Yaita T; Suzuki S; Shiwaku H Ultimate Selenium(IV) Monitoring and Removal from Water Using a New Class of Organic Ligand Based Composite Adsorbent. J. Hazard. Mater 2015, 291, 111–119. 10.1016/j.jhazmat.2015.02.066. [DOI] [PubMed] [Google Scholar]
- (185).Huang R; Ma X; Li X; Guo L; Xie X; Zhang M; Li J A Novel Ion-Imprinted Polymer Based on Graphene Oxide-Mesoporous Silica Nanosheet for Fast and Efficient Removal of Chromium (VI) from Aqueous Solution. J. Colloid Interface Sci 2018, 514, 544–553. 10.1016/j.jcis.2017.12.065. [DOI] [PubMed] [Google Scholar]
- (186).Pakade V; Cukrowska E; Darkwa J; Torto N; Chimuka L Selective Removal of Chromium (VI) from Sulphates and Other Metal Anions Using an Ion-Imprinted Polymer. Water SA 2011, 37 (4), 529–538–538. 10.4314/wsa.v37i4.11. [DOI] [Google Scholar]
- (187).Velempini T; Pillay K; Mbianda XY; Arotiba OA Epichlorohydrin Crosslinked Carboxymethyl Cellulose-Ethylenediamine Imprinted Polymer for the Selective Uptake of Cr(VI). Int. J. Biol. Macromol 2017, 101, 837–844. 10.1016/j.ijbiomac.2017.03.048. [DOI] [PubMed] [Google Scholar]
- (188).Qi X; Gao S; Ding G; Tang A-N Synthesis of Surface Cr (VI)-Imprinted Magnetic Nanoparticles for Selective Dispersive Solid-Phase Extraction and Determination of Cr (VI) in Water Samples. Talanta 2017, 162, 345–353. 10.1016/j.talanta.2016.10.040. [DOI] [PubMed] [Google Scholar]
- (189).Lu W; Li J; Sheng Y; Zhang X; You J; Chen L One-Pot Synthesis of Magnetic Iron Oxide Nanoparticle-Multiwalled Carbon Nanotube Composites for Enhanced Removal of Cr(VI) from Aqueous Solution. J. Colloid Interface Sci 2017, 505, 1134–1146. 10.1016/j.jcis.2017.07.013. [DOI] [PubMed] [Google Scholar]
- (190).Fang J; Gu Z; Gang D; Liu C; Ilton ES; Deng B Cr(VI) Removal from Aqueous Solution by Activated Carbon Coated with Quaternized Poly(4-Vinylpyridine). Environ. Sci. Technol 2007, 41 (13), 4748–4753. 10.1021/es061969b. [DOI] [PubMed] [Google Scholar]
- (191).Fan H-T; Fan X; Li J; Guo M; Zhang D; Yan F; Sun T Selective Removal of Arsenic(V) from Aqueous Solution Using A Surface-Ion-Imprinted Amine-Functionalized Silica Gel Sorbent. Ind. Eng. Chem. Res 2012, 51 (14), 5216–5223. 10.1021/ie202655x. [DOI] [Google Scholar]
- (192).Pearson RG Recent Advances in the Concept of Hard and Soft Acids and Bases. J. Chem. Educ 1987, 64 (7), 561. 10.1021/ed064p561. [DOI] [Google Scholar]
- (193).Essington ME Soil and Water Chemistry: An Integrative Approach; CRC Press, 2004. [Google Scholar]
- (194).Roos G; Loverix S; De Proft F; Wyns L; Geerlings P A Computational and Conceptual DFT Study of the Reactivity of Anionic Compounds: Implications for Enzymatic Catalysis. J. Phys. Chem. A 2003, 107 (35), 6828–6836. 10.1021/jp034376l. [DOI] [Google Scholar]
- (195).Mohanty D; Samal S Selective Removal of Toxic Metals like Copper and Arsenic from Drinking Water Using Phenol-Formaldehyde Type Chelating Resins. J. Chem 2009, 6 (4), 1035–1046. 10.1155/2009/195721. [DOI] [Google Scholar]
- (196).Chang R Chemistry, 10th ed.; McGraw-Hill, 2010. [Google Scholar]
- (197).Becke AD; Edgecombe KE A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys 1990, 92 (9), 5397–5403. 10.1063/1.458517. [DOI] [Google Scholar]
- (198).Moyer BA; Custelcean R; Hay BP; Sessler JL; Bowman-James K; Day VW; Kang S-O A Case for Molecular Recognition in Nuclear Separations: Sulfate Separation from Nuclear Wastes. Inorg. Chem 2013, 52 (7), 3473–3490. 10.1021/ic3016832. [DOI] [PubMed] [Google Scholar]
- (199).Luo W; Hirajima T; Sasaki K Selective Adsorption of Inorganic Anions on Unwashed and Washed Hexadecyl Pyridinium-Modified Montmorillonite. Sep. Purif. Technol 2017, 176, 120–125. 10.1016/j.seppur.2016.12.004. [DOI] [Google Scholar]
- (200).Marcus Y Thermodynamics of Solvation of Ions. J CHEM SOC FARADAY TRANS 1991, 87, 5. [Google Scholar]
- (201).Li L-L; Feng X-Q; Han R-P; Zang S-Q; Yang G Cr(VI) Removal via Anion Exchange on a Silver-Triazolate MOF. J. Hazard. Mater 2017, 321, 622–628. 10.1016/j.jhazmat.2016.09.029. [DOI] [PubMed] [Google Scholar]
- (202).Awual Md. R.; Jyo A Assessing of Phosphorus Removal by Polymeric Anion Exchangers. Desalination 2011, 281, 111–117. 10.1016/j.desal.2011.07.047. [DOI] [Google Scholar]
- (203).Hiemstra T; Rietra RPJJ; Van Riemsdijk WH Surface Complexation of Selenite on Goethite: MO/DFT Geometry and Charge Distribution. Croat. Chem. Acta 2007, 80 (3–4), 313–324. [Google Scholar]
- (204).Kubicki JD; Paul KW; Kabalan L; Zhu Q; Mrozik MK; Aryanpour M; Pierre-Louis A-M; Strongin DR ATR–FTIR and Density Functional Theory Study of the Structures, Energetics, and Vibrational Spectra of Phosphate Adsorbed onto Goethite. Langmuir 2012, 28 (41), 14573–14587. 10.1021/la303111a. [DOI] [PubMed] [Google Scholar]
- (205).Stachowicz M; Hiemstra T; van Riemsdijk WH Multi-Competitive Interaction of As(III) and As(V) Oxyanions with Ca2+, Mg2+, PO3−4, and CO2−3 Ions on Goethite. J. Colloid Interface Sci 2008, 320 (2), 400–414. 10.1016/j.jcis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- (206).Yan L; Song J; Chan T; Jing C Insights into Antimony Adsorption on {001} TiO2: XAFS and DFT Study. Environ. Sci. Technol 2017, 51 (11), 6335–6341. 10.1021/acs.est.7b00807. [DOI] [PubMed] [Google Scholar]
- (207).Paul KW; Kubicki JD; Sparks DL Sulphate Adsorption at the Fe (Hydr)Oxide?H 2 O Interface: Comparison of Cluster and Periodic Slab DFT Predictions. Eur. J. Soil Sci 2007, 58 (4), 978–988. 10.1111/j.1365-2389.2007.00936.x. [DOI] [Google Scholar]
- (208).He G; Pan G; Zhang M Studies on the Reaction Pathway of Arsenate Adsorption at Water–TiO2 Interfaces Using Density Functional Theory. J. Colloid Interface Sci 2011, 364 (2), 476–481. 10.1016/j.jcis.2011.08.040. [DOI] [PubMed] [Google Scholar]
- (209).Wei Z; Luo S; Xiao R; Khalfin R; Semiat R Characterization and Quantification of Chromate Adsorption by Layered Porous Iron Oxyhydroxide: An Experimental and Theoretical Study. J. Hazard. Mater 2017, 338, 472–481. 10.1016/j.jhazmat.2017.06.001. [DOI] [PubMed] [Google Scholar]
- (210).Kubicki JD; Kwon KD; Paul KW; Sparks DL Surface Complex Structures Modelled with Quantum Chemical Calculations: Carbonate, Phosphate, Sulphate, Arsenate and Arsenite. Eur. J. Soil Sci 2007, 58 (4), 932–944. 10.1111/j.1365-2389.2007.00931.x. [DOI] [Google Scholar]
- (211).Corum KW; Mason SE Using Density Functional Theory to Study Shape-Reactivity Relationships in Keggin Al-Nanoclusters. Water Res. 2016, 102, 413–420. 10.1016/j.watres.2016.06.043. [DOI] [PubMed] [Google Scholar]
- (212).Leung K; Criscenti LJ Lead and Selenite Adsorption at Water–Goethite Interfaces from First Principles. J. Phys. Condens. Matter 2017, 29 (36), 365101. 10.1088/1361-648X/aa7e4f. [DOI] [PubMed] [Google Scholar]
- (213).Wei Z; Zhang S; Pan Z; Liu Y Theoretical Studies of Arsenite Adsorption and Its Oxidation Mechanism on a Perfect TiO2 Anatase (101) Surface. Appl. Surf. Sci 2011, 258 (3), 1192–1198. 10.1016/j.apsusc.2011.09.069. [DOI] [Google Scholar]
- (214).Zhang N; Blowers P; Farrell J Evaluation of Density Functional Theory Methods for Studying Chemisorption of Arsenite on Ferric Hydroxides. Environ. Sci. Technol 2005, 39 (13), 4816–4822. 10.1021/es050271f. [DOI] [PubMed] [Google Scholar]
- (215).Johnston CP; Chrysochoou M Mechanisms of Chromate Adsorption on Hematite. Geochim. Cosmochim. Acta 2014, 138, 146–157. 10.1016/j.gca.2014.04.030. [DOI] [Google Scholar]
- (216).Shi Q; Yan L; Chan T; Jing C Arsenic Adsorption on Lanthanum-Impregnated Activated Alumina: Spectroscopic and DFT Study. ACS Appl. Mater. Interfaces 2015, 7 (48), 26735–26741. 10.1021/acsami.5b08730. [DOI] [PubMed] [Google Scholar]
- (217).Paul KW; Kubicki JD; Sparks DL Quantum Chemical Calculations of Sulfate Adsorption at the Al- and Fe-(Hydr)Oxide-H 2 O InterfaceEstimation of Gibbs Free Energies. Environ. Sci. Technol 2006, 40 (24), 7717–7724. 10.1021/es061139y. [DOI] [PubMed] [Google Scholar]
- (218).Acelas NY; Mejia SM; Mondragón F; Flórez E Density Functional Theory Characterization of Phosphate and Sulfate Adsorption on Fe-(Hydr)Oxide: Reactivity, PH Effect, Estimation of Gibbs Free Energies, and Topological Analysis of Hydrogen Bonds. Comput. Theor. Chem 2013, 1005, 16–24. 10.1016/j.comptc.2012.11.002. [DOI] [Google Scholar]
- (219).Kabengi NJ; Chrysochoou M; Bompoti N; Kubicki JD An Integrated Flow Microcalorimetry, Infrared Spectroscopy and Density Functional Theory Approach to the Study of Chromate Complexation on Hematite and Ferrihdyrite. Chem. Geol 2017, 464, 23–33. 10.1016/j.chemgeo.2017.01.017. [DOI] [Google Scholar]
- (220).Johnston CP; Chrysochoou M Investigation of Chromate Coordination on Ferrihydrite by in Situ ATR-FTIR Spectroscopy and Theoretical Frequency Calculations. Environ. Sci. Technol 2012, 46 (11), 5851–5858. 10.1021/es300660r. [DOI] [PubMed] [Google Scholar]
- (221).Johnston CP; Chrysochoou M Mechanisms of Chromate Adsorption on Boehmite. J. Hazard. Mater 2015, 281, 56–63. 10.1016/j.jhazmat.2014.05.067. [DOI] [PubMed] [Google Scholar]
- (222).Kubicki JD; Kabengi N; Chrysochoou M; Bompoti N Density Functional Theory Modeling of Chromate Adsorption onto Ferrihydrite Nanoparticles. Geochem. Trans 2018, 19 (1), 8. 10.1186/s12932-018-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Hu S; Yan L; Chan T; Jing C Molecular Insights into Ternary Surface Complexation of Arsenite and Cadmium on TiO 2. Environ. Sci. Technol 2015, 49 (10), 5973–5979. 10.1021/es5062903. [DOI] [PubMed] [Google Scholar]
- (224).Logadottir A; Rod TH; Nørskov JK; Hammer B; Dahl S; Jacobsen CJH The Brønsted–Evans–Polanyi Relation and the Volcano Plot for Ammonia Synthesis over Transition Metal Catalysts. J. Catal 2001, 197 (2), 229–231. 10.1006/jcat.2000.3087. [DOI] [Google Scholar]
- (225).Galli G Linear Scaling Methods for Electronic Structure Calculations and Quantum Molecular Dynamics Simulations. Curr. Opin. Solid State Mater. Sci 1996, 1 (6), 864–874. 10.1016/S1359-0286(96)80114-8. [DOI] [Google Scholar]
- (226).Butler KT; Davies DW; Cartwright H; Isayev O; Walsh A Machine Learning for Molecular and Materials Science. Nature 2018, 559 (7715), 547–555. 10.1038/s41586-018-0337-2. [DOI] [PubMed] [Google Scholar]
- (227).Lin H; Truhlar DG QM/MM: What Have We Learned, Where Are We, and Where Do We Go from Here? Theor. Chem. Acc 2006, 117 (2), 185. 10.1007/s00214-006-0143-z. [DOI] [Google Scholar]
- (228).Wu B; Wan J; Zhang Y; Pan B; Lo IMC Selective Phosphate Removal from Water and Wastewater Using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol 2020, 54 (1), 50–66. 10.1021/acs.est.9b05569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


