Abstract
From 1 January 1995 until 1 January 1996, we studied the molecular epidemiology of blood isolates of coagulase-negative staphylococci (CoNS) in the Neonatal Intensive Care Units (NICUs) of the Sophia Children’s Hospital (SCH; Rotterdam, The Netherlands) and the Wilhelmina Children’s Hospital (WCH; Utrecht, The Netherlands). The main goal of the present study was to detect putatively endemic clones of CoNS persisting in these NICUs. Pulsed-field gel electrophoresis was used to detect the possible presence of endemic clones of clinical significance. In addition, clinical data of patients in the SCH were analyzed retrospectively to identify risk factors for the acquisition of positive blood cultures. In both centers, endemic CoNS clones were persistently present. Thirty-three percent of the bacterial isolates derived from blood cultures in the SCH belonged to a single genotype. In the WCH, 45% of all bacterial strains belonged to a single clone. These clones were clearly different from each other, which implies that site specificity is involved. Interestingly, we observe that the clonal type in the SCH differed significantly from the incidentally occurring strains with respect to both the average pH and partial CO2 pressure of the patient’s blood at the time of bacterial culture. We found that the use of intravascular catheters, low gestational age, and a long hospital stay were important risk factors for the development of a putative CoNS infection. When the antibiotic susceptibility of the bacterial isolates was assessed, a clear correlation between the nature of the antibiotics most frequently used as a first line of defense versus the resistance profile was observed. We conclude that the intensive use of antibiotics in an NICU setting with highly susceptible patients causes selection of multiresistant clones of CoNS which subsequently become endemic.
Coagulase-negative staphylococci (CoNS), formerly regarded as harmless inhabitants of the skin and mucosal linings, are now recognized as a major cause of nosocomial infections in neonatal intensive care units (NICUs) in the United States as well as in Europe (7). The skin of newborn babies is not colonized by bacteria, although it acquires this flora quite rapidly during the first days after birth (9). In the period immediately after birth, bacterial colonization of the throat occurs, paving the way for subsequent invasive infections (5). The emergence of CoNS as important pathogens in the NICU setting is also due to major advances in perinatal and neonatal care. Recently, survival rates of premature, very low birth weight neonates have increased significantly. However, this is often accompanied by prolonged periods of hospitalization, frequent insertion of intravascular catheters, and the nearly unrestricted use of antibiotics. This leads not only to increasing infectious problems in the NICU but also to the development of antibiotic resistance in bacteria present in the environment, patients, and hospital personnel. Several recent studies have demonstrated a clear correlation between (extremely) low birth weight and the risk of acquisition of a nosocomial infection with CoNS (1, 12). The importance of the infections caused by CoNS is emphasized by the observation that these infections are associated with a huge increase in morbidity, whereas the directly attributable mortality may be as high as 14% (15).
The sites of origin and reservoirs of nosocomial bloodstream infection by CoNS are not well defined. Another matter of concern is whether these infections are caused by endemic clones of CoNS or by incidentally occurring bacterial strains of these ubiquitous species. The hospital environment, hospital personnel, and the nature of the patient population contribute to the complex balance between infection prevention and nosocomial endemicity and epidemics. It seems likely, but is difficult to prove, that differences in patient’s characteristics contribute significantly to the occurrence of CoNS infections (9). The fact that cross-infections occur is well established (10, 14), and the epidemiology of infections has been documented in detail in different clinical settings (11, 17). Persistence of CoNS types in single departments for periods ranging between 5 and 10 years has been documented (9, 13).
To provide answers to some of these questions, we performed a study to determine whether endemic clones persist in two of the largest Dutch NICUs. We compared the findings in the NICUs of the Sophia Children’s Hospital (SCH; Rotterdam, The Netherlands) and the Wilhelmina Children’s Hospital (WCH; Utrecht, The Netherlands). Since pulsed-field gel electrophoresis (PFGE) is suggested to be superior to both conventional and other molecular techniques (28), this technique was chosen as the typing method for CoNS isolates collected during the present study. In addition, risk factors were determined with respect to patient characteristics and different types of intravascular catheterization.
MATERIALS AND METHODS
Description of patients.
A retrospective study was performed in the SCH NICU. Medical charts and laboratory results of 502 patients admitted in 1995 were used as sources of information. A subgroup of 42 patients had one or more episodes of CoNS-positive blood cultures. Seventy strains isolated from blood cultures of these 42 patients were used for analysis. In addition, 31 strains from 22 patients with CoNS-positive blood cultures which occurred within 1 year in the WCH NICU were also analyzed to determine possible differences between the situations concerning CoNS endemicity in the Rotterdam and Utrecht NICUs.
Laboratory analysis.
Several clinical-chemical characteristics were determined for all of the blood samples. Two of these, the partial CO2 pressure (pCO2) and pH of the blood, turned out to be of particular importance (see Results). The pCO2 and pH values were determined electrochemically in an automated fashion by using Acid Base Laboratory equipment (Radiometer, Zoetermeer, The Netherlands). Electrodes were rinsed extensively after each assay, and calibration of the Acid Base Laboratory was performed three times per day for both pH and pCO2 measurement consistency.
The susceptibility of the bacterial isolates from the Rotterdam patients to the following antibiotics was assessed: penicillin, flucloxacillin, tobramycin, cefuroxime, erythromycin, clindamycin, vancomycin, fusidic acid, and rifampin. Assays for MIC determination were performed in accordance with National Committee for Clinical Laboratory Standards guidelines (16).
Blood cultures were performed with the BacTec 9240 System using pediatric bottles (Becton Dickinson, Meylan, France). Per bottle, between 1 and 3 ml of blood was inoculated. Bottles were incubated at 37°C for a maximum of 4 days, except for those cultures becoming positive at an earlier time. After Gram staining, staphylococci were identified to the species level on the basis of a Staphaurex test (Murex Biotech, Darford, United Kingdom) and Api Staph analysis (BioMerieux, Marcy l’Etoile, France). CoNS isolates were stored in glycerol-containing liquid media at −80°C in the microbiology laboratories of both hospitals. Prior to strain identification by PFGE, cultivation took place on Columbia blood agar plates at 37°C for 18 h. Culture purity was assessed by visual examination, and all further laboratory analyses were carried out at the Department of Medical Microbiology & Infectious Diseases, University Hospital Dijkzigt, Rotterdam, The Netherlands.
PFGE was used as a molecular typing technique. This procedure allows the separation of chromosomal DNA fragments differing in size by the use of rarely cutting restriction enzymes and the application of multidirectional electrical pulses through an agarose matrix (6). A protocol developed for CoNS and used in our laboratory on previous occasions was employed (26, 27). In short, a suspension of bacteria in 10 mM Tris-HCl (pH 8.0)–100 mM EDTA–10 mM EGTA was mixed with a solution of 1% InCert agarose (FMC Bioproducts, Rockland, Maine). Agarose plugs were incubated with lysostaphin. Spheroplasts were lysed by sodium dodecyl sulfate and proteinase K (Boehringer, Mannheim, Germany), washed six times for 30 min each time in 10 mM Tris-HCl (pH 8.0)–1 mM EDTA, and stored at 4°C until further use. Prior to electrophoresis, DNA was digested overnight with the restriction enzyme SmaI (Boehringer). PFGE was carried out with 1% SeaKem GTG agarose gels (FMC Bioproducts) in 0.5× Tris-borate-EDTA at 14°C in a contour-clamped homogeneous electric field mapper (Bio-Rad, Veenendaal, The Netherlands). The running time was 24 h. Gels were stained with ethidium bromide and photographed. Differences in banding patterns were documented by visual examination by two independent observers and indexed by capital lettering. Isolates were considered indistinguishable if there were no chromosomal band differences, related if they differed by one to three bands, and unrelated if they differed by four or more bands. Interpretation of the experimental data in accordance with these guidelines is supported by recent consensus publications (24, 25).
Risk factor assessment.
To determine risk factors, clinical characteristics of neonates without CoNS-positive blood cultures admitted to the SCH NICU in 1995 (n = 460) were collected and used as controls. They were compared with the characteristics of patients (n = 42) from whose blood CoNS strains were isolated. These data were analyzed by the nonparametric (two-tailed) Mann-Whitney test, since normality test (Kolmogorov-Smirnov one-sample test for normality) data, except for the catheter data, showed small P values. The catheter data were analyzed by the Chi-square test. P values smaller than 0.05 were considered to be significant. A logistic regression analysis was performed on the significant parameters. To relate laboratory results to clinical data, the data for SCH patients carrying the epidemic clone were identified and compared with the data from the other patients from whom incidentally occurring isolates were cultured, using Mann-Whitney tests to compare demographic and various clinical parameters. All statistical manipulations were performed with software from the Statistical Package for the Social Sciences.
RESULTS
Characteristics of patients.
Forty-six episodes of potential CoNS septicemia were identified in 42 patients. Sixteen patients had a single positive blood culture, and 26 infants had multiple positive blood cultures. Altogether, 70 strains were isolated. Of these cultures, blood samples for 12 isolates were drawn through arterial catheters, 3 were drawn through intravenous catheters, 1 was drawn through an umbilical venous catheter, and 1 was drawn through an umbilical arterial catheter. The remaining 53 samples were drawn from peripheral veins. Genuine episodes of septicemia are hard to define in this particular group of very small patients. The genotyping data as described in the present communication can be considered helpful. Clinically suspected sepsis syndrome could be experimentally corroborated in 10 of 16 cases: all bacterial isolates obtained from positive blood cultures derived from different samples displayed the same genotype. In 6 of these 16 cases, different genotypes were found, indicating the presence of mixed infections or, more likely, contamination of the culture medium with skin flora. Although strict guidelines for definition of sepsis were not implemented throughout the present study, it is suggested that on the basis of more than 60% of the positive blood cultures described here, patients could be diagnosed as having septicemia. To avoid miscommunication, the term sepsis will be avoided as much as possible and positive blood cultures will be discussed mainly.
Apnea, bradycardia, and temperature instability occurred most frequently as clinical symptoms of bacteremia. Demographic data and relevant laboratory parameters of the patients are shown in Table 1. Table 2 shows the percentage of patients needing ventilatory support or intravascular catheters. In addition, the duration of the intravascular residence of the catheters is noted. Three patients also had positive urine cultures, one of which was highly positive, identifying a genuine urinary tract infection. Finally, six patients also had other cultures from which CoNS were isolated, like a tube culture or a wound aspirate culture. In Table 3, antibiotic therapy prior to CoNS-positive blood cultures is listed. Broviac catheters were not taken into account, since they were used in a very small number of patients only.
TABLE 1.
Demographic data and laboratory values during CoNS positivity of blood cultures of 42 patients admitted to the SCH NICU in 1995
| Parameter | Mean (range; SD) |
|---|---|
| Gestational age (wk) | 30.9 (25.4–42.4; 4.4) |
| Birth wt (g) | 1,523 (550–4,625; 1,015) |
| Age at start bacteremia (days) | 17 (0–224; 35) |
| Total length of hospital stay (days) | 52 (5–270; 59) |
| Maximal complement-reactive protein level (mg/liter) | 66 (1–285; 66) |
| Leukocyte count (109/liter) | 13.8 (2.2–32.9; 7.5) |
| pH value | 7.31 (7.14–7.52; 0.08) |
| Thrombocyte count (109/liter) | 253 (20–821; 168) |
| Base excess | −2.1 (−10.8–9.8; 4,3) |
| CO2 (kPa) | 6.2 (2.7–8.7; 1.3) |
TABLE 2.
Ventilation and catheter types of 42 SCH patients with CoNS-positive blood cultures in 1995
| Treatment | % of neonates | Mean duration in days (range) |
|---|---|---|
| Ventilation | 74 | 23 (1–200) |
| Arterial catheter | 50 | 14.2 (1–99) |
| Umbilical venous catheter | 76 | 6.5 (1–18) |
| Umbilical arterial catheter | 50 | 5.4 (1–16) |
| Intravenous catheter | 38 | 10.2 (1–26) |
| Broviac catheter | 5 | 2 |
TABLE 3.
Antibiotic therapy of neonates at SCH NICU in 1995 before detection of positive blood cultures
| Antibiotic | No. of patients | Mean duration of therapy in days (range) |
|---|---|---|
| Amoxicillin | 36 | 4.1 (2–18) |
| Tobramycin | 34 | 3.4 (1–8) |
| Flucloxacillin | 5 | 2.8 (2–8) |
| Cefotaxime | 1 | 7 |
| Amikacin | 1 | 13 |
Laboratory analysis.
The antibiotic susceptibility data showed large proportions of the strains to be resistant to penicillin, flucloxacillin, tobramycin, and cefuroxime (99, 90, 89, and 90%, respectively). Various but limited numbers of strains appeared to be resistant to erythromycin, clindamycin, fusidic acid, and rifampin (44, 23, 6, and 9%, respectively). All strains were susceptible to vancomycin.
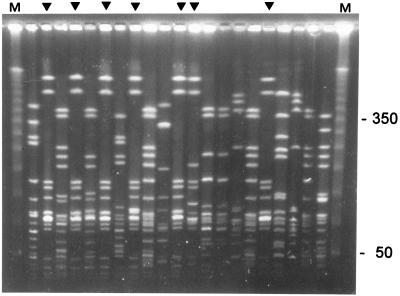
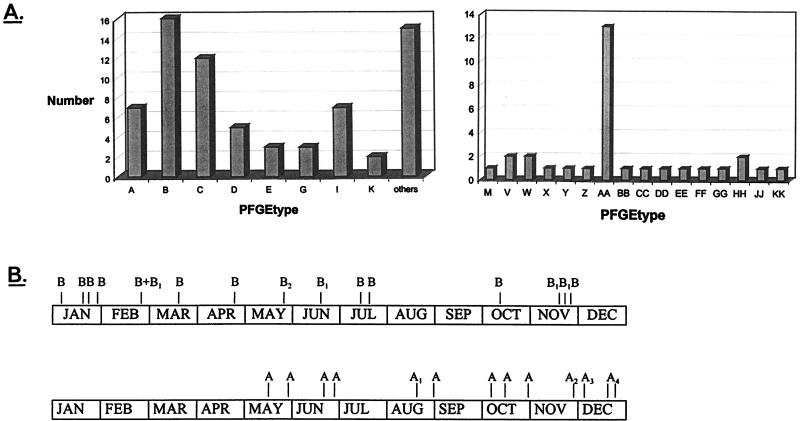
Figure 1 shows an example of the data obtained by PFGE. Endemic strains can be easily identified. The interpretation of the experimental data is summarized in Fig. 2A. The PFGE analysis revealed that 16 (23%) of 70 strains from the SCH were similar (either indistinguishable or related). These 16 strains were isolated from 14 different patients. Thus, this type (designated B) was responsible for one-third of all positive blood cultures in that year (14 of 42). Clone B and the less prevalent clone C together were responsible for 62% of all cases in which blood cultures grew CoNS in the NICU during the study period. The clinical data from cases with clone B were compared with those from the cases associated with the other types. Parameters analyzed were birth weight, gestational age, duration of ventilation, length of stay, clinical symptoms, and laboratory parameters such as complement-reactive protein, leukocyte counts, and base excess. No significant differences were found between these two groups with respect to these data. However, patients infected with clone B had a higher average pCO2 value in the blood (6.9 kPa) than did patients infected by one of the other types (5.8 kPa; Mann-Whitney test, P < 0.05). Moreover, clone B was also associated with increased blood acidity values. The pH during infection with this type was 7.26, on average, whereas during infection with another type, the average pH was 7.35 (Mann-Whitney test, P < 0.01). Finally, no differences between the two groups in duration of catheter stay (either arterial, intravenous, or umbilical) were found.
FIG. 1.
PFGE fingerprints for a random selection of the CoNS isolated from blood samples obtained from patients nursed in the SCH NICU. The pattern obtained for the endemic clone with PFGE type B can be observed in the lanes marked by a triangle. Some of the molecular sizes of the markers in lanes M are indicated on the right in kilobase pairs.
FIG. 2.
(A) Frequency distribution of the different PFGE types of CoNS in the SCH NICU (left) and the WCH NICU (right). PFGE types are identified by capital-letter codes. Bar height shows the number of clinical isolates identified in 1995. The “others” column accounts for the number of unique types encountered (i.e., those types occurring only once in 1995). (B) Longitudinal persistence of endemic clones of CoNS in the SCH NICU (top bar) and the WCH NICU (bottom bar). The period depicted covers the year 1995. The most prevalent strain of type AA is identified by the letter A. Note that this strain was introduced into the WCH NICU in the month of May and subsequently persisted.
In the WCH, as shown in Fig. 2, 13 (42%) of 31 strains were typed AA, indicating that 10 (45%) of 22 patients were infected with similar strains of CoNS. All other identified isolates were unique and were encountered only in individual patients. Figure 2B depicts how the epidemic clones in both hospitals persisted during the study period. It appears that strain AA was introduced into the WCH in May and persisted thereafter. Apparently, this strain has a significant ecological advantage over the other CoNS strains. In the SCH, the endemic strain was present all year round.
Risk factors.
Mann-Whitney U tests showed that gestational age and birth weight were significantly lower, whereas duration of ventilation and length of hospital stay were significantly longer in patients with CoNS-positive blood cultures (Table 4). The data on the use of the various catheters among neonates included in the study are shown in Table 5. Except for the arterial catheters, all catheters were used more frequently in neonates infected with CoNS than in neonates from the control group. However, no differences were found in the average length of stay of any of the catheters involved. A forward inclusion selection of variables was performed to identify the most important predictors for the development of blood cultures positive for CoNS. Variables were entered until no remaining candidate variable met a significance level of 0.05 or lower. Logistic regression analysis performed on the important parameters revealed that gestational age, hospital stay before onset of positive blood cultures, and insertion of a venous umbilical catheter significantly differed between the subgroup and the controls (P values of 0.01, 0.05, and 0.001, respectively). Table 6 summarizes the data on all of the catheters inserted. This shows the percentages of the respective catheter types colonized by CoNS.
TABLE 4.
Demographic dataa (SCH NICU, 1995)
| Parameter | Subgroup of neonates with CoNS-positive blood cultures | Control group (n = 460) |
|---|---|---|
| Gestational age (wk) | 30.9 | 34.0 |
| Birth wt (g) | 1,523 | 2,138 |
| Total length of hospital stay (days) | 52 | 10 |
| Length of stay before onset of culture positivity (days) | 16 | |
| Total duration of ventilation (days) | 23 | 4 |
| Ventilation time before onset of culture positivity (days) | 5 |
P < 0.05 (Mann-Whitney test).
TABLE 5.
Comparison of catheter data of all neonates admitted to the SCH NICU in 1995
| Catheter type | % of neonates receiving a catheter
|
P value using chi-square test | Mean duration of stay (days)
|
P value using Mann-Whitney test | ||
|---|---|---|---|---|---|---|
| Subgroupa | Control groupb | Subgroupa | Control groupb | |||
| Arterial | 50.0 | 37.4 | 0.108 | 10.7 | 5.6 | 0.273 |
| Arterial umbilical | 52.4 | 21.7 | <0.001 | 5.1 | 8.9 | 0.241 |
| Venous umbilical | 73.8 | 29.3 | <0.001 | 6.4 | 5.4 | 0.162 |
| Broviac | 4.8 | 0.7 | 0.010 | 4.5 | 42.7 | 0.200 |
| Intravenous | 38.1 | 10.9 | <0.001 | 10.9 | 9.6 | 0.316 |
n = 42.
n = 460.
TABLE 6.
Review of catheters in general at SCH NICU in 1995
| Catheter type | % (no./total no.) of:
|
|
|---|---|---|
| Neonates receiving a catheter of all neonates admitted in 1995 | Introduced catheters contaminated with CoNS | |
| Arterial | 38.4 (193/502) | 10.9 (21/193) |
| Arterial umbilical | 24.3 (122/502) | 18.0 (22/122) |
| Venous umbilical | 33.1 (166/502) | 18.7 (31/166) |
| Broviac | 1.0 (5/502) | 40.0 (2/5) |
| Intravenous | 13.1 (66/502) | 24.0 (16/66) |
DISCUSSION
CoNS are now recognized to be the most common cause of serious nosocomial infections in NICUs (23). Large-scale prevalence studies carried out in a concerted fashion in several European countries demonstrated an extremely high prevalence of nosocomial bloodstream infection by CoNS (22). The clinical data of neonates with CoNS infections in the SCH show that most of them were critically ill. Apnea, bradycardia, and temperature instability, which are strong indications of CoNS septicemia, frequently occurred. Although encountered on an occasional basis only, urine, wound fluid, and even CSF cultures demonstrating viable CoNS demonstrate that CoNS infections can cause serious invasive disease in these highly vulnerable neonates.
When studying the epidemiology of CoNS infections, it is advisable to analyze the bacterial strains by multiple typing procedures (18). It has been demonstrated on several occasions that phenotyping is less satisfactory than genotyping (4) and that with respect to genotyping, application of a single technique such as PFGE may suffice (26, 27). The PFGE results of the present study clearly show that certain strains of CoNS can become predominant in the NICU setting. PFGE genotypes B and C were responsible for two-thirds of all CoNS-positive blood cultures in the SCH NICU, while PFGE genotype AA was associated with nearly half (45%) of all cases in the WCH NICU. Such endemic strains seem to persist for prolonged periods, although they also appear to be NICU specific. The PFGE patterns of strains found in the SCH NICU were totally different from the PFGE patterns of strains found in the WCH NICU, indicating that the CoNS strains found in the two centers were not related to each other. Clonal persistence occurred in both centers, and even the persistence of multiple clusters in a single environment has recently been demonstrated (3).
Combinations of penicillins and aminoglycosides or expanded-spectrum cephalosporins and aminoglycosides are widely used as empiric antibiotic strategy in neonates. These regimens do not adequately cover infections by CoNS. This will cause selection of multiresistant CoNS strains, which, according to the current study, can become endemic in the NICU setting. The antibiograms of the prevalent strains precisely mirror the antibiotic therapy regimen executed in the departments (3). Standard therapy consists of a penicillin-tobramycin combination eventually followed by flucloxacillin and cefuroxime treatment. As stated in Results, most of the strains studied resist precisely these antibiotics. Clinical symptoms associated with infection by an endemic strain, however, do not seem to be different from those correlated with infection by nonendemic strains.
Assessment of risk factors showed that the more premature infants were at increased risk of acquiring CoNS bacteremia. Their stay in the NICU before detection of CoNS in the blood was significantly longer than the average hospital stay in the control group. This indicates that length of stay is a major risk factor for acquiring a CoNS strain that gives rise to bacteremia. This is in agreement with data of Martin et al. (15), who performed a study in the late 1980s, a period in which invasive CoNS were not as prevalent in the NICU setting as they are now.
CoNS account for a major part of nosocomial bacteremias, especially those events related to the insertion and maintenance of intravascular catheters (20). Although we did not systematically use the semiquantitative catheter tip culture technique for the diagnosis of catheter-related bacteremia (19), a catheter still remains one of the most obvious entry sites for CoNS in neonates. Previous studies indicated that the site of catheter insertion largely determines the risk for acquiring catheter-related septicemia. The subclavian site is considered favorable because of lower bacterial densities on the skin at this site (2). The fact that this skin site seems to be preferentially colonized by CoNS species may be contraindicative, although nothing is known about population densities. The present study clearly shows that insertion of a venous umbilical catheter is also a significant risk factor for the development of CoNS septicemia. Table 6 shows that 10 to 20% of all catheters become contaminated with CoNS in the end. Although continuous infusion of antibiotics such as teicoplanin or vancomycin through the catheter clearly reduces the incidence of bacteremia (21), this approach is not generally accepted due to the risk of selecting for (intermediately) vancomycin-resistant staphylococci (8).
Relatively little is known about the propensity of CoNS to induce clinically significant bacteremia. Specific virulence factors seem to play an important role (11), but general means of identification of potentially detrimental strains of CoNS have not been defined. It is interesting that the epidemic strains in the SCH seem to prefer or induce altered blood pH and pCO2 values in affected individuals. This observation warrants further analysis.
REFERENCES
- 1.Beck-Sague C M, Azimi P, Fonseca S N, Baltimore R S, Powell D A, Bland L A, Arduino M J, McAllister S K, Huberman R S, Sinkowitz R L. Bloodstream infections in neonatal intensive care unit patients: results of a multicenter study. Pediatr Infect Dis J. 1994;13:1110–1116. [PubMed] [Google Scholar]
- 2.Bertone S A, Fisher M C, Mortensen J E. Quantitative skin cultures at potential catheter sites in neonates. Infect Control Hosp Epidemiol. 1994;15:315–318. doi: 10.1086/646919. [DOI] [PubMed] [Google Scholar]
- 3.Burnie J P, Naderi-Nasab M, Loudon K W, Matthews R C. An epidemiological study of blood culture isolates of coagulase-negative staphylococci demonstrating hospital-acquired infection. J Clin Microbiol. 1997;35:1746–1750. doi: 10.1128/jcm.35.7.1746-1750.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degener J E, Heck M E O C, van Leeuwen W J, Heemskerk C, Crielaard A, Joosten P, Caesar P. Nosocomial infection by Staphylococcus haemolyticus and typing methods for epidemiological study. J Clin Microbiol. 1994;32:2260–2265. doi: 10.1128/jcm.32.9.2260-2265.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finelli L, Livengood J R, Saiman L. Surveillance of pharyngeal colonization: detection and control of serious bacterial illness in low birthweight infants. Pediatr Infect Dis J. 1994;13:854–859. [PubMed] [Google Scholar]
- 6.Goering R V. Molecular epidemiology of nosocomial infection: analysis of chromosomal restriction fragment patterns by pulsed-field gel electrophoresis. Infect Control Hosp Epidemiol. 1993;14:595–600. doi: 10.1086/646645. [DOI] [PubMed] [Google Scholar]
- 7.Hall S L. Coagulase-negative staphylococcal infections in neonates. Pediatr Infect Dis J. 1991;10:57–67. doi: 10.1097/00006454-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 9.Huebner J, Kropec A. Cross infections due to coagulase negative staphylococci in high risk patients. Zentralbl Bakteriol. 1995;283:169–174. doi: 10.1016/s0934-8840(11)80198-2. [DOI] [PubMed] [Google Scholar]
- 10.Huebner J, Pier G B, Maslow J N, Muller E, Shiro H, Parent M, Kropec A, Arbeit R D, Goldmann D A. Endemic nosocomial transmission of Staphylococcus epidermidis bacteremia isolates in a neonatal intensive care unit over 10 years. J Infect Dis. 1994;169:526–531. doi: 10.1093/infdis/169.3.526. [DOI] [PubMed] [Google Scholar]
- 11.Kacica M A, Horgan M J, Preston K E, Lepow M, Venezia R A. Relatedness of coagulase negative staphylococci causing bacteremia in low-birthweight infants. Infect Control Hosp Epidemiol. 1994;15:658–662. doi: 10.1086/646829. [DOI] [PubMed] [Google Scholar]
- 12.Khadilkar V, Tudehope D, Fraser S. A prospective study of nosocomial infection in a neonatal intensive care unit. J Pediatr Child Health. 1995;31:387–391. doi: 10.1111/j.1440-1754.1995.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 13.Low D E, Schmidt B K, Kirpalani H M, Moodie R, Kreiswirth B, Matlow A, Ford-Jones E L. An endemic strain of Staphylococcus haemolyticus colonizing and causing bacteremia in neonatal intensive care unit patients. Pediatrics. 1992;89:696–700. [PubMed] [Google Scholar]
- 14.Lyytikainen O, Valtonen V, Sivonen A, Ryhanen R, Vuopio-Varkila J. Molecular epidemiology of Staphylococcus epidermidis isolates in a hematological unit during a 4-month survey. Scand J Infect Dis. 1995;27:575–580. doi: 10.3109/00365549509047070. [DOI] [PubMed] [Google Scholar]
- 15.Martin M A, Pfaller M A, Wenzel R P. Coagulase negative staphylococcal bacteremia: mortality and hospital stay. Ann Intern Med. 1989;110:9–16. doi: 10.7326/0003-4819-110-1-9. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2nd ed. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 17.Neumeister B, Kastner S, Conrad S, Klotz G, Bartmann P. Characterization of coagulase negative staphylococci causing nosocomial infections in preterm infants. Eur J Clin Microbiol Infect Dis. 1995;14:856–863. doi: 10.1007/BF01691491. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim B A, Hartley J W, Lee W, Burnie J P. Outbreak of coagulase negative staphylococcus highly resistant to ciprofloxacin in a leukaemia unit. Br Med J. 1989;299:294–297. doi: 10.1136/bmj.299.6694.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rello J, Coll P, Prats G. Laboratory diagnosis of catheter-related bacteremia. Scand J Infect Dis. 1991;23:583–588. doi: 10.3109/00365549109105182. [DOI] [PubMed] [Google Scholar]
- 20.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 21.Spafford P S, Sinkin R A, Cox C, Reubens L, Powell K R. Prevention of central venous catheter related coagulase negative staphylococcal sepsis in neonates. J Pediatr. 1994;125:259–263. doi: 10.1016/s0022-3476(94)70208-x. [DOI] [PubMed] [Google Scholar]
- 22.Spencer R C. Predominant pathogens found in the European Prevalence of Infection in Intensive Care (EPIC) study. Eur J Clin Microbiol Infect Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- 23.St. Geme J W D, Bell L M, Baumgart S, Da C T, Harris M C. Distinguishing sepsis from blood culture contamination in young infants with blood cultures growing coagulase-negative staphylococci. Pediatrics. 1990;86:157–162. [PubMed] [Google Scholar]
- 24.Struelens M J, Bauernfeind A, van Belkum A, Blanc D, Cookson B D, Dijkshoorn L, El Solh N, Etienne J, Garaizar J, Gerner Smidt P, Legakis N, de Lencastre H, Nicolas M H, Pitt T L, Römling U, Rosdahl V, Witte W. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 25.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by PFGE: criteria for bacterial strains typing. J Clin Microbiol. 1996;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Belkum A, Kluijtmans J, van Leeuwen W, Goessens W, ter Averst E, Verbrugh H A. Investigation into the repeated recovery of coagulase negative staphylococci from blood taken at the end of cardiopulmonary by-pass. J Hosp Infect. 1995;31:285–293. doi: 10.1016/0195-6701(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 27.Van Belkum A, Kluijtmans J, van Leeuwen W, Goessens W, ter Averst E, Wielenga J, Verbrugh H A. Monitoring persistence of coagulase negative staphylococci in a hematology department using phenotypic and genotypic strategies. Infect Control Hosp Epidemiol. 1996;17:660–667. [PubMed] [Google Scholar]
- 28.Zaidi A K, Harrell L J, Rost J R, Reller L B. Assessment of similarity among coagulase-negative staphylococci from sequential blood cultures of neonates and children by pulsed-field gel electrophoresis. J Infect Dis. 1996;174:1010–1014. doi: 10.1093/infdis/174.5.1010. [DOI] [PubMed] [Google Scholar]