Abstract
Flowers are key organs in many ornamental plants, and various phases of flower development impact their economic value. The final stage of petal development is associated with flower senescence, which is an irreversible process involving programmed cell death, and premature senescence of cut flowers often results in major losses in quality during postharvest handling. Flower opening and senescence are two sequential processes. As flowers open, the stamens are exposed to attract pollinators. Once pollination occurs, flower senescence is initiated. Both the opening and senescence processes are regulated by a range of endogenous phytohormones and environmental factors. Ethylene acts as a central regulator for the ethylene-sensitive flowers. Other phytohormones, including auxin, gibberellin, cytokinin, jasmonic acid and abscisic acid, are also involved in the control of petal expansion and senescence. Water status also directly influences postharvest flower opening, while pollination is a key event in initiating the onset flower senescence. Here, we review the current understanding of flower opening and senescence, and propose future research directions, such as the study of interactions between hormonal and environmental signals, the application of new technology, and interdisciplinary research.
Keywords: Flower opening, Petal expansion, Petal senescence, Phytohormones, Environmental factors, Regulatory mechanisms
Introduction
By definition, flowers are of central importance in floral crops, and the processes of flower opening and senescence are key determinants of their ornamental quality and economic value (Srikanth and Schmid 2011). In angiosperms, flower opening is important for reproduction, often through the presentation of petals to attract animals that promote cross-pollination (van Doorn and Kamdee 2014). Once pollination is accomplished, petals complete their biological function and quickly enter the phase of senescence (Ketsa et al. 2001). Petal senescence can be affected by fruit development, which is necessary for plants to complete reproductive development. During postharvest shipping and storage, ethylene, and other harmful gases, can cause undesired opening of flowers and early senescence of petals, resulting in serious postharvest losses (Ma et al. 2005, 2006). Therefore, strategies to ensure desired flower opening and to delay petal senescence are important for optimizing postharvest flower quality and are topics of great interest in flower research.
In the current review, we summarize the molecular mechanisms known to be involved in flower opening and senescence, their regulation by phytohormones and environmental factors, and suggest future research directions.
Molecular control of postharvest flower opening
Flower opening is driven by cell division and expansion
Flower opening depends on petal expansion and movement and is a complex process, typically involving flower bud swelling, opening, bending outwards, folding, and finally development into a mature flower (Rizzo and Harampolis 2013). These floral organ movements lead to different types of flower opening, which vary between species and varieties. For instance: flower opening in poppy (P. somniferum) involves the release of mechanical constraints (Reid and Evans 1986); the loose flower structure of C. persicum is caused by petal expansion (Rolland-Lagan et al. 2003); and in Asiatic lily (Lilium), flower opening is mainly promoted by a change in the angle between the midrib and pedicel (Bieleski et al. 2000a). Differences in the rate, direction and anisotropy of cell expansion in different petal regions can all contribute to different types of flower opening (Rolland-Lagan et al. 2003). Petal expansion depends on a change in cell number and size, and flower opening is often accompanied by both cell division and cell expansion (Pei et al. 2013; van Doorn and Kamdee 2014). In rose (Rosa hybrida), flower opening driven by both cell division and cell expansion involves irreversible petal movement that is manifested in a transition from an entangled position to a horizontally expanded position (Yamada et al. 2009). In contrast, E. grandiflorum flower opening results from reversible asymmetric expansion of cells on the abaxial and adaxial side of petals (Ryo et al. 2016). Flower organ growth begins after formation of the flower primordium and all cells enter the stage of continuous division. Changes in the timing or rate of continuous cell division can result in changes in cell number, which in turn lead to changes in organ size (Gonzalez-Carranza et al. 2012; Czesnick and Lenhard 2016). Before flowers open, the petals complete organogenesis and most petal cells stop dividing, except for a limited number of the cells that maintain the capacity for division (Martin & Gerats, 1993; Huang and Irish 2016). After this time, petal expansion is mainly driven by increasing cell size (Ma et al. 2008).
Cell expansion depends on synergistic changes in cell wall metabolism, cell turgor and cytoskeletal reorganization (Zonia and Munnik 2007) and is a highly complex process. The spatially controlled synthesis, degradation, and restructuring of cell wall components results in the coordinated control of cell growth, a process that is strictly regulated by developmental and environmental factors (Cosgrove 2005). The irreversible ductility, or plastic deformation, of the cell wall is a key factor in the expansion of petal cells (Yamada et al. 2009), and to this end cell wall localized proteins, such as expansins and xyloglucan endotransglycosylase/hydrolases (XTHs), are involved in cell wall relaxation (van Sandt et al. 2007; Harada et al. 2011). Current models propose that expansins interfere with hydrogen bonds between polysaccharide molecules in the wall, thereby causing it to relax and making it more amenable to turgor-driven extension (Cosgrove 2015).
Cell turgor is the driving force for cell expansion, where a transmembrane osmotic potential gradient generated by intracellular solute accumulation causes water to move across the plasma membrane through aquaporins, and accumulate in the vacuole (Zonia and Munnik 2007). In Gentiana kochian and G. kochiana, flower opening mainly depends on the differential turgor-driven cell expansion on the two sides of the petal (Gookin et al. 2003; van Doorn and van Meeteren 2003).
Another key factor in cell expansion is remodeling of the cytoskeleton, an intracellular network composed of microtubules and microfilaments. Microtubules have been shown to contribute to the morphology of petal epidermal cells and to play a role in regulating the anisotropic shape of petals and the formation of cone cells (Panteris et al. 1994). The direction of cell expansion largely depends on the arrangement of microtubules. In the cell expansion stage, periplasmic microtubules at the outermost periphery of the protoplast are arranged perpendicular to the axis of cell elongation, and when these microtubules are arranged diagonally or longitudinally with the cell elongation direction, cell expansion is inhibited (Lloyd 2011). Thus, it is thought that microtubules control the direction of cell expansion through deposition of cellulose microfibrils, but the exact mechanism is still unclear (Wang and Jiao 2020).
Phytohormones control flower opening
Multiple phytohormones have a critical function in regulating organ development (Durbak et al. 2012) and affect flower opening through a series of sequential steps. As summarized below, it has been found that ethylene, gibberellin (GA), auxin, abscisic acid (ABA), jasmonic acid (JA) and brassinolide (BR) are all involved in flower opening.
Ethylene
Ethylene has been shown to promote flower opening in many ornamental plants, such as gladiola (Gladiolus), carnation (D. caryophyllus), petunia, orchid (Phalaenopsis), rose, wintersweet (Chimonanthus praecox), and others (Serek et al. 1994; Tang and Woodson 1996; Bui and O'Neill 1998; Jones and Woodson 1999; Ma et al. 2005; Sui et al. 2015). In the case of wintersweet, an exogenous ethylene treatment can rapidly promote flowers to reach the stage of full bloom, while exposure to 1-methylcyclopropene (1-MCP), an ethylene action inhibitor, significantly delays or inhibits flower opening, causing flowers to fail to open completely or remain partially open until wilting (Sui et al. 2015). Interestingly, ethylene can also promote flower opening in rose, but significantly reduces petal size by inhibiting petal cell elongation, suggesting that the effect of ethylene on rose flower opening may depend on the petal movement rather than the cell expansion (Ma et al. 2008).
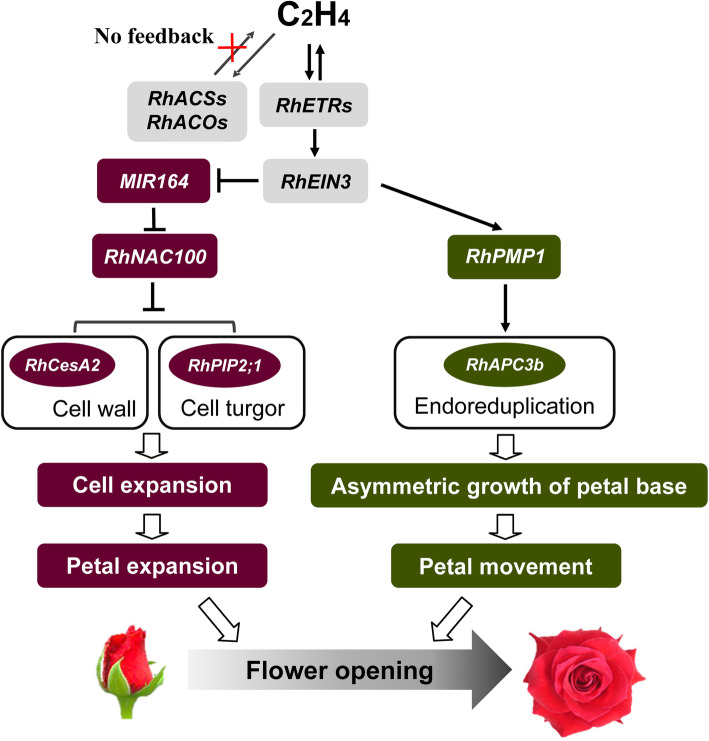
The mechanisms by which ethylene functions have been widely studied. Ethylene regulation of flower opening in rose (Fig. 1) first involves perception of ethylene rather than ethylene synthesis (Ma et al. 2005, 2006). That is to say, the effect of ethylene on rose flower opening is not through a positive feedback regulation of ethylene biosynthesis, but through up-regulation of genes encoding ethylene receptors. Of these, RhETR3 is the most important receptor gene in the mediation of ethylene signaling to promote flower opening (Ma et al. 2005, 2006, 2008; Tan et al. 2006). Among the five flower organs (sepals, petals, stamens, pistil and receptacle) in rose, the pistil is the first to respond to the ethylene signal (Ma et al. 2008). Notably, RhPIP2;1, a member of the aquaporin family, is involved in ethylene-regulated cell expansion of rose petals. Silencing of RhPIP2;1 results in a significant increase in the number of abaxial sub-epidermal (AbsE) cells in the petals, reduces the irregular shape of AbsE cells, and an obvious decrease in the length and width of the petals (Ma et al. 2008). Similarly, RhPIP1;1 is involved in the regulation of ethylene-induced petal cell expansion and its silencing results in decreased petal size and cell area, as well as reduced fresh weight (Chen et al. 2013). Another key gene involved in ethylene-regulated rose flower opening is the NAC transcription factor RhNAC100. Interestingly, it’s expression is controlled via microRNA164-dependent post-transcriptional regulation. RhNAC100 regulates petal expansion by modulating the expression of cell expansion-related genes, such as RhPIP2,1 and the cellulose synthase gene RhCesA2. Thus, ethylene regulates petal cell expansion by fine-tuning the microRNA164-RhNAC100 module, thereby promoting flower opening (Pei et al. 2013). Recently, the rose PETAL MOVEMENT-RELATED PROTEIN 1 (RhPMP1) transcription factor gene was identified as a direct target of ETHYLENE INSENSITIVE 3 (EIN3) which is a key transcription factor downstream of ethylene signaling. RhPMP1 could increase RhAPC3b expression and specifically activates cell endoreduplication in the parenchyma on the adaxial side of the petal base. The expression of cell expansion genes, such as RhBXL, RhPE and RhRCI3, was upregulated and induced asymmetric growth of the petal base. Therefore, a current model involves ethylene regulating a RhEIN3-RhPMP1-RhAPC3b transcriptional cascade to promote petal movement and flower opening (Cheng et al. 2021) (Fig. 1).
Fig. 1.
Proposed model of rose flower opening regulation by ethylene
Auxin
Auxins, such as indoleacetic acid (IAA) and naphthaleneacetic acid (NAA), promote flower opening and their exogenous application leads to an enhanced rate and angle of Iris flower opening (van Doorn et al. 2013), while treatment with auxin inhibitors has the opposite effect. Similar results have been reported for water lily (N. lotus), where treatment with an auxin inhibitor suppressed flower opening (Ke et al. 2018). During petal expansion and growth, auxin-related genes are up-regulated to promote cell elongation. Transcriptome analysis of chrysanthemum (C. morifolium) flowers showed that auxin-related genes, including signal transduction genes or transcription factors, regulate petal expansion through the TEOSINTEBRANCHED 1, CYCLOIDEA and PCF (TCP) transcription factor genes (Wang et al. 2017). Auxin response factor 8 (ARF8) was also shown to interact with BIGPETALp (BPEp) to modulate petal expansion by restricting mitotic growth in the early stages of petal development and cell expansion in the later stages (Varaud et al. 2011).
Gibberellins
The gibberellins (GA) class of phytohormones, of which there are several bioactive forms, function as promoters of flower opening in several species, including I. nil, Gerbera hybrida, and L. sinuatum (Steinitz and Cohen 1982; Raab and Koning 1987; Li et al. 2015). In G. hybrida, GA3 promotes the expansion of ray floret petals and an increase in cell size (Li et al. 2015), while in C. sativus, GA9 is naturally produced in the ovaries and moves to the sepals and petals, where it is converted into bioactive GA4, which has been shown to regulate organ growth (Lange and Lange 2016). GA concentration also increases sharply in Gaillardia petals when the corolla grows rapidly, and decreases significantly at later stages (Kening 1985).
GA regulates flower opening mainly through crosstalk with ethylene, and in rose, the ethylene-inhibited transcription factor RhNF-YC9 participates in regulating the expansion rate and size of petal cells by mediating GA synthesis. Accordingly, silencing RhNF-YC9 was observed to reduce expression of gibberellin acid biosynthetic gene RhGA20ox and increases gibberellin acid catabolic gene RhGA2ox transcripts level, resulting in a decreased rate of petal expansion (Chen et al. 2020). Crosstalk between GA and ethylene also regulates petal expansion by modulating DELLA gene expression and protein stability. Ethylene treatment induces the expression of the rose DELLA gene RhGAI1 via EIN3. Silencing of RhGAI1 promotes cell expansion in rose petals. RhGAI1 directly binds to the promotor of the cell wall cellulose synthesis gene RhCesA2 and repress its expression, thereby inhibiting flower opening (Luo et al. 2013).
Other hormones
Jasmonic acid (JA) also functions as a general promoter of flower opening, and a reduction in JA biosynthesis in A. thaliana was observed to cause delayed petal growth and flower opening (Ishiguro et al. 2001). The JA signal-insensitive tomato jai1–1 mutant has delayed flower opening (Niwa et al. 2018), and in E. grandiflorum, treatment with the JA analog methyl jasmonate (MeJA) significantly accelerates petal growth and flower opening, involving increased expression of cell wall modification associated genes (e.g. the expansin genes EgEXPA2, EgEXPA3 and the XTH gene EgXTH1) (Ochiai et al. 2013). JA has also been found to inhibit lateral tepal movement in Iris flowers and to prevent flower opening (van Doorn et al. 2013).
Finally, the brassinosteroid (BR) class of phytohormones promote flower opening, and in Gerbera, preferentially stimulate cell elongation in the middle and basal regions of petals to enhance petal growth (Huang et al. 2017).
Environmental factors influence flower opening
Plant growth and development is fundamentally affected by environmental factors, and those that are known to influence flower opening include water status, temperature, the dark-light cycle, carbohydrates and nutrients. As outlined below, each of these have been shown to adversely affect inter-related aspects of flower opening, hormone balance and cell growth (van Doorn and Kamdee 2014).
Water relations
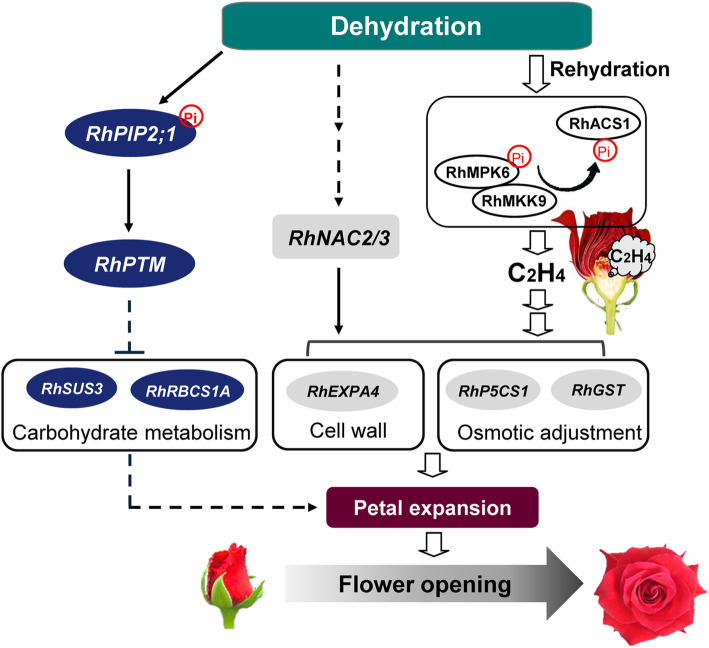
Maintaining an adequate water status is critical for ensuring high quality in open cut flowers and for prolonging their vase life. Under postharvest conditions, cut flowers often suffer from water deficit stress. Dehydration often leads to air embolism in the stem of cut flowers, which causes abnormal flower opening, petal wilting and early senescence, resulting in a loss of quality and economic value (Liu et al. 2013). Many studies have investigated the processes associated with water deficit stress and the consequent effects on flower opening and senescence at the transcriptional and post-translational levels (Fig. 2). In rose, the NAC transcription factor gene, RhNAC2, is up-regulated after dehydration stress, and its silencing reduces the recovery of intact petals and petal discs during subsequent rehydration. Biochemical and functional analyses have also shown that RhNAC2 is involved in regulating flower opening under dehydration stress by affecting cell expansion (Dai et al. 2012), and that the RhNAC2 protein can bind to the promoter of the expansin gene RhEXPA4. NAC3 may also participate in flower opening under water stress conditions and enhances the dehydration tolerance of petals by regulating the osmotic adjustment-associated genes, such as delta1-pyrroline-5-carboxylate synthase 1 (RhP5CS1) and glutathione S-transferase (RhGST) (Jiang et al. 2014). Water deficiency also increases abscisic acid (ABA) content and ethylene production in carnation flowers and shortens their longevity (Nukui et al. 2004), a process that was also observed in drought-induced senescence in daffodil (N. pseudonarcissus) (Hunter et al. 2004a) and daylily (Panavas et al. 1998). Interestingly, Fan et al. (2020) reported that a JA feedback loop mediated by an RhHB1/RhLOX4 regulatory module plays an important role in the dehydration tolerance in dehydrated rose flowers.
Fig. 2.
Proposed model of controlled postharvest rose flower opening
After a period of dehydration, rose flowers can quickly return to a normal petal expansion rate and fresh weight following the addition of water. During this rehydration phase, ethylene production increases rapidly and it is emitted as a transient burst by the gynoecium, coincident with a rapid increase in the expression of the protein kinase RhMPK6. The RhMPK6 protein phosphorylates and stabilizes RhACS1, a rate-limiting enzyme in ethylene biosynthesis. This burst in ethylene production is necessary to trigger the recovery response after a short period of rehydration. Thus, it has been proposed that the RhMPK6-RhACS1 module is central to sensing rehydration and transducing a signal to mediate ethylene regulated flower opening (Meng et al. 2014). Expression of the protein kinase RhMKK9 is also specifically and rapidly induced by rehydration in the gynoecium, and has been shown to function upstream of, and to activate, the RhMPK6-RhACS1 cascade (Chen et al. 2017) (Fig. 2). Similar results were found in a study of carnation, where removal of the gynoecium repressed the production of ethylene and delayed petal senescence (Shibuya et al. 2000).
Aquaporins play important roles in the water stress response and in maintaining water homeostasis by controlling water transport across membranes. In rose, the expression of two aquaporin genes, RhPIP1;1 and RhPIP2;1, partially inhibits petal cell expansion and affects water content during flower opening (Ma et al. 2008; Chen et al. 2013). A RhPIP2;1-RhPTM module has been found to regulate the trade-off between growth and stress (Zhang et al. 2019), and a recent study suggested that RhPIP2;1 functions as a dehydration sensor that is phosphorylated during water deficiency. This post-translational modification results in translocation of the RhPTM C terminus to the nucleus. Silencing of RhPTM greatly increases the expression of genes involved in starch and sucrose synthesis, including sucrose synthase 3 (RhSUS3) and ribulose bisphosphate carboxylase small-chain 1A (RhRBCS1A), and promotes petal expansion. A model reflecting dehydration and rehydration regulation of flower opening in rose is shown in Fig. 2.
Temperature
Different petal parts respond differently to temperature, resulting in the opening and closure movements of flowers. In Crocus, the optimum temperature required for cell expansion on the abaxial side is much lower than for the adaxial side. In a low-temperature environment, the growth rate of the abaxial side is greater than that of the adaxial side, resulting in petal closure and abnormal flower opening (Wood 1953). From an evolutionary perspective, differences in ambient temperature requirements for cell expansion on the adaxial and abaxial of petals is an adaptive mechanism that enables flowers to close petals when facing harsh conditions, such as cooling, precipitation and strong winds. Petal closure can protect the pistil and stamens to improve the rate of pollination (Abdusalam and Tan 2014; Franchi et al. 2014).
Temperature changes can cause petal movement by affecting petal cell turgor. In tulips (T. gesneriana), when the ambient temperature rises from 5 °C to 20 °C substantial amounts of water enter the petals and the flower rapidly opens; conversely, when ambient temperature drops returns to 5 °C, water flows out of the petals and the flowers close. The temperature-dependent opening and closure movement of tulip petals can be regulated by reversible phosphorylation of a plasma membrane aquaporin (PM-AQP) (Azad et al. 2004), which leads to an activate water channel composed of PM-AQP subunits. In contrast, in a low-temperature environment (5 °C), dephosphorylation of PM-AQP causes the inactivation of this water channel. Treatment with a calcium ion chelator and calcium ion channel blocker was reported to inhibit flower opening caused by higher temperature, indicating that calcium-dependent protein kinases (CDPKs) are involved in regulating water transport and flower opening and closure by phosphorylating PM-AQP proteins (Azad et al. 2004, 2007, 2008).
Dark-light cycle
Many ornamental flowering plants, such as E. grandiflorum, N. tetragona, T. cordata, E. oxypetalum, Nymphaea and Hemerocallis, show a diurnal rhythm of oscillating flower opening and closure (Bai and Saneyuki 2015; Ren et al. 2019). Time-lapse photography has been used to record flower opening in many species and it has been shown, in species such as morning glory (I. nil), Asiatic Lily (Lilium hybrida), and E. grandiflorum, that the time of flower opening is closely synchronized with the dark-light cycle (Bieleski et al. 2000b; Shinozaki et al. 2014; Bai and Saneyuki 2015). Other studies have indicated that flower opening is independent of light conditions, but is instead regulated by an internal circadian clock (Yon et al. 2016). In Nicotiana attenuate, silencing of the Late Elongated Hypocotyl (LHY) and ZEITLUPE (ZTL) genes which are known to be core clock components alters the internal rhythm, resulting in corolla opening and flower movement (Yon et al. 2016).
Carbohydrates and nutrients
Carbohydrate content is another environmental factor influence flower opening. In most plants, carbohydrates required to drive flower opening may derive from stored and/or imported carbohydrates (van Doorn and van Meeteren 2003). Young petals in some species, such as Lilium (Bieleski et al. 2000a) and Alstroemeria (Collier 1997), contain a large quantity of starch which is rapidly converted to glucose and fructose when flower opening. To date, most studies on the effects of carbohydrates on flower opening are at the physiological level, while the molecular mechanisms are rarely reported.
Nutrient status also affects postharvest flower opening. Among all the macroelements in nutrient solutions, calcium has the most beneficial effect on the retention of flower quality (Torre et al. 2001; Banijamali et al. 2018). In rose, CaCl2 treatment promoted bud opening and extended longevity (Halevy et al. 2001). Cytosolic calcium is considered to be second messenger in regulation of important cellular events, but the regulatory mechanism of calcium signaling on flower opening is still unclear. Therefore, molecular mechanism of nutrients influencing flower opening still needs further study.
Regulatory mechanisms underlying flower senescence
Flower senescence is an irreversible, highly coordinated process
Flower senescence generally refers to the process of programmed cell death that is associated with loss of petal function, and can be divided into two types: wilting senescence and abscission senescence. Flowers are a mixture of vegetative and reproductive organs. The main flower organs, the petals, are not directly involved in reproduction, but their function is to attract pollinators. Petals remain a ‘sink’ until fertilization and fruit set, then during senescence catabolic processes are activated and the petals convert into a ‘source’, although their nutrient recycling rate is much lower than that of leaves (Jones 2013). Petal senescence is also accompanied by orderly disintegration of internal cell structure, degradation of macromolecules and membrane systems, and recycling of various compounds. It is an irreversible process with several features that are unique to petals: 1) there are almost no chloroplasts in petal cells, while most contain chromoplasts; 2) within the petal metabolome there are low levels of few energy storage or transport compounds, such as carbohydrates, and most are secondary metabolites such as anthocyanins, carotenoids, and volatile components; 3) sugar levels in petals usually continuously decrease during senescence (van Doorn and Kamdee 2014). As is the case in fruit, petal senescence can broadly be divided into two types: ethylene sensitive and ethylene insensitive (Rogers 2013).
Senescence is the terminal stage of petal development and involves color change, loss of fragrance, wilting and shedding (Fischer 2012). Petal color in most flowering species fades during senescence, although in some the petals become blue (Schmitzer et al. 2010). In rose, flavonoid and anthocyanin levels increase in petals during senescence, and consequently the petal color develops to a deep blue shade (Lv et al. 2014). Unlike changes in morphology and color, floral fragrance in some species is not lost at the onset of senescence and volatiles emission continues for a period (Paul et al. 2019). This may be an advantage in prolonging the attraction of pollinators to other flowers of the same species.
Phytohormones orchestrate flower senescence
Ethylene
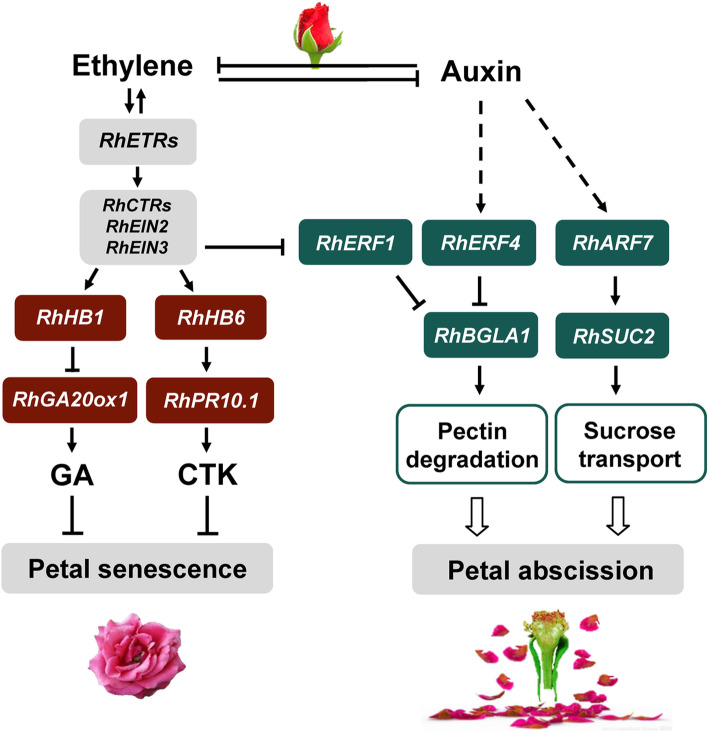
Ethylene is regarded as a centrally important phytohormone in the regulation of flower senescence, and has been shown to accelerate flower senescence in many ethylene sensitive ornamental plants (Ma et al. 2005; Ichimura et al. 2009). In most ethylene sensitive flowers, such as petunia and carnation, petal senescence is also accompanied by a rapid increase in ethylene production, which is similar to the positive feedback regulation of ethylene biosynthesis in climacteric fruits (Wang et al. 2018; Shibuya et al. 2002). However, in some ethylene sensitive flowers, such as rose and peony (P. suffruticosa), ethylene can promote the senescence of petals, but there is no typical positive feedback regulation of ethylene biosynthesis (Ma et al. 2005). Ethylene production in the stigma is thought to trigger the expression of ethylene biosynthetic genes, 1-aminocyclopropane-1-carboxylic acid synthesis genes (ACSs) and ACC oxidase genes (ACOs), in the onset of flower senescence (Ma et al. 2018). Ethylene can promote petal senescence through the induction of RhETR3 and the signal transduction RhCTR genes (Ma et al. 2005, 2006; Tan et al. 2006) (Fig. 3). Thus, there may be different modes of ethylene response and regulation in ethylene sensitive flowers. Regarding ethylene insensitive flowers, little is known about the role of ethylene in them, and more studies are needed to elucidate ethylene independent pathways (Reid and Jiang 2012).
Fig. 3.
Hormonal crosstalk during rose flower senescence
Auxin
Auxins have been found to delay senescence of some cut flowers, such as carnation and petunia (Halevy and Mayak 1981). Interestingly, in carnation the expression of Auxin/indole-3-acetic acid (Aux/IAA) transcription factor genes increases briefly in senescing petals, while the opposite is seen in M. jalapa (Xu et al. 2007; Price et al. 2008). Furthermore, the levels of endogenous auxins do not change during flower senescence in L. longiflorum, Ranunculus, Ipomoea and Hemerocallis flowers (Lombardi et al. 2015). Auxin is also associated with abscission-type of senescence. In rose, an Aux/IAA gene, RhIAA16, is upregulated in response to petal shedding and down-regulation of RhIAA16 accelerates petal abscission (Gao et al. 2016). The regulation of petal abscission is complex and involves a balance between auxin and ethylene signaling pathways. Expression of RhERF1 is inhibited by ethylene whereas RhERF4 is induced by auxin, and both RhERF1 and RhERF4 directly regulate expression of β-GALACTOSIDASE 1 (RhBGLA1), which encodes a pectin-metabolizing enzyme. A reduction in RhBGLA1 expression results in less pectin degradation and delayed petal abscission (Gao et al. 2019) (Fig. 3). A recent study found that auxin regulates the gene encoding the signaling protein RhARF7, which can bind to the promoter of the RhSUC2 sucrose transporter. Silencing of RhSUC2 or/and RhARF7 results in a reduction in sucrose levels in petals and promotes petal abscission. Fig. 3 shows the regulation of auxin-modulated sucrose transport during petal abscission by the RhAFR7-RhSUC2 module (Liang et al. 2020).
Cytokinin (CTK)
CTK is plays a critical role in delaying leaf senescence (Zwack et al. 2013), and the exogenous application of active CTKs is a widely used horticultural practice to delay plant senescence. In dahlia (Dahlia hybrida), treatment with the synthetic CTK 6-benzylaminopurine (BA) extends the vase life of cut flowers (Shimizu-Yumoto et al. 2020), and in Lilium, exogenous CTK can prolong flower life, but only if applied at the beginning of flower opening, since sensitivity to CTK gradually decreases after flower opening (Cubría-Radío et al. 2016). A CTK analogue thidiazuron dramatically extends flower longevity in ethylene insensitive Iris (Macnish et al. 2010). In rose, petunia and carnation, the CTK level is negatively correlated with the flower senescence process (Taverner et al. 1999; Chang et al. 2003; Wu et al. 2017). Recently, it was found that a microRNA inhibits CTK biosynthesis by downregulating expression of isopentenyl transferase (IPT) genes to promote leaf senescence (Zhang et al. 2020). Overexpression of IPT, driven by the SAG12 promoter, in petunia led to increased CTK levels in petals and extended flower longevity (Chang et al. 2003). In rose, silencing of RhPR10.1 resulted in lower CTK content, downregulation of the expression of three CTK signaling pathway genes, and accelerated petal senescence. The RhHB6-RhPR10.1 regulatory module antagonizes ethylene induced flower senescence by regulating the CTK content (Wu et al. 2017) (Fig. 3).
Other hormones
GA has anti-senescence effects and its exogenous application prolongs the vase life of cut flowers such as Hemerocallis and Iris (Hunter et al. 2004b; van Doorn and Woltering 2008). In addition, GA delays flower senescence in carnation, depending on the stage of flower development (Saks et al. 1992). Crosstalk between GA and ethylene can also affect flower senescence: in carnation, for example, GA can delay flower senescence through modifying the release of ethylene (Saks et al. 1992). In rose, the protein encoded by the ethylene-induced gene RhHB1 inhibits the expression of a key GA synthesis enzyme, RhGA20ox1, thereby reducing GA levels and promoting petal senescence (Lv et al. 2014) (Fig. 3).
Exogenous ABA treatments have been shown to accelerate flower senescence in some plants, including Hemerocallis and Gladiolus grandiflora (Panavas et al. 1998; Kumar et al. 2013), and in ethylene-sensitive flowers, such as carnation, ABA promotes petal senescence and wilting by increasing ethylene production (Mayak and Dilley 1976). The accumulation of ABA can be inhibited by blocking ethylene signal transduction during petal senescence (Ronen and Mayak 1981) and, conversely, ethylene-induced PhHD-ZIP expression induces the expression of ABA biosynthetic genes and promotes petal senescence in petunia (Chang et al. 2014).
Pollination is a key factor influencing flower senescence
Pollination results in a series of physiological and biochemical changes that often cause petal senescence. In many ethylene-sensitive flowering plants, including petunia, Eustoma, carnation, and orchid, pollination is accompanied by an increase in ethylene production, and there is also a burst in ethylene synthesis shortly after fertilization (Larsen et al. 1995; Halevy 1998; Xu and Hanson 2000). Pollination can obviously shorten the vase life of cut Eustoma (Shimizu-Yumoto and Ichimura 2006). In carnation, the ethylene precursor ACC produced from the pollinated stigma is translocated, via the style and ovary, to the petals. There, it up-regulates the expression of ethylene biosynthetic genes and induces the production of ethylene, accelerating petal senescence (ten Have and Woltering 1997). In gentian (Gentiana scabra), pollination significantly increases ethylene production of gynoecium, stamens and petals and promotes petal senescence (Shimizu-Yumoto and Ichimura 2012). Besides, pollination induces autophagy in petals via ethylene. In petunia, pollination induces the expression of autophagy-related gene 8 (ATG8) which is regulated by ethylene (Shibuya et al. 2013). Silencing of autophagy genes, PhATG6 and Phosphoinositide 3-Kinase (PhPI3K), accelerates petal senescence in petunia, thereby reducing flower longevity (Lin and Jones 2021).
Perspectives
The opening and senescence of flowers have evolved in angiosperms to enhance survival and reproduction. Flower opening, as a result of coordinated movements between flower organs, exposes stamens to attract pollinators and complete pollination (van Doorn and van Meeteren 2003). Many studies have shown that pollination can activate or promote petal senescence in some species, and this process is regulated by developmental signals. Thus, flower opening and senescence are closely linked during plant development (Stead 1992; Xu and Hanson 2000).
Given the diversity of flower morphology, it is not surprising that flower opening mechanisms also vary. However, at present much remains to be learned about the specific regulatory events involved. Petal movement is mainly driven by differences in cell expansion in different parts of the petal (Liang and Mahadevan 2011). The external environment can cause a series of changes in endogenous signals within the flower, and much research has focused on the influence of environmental factors and phytohormones on the asymmetric growth driving petal movement. A promising area of future research is the nature of the interactions between environmental factors and phytohormones. For example, is there a signal cascade amplification process that stimulates hormone levels or other regulatory factors, and do the signals triggered by the external environment and hormones act synergistically?
Another research hotspot is the initiation of petal senescence. Some studies have suggested that endogenous developmental signals and the precise balance between hormone synthesis and induction during development regulate senescence initiation, which may also involve epigenetic modifications (Woo et al. 2019). To elucidate the regulatory mechanisms of petal senescence initiation, potentially important approaches include: an investigation of petal senescence mechanisms initiated by developmental signals; the characterization of the effect of changes in hormone levels on the initiation of petal senescence; and the identification of coordinators that integrate developmental and hormone signals during petal senescence. Two other important areas also should be revealed: what are molecular regulatory mechanisms underlying the differences between 1) wilting and abscission types, and 2) ethylene sensitive and insensitive types.
Gene editing technology has emerged as a rapid and effective tool for modifying plant processes and the use of CRISPR/Cas9 technology will undoubtedly become widely used in plant molecular breeding research and functional verification of genes (Schindele et al. 2020; Wei et al. 2020). The advantage of the CRISPR technology is that it can generate true single gene knockout mutants or mutants in multiple genes, both of which can help elucidate the complex regulatory networks involved in flower opening and senescence. Flower development including opening and senescence sits at the intersection of esthetic and commercial spheres of society and the physical and life science fields. Multiple disciplines and technologies, including imaging, computer simulation and fluorescence tracking will all contribute to the development of models of flower development in different species and a greater understanding of this dynamic process.
Acknowledgments
We thank PlantScribe (www.plantscribe.com) for careful editing of this article.
Authors’ contributions
XS, NM and JG designed the framework of the manuscript. MQ, QY, ZH, YX, and YL prepared the initiated materials of the manuscript. XS and JG are major contributors in writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31730079, 31902059) and Science and Technology Program of Yunnan Province (Grant No. 202102AE090001).
Declaration
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdusalam A, Tan DY. Contribution of temporal floral closure to reproductive success of the spring-flowering Tulipa iliensis. J Syst Evol. 2014;52(2):186–194. doi: 10.1111/jse.12036. [DOI] [Google Scholar]
- Azad A, Sawa Y, Ishikawa T, Shibata H. Temperature-dependent stomatal movement in tulip petals controls water transpiration during flower opening and closing. Ann Appl Biol. 2007;150(1):81–87. doi: 10.1111/j.1744-7348.2006.00111.x. [DOI] [Google Scholar]
- Azad AK, Katsuhara M, Sawa Y, Ishikawa T, Shibata H. Characterization of four plasma membrane aquaporins in tulip petals: a putative homolog is regulated by phosphorylation. Plant Cell Physiol. 2008;49(8):1196–1208. doi: 10.1093/pcp/pcn095. [DOI] [PubMed] [Google Scholar]
- Azad AK, Sawa Y, Ishikawa T, Shibata H. Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol. 2004;45(5):608–617. doi: 10.1093/pcp/pch069. [DOI] [PubMed] [Google Scholar]
- Bai J, Saneyuki K. Regulation of diurnal rhythms of flower opening and closure by light cycles, wavelength, and intensity in Eustoma grandiflorum. Horticult J. 2015;84(2):148–155. doi: 10.2503/hortj.MI-019. [DOI] [Google Scholar]
- Banijamali SM, Feizian M, Bayat H, Mirzaei S. Effects of nitrogen forms and calcium amounts on growth and elemental concentration in Rosa hybrida cv. ‘Vendentta’. J Plant Nutr. 2018;41(9):1205–1213. doi: 10.1080/01904167.2018.1443127. [DOI] [Google Scholar]
- Bieleski R, Elgar J, Heyes J. Mechanical aspects of rapid flower opening in Asiatic lily. Ann Bot. 2000;86(6):1175–1183. doi: 10.1006/anbo.2000.1291. [DOI] [Google Scholar]
- Bieleski R, Elgar J, Heyes J, Woolf A. Flower opening in Asiatic lily is a rapid process controlled by dark-light cycling. Ann Bot. 2000;86(6):1169–1174. doi: 10.1006/anbo.2000.1289. [DOI] [Google Scholar]
- Bui AQ, O'Neill SD. Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol. 1998;116(1):419–428. doi: 10.1104/pp.116.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Jones ML, Banowetz GM, Clark DG. Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol. 2003;132(4):2174–2183. doi: 10.1104/pp.103.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Donnelly L, Sun D, Rao J, Reid MS, Jiang C-Z. A petunia homeodomain-leucine zipper protein, PhHD-Zip, plays an important role in flower senescence. PLoS One. 2014;9(2):e88320. doi: 10.1371/journal.pone.0088320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Hussain N, Wang YR, Li MT, Liu L, Qin MZ, Ma N, Gao J, Sun X. An ethylene-inhibited NF-YC transcription factor RhNF-YC9 regulates petal expansion in rose. Hortic Plant J. 2020;6(6):419–427. doi: 10.1016/j.hpj.2020.11.007. [DOI] [Google Scholar]
- Chen JW, Zhang Q, Wang QG, Feng M, Li Y, Meng YL, et al. RhMKK9, a rose MAP KINASE KINASE gene, is involved in rehydration-triggered ethylene production in rose gynoecia. BMC Plant Biol. 2017;17:51. 10.1186/s12870-017-0999-1. [DOI] [PMC free article] [PubMed]
- Chen W, Yin X, Wang L, Tian J, Yang R, Liu D, Yu Z, Ma N, Gao J. Involvement of rose aquaporin RhPIP1;1 in ethylene-regulated petal expansion through interaction with RhPIP2;1. Plant Mol Biol. 2013;83(3):219–233. doi: 10.1007/s11103-013-0084-6. [DOI] [PubMed] [Google Scholar]
- Cheng C, Yu Q, Wang Y, Wang H, Dong Y, Ji Y, Zhou X, Li Y, Jiang CZ, Gan SS, Zhao L, Fei Z, Gao J, Ma N. Ethylene-regulated asymmetric growth of the petal base promotes flower opening in rose (Rosa hybrida) Plant Cell. 2021;33(4):1229–1251. doi: 10.1093/plcell/koab031. [DOI] [PubMed] [Google Scholar]
- Collier DE. Changes in respiration, protein and carbohydrates of tulip tepals and Alstroemeria petals during development. J Plant Physiol. 1997;150(4):446–451. doi: 10.1016/S0176-1617(97)80096-X. [DOI] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6(11):850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol. 2015;25:162–172. doi: 10.1016/j.pbi.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubría-Radío M, Arrom L, Puig S, Munné-Bosch S. Hormonal sensitivity decreases during the progression of flower senescence in Lilium longiflorum. J Plant Growth Regul. 2016;36:402–412. doi: 10.1007/s00344-016-9648-4. [DOI] [Google Scholar]
- Czesnick H, Lenhard M. Antagonistic control of flowering time by functionally specialized poly(A) polymerases in Arabidopsis thaliana. Plant J. 2016;88(4):570–583. doi: 10.1111/tpj.13280. [DOI] [PubMed] [Google Scholar]
- Dai F, Zhang C, Jiang X, Kang M, Yin X, Lu P, et al. RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 2012;160(4):2064–2082. doi: 10.1104/pp.112.207720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbak A, Yao H, McSteen P. Hormone signaling in plant development. Curr Opin Plant Biol. 2012;15(1):92–96. doi: 10.1016/j.pbi.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu J, Zou J, Zhang X, Jiang L, Liu K, Lü P, Gao J, Zhang C. The RhHB1/RhLOX4 module affects the dehydration tolerance of rose flowers (Rosa hybrida) by fine-tuning jasmonic acid levels. Hortic Res. 2020;7(1):74. doi: 10.1038/s41438-020-0299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AM. The complex regulation of senescence. Crit Rev Plant Sci. 2012;31(2):124–147. doi: 10.1080/07352689.2011.616065. [DOI] [Google Scholar]
- Franchi G, Nepi M, Pacini E. Is flower/corolla closure linked to decrease in viability of desiccation-sensitive pollen? Facts and hypotheses: a review of current literature with the support of some new experimental data. Plant Syst Evol. 2014;300(4):577–584. doi: 10.1007/s00606-013-0911-x. [DOI] [Google Scholar]
- Gao Y, Liu C, Li X, Xu H, Liang Y, Ma N, et al. Transcriptome profiling of petal abscission zone and functional analysis of an Aux/IAA family gene RhIAA16 involved in petal shedding in rose. Front Plant Sci. 2016;7:1375. doi: 10.3389/fpls.2016.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Liu Y, Liang Y, Lu J, Jiang C, Fei Z, Jiang CZ, Ma C, Gao J 2019. Rosa hybrida RhERF1 and RhERF4 mediate ethylene-and auxin-regulated petal abscission by influencing pectin degradation. Plant J 99: 1159–1171, DOI: 10.1111/tpj.14412. [DOI] [PubMed]
- Gonzalez-Carranza ZH, Shahid AA, Zhang L, Liu Y, Ninsuwan U, Roberts JA. A novel approach to dissect the abscission process in Arabidopsis. Plant Physiol. 2012;160(3):1342–1356. doi: 10.1104/pp.112.205955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gookin TE, Hunter DA, Reid MS. Temporal analysis of alpha and beta-expansin expression during floral opening and senescence. Plant Sci. 2003;164(5):769–781. doi: 10.1016/S0168-9452(03)00063-3. [DOI] [Google Scholar]
- Halevy A, Mayak S. Senescence and postharvest physiology of cut flower- part 2. Hortic Sci. 1981;3:59–143. [Google Scholar]
- Halevy AH. Recent advance in postharvest physiology of flowers. Korean J Hortic Sci. 1998;16:49. [Google Scholar]
- Halevy AH, Torre S, Borochov A, Porat R, Philosoph-Hadas S, Meir S, et al. Calcium in regulation of postharvest life of flowers. Acta Horti. 2001;543:345–351. doi: 10.17660/ActaHortic.2001.543.42. [DOI] [Google Scholar]
- Harada T, Torii Y, Morita S, Onodera R, Hara Y, Yokoyama R, Nishitani K, Satoh S. Cloning, characterization, and expression of xyloglucan endotransglucosylase/hydrolase and expansin genes associated with petal growth and development during carnation flower opening. J Exp Bot. 2011;62(2):815–823. doi: 10.1093/jxb/erq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Han M, Yao W, Wang Y. Transcriptome analysis reveals the regulation of brassinosteroids on petal growth in Gerbera hybrida. PeerJ. 2017;5:e3382. doi: 10.7717/peerj.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Irish VF. Gene networks controlling petal organogenesis. J Exp Bot. 2016;67(1):61–68. doi: 10.1093/jxb/erv444. [DOI] [PubMed] [Google Scholar]
- Hunter DA, Ferrante A, Vernieri P, Reid MS. Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus ‘Dutch Master’) Physiol Plant. 2004;121(2):313–321. doi: 10.1111/j.0031-9317.2004.0311.x. [DOI] [PubMed] [Google Scholar]
- Hunter DA, Yi M, Xu X, Reid MS. Role of ethylene in perianth senescence of daffodil (Narcissus pseudonarcissus L. ‘Dutch master’) Postharvest Biol Technol. 2004;32(3):269–280. doi: 10.1016/j.postharvbio.2003.11.013. [DOI] [Google Scholar]
- Ichimura K, Shimizu-Yumoto H, Goto R. Ethylene production by gynoecium and receptacle is associated with sepal abscission in cut Delphinium flowers. Postharvest Biol Technol. 2009;52(3):267–272. doi: 10.1016/j.postharvbio.2008.12.008. [DOI] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther DEHISCENCE, and flower opening in Arabidopsis. Plant Cell. 2001;13(10):2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang C, Lv P, Jiang G, Liu X, Dai F, et al. RhNAC3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol J. 2014;12(1):38–48. doi: 10.1111/pbi.12114. [DOI] [PubMed] [Google Scholar]
- Jones ML. Mineral nutrient remobilization during corolla senescence in ethylene-sensitive and -insensitive flowers. AoB Plants. 2013;5:plt023. doi: 10.1093/aobpla/plt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Woodson WR. Differential expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in carnation. Plant Physiol. 1999;119(2):755–764. doi: 10.1104/pp.119.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke M, Gao Z, Chen J, Qiu Y, Zhang L, Chen X. Auxin controls circadian flower opening and closure in the waterlily. BMC Plant Biol. 2018;18(1):143. doi: 10.1186/s12870-018-1357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kening . Gaillardia. In: Halevy AH, editor. Handbook of flowering. Boca Raton: CRC, Press; 1985. pp. 117–126. [Google Scholar]
- Ketsa S, Bunya-atichart K, van Doorn WG. Ethylene production and post-pollination development in dendrobium flowers treated with foreign pollen. Funct Plant Biol. 2001;28(5):409–415. doi: 10.1071/PP00048. [DOI] [Google Scholar]
- Kumar M, Singh VP, Arora A, Singh N. The role of abscisic acid (ABA) in ethylene insensitive Gladiolus (Gladiolus grandiflora Hort.) flower senescence. Acta Physiol Plant. 2013;36:151–159. doi: 10.1007/s11738-013-1395-6. [DOI] [Google Scholar]
- Lange MJP, Lange T. Ovary-derived precursor gibberellin A9 is essential for female flower development in cucumber. Development. 2016;143:4425–4429. doi: 10.1242/dev.135947. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Ashworth EN, Jones ML, Woodson WR. Pollination-induced ethylene in carnation (role of pollen tube growth and sexual compatibility) Plant Physiol. 1995;108(4):1405–1412. doi: 10.1104/pp.108.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang W, Zhang L, Li N, Peng J, Wang Y, et al. Transcriptomic insights into antagonistic effects of gibberellin and abscisic acid on petal growth in Gerbera hybrida. Front Plant Sci. 2015;6:168. doi: 10.3389/fpls.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Mahadevan L. Growth, geometry, and mechanics of a blooming lily. Proc Natl Acad Sci U S A. 2011;108(14):5516–5521. doi: 10.1073/pnas.1007808108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Jiang C, Liu Y, Gao Y, Lu J, Aiwaili P, Fei Z, Jiang CZ, Hong B, Ma C, Gao J. Auxin regulates sucrose transport to repress petal abscission in rose (Rosa hybrida) Plant Cell. 2020;32(11):3485–3499. doi: 10.1105/tpc.19.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YY, Jones ML. Silencing ATG6 and PI3K accelerates petal senescence and reduces flower number and shoot biomass in petunia. Plant Sci. 2021;302:110713. doi: 10.1016/j.plantsci.2020.110713. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu X, Meng Y, Sun C, Tang H, Jiang Y, Khan MA, Xue J, Ma N, Gao J. An organ-specific role for ethylene in rose petal expansion during dehydration and rehydration. J Exp Bot. 2013;64(8):2333–2344. doi: 10.1093/jxb/ert092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C. Dynamic microtubules and the texture of plant cell walls. Int Rev Cell Mol Biol. 2011;287:287–329. doi: 10.1016/B978-0-12-386043-9.00007-4. [DOI] [PubMed] [Google Scholar]
- Lombardi L, Arrom L, Mariotti L, Battelli R, Picciarelli P, Kille P, Stead T, Munné-Bosch S, Rogers HJ. Auxin involvement in tepal senescence and abscission in Lilium: a tale of two lilies. J Exp Bot. 2015;66(3):945–956. doi: 10.1093/jxb/eru451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Ma N, Pei H, Chen J, Li J, Gao J. A DELLA gene, RhGAI1, is a direct target of EIN3 and mediates ethylene-regulated rose petal cell expansion via repressing the expression of RhCesA2. J Exp Bot. 2013;64(16):5075–5084. doi: 10.1093/jxb/ert296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv P, Zhang C, Liu J, Liu X, Jiang G, Jiang X, et al. RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J. 2014;78:578–590. doi: 10.1111/tpj.12494. [DOI] [PubMed] [Google Scholar]
- Ma N, Cai L, Lu W, Tan H, Gao J. Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Sci China C Life Sci. 2005;48(5):434–444. doi: 10.1360/062004-37. [DOI] [PubMed] [Google Scholar]
- Ma N, Ma C, Liu Y, Shahid MO, Wang CP, Gao JP. Petal senescence: a hormone view. J Exp Bot. 2018;69(4):719–732. doi: 10.1093/jxb/ery009. [DOI] [PubMed] [Google Scholar]
- Ma N, Tan H, Liu X, Xue J, Li Y, Gao J. Transcriptional regulation of ethylene receptor and CTR genes involved in ethylene-induced flower opening in cut rose (Rosa hybrida) cv. Samantha. J Exp Bot. 2006;57(11):2763–2773. doi: 10.1093/jxb/erl033. [DOI] [PubMed] [Google Scholar]
- Ma N, Xue J, Li Y, Liu X, Dai F, Jia W, Luo Y, Gao J 2008. Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol 148: 894–907, DOI: 10.1104/pp.108.120154. [DOI] [PMC free article] [PubMed]
- Macnish AJ, Jiang CZ, Reid MS. Treatment with thidiazuron improves opening and vase life of iris flowers. Postharvest Biol Technol. 2010;56(1):77–84. doi: 10.1016/j.postharvbio.2009.11.011. [DOI] [Google Scholar]
- Martin C, Gerats T. Control of pigment biosynthesis genes during petal development. Plant Cell. 1993;5(10):1253–1264. doi: 10.2307/3869778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S, Dilley DR. Regulation of senescence in carnation (Dianthus caryophyllus): effect of abscisic acid and carbon dioxide on ethylene production. Plant Physiol. 1976;58(5):663–665. doi: 10.1104/pp.58.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Ma N, Zhang Q, You Q, Li N, Ali Khan M, Liu X, Wu L, Su Z, Gao J. Precise spatio-temporal modulation of ACC synthase by MPK6 cascade mediates the response of rose flowers to rehydration. Plant J. 2014;79(6):941–950. doi: 10.1111/tpj.12594. [DOI] [PubMed] [Google Scholar]
- Niwa T, Suzuki T, Takebayashi Y, Ishiguro R, Higashiyama T, Sakakibara H, Ishiguro S. Jasmonic acid facilitates flower opening and floral organ development through the upregulated expression of SIMYB21 transcription factor in tomato. Biosci Biotechnol Biochem. 2018;82(2):292–303. doi: 10.1080/09168451.2017.1422107. [DOI] [PubMed] [Google Scholar]
- Nukui H, Kudo S, Yamashita A, Satoh S. Repressed ethylene production in the gynoecium of long-lasting flowers of the carnation ‘white candle’: role of the gynoecium in carnation flower senescence. J Exp Bot. 2004;55(397):641–650. doi: 10.1093/jxb/erh081. [DOI] [PubMed] [Google Scholar]
- Ochiai M, Matsumoto S, Yamada K. Methyl jasmonate treatment promotes flower opening of cut Eustoma by inducing cell wall loosening proteins in petals. Postharvest Biol Technol. 2013;82:1–5. doi: 10.1016/j.postharvbio.2013.02.018. [DOI] [Google Scholar]
- Panavas T, Reid PD, Rubinstein B. Programmed cell death of daylily petals: activities of wall-based enzymes and effects of heat shock. Plant Physiol Biochem. 1998;36(5):379–388. doi: 10.1016/S0981-9428(98)80079-X. [DOI] [Google Scholar]
- Panteris E, Apostolakos P, Galatis B. Sinuous ordinary epidermal-cells: behind several patterns of waviness, a common morphogenetic mechanism. New Phytol. 1994;127(4):771–780. doi: 10.1111/j.1469-8137.1994.tb02981.x. [DOI] [PubMed] [Google Scholar]
- Paul I, Chatterjee A, Maiti S, Bhadoria PBS, Mitra A. Dynamic trajectories of volatile and non-volatile specialised metabolites in ‘overnight’ fragrant flowers of Murraya paniculata. Plant Biol. 2019;21(5):899–910. doi: 10.1111/plb.12983. [DOI] [PubMed] [Google Scholar]
- Pei H, Ma N, Tian J, Luo J, Chen J, Li J, Zheng Y, Chen X, Fei Z, Gao J. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol. 2013;163(2):775–791. doi: 10.1104/pp.113.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AM, Aros Orellana DF, Salleh FM, Stevens R, Acock R, Buchanan-Wollaston V, Stead AD, Rogers HJ. A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol. 2008;147(4):1898–1912. doi: 10.1104/pp.108.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab MM, Koning RE. Interacting roles of gibberellin and ethylene in corolla expansion of Ipomoea nil (Convolvulaceae) Am J Bot. 1987;74(6):921–927. doi: 10.1002/j.1537-2197.1987.tb08696.x. [DOI] [Google Scholar]
- Reid MS, Evans RY. Control of cut flower opening. Acta Hortic. 1986;181:45–54. doi: 10.17660/ActaHortic.1986.181.4. [DOI] [Google Scholar]
- Reid MS, Jiang C-Z. Postharvest biology and technology of cut flowers and potted plants. Horti Rev. 2012. 10.1002/9781118351871.ch1.
- Ren Y, Gao YK, Gao SY, Yuan L, Wang XQ, Zhang QX. Genetic characteristics of circadian flowering rhythm in Hemerocallis. Sci Hortic. 2019;250:19–26. doi: 10.1016/j.scienta.2019.01.052. [DOI] [Google Scholar]
- Rizzo J, Harampolis A. The flower recipe book. (Artisan) 2013. [Google Scholar]
- Rogers HJ. From models to ornamentals: how is flower senescence regulated? Plant Mol Biol. 2013;82(6):563–574. doi: 10.1007/s11103-012-9968-0. [DOI] [PubMed] [Google Scholar]
- Rolland-Lagan AG, Bangham JA, Coen E. Growth dynamics underlying petal shape and asymmetry. Nature. 2003;422(6928):161–163. doi: 10.1038/nature01443. [DOI] [PubMed] [Google Scholar]
- Ronen M, Mayak S. Interrelationship between abscisic-acid and ethylene in the control of senescence processes in carnation flowers. J Exp Bot. 1981;32(4):759–765. doi: 10.1093/jxb/32.4.759. [DOI] [Google Scholar]
- Ryo N, Takehiko S, Kazuo I. Cell division and expansion in petals during flower development and opening in Eustoma grandiflorum. Horticult J. 2016;85:154–160. doi: 10.2503/hortj.MI-071. [DOI] [Google Scholar]
- Saks Y, Vanstaden J, Smith MT. Effect of gibberellic-acid on carnation flower senescence: evidence that the delay of carnation flower senescence by gibberellic-acid depends on the stage of flower development. Plant Growth Regul. 1992;11(1):45–51. doi: 10.1007/BF00024432. [DOI] [Google Scholar]
- Schindele A, Dorn A, Puchta H. CRISPR/Cas brings plant biology and breeding into the fast lane. Curr Opin Biotechnol. 2020;61:7–14. doi: 10.1016/j.copbio.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Schmitzer V, Veberic R, Osterc G, Stampar F. Color and phenolic content changes during flower development in groundcover rose. J Am Soc Hortic Sci. 2010;135(3):195–202. doi: 10.21273/JASHS.135.3.195. [DOI] [Google Scholar]
- Serek M, Jones RB, Reid MS. Role of ethylene in opening and senescence of Gladiolus sp. flowers. J Am Soc Hortic Sci. 1994;119(5):1014–1019. doi: 10.21273/JASHS.119.5.1014. [DOI] [Google Scholar]
- Shibuya K, Nagata M, Tanikawa N, Yoshioka T, Hashiba T, Satoh S. Comparison of mRNA levels of three ethylene receptors in senescing flowers of carnation (Dianthus caryophyllus L.) J Exp Bot. 2002;53(368):399–406. doi: 10.1093/jexbot/53.368.399. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Niki T, Ichimura K. Pollination induces autophagy in petunia petals via ethylene. J Exp Bot. 2013;64(4):1111–1120. doi: 10.1093/jxb/ers395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Yoshioka T, Hashiba T, Satoh S. Role of the gynoecium in natural senescence of carnation (Dianthus caryophyllus L.) flowers. J Exp Bot. 2000;51(353):2067–2073. doi: 10.1093/jexbot/51.353.2067. [DOI] [PubMed] [Google Scholar]
- Shimizu-Yumoto H, Ichimura K. Senescence of Eustoma flowers as affected by pollinated area of the stigmatic surface. J JPN Soc Hortic Sci. 2006;75(1):66–71. doi: 10.2503/jjshs.75.66. [DOI] [Google Scholar]
- Shimizu-Yumoto H, Ichimura K. Effects of ethylene, pollination, and ethylene inhibitor treatments on flower senescence of gentians. Postharvest Biol Technol. 2012;63(1):111–115. doi: 10.1016/j.postharvbio.2011.08.009. [DOI] [Google Scholar]
- Shimizu-Yumoto H, Tsujimoto N, Naka T. Acid invertase activities of dahlia ‘Kokucho’ petals during flower opening and following cutting and treatment with 6-benzylaminopurine. Sci Hortic. 2020;272:109525. doi: 10.1016/j.scienta.2020.109525. [DOI] [Google Scholar]
- Shinozaki Y, Tanaka R, Ono H, Ogiwara I, Kanekatsu M, van Doorn WG, Yamada T. Length of the dark period affects flower opening and the expression of circadian-clock associated genes as well as xyloglucan endotransglucosylase/hydrolase genes in petals of morning glory (Ipomoea nil) Plant Cell Rep. 2014;33(7):1121–1131. doi: 10.1007/s00299-014-1601-z. [DOI] [PubMed] [Google Scholar]
- Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci. 2011;68(12):2013–2037. doi: 10.1007/s00018-011-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead AD. Pollination-induced flower senescence: a review. Plant Growth Regul. 1992;11(1):13–20. doi: 10.1007/BF00024427. [DOI] [Google Scholar]
- Steinitz B, Cohen A. Gibberellic acid promotes flower bud opening on detached flower stalks of statice (Limonium sinuatum L.) Hort Sci. 1982;17:903–904. [Google Scholar]
- Sui SZ, Luo JH, Liu DF, Ma J, Men WT, Fan L, et al. Effects of hormone treatments on cut flower opening and senescence in wintersweet (Chimonanthus praecox) Hort Sci. 2015;50:1365–1369. [Google Scholar]
- Tan H, Liu XH, Ma N, Xue JQ, Lu WJ, Bai JH, Gao J. Ethylene-influenced flower opening and expression of genes encoding ETRs, CTRs, and EIN3s in two cut rose cultivars. Postharvest Biol Technol. 2006;40(2):97–105. doi: 10.1016/j.postharvbio.2006.01.007. [DOI] [Google Scholar]
- Tang X, Woodson WR. Temporal and spatial expression of 1-aminocyclopropane-1-carboxylate oxidase mRNA following pollination of immature and mature petunia flowers. Plant Physiol. 1996;112(2):503–511. doi: 10.1104/pp.112.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverner E, Letham DS, Wang J, Cornish E, Willcocks DA. Influence of ethylene on cytokinin metabolism in relation to petunia corolla senescence. Phytochemistry. 1999;51(3):341–347. doi: 10.1016/S0031-9422(98)00757-2. [DOI] [Google Scholar]
- ten Have A, Woltering EJ. Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Mol Biol. 1997;34(1):89–97. doi: 10.1023/A:1005894703444. [DOI] [PubMed] [Google Scholar]
- Torre S, Fjeld T, Gislerod HR. Effects of air humidity and K/ca ratio in the nutrient supply on growth and postharvest characteristics of cut roses. Sci Hortic. 2001;90(3-4):291–304. doi: 10.1016/S0304-4238(01)00230-8. [DOI] [Google Scholar]
- van Doorn WG, Dole I, Celikel FG, Harkema H. Opening of Iris flowers is regulated by endogenous auxins. J Plant Physiol. 2013;170(2):161–164. doi: 10.1016/j.jplph.2012.09.014. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Kamdee C. Flower opening and closure: an update. J Exp Bot. 2014;65(20):5749–5757. doi: 10.1093/jxb/eru327. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, van Meeteren U. Flower opening and closure: a review. J Exp Bot. 2003;54(389):1801–1812. doi: 10.1093/jxb/erg213. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence. J Exp Bot. 2008;59(3):453–480. doi: 10.1093/jxb/erm356. [DOI] [PubMed] [Google Scholar]
- van Sandt VS, Suslov D, Verbelen JP, Vissenberg K. Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot. 2007;100(7):1467–1473. doi: 10.1093/aob/mcm248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaud E, Brioudes F, Szecsi J, Leroux J, Brown S, Perrot-Rechenmann C, et al. AUXIN RESPONSE FACTOR8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. Plant Cell. 2011;23(3):973–983. doi: 10.1105/tpc.110.081653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chang X, Lin J, Chang Y, Chen JC, Reid MS, Jiang CZ. Transcriptome profiling reveals regulatory mechanisms underlying corolla senescence in petunia. Hortic Res. 2018;5(1):16. doi: 10.1038/s41438-018-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang H, Ding L, Song A, Shen F, Jiang J, Chen S, Chen F. Transcriptomic and hormone analyses reveal mechanisms underlying petal elongation in Chrysanthemum morifolium ‘Jinba’. Plant Mol Biol. 2017;93(6):593–606. doi: 10.1007/s11103-017-0584-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiao Y. Cellulose microfibril-mediated directional plant cell expansion: gas and brake. Mol Plant. 2020;13(12):1670–1672. doi: 10.1016/j.molp.2020.10.010. [DOI] [PubMed] [Google Scholar]
- Wei T, Cheng Q, Min Y-L, Olson EN, Siegwart DJ. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 2020;11:1–12. doi: 10.1038/s41467-020-17029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HR, Kim HJ, Lim PO, Nam HG. Leaf senescence: systems and dynamics aspects. Annu Rev Plant Biol. 2019;70(1):347–376. doi: 10.1146/annurev-arplant-050718-095859. [DOI] [PubMed] [Google Scholar]
- Wood WML. Thermonasty in tulip and crocus flowers. J Exp Bot. 1953;4(1):65–77. doi: 10.1093/jxb/4.1.65. [DOI] [Google Scholar]
- Wu L, Ma N, Jia Y, Zhang Y, Feng M, Jiang CZ, Ma C, Gao J. An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol. 2017;173(1):853–862. doi: 10.1104/pp.16.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Gookin T, Jiang CZ, Reid M. Genes associated with opening and senescence of Mirabilis jalapa flowers. J Exp Bot. 2007;58(8):2193–2201. doi: 10.1093/jxb/erm058. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hanson MR. Programmed cell death during pollination-induced petal senescence in petunia. Plant Physiol. 2000;122(4):1323–1333. doi: 10.1104/pp.122.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Norikoshi R, Suzuki K, Nishijima T, Imanishi H, Ichimura K. Cell division and expansion growth during rose petal development. J JPN Soc Hortic Sci. 2009;78(3):356–362. doi: 10.2503/jjshs1.78.356. [DOI] [Google Scholar]
- Yon F, Joo Y, Cortes Llorca L, Rothe E, Baldwin IT, Kim SG. Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers. New Phytol. 2016;209(3):1058–1066. doi: 10.1111/nph.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Feng M, Chen W, Zhou X, Lu J, Wang Y, Li Y, Jiang CZ, Gan SS, Ma N, Gao J. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat Plants. 2019;5(3):290–299. doi: 10.1038/s41477-019-0376-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yin S, Tu Y, Mei H, Yang Y. A novel microRNA, SlymiR208, promotes leaf senescence via regulating cytokinin biosynthesis in tomato. Physiol Plant. 2020;169(2):143–155. doi: 10.1111/ppl.13068. [DOI] [PubMed] [Google Scholar]
- Zonia L, Munnik T. Life under pressure: hydrostatic pressure in cell growth and function. Trends Plant Sci. 2007;12(3):90–97. doi: 10.1016/j.tplants.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Zwack PJ, Robinson BR, Risley MG, Rashotte AM. Cytokinin response factor 6 negatively regulates leaf senescence and is induced in response to cytokinin and numerous abiotic stresses. Plant Cell Physiol. 2013;54(6):971–981. doi: 10.1093/pcp/pct049. [DOI] [PubMed] [Google Scholar]