Abstract
Prolonged exposure to an excess of glucocorticosteroids (GCs), both endogenous and exogenous, leads to a wide range of comorbidities, including cardiovascular, metabolic, psychiatric, and musculoskeletal disorders. The latter comprise osteopenia and osteoporosis leading to skeletal fractures and myopathy.
Although endogenous hypercortisolemia is a rare disorder, GCs are among the most frequently prescribed drugs, often administered chronically and despite multiple side effects, impossible to taper off due to therapeutic reasons. The pathophysiology of the effect of GC excess on bone often leads to fractures despite normal or low-normal bone mineral density and it includes direct (mainly disturbance in bone formation processes, through inactivation of the Wnt/β-catenin signalling pathway) and indirect mechanisms (through suppressing the gonadal and somatotrophic axis, and also through antagonizing vitamin D actions). Glucocorticosteroid-induced fast-twitch, glycolytic muscles atrophy occurs due to increased protein catabolism and impaired synthesis. Protein degradation is a result of activation of the ubiquitin proteasome and the lysosomes stimulated through overexpression of several atrogenes (such as FOXO-1 and atrogin-1).
This review will discuss pathophysiology, clinical presentation, prevention, and management of GC-induced osteoporosis (including calcium and vitamin D supplementation, and bisphosphonates) and myopathy associated with GC excess.
Keywords: muscular diseases, osteoporosis, glucocorticosteroids, Cushing syndrome
Introduction
Cushing syndrome (CS) is a consequence of prolonged exposure to excessive amounts of circulating glucocorticosteroids (GCs). Chronic administration of GCs is the most common cause of CS while pituitary neuroendocrine tumour (PitNET) secreting adrenocorticotropic hormone (ACTH), known as Cushing disease (CD), remains the most frequent cause of endogenous cortisol hypersecretion, followed by ACTH-independent cases (predominantly adrenal adenoma) and ectopic corticotropin-releasing hormone (CRH)/ACTH-producing neoplasms (e.g., bronchial neuroendocrine neoplasms) [1]. The annual incidence of CS ranges between 1.8 and 3.2 cases per million [1–3].
Despite scientific progress since 1932, when Harvey Cushing described the first case of the clinical syndrome that now bears his name, the diagnosis of endogenous CS is often delayed (mean time to diagnosis 34 months) and poses a significant diagnostic challenge [4]. Cushing syndrome is characterized by increased mortality and various comorbidities with a pronounced impact on quality of life, e.g., metabolic and neuropsychiatric disorders, impairment of reproductive and sexual function, dermatological manifestations, but also musculoskeletal complications [5]. The latter including osteoporosis, skeletal fractures, and myopathy [5, 6].
Cushing syndrome may damage the musculoskeletal system both structurally and functionally and the level of impairment depends mainly on the time of exposure and excessive concentration of GCs [7]. Although endogenous CS remains a rare entity, GCs are among the most frequently prescribed drugs: the weighted prevalence of oral GC use was 1.2% between 1999 and 2008 in the United States [8]. Interestingly, only 37.9% of oral GCs users reported concomitant use of any anti-osteoporotic agent (bisphosphonates, calcitonin, calcium, hormone replacement therapies, teriparatide, and vitamin D) [8]. Thus, early identification of patients at risk of musculoskeletal complications of excess of GCs and adequate treatment remains essential.
In this review, we discuss the pathophysiology, clinical presentation, prevention, and management of musculoskeletal complications of prolonged exposure to GCs.
Skeletal complications of glucocorticosteroids excess
Epidemiology
Any chronic excess of GCs, regardless of cause, leads to bone loss and increased risk of fractures. Deterioration of bone status has been described in 64–100% of patients with endogenous CS, osteopenia occurs in 40–78%, osteoporosis in 22–57%, and skeletal fractures in 11–76% of patients [5]. More than 10% of patients who receive long-term treatment of GC are diagnosed with a clinical fracture, and 30–40% have radiographic evidence of vertebral fractures [9].
The risk of fractures induced by oral GCs is related mainly to the daily dose, not to the cumulative dose of GCs [10]. In endogenous CS, bone status largely correlates with severity of hypercortisolemia; therefore patients with ectopic ACTH secretion have lower bone mineral density (BMD) and a higher risk of fractures compared to patients with pituitary or adrenal CS [11]. Patients with adrenal hypercortisolemia experience more severe bone loss than patients with CD because of the protective role of adrenal androgens (including dehydroepiandrosterone sulfate – DHEA-S), which are higher in CD because of ACTH stimulation [12, 13]. Men with endogenous CS are more likely to suffer from osteoporosis and vertebral fractures than women, which suggests that testosterone deficiency additionally negatively affects the bone condition in CS [14].
The excess of GCs impairs mainly the trabecular bone, leading to an increased risk of osteoporotic fractures mainly of the spine, which are often the first symptom of CS. The results of the Danish Cohort Study showed that the risk of fractures of patients with endogenous CS increased particularly within the 3 years prior to diagnosis and treatment and became lower with longer follow-up duration [15]. These data indicate that endogenous CS is an insidiously evolving disease. Therefore, prompt diagnosis and treatment of CS are crucial to limit bone damage. In exogenous GCs excess the highest rate of bone loss occurs predominantly within the first 3–6 months of GCs treatment, even before significant loss of BMD, and a slower decline continues with persistent use [16].
Pathophysiology – direct effects of glucocorticosteroids on bone status
Knowledge on the effect of GCs on the bone status through direct and indirect mechanisms and the pathogenesis of GC-induced osteoporosis (GIO) comes mainly from studies of patients treated with exogenous GCs and experimental animal model testing.
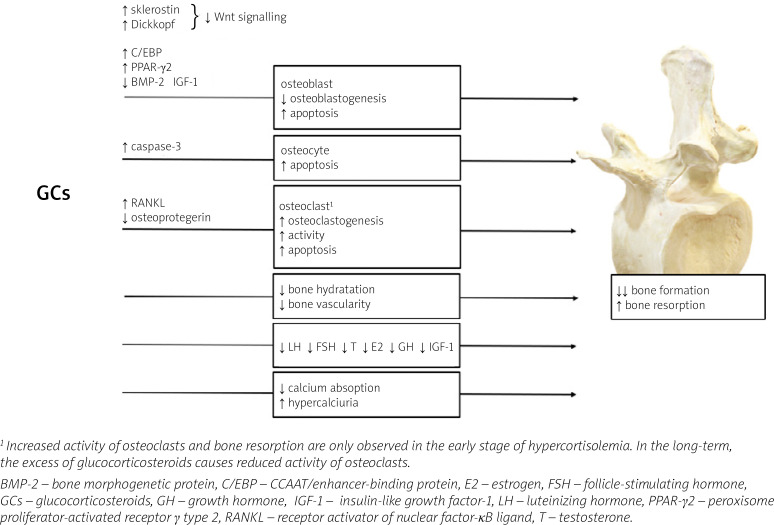
At the tissue level, GCs mainly disturb bone formation processes, in contrast to postmenopausal osteo-porosis or hyperparathyroidism, in which bone resorption dominates. The decrease in bone formation is the result of inhibition of osteoblast differentiation, function and lifespan which is attributed to inactivation of the Wnt/β-catenin signaling pathway (a critical pathway for osteoblastogenesis), the induction of nuclear factors of the CCAAT/enhancer-binding protein (C/EBP) family and peroxisome proliferator-activated receptor γ (PPAR-γ) type 2, as well as repression of bone morphogenetic protein (BMP-2) [17]. Glucocorticosteroids may also enhance bone marrow stromal cell development toward the adipocyte lineage rather than toward osteoblasts [18].
In addition, GCs also promote apoptosis in osteoblasts and osteocytes due to activation of caspase-3 [19] and may also affect the metabolism and function of osteocytes, modifying the elastic modulus surrounding osteocyte lacunae and causing reduced mineral-to-matrix ratios in the same areas with an increase in lacunar size [20]. These effects of GCs on osteoblasts and osteocytes might account for a disproportionate loss of bone strength in relation to bone mass. In fact, in both endogenous and exogenous hypercortisolism, fractures may develop even in the presence of normal or low-normal BMD, measured by dual-energy X-ray absorptiometry (DXA) [21].
Apart from the effect on osteoblasts and osteocytes, GCs can also intensify the process of bone resorption by disturbing the balance between receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG). RANKL produced by osteoblasts and osteocytes, is essential for osteoclast maturation, activation and survival [22, 23]. The effects of RANKL are counteracted by osteoprotegerin, a neutralizing decoy receptor which thus inhibits maturation and differentiation of the osteoclasts. The balance between RANKL and OPG during GCs treatment is disturbed and favors of RANKL leading to an initial increase in bone resorption. However, owing to the potent suppressive effects of GCs on osteoblasts, the increase in RANKL is only transient [24].
Glucocorticosteroid-induced bone loss occurs in two phases: a rapid, early phase in which bone mass is lost due to excessive bone resorption and a slower, later phase in which bone is lost due to inadequate bone formation. These two phases of action of GCs were confirmed by Yao et al. [25]: in this study, gene expression and changes in bone microarchitecture were assessed in GC-treated mice on days 0, 7, 28 and 56: GC excess induced early up-regulation of genes involved in osteoclast activation, function, and adipogenesis, which peaked on day 7; from day 28 to 56, genes associated with osteoblast maturation and activation significantly decreased from the baseline values, and Wnt/β-catenin antagonists including Dickkopf-1 and sclerostin were increased. These changes in gene expression were consistent with changes in the biochemical markers of bone turnover and bone mass, with an initial increase in bone resorption markers such as serum cross-linked C-telopeptide of type I collagen (CTX-I) and a reduction in trabecular bone volume of the spine, followed by a decrease in the bone formation marker osteocalcin [25].
Furthermore, studies in GC-treated mice have shown a decrease in bone hydration accompanied by a reduction in bone vascularity and blood flow compared to control mice, which may be an additional mechanism whereby GCs reduce bone strength [26, 27]. Moreover, GCs mediate changes in synthesis and binding of receptors or growth factor binding proteins present in the bone microenvironment [17]. Glucocorticosteroids inhibit the expression of IGF-1 in osteoblasts, which stimulates bone formation and type I collagen synthesis and reduces bone collagen degradation and osteoblast apoptosis [28].
One of the features of GIO that is still not sufficiently explored is the high interindividual variability of its clinical course and severity. This variable effect of GCs on bone status may be the result of the glucocorticosteroid receptor (GR) polymorphisms associated with increased (BclI and N363S) or decreased (ER22/23EK and A3669G) susceptibility to GCs or possibly local 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) activity [29, 30]. Osteoblastic 11β-HSD1 can effectively convert prednisone to active prednisolone with kinetics similar to cortisone to cortisol conversion [31]. This suggests that osteoblastic 11β-HSD1 may influence the sensitivity of bone to therapeutic GCs.
Glucocorticosteroids increase the activity of 11β-HSD1, but also pro-inflammatory cytokines, which significantly induces the sensitivity of bones to GCs in the course of an inflammatory diseases [32].
Indirect effects of glucocorticosteroids on bone status
Indirect effects of GCs on bone status include mainly disturbances in the secretion and action of hormones. Both exogenous and endogenous GC excess inhibit the hypothalamic–pituitary–gonadal axis. Decreased testosterone and estrogen production may be an additional factor in the pathogenesis of GIO [33].
Glucocorticosteroids also inhibit the IGF-1 axis (GH–IGF-1). Growth hormone directly and through IGF-1 stimulates the process of bone formation [17]. Functional GH deficiency was observed even when the GCs excess was mild, as in patients treated with inhaled GC and in those with “subclinical” endogenous hypercortisolism [17, 34, 35].
Another indirect mechanism for the adverse skeletal effects of GCs is a decrease in intestinal calcium absorption as a result of inhibition of vitamin D actions, decrease of the expression of specific calcium channels in the duodenum and increase in renal calcium excretion. Physiologically in this situation one would expect to find evidence of a secondary increase in parathormone (PTH). However, no significant change in PTH levels was found in most CS patients.
Furthermore, direct histomorphometric analysis of bone biopsies demonstrated reduced bone turnover in CS, in contrast to the increased bone turnover that characterizes hyperparathyroidism [25]. This indicates a relatively minor role of PTH in the bone loss associated with GCs use. Nevertheless, GCs have a significant effect on the dynamics of PTH secretion, with a decrease in tonic PTH release and an increase in pulsatile releases of this hormone [36]. In addition, GCs have been reported to increase the expression and availability of PTH receptors on osteoblasts, which may be associated with increased sensitivity of skeletal cells to PTH [37].
The mechanisms of GCs excess action on bone are summarized in Figure 1.
Fig. 1.
Mechanisms of glucocorticosteroid action leading to the development of glucocorticoid-induced osteoporosis.
Clinical presentation
Given that GIO is an example of a bone disorder in which fractures often develop in patients with normal or low normal BMD, standard DXA alone is not a sufficient tool to assess bone status in CS, in which, mainly bone quality is impaired. Bone microarchitecture, probably the most important determinant of bone quality, may explain bone fragility in CS.
It was originally studied by histomorphometry, but it can currently be evaluated by computed tomography systems [high resolution peripheral quantitative computed tomography (HRpQCT) and central quantitative computed tomography (QCT)] or micromagnetic resonance imaging (microMRI), which are not readily available in a clinical setting [38]. A non-invasive technique that correlates with HRQCT and QCT measurements and has gained acceptance in assessing bone micro-architecture is the trabecular bone score (TBS) [39]. The lumbar spine TBS is a grey-level texture measurement based on the use of experimental variograms that can be extracted from the two-dimensional lumbar spine DXA image. Low TBS values are associated with thin trabeculae, low trabecular number, and low connectivity density, which reflects poor micro-architectural bone quality [40].
Recent studies showing patients receiving GCs have lower TBS Z-scores than GCs-naïve patients without differences in their BMD Z-scores [41, 42].
In addition to the assessment of bone quality, morphometric vertebral assessment is recommended in patients with CS. Vertebral fractures are often overlooked, being frequently asymptomatic [43]. Height loss greater than 3–5 cm is an indicator of possible pre-existing vertebral fractures [44].
Treatment
Most studies have shown that GC bone loss and GIO are potentially reversible after successful surgical treatment, although the time to bone recovery is relatively long and variable and restoration is not always complete [45–49]. An improvement in BMD was noted 3 to 6 months after hypercortisolism remission, generally slower at the femoral neck than at the lumbar spine [46, 49]. A long-term prospective study showed normalization of BMD at the lumbar spine and femoral neck in patients with endogenous CS after successful surgical treatment after a mean follow-up of 71 months [45]. While in endogenous CS, resolution of the underlying disease can resolve the problem of bone damage, in exogenous GIO, patients are often unable to taper off therapeutic doses of GC.
Oral administration of each GC in a dose equivalent to 2.5 mg of prednisone or higher daily over 3 months or longer increases the risk of fracture [50]. Previous studies suggested a less negative impact on bone status of deflazacort than other GCs [51, 52]. However, a recent retrospective study on GC-treated boys with Duchenne muscular dystrophy (DMD) reported higher fracture risk of the group treated with daily deflazacort compared with the group treated with daily prednisolone [53].
The severity of GIO is less pronounced in patients treated with high dose 6-methylprednisolone intravenous pulse therapy than daily oral GC therapy [54]. It is noteworthy that even high dose of inhaled GC (≥ 1,000 μg of fluticasone daily or equivalent) for more than 4 years increases the risk of fracture [55].
All orally administered GCs (intermediate- and long-acting) should be administered in a single morning dose to adjust to the physiological cortisol circadian rhythm. However, the use of hydrocortisone (short-acting GC) in doses higher than 30 mg in replacement therapy in adrenal insufficiency was proven to induce bone loss [56].
All patients exposed to excess GCs should be treated with adequate vitamin D and calcium supplementation. Lifestyle modification (balanced diet, smoking cessation, regular weight-bearing or resistance training exercise) is also necessary. The guidelines recommend 1,200–1,500 mg of calcium daily, including all sources (supplements, dietary intake). The clinical goal of vitamin D supplementation is to achieve a minimum concentration of 30 ng/ml [57].
In addition to correcting concomitant risk factors for fractures, treatment with anti-osteoporosis drugs may be necessary in persistent endogenous CS. However, evidence-based knowledge on the use of anti-osteoporosis treatments in endogenous CS is scarce and no specific guidelines are available in this area.
In contrast to this group of patients, there are specific guidelines for exogenous GIO that can be only partially translated to patients with endogenous CS [9, 58]. The guidelines recommend modulation of treatment with exogenous GIO depending on individual fracture risk, which should be assessed in all patients receiving prednisone (or equivalent) at a daily dose > 2.5 mg for > 3 months. The most important elements of this evaluation are age, previous history of fractures, daily dose and duration of GCs used and individual fracture risk profile (calculated by the Fracture Risk Assessment Tool – FRAX). Oral bisphosphonates are recommended in patients at moderate to high risk of fracture and in any patient with prevalent fracture.
According to Polish recommendations, this procedure should also be initiated obligatorily in patients over 65 years of age, receiving ≥ 7.5 mg/day prednisone, even if they present no other fracture risk factors [59]. Oral bisphosphonates are preferred as first-line drugs due to safety, cost, and lack of evidence for superior anti-fracture effects of other anti-osteoporosis medications, in accordance with the relevant guidelines.
Other therapies are recommended if oral bisphosphonates are not appropriate, in order of preference: bisphosphonates intravenously, teriparatide, denosumab (anti-RANKL monoclonal antibody) and raloxifene (selective estrogen receptor modulator [SERM] for postmenopausal women in whom none of the medications listed above is appropriate). However, the results of multivariate random-effects network meta-analyses suggest that teriparatide and denosumab are more effective and reduce the risk of vertebral fractures more than oral bisphosphonates [60]. In patients at moderate or high risk of fracture who are being treated with anti-osteoporosis medication and discontinuing GCs, it is recommended that the treatment course should be continued until the fracture risk is reduced to a low level; then treatment should be ceased [9].
In endogenous CS, anti-osteoporotic drugs should be reserved for patients with persistent hypercortisolism not adequately corrected by specific treatments [61]. There is evidence that bisphosphonates can induce an improvement in BMD and could be useful in patients with persistent post-surgical hypercortisolism to prevent further bone loss [62]. However, it is still unknown how bisphosphonates with long-term antiresorptive effects can affect bone remodeling after successful treatment of endogenous CS. The diagnosis of CS is usually delayed, and at this stage the bone condition is usually characterized by low bone turnover, which may be further suppressed by antiresorptive drugs.
Therefore, based on the knowledge of the pathophysiology of the effect of GCs on bone, it seems that other anti-osteoporosis drugs may have a better effect in the treatment of patients with skeletal fragility caused by endogenous CS.
Denosumab has been shown to reverse the effects of GCs on bones and due to its rapidly reversible effect after discontinuation, it may be an attractive drug for short-term use in GIO caused by endogenous hypercortisolism, when curative surgery is expected [21, 63]. However, the main pathophysiological role of reduced bone formation caused by GC provides a rationale for the use of an anabolic drug such as teriparatide. Nevertheless, evidence for the safety and efficacy of teriparatide in patients with endogenous CS is still lacking.
Muscular complications of glucocorticosteroid excess
Epidemiology
Myopathy associated with GCs excess remains one of the most prominent features of CS, with the prevalence ranging from 42% to 83% [5, 64]. However, detailed examination identifies myopathy in nearly every patient with florid hypercortisolemia [6]. Interestingly, GC-induced myopathy (GIM) is slightly more common in ectopic than in adrenal hypercortisolemia [64]. Among patients with exogenous GC excess, severe muscle weakness affects 2.4% to 21% [65].
The development of GIM depends on several factors. In patients treated with exogenous GCs, the use of prednisone in doses lower than 10 mg per day (or equivalent) is usually not associated with GIM. Higher GC doses lead to a more rapid onset of clinically significant muscle weakness, which can develop within 2 weeks after the introduction of therapy with GCs [66]. The administration of 40–60 mg of prednisone per day (or equivalent) for 1 month may lead to some muscle weakness [66]. The risk of GIM is higher in patients treated with fluorinated GCs (e.g., betamethasone, dexamethasone, triamcinolone) than in patients treated with non-fluorinated GCs (e.g., prednisone and prednisolone) [66].
The way of GC administration also influences the risk of muscle damage: in the case of inhaled or epidurally injected GCs, GIM has rarely been reported [67, 68]. Other factors which may increase the risk of GIM development include older age, hyperglycemia, presence of malignancy, respiratory muscles disorder, negative nitrogen balance before the introduction of GC treatment and lack of physical activity [6, 65].
Interindividual susceptibility to GIM in endogenous CS may be attributed to various factors e.g., to GR polymorphisms. In the study of Müller et al. [69] decreased handgrip strength in hypercortisolemia was more pronounced in A3669G wild type than in A3669G minor allele carriers.
Pathophysiology
Glucocorticosteroids excess alters the structure and function of skeletal muscle in multiple mechanisms; GCs cause fast-twitch (type II) muscle fibers (mainly type IIx and IIb) atrophy with a limited or no impact on type I fibers [70, 71]. GR was proven to be essential for the development of GC-induced myopathy – the study of Watson et al. [72] showed that muscle-specific GR-knock-out mice are resistant to the effect of excess GC on muscle.
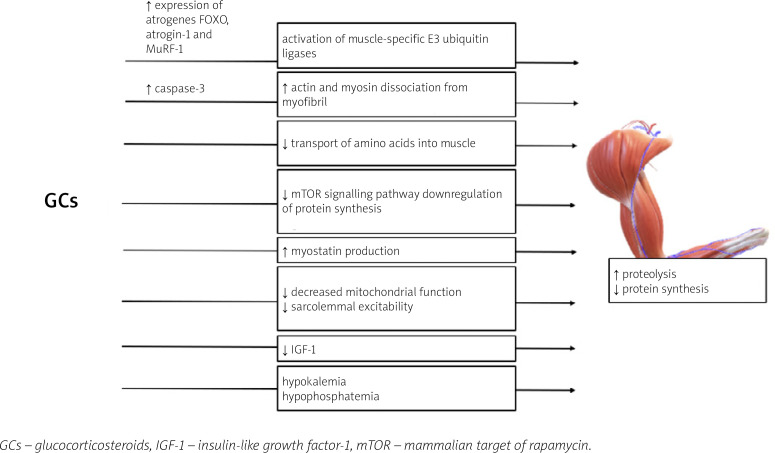
Glucocorticosteroid-induced myopathy is a consequence of increased protein catabolism and decreased synthesis [70]. Induction of muscle proteolysis occurs mainly due to the activation of ubiquitin proteasome, lysosomal systems, and calcium-mediated systems (calpains) [71]. The activation of the two former catabolic systems is regulated by the genomic actions of GC: the increased expression of numerous atrogenes (genes involved in atrophy), e.g., FOXO, atrogin-1 and MuRF-1 genes [70, 73].
The role of Forkhead box O (FOXO) transcriptional factors in the development of GC-induced muscle atrophy has been established in multiple studies [73–76]. Overexpression of FOXO leads to the activation of muscle RING-finger protein-1 (MuRF-1) and atrogin-1, which are muscle-specific E3 ubiquitin ligases, and results in increased muscle wasting [70, 76, 77]. Interestingly, the ubiquitin proteasome system is not capable of intact myofibril degradation; thus the entire process requires the activation of caspase-3 for actin and myosin dissociation [78].
The anti-anabolic effect of the excess of GC on muscles is also a result of different processes [70]. Glucocorticosteroids inhibit the transport of amino acids into muscle [70]. Furthermore, GC repress the mammalian target of rapamycin (mTOR) kinase signalling pathway, responsible for phosphorylation of eIF4E-binding protein 1 (4E-BP1) and the ribosomal protein S6 kinase 1 (S6K1), leading to downregulation of protein synthesis [79].
Glucocorticosteroids may also hinder muscle development through alterations in local growth factor production [70]. Also GCs suppress the muscle IGF-1 production [80] and IGF-1 has an ability to activate phosphoinositide 3-kinases/protein kinase and the B/mammalian target of rapamycin kinase (PI3K/Akt/mTOR) pathway, repress the transcription of FOXO, and decrease GC-induced atrophy (GIMA) [70, 81, 82].
Muscle mass development in patients with GCs excess is also affected by stimulation of myostatin (member of the transforming growth factor β [TGF-β] superfamily), production which results in decreased proliferation and differentiation of satellite cells [83]. Other postulated mechanisms leading to GIMA include decreased mitochondrial function and sarcolemmal excitability [5, 70]. Furthermore, GCs with high mineralocorticoid activity may induce hypokalemia and hypophosphatemia which may contribute to muscle weakness [65].
The mechanisms of GC excess leading to the development of GC-induced myopathy are presented in Figure 2.
Fig. 2.
Mechanisms of glucocorticosteroid action leading to the development of glucocorticosteroidinduced myopathy.
Clinical presentation
Glucocorticosteroid-induced myopathy occurs in two clinical forms. Acute GIM is a rapidly progressive condition affecting proximal and distal muscle groups; respiratory muscles also can be affected [84]. This form of GC-induced myopathy is often observed in patients of intensive care units, treated with high doses of GCs [66].
Chronic GIM is characterized by painless (or mildly painful), slowly progressive proximal myopathy affecting predominantly the pelvic girdle muscles, rarely distal muscles [66]. The patients usually report difficulties while rising from a seated position, climbing stairs, and performing overhead activities [6]. Laboratory tests, including creatine phosphokinase (CPK), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and aldolase, are usually within the reference range, but mild elevation may be observed in the early stage of the disease and in the acute form of GIM [66, 85]. Muscle biopsy reveals nonspecific atrophy of type IIb fibers, no inflammatory infiltrate, rarely necrosis [66]. The electroneuromyography (EMG) result is within the normal range in the early stages, but as the disease progresses, a myopathic pattern with small-amplitude short-duration polyphasic action potentials, without spontaneous activity upon needle insertion, may be observed [66, 86]. Magnetic resonance imaging (MRI) may be helpful in differential diagnosis, to exclude inflammatory myopathies [66].
Treatment
Undeniably, in patients with endogenous hypercortisolemia and GIM, restoring the normal cortisol level through operative or pharmacological treatment is an optimal way to reverse muscle damage. In prevention and management of GIM, adequate protein intake and physical therapy (aerobic and resistance exercises) are of proven efficacy [87, 88]. Since the increased expression of IGF-1 was shown to play a protective role in GIM, it seems to be a promising therapeutic target. In an animal model, stimulation of IGF-1 and deletion of myostatin gene prevented GIM [82, 89]. Among other growth factors, ghrelin, famously known as the “hunger hormone”, produced mainly by the stomach, may also inhibit GIM [90]. Due to the hypertrophic effect on muscles, it is to be expected that androgens may also prevent GIM.
The administration of testosterone, nandrolone, selective androgen receptor modulator (SARM) and DHEA was reported to prevent the reduction of muscle strength and mass induced by GCs [91–94]. Among other agents, branched chain amino acids (BCAAs), glutamine, taurine, creatine, carbenoxolone (inhibitor of 11β-HSD1 regulating conversion of cortisone to cortisol, which decreases active GCs availability) and vitamin D have been reported to prevent GC-muscle atrophy [95–100]. However, these interventions were not conclusively evaluated in randomized controlled studies in humans and are not currently recommended. A summary of characteristics of GIO and GIM discussed in the review is presented in Table I.
Table I.
Summary of features of glucocorticosteroid-induced osteoporosis and glucocorticosteroid-induced myopathy described in the review
| Glucocorticosteroid-induced osteoporosis | Glucocorticosteroid-induced myopathy | |
|---|---|---|
| Epidemiology | Endogenous hypercortisolemia:
In patients receiving long-term GC: up to 40% radiological evidence of vertebral fractures |
Endogenous hypercortisolemia: 42% to 83% of patients In exogenous GC excess, severe muscle weakness: 2.4% to 21% of patients |
| Pathophysiology | Disturbance in bone formation processes rather than bone resorption Direct mechanisms:
Indirect mechanisms:
|
Atrophy of fast-twitch (type II) muscle fibers (mainly type IIx and IIb), limited impact on type I fibers Increased protein catabolism through:
Impaired protein synthesis due to:
Alterations in local growth factor production:
GC-induced dyselectrolytemia:
|
| Clinical presentation |
|
|
| Treatment |
|
|
AST – aspartate aminotransferase, BMD – bone mineral density, BMP-2 – bone morphogenetic protein 2, C/EBP – CCAAT/enhancerbinding protein, CPK – creatine phosphokinase, GCs – glucocorticosteroids, GH – growth hormone, HRpQCT – high resolution peripheral quantitative computed tomography, IGF-1 – insulin-like growth factor-1, LDH – lactate dehydrogenase, mTOR – mammalian target of rapamycin, OPG – osteoprotegerin, RANKL – nuclear factor-κB ligand, TBS – trabecular bone score.
Conclusions
Musculoskeletal complications of CS contribute to the increased mortality and impaired quality of life. However, successful treatment of endogenous CS or in the case of exogenous CS discontinuation of GC therapy (if feasible) leads to improvement of the clinical course. Since the diagnosis is often delayed, it is crucial to identify patients with endogenous hypercortisolemia early and restore the normal cortisol level.
For the patients who are administered GC because of therapeutic purposes, early assessment, and introduction of pharmacological (calcium and vitamin D supplementation and bisphosphonates), behavioral (physical therapy) or dietary (adequate protein intake) interventions may help to reduce musculoskeletal complications.
Since the pathophysiology of the effect of GC excess on the musculoskeletal system is a complex interplay of different factors, to large extent unexplained, there is a need for studies providing high-quality data, which may unveil new therapeutic targets.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hakami OA, Ahmed S, Karavitaki N. Epidemiology and mortality of Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab 2021; 35: 101521, DOI: 10.1016/j.beem.2021.101521. [DOI] [PubMed] [Google Scholar]
- 2.Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf) 2011; 75: 436–442, DOI: 10.1111/j.1365-2265.2011.04124.x. [DOI] [PubMed] [Google Scholar]
- 3.Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol (Oxf) 2019; 91: 263–270, DOI: 10.1111/cen.14014. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein G, Osswald A, Hoster E, et al. Time to Diagnosis in Cushing’s Syndrome: A Meta-Analysis Based on 5367 Patients. J Clin Endocrinol Metab 2020; 105: dgz136, DOI: 10.1210/clinem/dgz136. [DOI] [PubMed] [Google Scholar]
- 5.Pivonello R, Isidori AM, De Martino MC, et al. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol 2016; 4: 611–629, DOI: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 6.Reincke M. Cushing Syndrome Associated Myopathy: It Is Time for a Change. Endocrinol Metab (Seoul) 2021; 36: 564–571, DOI: 10.3803/EnM.2021.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci 2002; 966: 73–81, DOI: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 8.Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken) 2013; 65: 294–298, DOI: 10.1002/acr.21796. [DOI] [PubMed] [Google Scholar]
- 9.Buckley L, Guyatt G, Fink HA, et al. 2017. American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol 2017; 69: 1521–1537, DOI: 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 10.van Staa TP, Leufkens HG, Abenhaim L, et al. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000; 39: 1383–1389, DOI: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 11.Tauchmanova L, Pivonello R, Di Somma C, et al. Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab 2006; 91: 1779–1784, DOI: 10.1210/jc.2005-0582. [DOI] [PubMed] [Google Scholar]
- 12.Minetto M, Reimondo G, Osella G, et al. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos Int 2004; 15: 855–861, DOI: 10.1007/s00198-004-1616-3. [DOI] [PubMed] [Google Scholar]
- 13.Ohmori N, Nomura K, Ohmori K, et al. Osteoporosis is more prevalent in adrenal than in pituitary Cushing’s syndrome. Endocr J 2003; 50: 1–7, DOI: 10.1507/endocrj.50.1. [DOI] [PubMed] [Google Scholar]
- 14.Pecori Giraldi F, Moro M, Cavagnini F, Study Group on the Hypothalamo–Pituitary–Adrenal Axis of the Italian Society of E. Gender-related differences in the presentation and course of Cushing’s disease. J Clin Endocrinol Metab 2003; 88: 1554–1558, DOI: 10.1210/jc.2002-021518. [DOI] [PubMed] [Google Scholar]
- 15.Dekkers OM, Horvath-Puho E, Jorgensen JO, et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab 2013; 98: 2277–2284, DOI: 10.1210/jc.2012-3582. [DOI] [PubMed] [Google Scholar]
- 16.Laan RF, van Riel PL, van de Putte LB, et al. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med 1993; 119: 963–968, DOI: 10.7326/0003-4819-119-10-199311150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Mazziotti G, Frara S, Giustina A. Pituitary Diseases and Bone. Endocr Rev 2018; 39: 440–488, DOI: 10.1210/er.2018-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ito S, Suzuki N, Kato S, et al. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone 2007; 40: 84–92, DOI: 10.1016/j.bone.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien CA, Jia D, Plotkin LI, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 2004; 145: 1835–1841, DOI: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 20.Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res 2006; 21: 466–476, DOI: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scillitani A, Mazziotti G, Di Somma C, et al. Treatment of skeletal impairment in patients with endogenous hypercortisolism: when and how? Osteoporos Int 2014; 25: 441–446, DOI: 10.1007/s00198-013-2588-y. [DOI] [PubMed] [Google Scholar]
- 22.Suda T, Takahashi N, Udagawa N, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999; 20: 345–357, DOI: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 23.Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med 2011; 17: 1235–1241, DOI: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18: 1319–1328, DOI: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 25.Yao W, Cheng Z, Busse C, et al. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum 2008; 58: 1674–1686, DOI: 10.1002/art.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein RS, Wan C, Liu Q, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 2010; 9: 147–161, DOI: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan G, Lay EY, Berka H, et al. A Novel Hybrid Compound LLP2A-Ale Both Prevented and Rescued the Osteoporotic Phenotype in a Mouse Model of Glucocorticoid-Induced Osteoporosis. Calcif Tissue Int 2017; 100: 67–79, DOI: 10.1007/s00223-016-0195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 1989; 83: 60–65, DOI: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manenschijn L, van den Akker EL, Lamberts SW, van Rossum EF. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann N Y Acad Sci 2009; 1179: 179–198, DOI: 10.1111/j.1749-6632.2009.05013.x. [DOI] [PubMed] [Google Scholar]
- 30.Lane NE. Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Curr Osteoporos Rep 2019; 17: 1–7, DOI: 10.1007/s11914-019-00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper MS, Bujalska I, Rabbitt E, et al. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 2001; 16: 1037–1044, DOI: 10.1359/jbmr.2001.16.6.1037. [DOI] [PubMed] [Google Scholar]
- 32.Kaur K, Hardy R, Ahasan MM, et al. Synergistic induction of local glucocorticoid generation by inflammatory cytokines and glucocorticoids: implications for inflammation associated bone loss. Ann Rheum Dis 2010; 69: 1185–1190, DOI: 10.1136/ard.2009.107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiodini I, Torlontano M, Carnevale V, et al. Skeletal involvement in adult patients with endogenous hypercortisolism. J Endocrinol Invest 2008; 31: 267–276, DOI: 10.1007/BF03345601. [DOI] [PubMed] [Google Scholar]
- 34.Malerba M, Bossoni S, Radaeli A, et al. Growth hormone response to growth hormone-releasing hormone is reduced in adult asthmatic patients receiving long-term inhaled corticosteroid treatment. Chest 2005; 127: 515–521, DOI: 10.1378/chest.127.2.515. [DOI] [PubMed] [Google Scholar]
- 35.Terzolo M, Bossoni S, Ali A, et al. Growth hormone (GH) responses to GH-releasing hormone alone or combined with arginine in patients with adrenal incidentaloma: evidence for enhanced somatostatinergic tone. J Clin Endocrinol Metab 2000; 85: 1310–1315, DOI: 10.1210/jcem.85.3.6531. [DOI] [PubMed] [Google Scholar]
- 36.Bonadonna S, Burattin A, Nuzzo M, et al. Chronic glucocorticoid treatment alters spontaneous pulsatile parathyroid hormone secretory dynamics in human subjects. Eur J Endocrinol 2005; 152: 199–205, DOI: 10.1530/eje.1.01841. [DOI] [PubMed] [Google Scholar]
- 37.Rubin MR, Bilezikian JP. Clinical review 151: The role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 2002; 87: 4033–4041, DOI: 10.1210/jc.2002-012101. [DOI] [PubMed] [Google Scholar]
- 38.dos Santos CV, Vieira Neto L, Madeira M, et al. Bone density and microarchitecture in endogenous hypercortisolism. Clin Endocrinol (Oxf) 2015; 83: 468–474, DOI: 10.1111/cen.12812. [DOI] [PubMed] [Google Scholar]
- 39.Silva BC, Walker MD, Abraham A, et al. Trabecular bone score is associated with volumetric bone density and microarchitecture as assessed by central QCT and HRpQCT in Chinese American and white women. J Clin Densitom 2013; 16: 554–561, DOI: 10.1016/j.jocd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 2014; 29: 518–530, DOI: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 41.Vinolas H, Grouthier V, Mehsen-Cetre N, et al. Assessment of vertebral microarchitecture in overt and mild Cushing’s syndrome using trabecular bone score. Clin Endocrinol (Oxf) 2018; 89: 148–154, DOI: 10.1111/cen.13743. [DOI] [PubMed] [Google Scholar]
- 42.Paggiosi MA, Peel NF, Eastell R. The impact of glucocorticoid therapy on trabecular bone score in older women. Osteoporos Int 2015; 26: 1773–1780, DOI: 10.1007/s00198-015-3078-1. [DOI] [PubMed] [Google Scholar]
- 43.Frara S, di Filippo L, Doga M, et al. Novel approaches to bone comorbidity in Cushing’s disease: an update. Pituitary 2022; 25: 754–759, DOI: 10.1007/s11102-022-01252-w. [DOI] [PubMed] [Google Scholar]
- 44.Seibel MJ, Cooper MS, Zhou H. Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol 2013; 1: 59–70, DOI: 10.1016/S2213-8587(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 45.Kristo C, Jemtland R, Ueland T, et al. Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing’s syndrome: a prospective, long-term study. Eur J Endocrinol 2006; 154: 109–118, DOI: 10.1530/eje.1.02067. [DOI] [PubMed] [Google Scholar]
- 46.Kawamata A, Iihara M, Okamoto T, Obara T. Bone mineral density before and after surgical cure of Cushing’s syndrome due to adrenocortical adenoma: prospective study. World J Surg 2008; 32: 890–896, DOI: 10.1007/s00268-007-9394-7. [DOI] [PubMed] [Google Scholar]
- 47.Randazzo ME, Grossrubatscher E, Dalino Ciaramella P, et al. Spontaneous recovery of bone mass after cure of endogenous hypercortisolism. Pituitary 2012; 15: 193–201, DOI: 10.1007/s11102-011-0306-3. [DOI] [PubMed] [Google Scholar]
- 48.Futo L, Toke J, Patocs A, et al. Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing’s syndrome. Osteoporos Int 2008; 19: 941–949, DOI: 10.1007/s00198-007-0514-x. [DOI] [PubMed] [Google Scholar]
- 49.Hermus AR, Smals AG, Swinkels LM, et al. Bone mineral density and bone turnover before and after surgical cure of Cushing’s syndrome. J Clin Endocrinol Metab 1995; 80: 2859–2865, DOI: 10.1210/jcem.80.10.7559865. [DOI] [PubMed] [Google Scholar]
- 50.Messina OD, Vidal LF, Wilman MV, et al. Management of glucocorticoid-induced osteoporosis. Aging Clin Exp Res 2021; 33: 793–804, DOI: 10.1007/s40520-021-01823-0. [DOI] [PubMed] [Google Scholar]
- 51.Loftus J, Allen R, Hesp R, David J, et al. Randomized, double-blind trial of deflazacort versus prednisone in juvenile chronic (or rheumatoid) arthritis: a relatively bone-sparing effect of deflazacort. Pediatrics 1991; 88: 428–436. [PubMed] [Google Scholar]
- 52.Ferraris JR, Pasqualini T, Legal S, et al. Effect of deflazacort versus methylprednisone on growth, body composition, lipid profile, and bone mass after renal transplantation. The Deflazacort Study Group. Pediatr Nephrol 2000; 14: 682–688, DOI: 10.1007/s004670000337. [DOI] [PubMed] [Google Scholar]
- 53.Joseph S, Wang C, Bushby K, et al. Fractures and Linear Growth in a Nationwide Cohort of Boys With Duchenne Muscular Dystrophy With and Without Glucocorticoid Treatment: Results From the UK NorthStar Database. JAMA Neurol 2019; 76: 701–709, DOI: 10.1001/jamaneurol.2019.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frediani B, Falsetti P, Bisogno S, et al. Effects of high dose methylprednisolone pulse therapy on bone mass and biochemical markers of bone metabolism in patients with active rheumatoid arthritis: a 12-month randomized prospective controlled study. J Rheumatol 2004; 31: 1083–1087. [PubMed] [Google Scholar]
- 55.Gonzalez AV, Coulombe J, Ernst P, Suissa S. Long-term Use of Inhaled Corticosteroids in COPD and the Risk of Fracture. Chest 2018; 153: 321–328, DOI: 10.1016/j.chest.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Wichers M, Springer W, Bidlingmaier F, Klingmuller D. The influence of hydrocortisone substitution on the quality of life and parameters of bone metabolism in patients with secondary hypocortisolism. Clin Endocrinol (Oxf) 1999; 50: 759–765, DOI: 10.1046/j.1365-2265.1999.00723.x. [DOI] [PubMed] [Google Scholar]
- 57.Mazziotti G, Formenti AM, Adler RA, et al. Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelines. Endocrine 2016; 54: 603–611, DOI: 10.1007/s12020-016-1146-8. [DOI] [PubMed] [Google Scholar]
- 58.Lekamwasam S, Adachi JD, Agnusdei D, et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 2012; 23: 2257–2276, DOI: 10.1007/s00198-012-1958-1. [DOI] [PubMed] [Google Scholar]
- 59.Lorenc R, Gluszko P, Franek E, et al. Guidelines for the diagnosis and management of osteoporosis in Poland: Update 2017. Endokrynol Pol 2017; 68: 604–609, DOI: 10.5603/EP.2017.0062. [DOI] [PubMed] [Google Scholar]
- 60.Ding L, Hu J, Wang D, et al. Efficacy and Safety of First- and Second-Line Drugs to Prevent Glucocorticoid-Induced Fractures. J Clin Endocrinol Metab 2020; 105: dgz023, DOI: 10.1210/clinem/dgz023. [DOI] [PubMed] [Google Scholar]
- 61.Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol 2021; 9: 847–875, DOI: 10.1016/S2213-8587(21)00235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Somma C, Colao A, Pivonello R, et al. Effectiveness of chronic treatment with alendronate in the osteoporosis of Cushing’s disease. Clin Endocrinol (Oxf) 1998; 48: 655–662, DOI: 10.1046/j.1365-2265.1998.00486.x. [DOI] [PubMed] [Google Scholar]
- 63.Dore RK, Cohen SB, Lane NE, et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis 2010; 69: 872–875, DOI: 10.1136/ard.2009.112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valassi E, Santos A, Yaneva M, et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol 2011; 165: 383–392, DOI: 10.1530/EJE-11-0272. [DOI] [PubMed] [Google Scholar]
- 65.Gupta A, Gupta Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab 2013; 17: 913–916, DOI: 10.4103/2230-8210.117215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine 2011; 78: 41–44, DOI: 10.1016/j.jbspin.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Herzog AG. Proximal myopathy associated with inhaled steroids. JAMA 1999; 281: 37, DOI: 10.1001/jama.281.1.37-c. [DOI] [PubMed] [Google Scholar]
- 68.Boonen S, Van Distel G, Westhovens R, Dequeker J. Steroid myopathy induced by epidural triamcinolone injection. Br J Rheumatol 1995; 34: 385–386, DOI: 10.1093/rheumatology/34.4.385. [DOI] [PubMed] [Google Scholar]
- 69.Muller LM, Kienitz T, Deutschbein T, et al. Glucocorticoid Receptor Polymorphisms Influence Muscle Strength in Cushing’s Syndrome. J Clin Endocrinol Metab 2020; 105: dgz052, DOI: 10.1210/clinem/dgz052. [DOI] [PubMed] [Google Scholar]
- 70.Schakman O, Kalista S, Barbe C, et al. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 2013; 45: 2163–2172, DOI: 10.1016/j.biocel.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 71.Fournier M, Huang ZS, Li H, et al. Insulin-like growth factor I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol 2003; 285: R34–43, DOI: 10.1152/ajpregu.00177.2002. [DOI] [PubMed] [Google Scholar]
- 72.Watson ML, Baehr LM, Reichardt HM, et al. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab 2012; 302: E1210–1220, DOI: 10.1152/ajpendo.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho JE, Fournier M, Da X, Lewis MI. Time course expression of Foxo transcription factors in skeletal muscle following corticosteroid administration. J Appl Physiol (1985) 2010; 108: 137–145, DOI: 10.1152/japplphysiol.00704.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imae M, Fu Z, Yoshida A, et al. Nutritional and hormonal factors control the gene expression of FoxOs, the mammalian homologues of DAF-16. J Mol Endocrinol 2003; 30: 253–262, DOI: 10.1677/jme.0.0300253. [DOI] [PubMed] [Google Scholar]
- 75.Kamei Y, Miura S, Suzuki M, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 2004; 279: 41114–41123, DOI: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 76.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004; 117: 399–412, DOI: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jagoe RT, Redfern CP, Roberts RG, et al. Skeletal muscle mRNA levels for cathepsin B, but not components of the ubiquitin-proteasome pathway, are increased in patients with lung cancer referred for thoracotomy. Clin Sci (Lond) 2002; 102: 353–361. [PubMed] [Google Scholar]
- 78.Wang XH, Zhang L, Mitch WE, et al. Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J Biol Chem 2010; 285: 21249–21257, DOI: 10.1074/jbc.M109.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jellyman JK, Martin-Gronert MS, Cripps RL, et al. Effects of cortisol and dexamethasone on insulin signalling pathways in skeletal muscle of the ovine fetus during late gestation. PLoS One 2012; 7: e52363, DOI: 10.1371/journal.pone.0052363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inder WJ, Jang C, Obeyesekere VR, Alford FP. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1 – implications for steroid-induced myopathy. Clin Endocrinol (Oxf) 2010; 73: 126–132, DOI: 10.1111/j.1365-2265.2009.03683.x. [DOI] [PubMed] [Google Scholar]
- 81.Latres E, Amini AR, Amini AA, et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 2005; 280: 2737–2744, DOI: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- 82.Schakman O, Gilson H, de Coninck V, et al. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 2005; 146: 1789–1797, DOI: 10.1210/en.2004-1594. [DOI] [PubMed] [Google Scholar]
- 83.McCroskery S, Thomas M, Maxwell L, et al. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 2003; 162: 1135–1147, DOI: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Balkom RH, van der Heijden HF, van Herwaarden CL, Dekhuijzen PN. Corticosteroid-induced myopathy of the respiratory muscles. Neth J Med 1994; 45: 114–122. [PubMed] [Google Scholar]
- 85.Bodine SC, Furlow JD. Glucocorticoids and skeletal muscle. Adv Exp Med Biol 2015; 872: 145–176, DOI: 10.1007/978-1-4939-2895-8_7. [DOI] [PubMed] [Google Scholar]
- 86.Ruff RL, Weissmann J. Endocrine myopathies. Neurol Clin 1988; 6: 575–592. [PubMed] [Google Scholar]
- 87.Alshekhlee A, Kaminski HJ, Ruff RL. Neuromuscular manifestations of endocrine disorders. Neurol Clin 2002; 20: 35–58, v-vi, DOI: 10.1016/s0733-8619(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 88.Braith RW, Welsch MA, Mills RM, Jr., et al. Resistance exercise prevents glucocorticoid-induced myopathy in heart transplant recipients. Med Sci Sports Exerc 1998; 30: 483–489, DOI: 10.1097/00005768-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Gilson H, Schakman O, Combaret L, et al. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology 2007; 148: 452–460, DOI: 10.1210/en.2006-0539. [DOI] [PubMed] [Google Scholar]
- 90.Porporato PE, Filigheddu N, Reano S, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Invest 2013; 123: 611–622, DOI: 10.1172/JCI39920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones A, Hwang DJ, Narayanan R, et al. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology 2010; 151: 3706–3719, DOI: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 92.Van Balkom RH, Dekhuijzen PN, Folgering HT, et al. Anabolic steroids in part reverse glucocorticoid-induced alterations in rat diaphragm. J Appl Physiol (1985) 1998; 84: 1492–1499, DOI: 10.1152/jappl.1998.84.5.1492. [DOI] [PubMed] [Google Scholar]
- 93.Crawford BA, Liu PY, Kean MT, et al. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 2003; 88: 3167–3176, DOI: 10.1210/jc.2002-021827. [DOI] [PubMed] [Google Scholar]
- 94.Robinzon B, Cutolo M. Should dehydroepiandrosterone replacement therapy be provided with glucocorticoids? Rheumatology (Oxford) 1999; 38: 488–495, DOI: 10.1093/rheumatology/38.6.488. [DOI] [PubMed] [Google Scholar]
- 95.Aversa Z, Alamdari N, Castillero E, et al. β-Hydroxy-β-methylbutyrate (HMB) prevents dexamethasone-induced myotube atrophy. Biochem Biophys Res Commun 2012; 423: 739–743, DOI: 10.1016/j.bbrc.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hickson RC, Wegrzyn LE, Osborne DF, Karl IE. Alanyl-glutamine prevents muscle atrophy and glutamine synthetase induction by glucocorticoids. Am J Physiol 1996; 271: R1165–1172, DOI: 10.1152/ajpregu.1996.271.5.R1165. [DOI] [PubMed] [Google Scholar]
- 97.Uozumi Y, Ito T, Takahashi K, et al. Myogenic induction of taurine transporter prevents dexamethasone-induced muscle atrophy. Adv Exp Med Biol 2006; 583: 265–270, DOI: 10.1007/978-0-387-33504-9_29. [DOI] [PubMed] [Google Scholar]
- 98.Menezes LG, Sobreira C, Neder L, et al. Creatine supplementation attenuates corticosteroid-induced muscle wasting and impairment of exercise performance in rats. J Appl Physiol (1985) 2007; 102: 698–703, DOI: 10.1152/japplphysiol.01188.2005. [DOI] [PubMed] [Google Scholar]
- 99.Biedasek K, Andres J, Mai K, et al. Skeletal muscle 11beta-HSD1 controls glucocorticoid-induced proteolysis and expression of E3 ubiquitin ligases atrogin-1 and MuRF-1. PLoS One 2011; 6: e16674, DOI: 10.1371/journal.pone.0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karnia MJ, Korewo D, Myslinska D, et al. The positive impact of vitamin D on glucocorticoid-dependent skeletal muscle atrophy. Nutrients 2021; 13: 936, DOI: 10.3390/nu13030936. [DOI] [PMC free article] [PubMed] [Google Scholar]