Glioblastoma (GBM) is a lethal brain tumor that has a median survival of 14 to 15 mo despite aggressive treatment with surgical resection, radiation, and chemotherapy (1). Studying the molecular genetics and epigenetics of GBM has not only revealed it to be a very heterogenous tumor with inter- and intrapatient variability, but also has given us glimpses into molecular vulnerabilities that can be harnessed for diagnosis and treatment planning (2). Isocitrate dehydrogenase 1 (IDH1) and IDH2 mutations in tumors can predict better outcomes than wild-type IDH tumors (3). The presence of methylguanine methyltransferase promoter methylation has been shown to improve temozolamide treatment in GBM (4). Epidermal growth factor receptor (EGFR) is yet another oncogene that is mutated, over-expressed, and amplified in GBM (5). EGFR can be amplified in extrachromosomal DNA as double minutes and lead to greater copy number of the DNA for the gene (6) or deletion of a region from exon 2 to 7 can lead to a truncated version called EGFRvIII that is constitutively active independent of ligand (7). While inhibitors of the EGFR pathway should ideally target oncogene addiction of GBMs, attempts to target EGFR with antibodies or small molecules have not led to clinical success (8, 9). Unlike nonsmall cell lung cancers (NSCLC) where EGFR mutations are found in the intracellular kinase domains keeping them in a constitutively active conformation that can be bound and inhibited by tyrosine kinase inhibitors such as erlotinib (TKIs), mutations in EGFR in GBM are in the extracellular domain, leading to only a partly active conformation of its kinase domain and rapid dissociation of erlotinib (10).
Given the bleak prognosis and challenges associated with treating GBMs with the traditional approach, alternative approaches to boost immunotherapy are critically needed. While the use of immune checkpoint inhibitors to block inhibitory proteins such as PD-1 (programmed cell death protein-1) and cytotoxic T-lymphocyte-associated protein 4 has become a major pillar of immunotherapy approaches in the treatment of melanoma, NSCLC, and urologic cancers, trials of anti-PD-1 therapy in GBM have been disappointing [CheckMate 143 trials (11)], despite combination with chemotherapy or radiation [CheckMate 498 and CheckMate 548 trials (12, 13)]. Paucity of CD8 T cells in GBM, and the upregulation of PD-L1 (Programmed death-ligand 1) on GBM cells can contribute to failure of therapy. Therefore, alleviating T cell exhaustion and increasing CD8 T cell persistence in the tumor can overcome the immunologically “cold” phenotype.
Zeng et al.’s work tying mutation-based therapy with immunotherapy can help target key vulnerabilities in EGFR-amplified GBM and provide a precedent for future studies delving into coamplified genes in the context of other tumors.
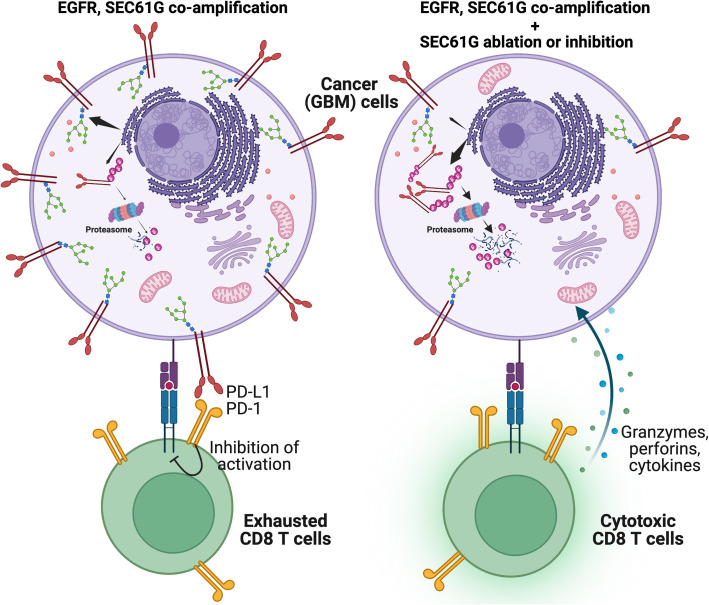
Targeting the genetic vulnerability of cancers while reducing checkpoint engagement in the tumor can boost antitumor activity of the already scant intratumoral CD8 T cells in GBM. Writing in PNAS, Zeng and colleagues demonstrate that targeting SEC61G, one of the genes that is coamplified at the 7p11 locus along with EGFR, can boost CD8 immune response against EGFR-amplified GBM (14). They show that SEC16G is one of the most frequently coamplified neighboring genes with EGFR and promotes stability and membrane presentation of checkpoint ligands such as PD-L1, PD-L2, and poliovirus receptor on GBM cells (Fig. 1). As part of the translocon complex secretory 61 (SEC61), SEC61G enables their translocation to ER for posttranslational N-glycosylation. Ablation of SEC16G prevented glycosylation of the checkpoint ligands and promoted their ubiquitination and ER-associated protein degradation, reducing membrane localization. Due to this, CD8 T cells had increased cytotoxicity against GL-26 cells expressing SEC61G shRNA (and diminished PD-L1 expression) and greater infiltration in the tumors.
Fig. 1.
Along with EGFR, SEC61G can be coamplified in 7p11 and promote immune escape of GBM cells. Checkpoint ligands such as PD-L1 can be glycosylated and stabilized by SEC61G-mediated translocon complex, leading to surface expression on GBM cells (Left). Ablating SEC61G or preventing SEC61 translocon can prevent PD-L1 glycosylation, promote their ubiquitination and degradation, and allow CD8 T cells to exert cytotoxic activity. (Created with Biorender).
Treating newly diagnosed GBM patients with EGFR TKIs has shown limited efficacy (9). Since EGFR amplification is found in more than 40% of GBMs, and half of these patients have EGFRvIII mutations, selecting patients based on EGFR status was expected to improve treatment with EGFR TKIs (7). But Erlotinib, a first-generation EGFR TKI, showed minimal efficacy despite high protein expression of EGFR, EGFRvIII, and PTEN in patients with recurrent GBM (8). Dacomitinib, a second-generation irreversible EGFR TKI, also showed limited activity in patients with EGFR-amplified recurrent GBM (15). There are studies that have implicated EGFR amplification as a possible resistance mechanism to EGFR TKIs (16). In this context, it is imperative to find strategies that can leverage EGFR amplification in tumors to find sustainable treatment options. In this issue of PNAS, Zeng and colleagues identify EGFR-co-amplified gene, SEC61G as a target to improve EGFR TKI efficacy and to boost lymphoid immune response in EGFR amplified GBM (14). Using intracranial tumor model in mice, they showed that SEC61G ablation in GBM cells along with erlotinib had the most robust inhibition of tumor growth, compared to SEC16G ablation or erlotinib alone. This was accompanied by a robust presence of anti-tumor CD8 T cells in the tumor, due to the downregulation of checkpoint ligands on GBM cells. The authors also used a small molecular inhibitor of the SEC61 translocon, Eeyarestatin I, which prevents protein translocation at the ER, to show diminished tumor growth in a flank tumor model when used as a monotherapy and a near-complete abrogation of tumor formation when used together with erlotinib. Increasing blood–brain barrier permeability of SEC61 inhibitors can open new avenues for the treatment of EGFR-amplified tumors, such as GBM and NSCLC. Currently, such an inhibitor is being tested in preclinical studies and being evaluated in a Phase I clinical trial (17).
By tying together mutation-targeted therapies and immunotherapy, these findings hold immense implications for treatment of EGFR-amplified GBM and improvement of immunotherapy strategies. While PD-L1 antibodies such as atezolizumab have not demonstrated clinical response (18), using SEC61G blocking agents could preclude the upregulation of alternative checkpoint ligands following PD-L1 blockade due to resistance mechanisms and clonal selection, since multiple checkpoint ligands rely on SEC61G translocon. Testing combination of SEC61G/EGFR blockade with radiation and/or chemotherapy and immunotherapy in clinical trials can further improve our understanding of the immune response and resistance mechanisms that can still emerge. Zeng and colleagues had identified multiple genes that were coamplified with EGFR in the 7p11 locus. These include genes that have been shown to increase resistance to EGFR TKIs in EGFR-mutant cancer cells (LANCL2) (19), resistance to apoptosis (VOPP1) (20), mitochondrial respiration and hypoxia-induced invasion (CHCHD2) (21), cancer cell proliferation (CCT6A) (22), and invasion (MRPS17 and PSPH) (23, 24). Therefore, the genes in 7p11 locus can promote tumor growth and suppress immune system in various ways. Zeng et al.’s work tying mutation-based therapy with immunotherapy can help target key vulnerabilities in EGFR-amplified GBM and provide a precedent for future studies delving into coamplified genes in the context of other tumors.
Acknowledgments
The author’s research is supported by the National Institutes of Health, Grants R01 CA230285-03 and R01 5R01NS121404-02.
Author contributions
A.P. and M.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
See companion article, “SEC61G assists EGFR-amplified glioblastoma to evade immune elimination,” 10.1073/pnas.2303400120.
References
- 1.Hanif F., Muzaffar K., Perveen K., Malhi S. M., Simjee S. U., Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J. Cancer Prev. 18, 3–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puchalski R. B., et al. , An anatomic transcriptional atlas of human glioblastoma. Science 360, 660–663 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H., et al. , IDH1 and IDH2 mutations in gliomas. N Engl. J. Med. 360, 765–773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegi M. E., et al. , MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl. J. Med. 352, 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Hatanpaa K. J., Burma S., Zhao D., Habib A. A., Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 12, 675–684 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner K. M., et al. , Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 543, 122–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellinghoff I. K., et al. , Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl. J. Med. 353, 2012–2024 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Gallego O., et al. , Efficacy of erlotinib in patients with relapsed gliobastoma multiforme who expressed EGFRVIII and PTEN determined by immunohistochemistry. J. Neurooncol. 116, 413–419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarti A., et al. , RTOG 0211: A phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int. J. Radiation Oncol. Biol. Phys. 85, 1206–1211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivanco I., et al. , Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2, 458–471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reardon D. A., et al. , Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The checkMate 143 phase 3 randomized clinical trial. JAMA Oncol. 6, 1003–1010 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omuro A., et al. , Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro Oncol. 25, 123–134 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim M., et al. , Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 24, 1935–1949 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng K., et al. , SEC61G assists EGFR-amplified glioblastoma to evade immune elimination. Proc. Natl. Acad. Sci. U.S.A. 120, e2303400120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sepúlveda-Sánchez J. M., et al. , Phase II trial of dacomitinib, a pan–human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro-Oncol. 19, 1522–1531 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H., et al. , EGFR amplification is a putative resistance mechanism for NSCLC–LM patients with TKI therapy and is associated with poor outcome. Front. Oncol. 12, 902664 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauwels E., Schülein R., K. Vermeire, Inhibitors of the Sec61 Complex and novel high throughput screening strategies to target the protein translocation pathway. Int. J. Mol. Sci. 22, 12007 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukas R. V., et al. , Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J. Neurooncol. 140, 317–328 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Lou Y., et al. , Akt kinase LANCL2 functions as a key driver in EGFR-mutant lung adenocarcinoma tumorigenesis. Cell Death Dis. 12, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S., James C. D., ECop (EGFR-Coamplified and overexpressed protein), a novel protein, regulates NF-κB transcriptional activity and associated apoptotic response in an IκBα-dependent manner. Oncogene 24, 2495–2502 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Lumibao J. C., et al. , CHCHD2 mediates glioblastoma cell proliferation, mitochondrial metabolism, hypoxia-induced invasion, and therapeutic resistance. bioRxiv (2022): 2022-07. [DOI] [PMC free article] [PubMed]
- 22.Zeng G., et al. , Overexpressing CCT6A contributes to cancer cell growth by affecting the G1-To-S phase transition and predicts a negative prognosis in hepatocellular carcinoma. Onco Targets Ther. 12, 10427–10439 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W., et al. , MRPS17 promotes invasion and metastasis through PI3K/AKT signal pathway and could be potential prognostic marker for gastric cancer. J. Cancer 12, 4849–4861 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawat V., et al. , PSPH promotes melanoma growth and metastasis by metabolic-deregulation–mediated transcriptional activation of NR4A1. Oncogene 40, 2448–2462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]