Abstract

Developing new methods of catalyst preparation is one of the most important tasks in the field of catalysis. A simple one-tube vapor deposition (VD) is provided in this paper for preparing the supported Ni catalyst. Ni(acac)2 was used as the Ni precursor. This preparation method was successfully applied to three types of catalytic supports, that is, Al2O3 and zeolites 5A and Hβ. Varying Ni contents of less than 8 wt % can be obtained by employing different conditions. The Ni content, depending on different deposition conditions, was preliminarily explored. The catalytic performance for oxidative dehydrogenation of ethane (ODHE) was tested for the prepared Ni catalysts by the VD method. Several cases of catalytic tests showed that for the same Ni content, the VD-prepared Ni catalyst presented better performance for ODHE than the one prepared by a traditional impregnation method. Besides the improvement in catalytic performance, several advantages of our VD preparation method for catalysis are discussed.
1. Introduction
Nickel-based catalysts, especially in the form of supported nickel catalysts, are widely used in the process of hydrogenation,1,2 methane reforming,3,4 hydrogenated cracking,5−7 and oxidative dehydrogenation (ODH) of lower alkanes in general8−23 and of ethane (ODHE) in particular.8−21 Developing a new preparation method for supported Ni catalysts could have potential significance in the following aspects. It may lead to cost saving for catalyst preparation on the one hand and lead to improvement in catalytic performance for certain reactions on the other hand. From a fundamental research point of view, it provides a new tool for a researcher to tune the status of the nickel component on the surface of the catalytic support.
Solution-based methods24 like wetness impregnation, precipitation, sol–gel, and ion-exchange are common methods to prepare supported Ni catalysts. For example, Nieto and co-workers14 used two different synthetic methods to synthesize Al2O3-supported nickel oxide catalysts to elucidate the chemical properties of NiO catalysts for oxidative dehydrogenation of ethane. (1) Oxalic acid was added to NiO/Al2O3 system as an organic additive. (2) Nb5+ was introduced as a dopant in the preparation of Al2O3 support. He and co-workers25 used the ligand unsaturated Al3+ center on mesoporous Al2O3 as a defect to induce Ni2+ anchoring on the surface of Al2O3 under alkaline conditions. Mesoporous Al2O3 undergoes structural reconstruction under alkaline induction, prompting Ni to form a small, uniform dispersion on the surface of Al2O3.
Compared to the solution-based methods, vapor deposition (VD)-based methods26 were used far less often for Ni catalysts. As a consequence, the catalytic properties of VD-based Ni catalysts are poorly understood. Ni(CO)4 was found to be a convenient precursor for the preparation of the Ni film with VD as early as 1890, and these films were active hydrogenation catalysts.27,28 Derouane et al.29 prepared supported Ni catalysts by bringing the Ni(CO)4 vapor to several catalytic supports. However, metal carbonyl compounds are highly toxic and explosive, which limits the application of this method. Lindblad et al.30 reported an atomic layer epitaxy (ALE) method for growing Ni particles on the Al2O3 support using Ni(acac)2 (acac = acetalacetonate) as the Ni precursor. The whole setup was somewhat complicated, which contained three chambers storing different solid/liquid/gas reactants. The vaporized Ni(acac)2 was brought to the Al2O3 support at a low pressure by the carrier gas. From the first ALE cycle, the atomic level of nickel dispersion was obtained; however, this catalyst did not present activity of toluene hydrogenation. More ALE cycles led to an increase of Ni content and the size of the Ni cluster, improving the catalytic activity. Medlin and co-workers31 have reported the atomic layer deposition (ALD) preparation of Al2O3-supported Ni catalyst using H2 and Ni(cp)2 as the precursors. The ALD-formed Ni component had a higher dispersion than that on the wetness impregnation catalysts. The ALD catalyst gave an obvious improvement in the selectivity to propylene hydrogenolysis instead of propylene hydrogenation compared with the impregnation catalyst. However, as is known, the cost of preparation for ALD/ALE is quite high.
Taghavi et al.32 used the evaporation impregnation method to impregnate Ni on the H-Beta support. First, the Ni precursor was dissolved in distilled water in the flask, and the H-Beta support was added to the solution. It was impregnated in a rotary evaporator, a water jet vacuum pump was used to evaporate the aqueous solution in a rotating steam, and finally, the obtained catalyst powder was dried and calcined. Although this method has the characteristics of VD, it still cannot avoid the treatment of waste liquid in the preparation.
In a previous work,33 we had used a simple one-tube VD method for preparing Al2O3-supported Fe catalysts (VD-Fe/Al2O3). Fe(cp)2 was used as the Fe precursor for deposition, and N2 was used as the carrier gas. VD-Fe/Al2O3 gave a much better catalytic performance for ODHE compared to the Fe/Al2O3 catalyst prepared by wetness impregnation (IMP-Fe/Al2O3). The catalytic performances were compared on the basis of the conversions of ethane [X(C2H6)], the conversion of oxygen [X(O2)], and the yield of ethylene [Y(C2H4)]. The results from the catalyst characterization showed a higher dispersion of the Fe components on VD-Fe/Al2O3 than on IMP-Fe/Al2O3.
However, as is known, Fe-based catalysts are usually not good ones for ODHE, especially the selectivities to ethylene [S(C2H4)] are always not high.12,34 Compared with supported Fe-based catalysts, supported Ni-based catalysts always present desirably high S(C2H4) for ODHE reations.8−13,15−21 On the other hand, the catalytic performance of a certain catalyst highly depends on the method of preparation. Therefore, in this context, two interesting questions are open. The first is whether the simple one-tube VD method is suitable for use in preparing supported Ni catalysts. The second is whether this VD method can lead to an improved catalytic performance for ODHE compared to the solution-based method such as traditional wetness impregnation (IMP). To answer these two questions in this work, we employed a one-tube VD method to prepared supported Ni catalysts using Al2O3, zeolite 5A, and zeolite Hβ as supports and using nickel acetylacetonate [Ni(acac)2] as the Ni precursor. Then, the Ni loading, depending on the preparation conditions, was examined. The catalytic performances of the supported Ni catalysts were compared with the VD and IMP methods.
2. Experimental Section
2.1. Catalyst Preparation
2.1.1. Preparation of the Catalytic Supports
In order to verify the universality of our one-tube VD preparation method, three kinds of catalytic supports were prepared before the VD process. These three were Al2O3, zeolite 5A, and zeolite Hβ.
In a typical preparation of the Al2O3 support,35 200 g of Al(OH)3 powder (≥97.0 wt %, Henghui Chemicals Co. Ltd., Yantai), 6.0 g of sesbania powder (biomass-derived macromolecular compound mixture with an average molecular weight of ∼200,000, served as glue and template for pores in the shaped Al2O3, Henghui Chemicals), 9.2 g of HNO3 (analytical purity with HNO3 wt % = 65%, Aladdin Reagents Company, Shanghai) solution, and 140 g of deionized water were uniformly mixed. Then, the mixture was extruded into strips with a 15 mm diameter by an extruding machine. The obtained strips were dried in air at room temperature for 24 h and then at 120 °C for another 24 h. The dried strips were calcined in air at 500 °C for 4 h to form γ-Al2O3, simply Al2O3 in this paper. Then, the Al2O3 strips were ground into 20–40 mesh particles.
The zeolite 5A (Si/Al ratio = 2.4) support was bought from Nankai Catalyst Company (Tianjing) and was ground into 20–40 mesh particles.
The zeolite Hβ (Si/Al ratio = 12) support was prepared by an ion-exchange process, followed by a shaping process. Zeolite Hβ powder (Nankai Catalyst Company) of 6 g and 200 mL of 0.4 mol/L NH4NO3 (≥98.5 wt %, Aladdin Reagents Company) solution were mixed. The slurry mixture was stirred at 80 °C for 6 h. The slurry was filtered, and the obtained powder was washed with a large amount of water. Then, the powder was dried in air at 110 °C for 4 h and calcined at 450 °C for 4 h. The obtained powder was further mixed with the Al(OH)3 powder (w/w = 9/1). The shaping process, including extruding and grinding, was the same as that for the Al2O3 support.
2.1.2. Preparation of Supported Ni Catalysts by VD
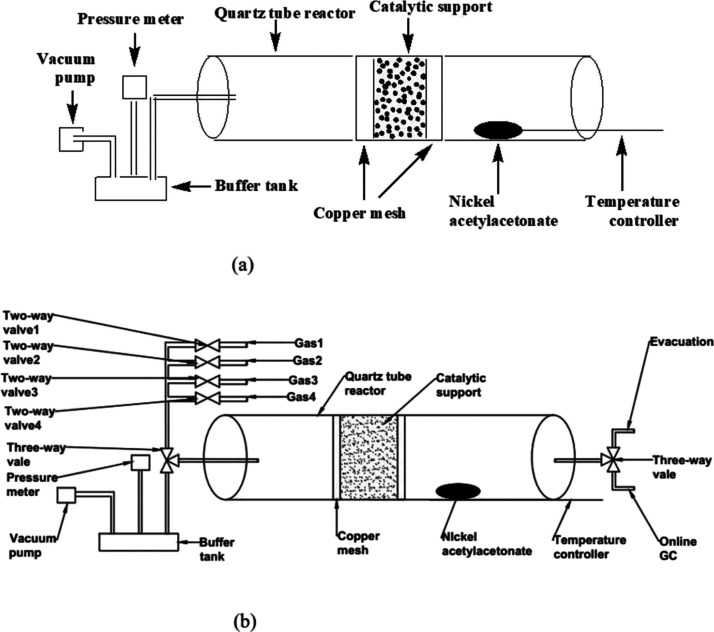
Figure 1a shows the homemade device for the one-tube VD preparation of supported Ni catalysts. This device was a revised version of the one used previously for the Fe catalyst.27 In a typical preparation, 1.0 g of a certain catalytic support (see Section 2.1.1) was fixed in the middle of a quartz tube with two pieces of copper mesh (60 mesh). A certain amount of Ni(acac)2 (Aladdin Reagents Company) was placed on one side of the support with a center distance of ∼5 cm. The mass of Ni(acac)2 is denoted as mpre hereafter. A vacuum pump was connected to the quartz tube on the other side of the support via a buffer tank. The pressure of the quartz tube was monitored by a pressure meter. This quartz tube was horizontally placed into a tubular furnace equipped with a temperature controller. The thermocouple of this controller monitored the temperature of the Ni(acac)2 pile. The pressure in the tube was kept lower than 1 kPa, and the temperature of Ni(acac)2 was kept at a value in the range of 200–300 °C (this value is denoted as TD) for 1 h.
Figure 1.
(a) Experimental device for preparing supported Ni catalysts by a one-tube VD method. The right side of the quartz tube was sealed by a two-way on/off valve. (b) Proposed combined device of catalyst preparation and catalytic reaction processes based on the device shown in (a).
The deposition procedure can be performed more than once to increase the Ni content on the catalyst. The number of operations is denoted as n in this paper. After the deposition procedure, the Ni-deposited catalytic support was moved to a muffle oven and calcined in the atmospheric air at 600 °C for 4 h for further use. A temperature increase rate of 10 K/min was set before the muffle oven, and the target calcination temperature was 600 °C. The catalyst prepared with the above-described procedure was denoted as VD-Ni/support, where “support” is one of Al2O3, 5A, or Hβ.
2.1.3. Preparation of Supported Ni Catalysts by Wetness Impregnation
After a certain supported Ni catalyst was prepared by the VD method, the Ni content of this catalyst was measured (vide infra). According to the measured Ni content of the prepared VD-Ni catalysts, a traditional equal-volume wetness impregnation method was also used to prepare supported Ni catalysts36 (denoted as IMP-Ni catalysts). The impregnating solution was aqueous Ni(NO3)2 solution, and the concentration of this solution was controlled in order to obtain the same Ni content as a certain VD-Ni catalyst. The same calcination process used for preparation of the VD-Ni catalysts was applied when preparing the IMP-Ni catalysts.
2.2. Catalyst Characterization
A known amount (∼0.1 g) of Ni catalyst was dissolved in a hot solution containing 2 mL of HClO4 (wt % = 70–72%, Aladdin Reagents Company), 6 mL of HNO3 (wt % = 65%), and 2 mL of HF (wt % = 40%, Aladdin Reagents Company). The amount of Ni in the solution was measured by a classical method of ultraviolet–visible spectroscopy.37 Dimethylglyoxime was used as the chromogenic agent for Ni2+ in the solution. The UV–vis absorbance was measured with an incident light of 465 nm on a SPECORD-205 (Jena, Germany) spectrometer.
The X-ray diffraction (XRD) patterns of the prepared catalysts were recorded by an X’pert PRO MPD diffractometer (PANalytical Company, Netherlands) with Cu–K radiation (40 kV, 40 mA) at a speed of 5 K/min.
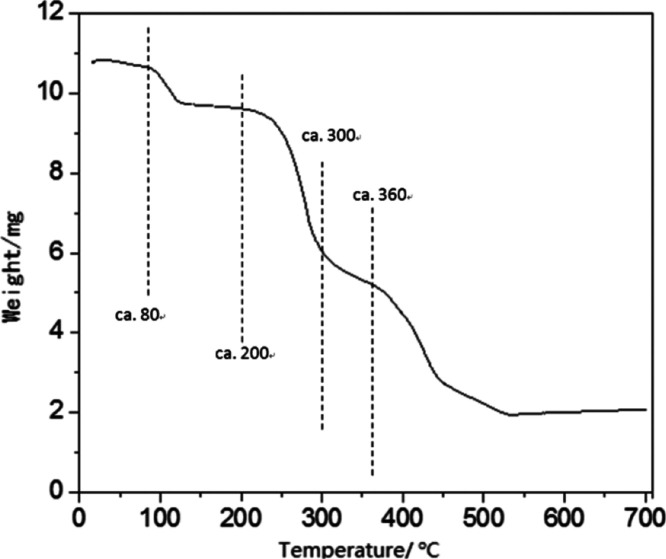
The thermogravimetric (TG) curve of Ni(acac)2 was recorded by a WCT-2D thermogravimeter (Beijing Manufactory of Optical Devices) in air with a temperature increasing rate of 10 K/min from room temperature.
2.3. Catalytic Performance Test of the Supported Ni Catalysts for ODHE
Catalytic ODHE was carried out in a tubular quartz fixed-bed reactor (i.d. of ∼10 mm) operating at atmospheric pressure. The as-prepared Ni catalyst was further sieved to the 420-to-840 μm particles, and such a catalyst of 0.5 g was diluted with 1 g of inert quartz particles having the same size range. The mixed particles were packed in a fixed-bed reactor. The reactant feed flow was a mixture of 11.0 sccm C2H6, 7.3 sccm O2, 62.3 sccm He, and 4.6 sccm N2 (O2, N2, He, and C2H6 were bought from Tianyuan Gas Co., Qingdao. O2, N2, and He had a purity of ≥99.99%, and C2H6 had a purity of ≥99.7%). The temperature of the center of the catalyst bed was controlled by a temperature controller. After the temperature reached an interested value, it was kept at this value for 1 h. The product flow was analyzed by an online gas chromatograph equipped with a thermal conductivity detector for the analysis of O2, N2, CO, and CO2 and a flame ionization detector for the analysis of gas hydrocarbon compounds. The carbon balance was 95–100%. The catalytic performance of a supported Ni catalyst for ODHE was calculated by the following equations:
| 1 |
| 2 |
| 3 |
| 4 |
where “MF( )” and “MF( )0” represent the molar fraction of a certain substance in the product flow and in the reactant flow, respectively.
3. Results and Discussion
3.1. Catalyst Preparation by the VD Method
3.1.1. Effect of Deposition Temperature on Ni Content
Figure 2 shows the Ni content of the VD-Ni/Al2O3 catalysts prepared at different Td values under the conditions of mpre = 1.0 g and n = 1 (see parameter definition in Section 2.1.2). The Ni content has a maximum loading of ∼7% at 240 °C. The existence of a maximum can be accounted for the decomposition of Ni(acac)2 occurring at the temperature higher than 240 °C. For example, when Td was 240 °C, almost all of the original Ni(acac)2 pile disappeared because of thermal vaporization; however, when Td was 280 °C, a noticeable portion of the Ni(acac)2 pile stayed and appeared sintered. At Td lower than 200 °C, the color of Al2O3 particles was not quite uniform, showing that the distribution of the deposited Ni precursor was not uniform.
Figure 2.
Dependence of Ni content (in wt %) on the deposition temperature (TD) for the prepared VD-Ni/Al2O3 catalysts. Other conditions: number of deposition times of 1 and mass of Ni(acac)2 of 1 g.
In our previous study,33 a maximum Fe content was also observed when preparing Fe/Al2O3 catalyst through VD using Fe(cp)2 as the Fe precursor. In that case, Fe(cp)2 was brought to Al2O3 particles through both thermal evaporation and carrier gas flow. Further characterization showed that Fe(cp)2 resided on the surface of Al2O3 mostly through physical instead of chemical adsorption, which accounted for the maximum. It had been shown in the literature that Ni(acac)2 interacts with the Al2O3 surface more favorably via physical adsorption.30 However, there was no carrier gas flow when Ni catalysts were prepared in this work. In order to better understand the deposition process, the TG curve of Ni(acac)2 was measured, which is presented in Figure 3. A slight weight loss starting at ∼100 °C was due to the loss of residue water. The weight loss in the range of 200–300 °C can be due to both physical evaporation and chemical decomposition of Ni(acac)2.38 The weight loss starting at ∼380 °C can be due to a further chemical decomposition of the compound form from the previous decomposition of Ni(acac)2. There was no observable weight loss after ∼530 °C, which supports our selection of a calcination temperature of 600 °C.
Figure 3.
Thermogravimetric curve of Ni(acac)2.
In general, according to the classic Clausius–Clapeyron equation, the saturated vapor pressure of a solid or liquid substance increases exponentially with −1/T, where T is the temperature of this substance. Therefore, during the course of the deposition process, the increasing Td was advantageous for increasing the vapor pressure of Ni(acac)2, which favored the Ni content on the catalyst, and was also advantageous for the chemical decomposition of Ni(acac)2 before deposition, which disfavored the Ni content.
3.1.2. Effect of the Type of Catalytic Support on the Ni Content
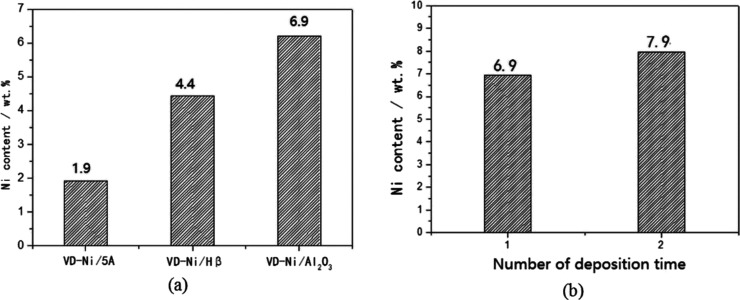
Figure 4a shows that the vapor-deposited Ni content varied with the type of catalytic support, which followed the order Al2O3 > Hβ > 5A at the conditions of TD = 1, mpre = 1.0 g, and n = 1. This variation may be accounted for different pore sizes in these catalytic supports, which influenced the diffusion behavior of the Ni precursor. All these supports are porous materials. Al2O3 has pores with the size ∼7 nm according to a previous BET result.33 Zeolite Hβ has ordered 1D subnanometer pores of ∼0.6 nm.39 Zeolite 5A has a faujasite-type structure containing three different types of cages at the subnanometer scale.39
Figure 4.
Dependence of Ni content (in wt %) on the (a) type of catalytic support for the supported VD-Ni catalysts and (b) number of deposition times for the VD-Ni/Al2O3 catalysts. Other conditions: (a) number of deposition time = 1, mass of Ni(acac)2 = 1 g, and deposition temperature = 240 °C; (b) mass of Ni(acac)2 = 1 g and deposition temperature = 240 °C.
As is known, the catalytic performance may be largely influenced by the catalytic support for a certain supported catalyst. Obviously the success of Ni catalyst preparation on different supports broadens the application of our one-tube VD method. During our exploration, it was found that the particle size of the catalytic support was the key for the successful preparation. For example, for the Hβ support, it was mixed with a small portion of Al2O3 and shaped to be 20–40 mesh particles. When the original Hβ powder was used instead, no noticeable Ni content was observed after the VD process.
3.1.3. Effect of the Number of Deposition Times on the Ni Content
Figure 4b shows that an increase of the number of deposition times (n) led to an increased Ni content. The Ni content introduced by the second-time deposition was obviously less than that introduced by the first-time deposition. This also reflected that for the first-time deposition, most of the deposited Ni(acac)2 existed on the surface of the support through physical adsorption, and a small portion changed its chemical status to be a nonvolatile Ni component. For the second-time deposition, the physically adsorbed Ni precursor can leave the surface of Al2O3 and a new Ni precursor deposited on the surface at the same time.
It can be anticipated reasonably that catalysts with a larger Ni content can be obtained by the one-tube VD method with more deposition times (larger n). However, in the following study of catalytic performance test for ODHE, it was found that the VD-Ni catalysts did not present better performance than the IMP-Ni catalysts when the Ni content was larger than 6%. Therefore, the VD-Ni catalysts prepared with n > 2 were not used in this work.
3.2. XRD Measurement
Figure 5 presents the XRD patterns of several supported Ni catalysts prepared by the VD and IMP methods. The selected Ni catalysts were the ones which would be used to perform catalytic reaction tests later (in Section 3.3). These XRD data show that the prepared Ni catalysts with both methods did not have observable features other than the catalytic supports themselves. This phenomenon implicated that the Ni component on the prepared Ni catalysts existed in small particles with the size of several nanometers or smaller since typically particles with the size larger than 5 nm can usually be observed with XRD.
Figure 5.
X-ray diffraction patterns of the supported Ni catalysts. Catalytic supports are (a) Al2O3, (b) zeolite 5A, and (c) zeolite Hβ. The XRD patterns of the undeposited catalytic supports are also shown for comparison.
From the commonsense of the catalytic chemistry, the high dispersion of the active metal component is one of the keys for a supported metal catalyst to present good catalytic performance. Therefore, the above XRD results intrigued us to compare the catalytic performance of the VD-Ni catalysts with that of the IMP-Ni ones.
3.3. Catalytic Performance of the VD-Ni and IMP-Ni Catalysts for ODHE
The success in the one-tube VD preparation of supported Ni catalysts on different supports intrigued us to explore the catalytic performances of these catalysts. A noticeable amount of reports in the literature in the recent years8−21 show that Ni-based catalysts are usually able to present desirable catalytic performance for ODHE. Therefore, comparisons between the VD- and IMP-Ni catalysts with different supports were done by testing their catalytic performance for ODHE.
Figure 6 presents the results for the catalytic test of the VD- and IMP-prepared Ni/Al2O3 catalysts with the same Ni content of 4.1% (prepared with TD = 240 °C, mpre = 0.5 g, and n = 1). The catalytic performances of these two catalysts are quite comparable to each other. The conversion of the two reactants, X(C2H6) and X(O2), were slightly higher from the VD-Ni/Al2O3 catalyst than those from the IMP-Ni/Al2O3 one at both temperatures. Although the former presented a slight lower selectivity to C2H4 [S(C2H4)] than the latter, the former still presented a slightly higher yield of C2H4. These trends were also observed for the VD-Fe/Al2O3 and IMP-Fe/Al2O3 catalysts in our previous study,33 although the Fe catalysts had much lower S(C2H4) than the Ni ones.
Figure 6.
Comparison between the catalytic performances for the ODHE given by the VD-Ni/Al2O3 and IMP-Ni/Al2O3 catalysts. See details for the catalytic test in Section 2.3. The Ni content was 4.1%. Reactant flow: 11.0 sccm C2H6, 7.3 sccm O2, 62.3 sccm He, and 4.6 sccm N2. Reaction temperature: (a) 510 °C and (b) 600 °C.
When the 5A zeolite was used as the catalytic support, the catalytic performance of the VD-Ni/5A catalyst (prepared with TD = 240 °C, mpre = 1 g and n = 1) was noticeably better than the IMP-Ni/5A one. Figure 7 presents the results for the catalytic test of the VD- and IMP-prepared Ni/5A catalysts with the same Ni content of 1.9%. For example, when the reaction temperature was 510 °C (Figure 7a), X(O2) was improved by more than 8%, and S(C2H4) was improved by ∼15%. When the temperature was increased to 600 °C, the improvements of the X(O2) and S(C2H4) values were decreased. However, the VD-Ni/5A still had a noticeably higher S(C2H4) and Y(C2H4) by 8.4 and 2.1%, respectively.
Figure 7.
Comparison between the catalytic performances for the oxidation dehydrogenation of ethane given by the VD-Ni/5A and IMP-Ni/5A catalysts. See details for catalytic test in Section 2.3. The Ni content was 1.9%. Reactant flow: 11.0 sccm C2H6, 7.3 sccm O2, 62.3 sccm He, and 4.6 sccm N2. Reaction temperature: (a) 510 and (b) 600 °C.
The above two comparison cases reinforced that S(C2H4) usually increases with the temperature for the Ni-based catalysts.11,12 Severe coking was observed on the VD-Ni/Hβ catalyst (prepared with TD = 240 °C, mpre = 0.5 g and n = 1) at temperatures higher than 500 °C. The coking process can be mainly due to the complete consumption of O2 in the reaction system (since the reaction feed was C2H6 rich and formation of CO2 consumed a large portion of O2) and partly due to the high acidity of the Hβ zeolite.40 At a lower temperature of 475 °C, the VD-Ni/Hβ catalyst also presented higher X(C2H6), X(O2), S(C2H4), and Y(C2H4) values than the IMP-Ni/Hβ catalyst (see Figure 8).
Figure 8.
Comparison between the catalytic performances for the ODHE given by the VD-Ni/Hβ and IMP-Ni/Hβ catalysts at 475 °C. The Ni content was 2.2%. See details for catalytic test in Section 2.3. The Ni content was 1.9%. Reactant flow: 11.0 sccm C2H6, 7.3 sccm O2, 62.3 sccm He, and 4.6 sccm N2..
The catalytic performance for ODHE, especially the C2H4 selectivity, may be closely related to the explicit dispersion of the Ni component in a subnanometer-to-several nanometer scale.15 In the present stage, it still requires further explicit characterization on the Ni dispersion to gain a deeper understanding of the underlying reason for the better catalytic performance presented by the VD-Ni catalysts than that by the IMP-Ni ones. However, such an investigation is out of the scope of this paper. The results in this paper reinforce that the development of a new method for catalyst preparation can result in a good chance to improve the catalytic performance of important reactions in the chemical industry. It would be of great interest to further discover the catalytic properties of the VD-Ni catalysts prepared in this paper.
Further Discussion about the Advantage of the One-Tube VD Preparation Method
Section 3.3 has demonstrated that the VD-Ni catalysts can provide improved catalytic performance for ODHE compared with the IMP-Ni ones. Besides this advantage, the one-tube VD preparation method provided in this paper has several other advantages as follows.
First, compared to the traditional solution-based method, such as impregnation, our VD method is a solution-free one. As-supported Ni catalysts are widely used in the chemical industry; a solution-free method may avoid preparing huge amounts of solution and disposal of waste liquid, which is of large economic significance from an industrial application point of view.
Second, our VD method is potentially appropriate for large-scale production. Inspection of Figure 1 for the preparation device shows that each unit of this device is a commonly used one, and the whole set up is suitable for magnification. It is known that ALE/ALD-based methods30,31 require very expensive setup. In contrast, our method is obviously cheaper.
Third, our VD method for preparing supported Ni catalyst does not need carrier gas or reacting gas compared with the ALE/ALD-based methods. The only two starting materials are Ni(acac)2 and a certain type of catalytic support. This makes our method greener and more competitive since there is no exhaust gas issue.
Fourth, from the aspect of catalytic application, if one aims at using the prepared Ni catalysts for catalytic reactions like ODHE in special and selective conversion of light alkanes in general, another advantage for the new preparation method can be found. This advantage is that it is technically feasible to combine the catalyst preparation process with a catalytic reaction with one device. The “combined device” can be obtained by making two changes from the device shown in Figure 1a, which is shown in Figure 1b. The first change is to add a three-way valve to the left side of the quartz tube for switching between the buffer tank for catalyst preparation and the feed of the reactant mixture for the catalytic reaction. The second change is to connect to the two-way valve at the right side of the quartz tube with a product collecting system or a GC to analyze the product mixture. The proposed combined device may help one save the device cost on the one hand and also save the operation cost on the other hand.
4. Conclusions
Recalling the two open questions raised in the introduction section, one can see that the significance of this work is two-fold. First, we provide a simple one-tube VD method for the preparation of supported Ni catalyst with Ni(acac)2 as the Ni precursor. This preparation method was successful to apply to different types of supports (Al2O3, zeolite 5A, and zeolite Hβ). The varying Ni content less than 8 wt % can be obtained by using different deposition conditions. The Ni content depending on different deposition conditions was preliminarily explored. Second, three cases are shown where a VD-prepared Ni catalyst presented better catalytic performance for ODHE than the IMP-prepared one having the same Ni content. Based on this result, several advantages of our preparation method for catalysis are discussed.
Acknowledgments
Support from the National Natural Science Foundation of China (21576291), the Fundamental Research Funds for the Central Universities (22CX06012A) presented by Dr. Lishuang Ma, and the Natural Science Foundation of Rizhao City (RZ2021ZR40) is gratefully acknowledged.
The authors declare no competing financial interest.
References
- Feng J.; Wang Q.; Fan D. Nickel-based xerogel catalysts: synthesis via fast sol-gel method and application in catalytichydrogenation of p-nitrophenol to p-aminophenol. Appl. Surf. Sci. 2016, 382, 135–143. 10.1016/j.apsusc.2016.04.125. [DOI] [Google Scholar]
- Yang Y.; Ma J.; Jia X. Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol on alkaline earth metal modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. 10.1039/C6RA05680F. [DOI] [Google Scholar]
- Zagaynov I. V.; Loktev A. S.; Arashanova A. L. Ni(Co)-Gd0.1Ti0.1Zr0.1Ce0.7O2 mesoporous materials in partial oxidation and dry reforming of methane into synthesis gas. Chem. Eng. J. 2016, 290, 193–200. 10.1016/j.cej.2016.01.066. [DOI] [Google Scholar]
- Oemar U.; Kathiraser Y.; Mo L. CO2 reforming of methane over highly active La-promoted Ni supported on SBA-15 catalysts: mechanism and kinetic modeling. Catal. Sci. Technol. 2016, 6, 1173–1186. 10.1039/C5CY00906E. [DOI] [Google Scholar]
- Escola J. M.; Serrano D. P.; Aguado J. Hydroreforming of the LDPE thermal cracking oil over hierarchical Ni/Beta catalysts with different Ni particle size distributions. Ind. Eng. Chem. Res. 2015, 54, 6660–6668. 10.1021/acs.iecr.5b01160. [DOI] [Google Scholar]
- Aguado J.; Serrano D. P.; Escola J. M. Deactivation and regeneration of a Ni supported hierarchical Beta zeolite catalyst used in the hydroreforming of the oil produced by LDPE thermal cracking. Fuel 2013, 109, 679–686. 10.1016/j.fuel.2013.03.011. [DOI] [Google Scholar]
- Escola J. M.; Aguado J.; Serrano D. P. Conversion of polyethylene into transportation fuels by the combination of thermal cracking and catalytic hydroreforming over Ni-supported hierarchical beta zeolite. Energy Fuels 2012, 26, 3187–3195. 10.1021/ef300938r. [DOI] [Google Scholar]
- Zhang X.; Liu J.; Xie Y. Support effects on the catalytic behavior of NiO/Al2O3 for oxidative dehydrogenation of ethane to ethylene. Appl. Catal., A 2003, 240, 143–150. 10.1016/S0926-860X(02)00426-X. [DOI] [Google Scholar]
- Heracleous E.; Lemonidou A. A. Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part I: Characterization and catalytic performance. J. Catal. 2006, 237, 162–174. 10.1016/j.jcat.2005.11.002. [DOI] [Google Scholar]
- Cavani F.; Ballarini N.; Cericola A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation. Catal. Today 2007, 127, 113–131. 10.1016/j.cattod.2007.05.009. [DOI] [Google Scholar]
- Lin X.; Hoel C. A.; Sachtler W. M. H.; Poeppelmeier K. R.; Weitz E. Oxidative dehydrogenation (ODH) of ethane with O2 as oxidant on selected transition metal-loaded zeolites. J. Catal. 2009, 265, 54–62. 10.1016/j.jcat.2009.04.007. [DOI] [Google Scholar]
- Lin X.; Poeppelmeier K. R.; Weitz E. Oxidative dehydrogenation of ethane with oxygen catalyzed by K-Y zeolite supported first-row transition metals. Appl. Catal., A 2010, 381, 114–120. 10.1016/j.apcata.2010.03.049. [DOI] [Google Scholar]
- Solsona B.; López Nieto J. M.; Concepción P.; Dejoz A.; Ivars F.; Vázquez M. I. Oxidative dehydrogenation of ethane over Ni–W–O mixed metal oxide catalysts. J. Catal. 2011, 280, 28–39. 10.1016/j.jcat.2011.02.010. [DOI] [Google Scholar]
- Abdelbaki Y.; Arriba A. D.; Solsona B.; Delgado D.; García-Gonzalez E.; Issaadi R.; López Nieto J. M. The nickel-support interaction as determining factor of the selectivity to ethylene in the oxidative dehydrogenation of ethane over nickel oxide/alumina catalysts. Appl. Catal., A 2021, 623, 118242 10.1016/j.apcata.2021.118242. [DOI] [Google Scholar]
- Rodriguez-Castelljn E.; Delgado D.; Dejoz A.; Vazquez I.; Agouram S.; Cecilia J. A.; Solsona B.; Nieto J. M. L. Enhanced NiO dispersion on a high surface area pillared heterostructure covered by niobium leads to optimal behaviour in the oxidative dehydrogenation of ethane. Chem.—Eur. J. 2020, 26, 9371–9381. 10.1002/chem.202000832. [DOI] [PubMed] [Google Scholar]
- Xu X.; Megarajan S. K.; Xia X.; Toghan A.; Feldhof A.; Zhang Y.; Jiang H. Q. Effect of reduction temperature on the structure and catalytic performance of mesoporous Ni–Fe–Al2O3 in oxidative dehydrogenation of ethane. New J. Chem. 2020, 44, 18994. 10.1039/D0NJ02618B. [DOI] [Google Scholar]
- Abdelbaki Y.; De Arriba A.; Solsona B.; Delgado D.; García-Gonzalez E.; Issaadi R.; Nieto J. M. L. The nickel-support interaction as determining factor of the selectivity to ethylene in the oxidative dehydrogenation of ethane over nickel oxide/alumina catalysts. Appl. Catal., A 2021, 623, 118242 10.1016/j.apcata.2021.118242. [DOI] [Google Scholar]
- Li X. Q.; Yang Z. Q.; Zhang L.; He Z. Q.; Fang R. M.; Wang Z. Q.; Yan Y. F.; Ran J. Y. Effect of Pd doping in (Fe/Ni)/CeO2 catalyst for the reaction path in CO2 oxidative ethane dehydrogenation/reforming. Energy 2021, 234, 121261 10.1016/j.energy.2021.121261. [DOI] [Google Scholar]
- Brussino P.; Mehring E. L.; Ulla M. A.; Bortolozzi J. P. Tuning the properties of NiO supported on silicon-aluminum oxides: Influence of the silica amount in the ODH of ethane. Catal. Today 2022, 394–396, 133–142. 10.1016/j.cattod.2021.10.017. [DOI] [Google Scholar]
- Wang C. J.; Tian M.; Han Y. J.; Zong T.; Wang N. X.; Li L.; Lin J.; Wang X. D. Dual Ni active sites mediated by In to separate ethane activation and oxidation for enhanced ethene production via chemical looping scheme. Appl. Catal., B 2023, 325, 122334 10.1016/j.apcatb.2022.122334. [DOI] [Google Scholar]
- Kong L.; Li D.; Bi J. Y.; Fan X. Q.; Xie Z. A.; Xiao X.; Zhao Z. Template-induced mesoporous Ni–Al oxide catalysts with tuned physico–chemical properties for the oxidative dehydrogenation of ethane. Chem. Eng. J. 2023, 452, 139247 10.1016/j.cej.2022.139247. [DOI] [Google Scholar]
- Li J. H.; Wang C. C.; Huang C. J.; Sun Y. F.; Weng W. Z.; Wan H. L. Mesoporous nickel oxides as effective catalysts for oxidative dehydrogenation of propane to propene. Appl. Catal., A 2010, 382, 99–105. 10.1016/j.apcata.2010.04.034. [DOI] [Google Scholar]
- Fukudome K.; Kanno A.; Ikenaga N.; Miyake T.; Suzuki T. The oxidative dehydrogenation of propane over NiO–ZrO2 catalyst. Catal. Lett. 2011, 141, 68–77. 10.1007/s10562-010-0461-6. [DOI] [Google Scholar]
- Gallei E. F.; Hesse M.; Schwab E. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl G., Knözinger H., Schüth F., Weitkamp J., Eds.; Wiley-VCH, 2012; pp 57–719. [Google Scholar]
- Liu X.; Zhang L.; Zheng X.; Zhang Y.; He D.; Luo Y. Highly dispersed Ni/Al2O3 catalysts for dry reforming of methane prepared by alkalineinduced adsorption process. Int. J. Hydrogen Energy 2022, 47, 30937–30949. 10.1016/j.ijhydene.2022.01.217. [DOI] [Google Scholar]
- Serp P.; Kalck P. Chemical vapor deposition methods for the controlled preparation of supported catalytic materials. Chem. Rev. 2002, 102, 3085–3128. 10.1021/cr9903508. [DOI] [PubMed] [Google Scholar]
- Mond L.; Langer C.; Quincke F. Action of carbon monoxide on nickel. J. Chem. Soc. 1890, 57, 749–753. 10.1039/CT8905700749. [DOI] [Google Scholar]
- Baker L. L. Jr; Bernstein R. B. Carbonyl nickel films as hydrogenation catalysts. J. Am. Chem. Soc. 1951, 73, 4434–4436. 10.1021/ja01153a118. [DOI] [Google Scholar]
- Derouane E. G.; Nagy J. B.; Védrine J. C. Adsorption and thermal decomposition of Ni(CO)4 on various oxides: A novel preparation of dispersed metallic nickel catalysts. J. Catal. 1977, 46, 434. 10.1016/0021-9517(77)90231-7. [DOI] [Google Scholar]
- Lindblad M.; Lindfors L. P.; Suntola T. Preparation of Ni/Al2O3 catalysts from vapor phase by atomic layer epitaxy. Catal. Lett. 1994, 27, 323–336. 10.1007/BF00813919. [DOI] [Google Scholar]
- Gould T. D.; Lubers A. M.; Neltner B. T.; Carrier J. V.; Weimer A. W.; Falconer J. L.; Will Medlin J. Synthesis of supported Ni catalysts by atomic layer deposition. J. Catal. 2013, 303, 9–15. 10.1016/j.jcat.2013.03.013. [DOI] [Google Scholar]
- Taghavi S.; Mki-Arvela P.; Vajglová Z.. One-Pot Transformation of citronellal to menthol over H-Beta zeolite supported Ni catalyst: effect of catalyst support acidity and Ni loading Catal. Lett. 2023, 153, 2674, 10.1007/s10562-022-04178-x. [DOI] [Google Scholar]
- Xu L.; Lin X.; Xi Y.; Lu X.; Wang C.; Liu C. Alumina-supported Fe catalyst prepared by vapor deposition and its catalytic performance for oxidative dehydrogenation of ethane. Mater. Res. Bull. 2014, 59, 254–260. 10.1016/j.materresbull.2014.07.023. [DOI] [Google Scholar]
- Schuurman Y.; Ducarme V.; Chen T.; Li W.; Mirodatos C.; Martin G. A. Low temperature oxidative dehydrogenation of ethane over catalysts based on group VIII metals. Appl. Catal., A 1997, 163, 227–235. 10.1016/S0926-860X(97)00147-6. [DOI] [Google Scholar]
- Liu B.; Chai Y. M.; Wang Y. J.; Zhang T. T.; Liu Y. Q.; Liu C. G. A simple technique for preparation of presulfided eggshell MoS2/Al2O3 catalysts and kinetics approach for highly selective hydrodesulfurization of FCC gasoline. Appl. Catal., A 2010, 388, 248–255. 10.1016/j.apcata.2010.08.059. [DOI] [Google Scholar]
- Hoang D. L.; Berndt H.; Vtilter J. Nickel modified H-ZSM-5 catalysts. Appl. Catal., A 1994, 114, 295–311. 10.1016/0926-860X(94)80181-9. [DOI] [Google Scholar]
- Wang Y. Determination of Ni in nickelferrite by ammonium persulfate–dimethylglyoxime differential spectrophotometry method. J. Northeast For. Univ. 2001, 29, 82–83. [Google Scholar]
- Moravec P.; Smolik J.; Keskinen H.; Mm Makela J.; Bakardjieva S. NiOx Nanoparticle synthesis by chemical vapor deposition from nickel acetylacetonate. Mater. Sci. Appl. 2011, 2, 258–264. 10.4236/msa.2011.24033. [DOI] [Google Scholar]
- Baerlocher C.; McCusker L. B.; Olson D. H. In Atlas of zeolite framework types, 6th ed.; Elsevier, 2007. [Google Scholar]
- Lin X.; Lv Y.; Xi Y.; Qu Y.; Phillips D. L.; Liu C. Hydrogenolysis of glycerol by the combined use of zeolite and Ni/Al2O3 as catalysts: a route for achieving high selectivity to 1-propanol. Energy Fuels 2014, 28, 3345–3351. 10.1021/ef500147k. [DOI] [Google Scholar]