Abstract
Petals and leaves share common evolutionary origins but have different phenotypic characteristics, such as the absence of stomata in the petals of most angiosperm species. Plant NAC transcription factor, NAP, is involved in ABA responses and regulates senescence-associated genes, and especially those that affect stomatal movement. However, the regulatory mechanisms and significance of NAP action in senescing astomatous petals is unclear. A major limiting factor is failure of flower opening and accelerated senescence. Our goal is to understand the finely regulatory mechanism of dehydration tolerance and aging in rose flowers. We functionally characterized RhNAP, an AtNAP-like transcription factor gene that is induced by dehydration and aging in astomatous rose petals. Cytokinins (CKs) are known to delay petal senescence and we found that a cytokinin oxidase/dehydrogenase gene 6 (RhCKX6) shares similar expression patterns with RhNAP. Silencing of RhNAP or RhCKX6 expression in rose petals by virus induced gene silencing markedly reduced petal dehydration tolerance and delayed petal senescence. Endogenous CK levels in RhNAP- or RhCKX6-silenced petals were significantly higher than those of the control. Moreover, RhCKX6 expression was reduced in RhNAP-silenced petals. This suggests that the expression of RhCKX6 is regulated by RhNAP. Yeast one-hybrid experiments and electrophoresis mobility shift assays showed that RhNAP binds to the RhCKX6 promoter in heterologous in vivo system and in vitro, respectively. Furthermore, the expression of putative signal transduction and downstream genes of ABA-signaling pathways were also reduced due to the repression of PP2C homolog genes by RhNAP in rose petals. Taken together, our study indicates that the RhNAP/RhCKX6 interaction represents a regulatory step enhancing dehydration tolerance in young rose petals and accelerating senescence in mature petals in a stomata-independent manner.
Supplementary Information
The online version contains supplementary material available at 10.1186/s43897-021-00016-7.
Keywords: Rosa hybrida, RhNAP, RhCKX6, Dehydration, Petal senescence, Cytokinins
Core
Cytokinins (CKs) play an important role in the regulation of environmental stress responses and organ senescing processes through keeping at the appropriate level of CK activity. Here we reveal that the dehydration- and aging-induced RhNAP physically binds to the promoter of RhCKX6, promoting CK catabolism. The RhNAP/RhCKX6 interaction represents a regulatory step enhancing dehydration tolerance in young rose petals and accelerating senescence in mature petals in a stomata-independent manner.
Introduction
Plant organ senescence is a finely tuned developmental process during which the constituent cells undergo dramatic changes in metabolism, structure and gene expression (Woo et al. 2018; Ma et al. 2018) and, ultimately, programmed cell death (PCD) (van Doorn and Woltering 2008; Kabbage et al. 2017). Developmentally regulated senescence has been studied in leaves (Zhang and Gan 2012; Jiang et al. 2014), fruits (Jiang et al. 2017) and flowers (Wu et al. 2017; Lü et al. 2014), and it has also been shown that environmental conditions, such as drought, darkness, high temperature and salinity, as well as pathogen challenge, can trigger organ senescence (Sade et al. 2018; Patharkar and Walker 2019). However, the mechanistic relationships between senescence that is developmentally programmed or that which is environmentally induced, or the nature of any shared signaling pathways, are not well understood (Guo and Gan 2012).
It has been reported that senescence programs in both leaves and flowers involve the preferential expression of a specific set of senescence associated genes (SAGs), which include regulatory transcription factors (TFs) and structural proteins (Gao et al. 2016; Li et al. 2018; Sun et al. 2021). Among these, different classes of TF genes have been functionally associated with leaf and/or flower senescence, including MYB, MYC, C2H2-type zinc-finger, AP2/EREBP, MADS-Box, NAC and WRKY domain TFs, as well as homeodomain proteins (Li et al. 2018; Shahri and Tahir 2014). As an example, the A. thaliana NAC family gene NAC-LIKE, ACTIVATED BY AP3/PI (AtNAP) is substanially upregulated in senescing leaves, and it has been found that AtNAP overexpression results in precocious leaf, while loss-of-function mutants show significantly delayed senescence (Guo and Gan 2006). AtNAP is also known to act together with a protein phosphatase 2C (PP2C) to mediate the abscisic acid (ABA) regulated promotion of stomatal opening and water loss in senescing leaves, as part of the ABA-AtNAP-SAG113 PP2C signal transduction pathway (Zhang and Gan 2012). AtNAP homologs have also been identified in rice (O. sativa) (Liang et al. 2014) and morning glory (I. nil) (Shinozaki et al. 2014).
In addition to TFs, senescence is also controlled by the contrasting actions of a number of phytohormones (Sakuraba et al. 2014). For example, while ethylene and ABA promote senescence, it is substantially delayed by treatment with cytokinins (CKs). In addition to influencing organ and whole plant senescence, CKs regulate many physiological and developmental processes, including cell division (cytokinesis), nutrient mobilization, shoot apical meristem activity, floral development, chloroplast development and differentiation (Bartrina et al. 2011). CK biosynthesis is controlled by the ATP/ADP isopentenyltransferase (IPT) genes, which encode rate-limiting enzymes in CK biosynthesis. Conversely, CK degradation is mediated by cytokinin oxidase/dehydrogenase (CKX), which irreversibly degrades active CKs into adenine or adenosine and side chains (Frébort et al. 2011; Hwang et al. 2012). Changes in the expression levels of CK metabolism genes presumably help maintain the appropriate level of CK activity; however, the means by which this control is exerted during developmentally or environmentally controlled senescence is not known.
CKs are known to delay flower senescence in a number of important ornamental species, including carnation (D. caryophyllus) (Eisinger 1977), petunia (P. hybrida) (Taverner et al. 1999) and rose (Rosa hybrida) (Mayak and Halevy 1970, 1974). An inverse relationship between CK content and senescence has been identified floral tissues (Van Staden et al. 1988) and the importance of CK levels for flower senescence is also suggested by a number of other observations. For example, the longevity of petunia flowers was greatly extended in plants expressing a bacterial IPT gene that provides precursors for CK biosynthesis, under the control of the senescence-associated SAG12 promoter (Chang et al. 2003). Moreover, the exogenous application of 6-methylpurine, a CKX inhibitor, was reported to substantially increase the life span of carnation petals (Taverner et al. 2000). A reported increase in the mRNA abundance of two CKX genes during carnation petal senescence is also suggestive of accelerated CK breakdown (Hoeberichts et al. 2007). Such observations indicate a function for CKs in delaying petal senescence, but the means by which CK levels are regulated has not been established.
There is increasing evidence that CKs play an important role in the regulation of environmental stress responses, involving intensive interactions and crosstalk with ABA (Verslues 2016; Nishiyama et al. 2011; Verma et al. 2016). CKs and ABA exert antagonistic activities during several developmental and physiological processes, including plant adaptation to environmental stresses, stomatal closure and leaf senescence (Gan and Amasino 1995; Chang et al. 2003; Nishiyama et al. 2011). When plants are exposed to stress, the accumulation of ABA specifically promotes stomatal closure to minimize water loss and accelerates leaf senescence. Conversely, CKs trigger responses to delay both stomatal closure and leaf senescence (Pospisilova 2003; Pospisilova et al. 2005), although it is worth noting that differences in drought related phenotypes are not always associated with stomata-related traits (Bartels and Sunkar 2005; Fujita et al. 2005; Hirayama and Shinozaki 2010). For instance, the AREB1ΔQT transgenic A. thaliana plants showed enhanced drought tolerance but no differences in stomatal movement or function (Fujita et al. 2005).
Shared regulation mechanisms exist between CK and ABA metabolism and signaling during different processes that involve plant adaptation to stresses, as well as plant growth and development (Nishiyama et al. 2011). However, the molecular pathways that govern the antagonistic actions of CK and ABA in organ senescence and dehydration tolerance are still unclear, particularly in petals that have no stomata. Similarly, while NAP is involved in ABA responses and regulates senescence-associated genes, and especially those that affect stomatal movement (Zhang and Gan 2012; Liang et al. 2014; Hu et al. 2021), the regulatory mechanisms and significance of NAP action in senescing astomatous petals is unclear (van Doorn 1997).
In this current study we isolated a dehydration- and senescence-induced AtNAP-like gene, RhNAP, from rose petals. We found that RhNAP shares similar expression patterns with a rose cytokinin oxidase/dehydrogenase gene (RhCKX6) in senescing petals or those undergoing dehydration. Silencing of RhNAP or RhCKX6 expression decreased young petal dehydration tolerance and delayed mature petal senescence. RhNAP was found to physically bind to the RhCKX6 promoter both in vivo and in vitro. Together with ABA signaling cascade mediated by RhNAP-RhPP2C interactions, we propose that RhNAP-RhCKX6 associations regulate petal dehydration tolerance and senescence in rose flowers.
Results
RhNAP and RhCKX6 are co-expressed in response to dehydration and during petal senescence
Based on our previous microarray results (Dai et al. 2012), we identified a dehydration-induced unigene, JK619941, encoding a rose NAC family TF. Phylogenetic analysis and protein sequence alignments showed that this NAC protein is closely related to A. thaliana AtNAP (Fig. S1) and so the unigene was named RhNAP. A GAL4 transient expression assay of RhNAP, also in Arabidopsis protoplasts, indicated that RhNAP functions as a transcriptional activator with a transactivation domain at the C-terminus (Fig. S2). Our previous microarray analysis also indicated the involvement of CK metabolism in the dehydration response and a CYTOKININ OXIDASE/DEHYDROGENASE (CKX) gene (JK618028) was observed to be highly up-regulated during dehydration (Dai et al. 2012). Phylogenetic analysis indicated that this gene is a homolog of CKX genes from F. vesca (FvCKX6) and A. thaliana (AtCKX6) (Fig. S3). We designated JK618028 as RhCKX6.
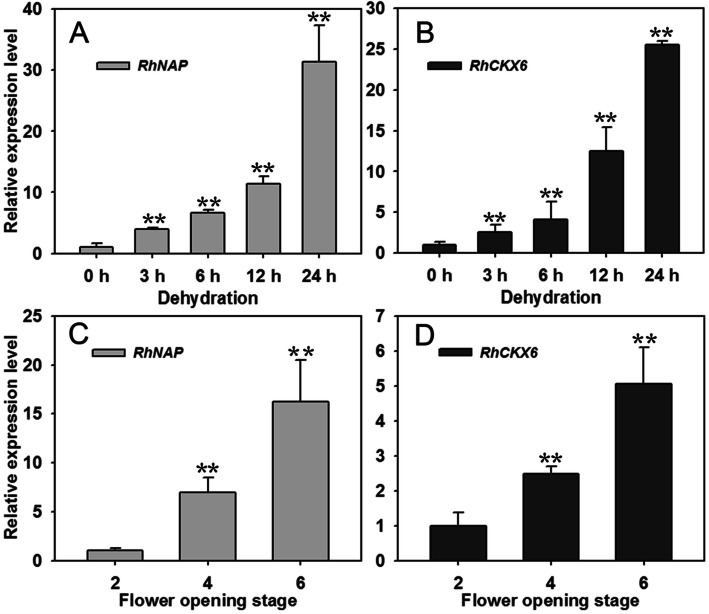
We further confirmed the expression profiles of both RhNAP and RhCKX6 in rose petals subjected to a dehydration treatment by quantitative RT-PCR assays. The transcript levels of RhNAP and RhCKX6 increased substantially in rose petals after 3 h of dehydration and were 31-fold and 26-fold greater, respectively, than levels in control petals after 24 h of dehydration (Fig. 1A, B). Dehydration typically results in ABA accumulation in rose petals (Le Page-Degivry et al. 1991), so we also tested the expression levels of RhNAP and RhCKX6 in petals following treatment with ABA. Both genes were express at higher levels in ABA treated petals than in controls (Fig. S4).
Fig. 1.
Both RhNAP and RhCKX6 are induced by dehydration and petal senescence. qRT-PCR analysis of RhNAP and RhCKX6 expression in rose petals in response to dehydration treatment (A, B). qRT-PCR analysis of RhNAP and RhCKX6 expression in rose petals at different opening stages (C, D). RhUBI1 was used as an internal control. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01
AtNAP is a key regulator of leaf senescence (Guo and Gan 2006; Zhang and Gan 2012) and dehydration stress can trigger the senescence of plant organs (Zhang and Gan 2012). We therefore assessed the expression of RhNAP and RhCKX6 during petal senescence. qRT-PCR showed that the expression levels of both genes increased substantially in rose petals in parallel with flower aging from a full opened flower (opening stage 4) to the onset of petal wilting (opening stage 6), supporting a functional association of both genes with petal senescence (Fig. 1C, D).
Functional analysis of RhNAP and RhCKX6 in association with dehydration tolerance in rose petals and A. thaliana seedlings
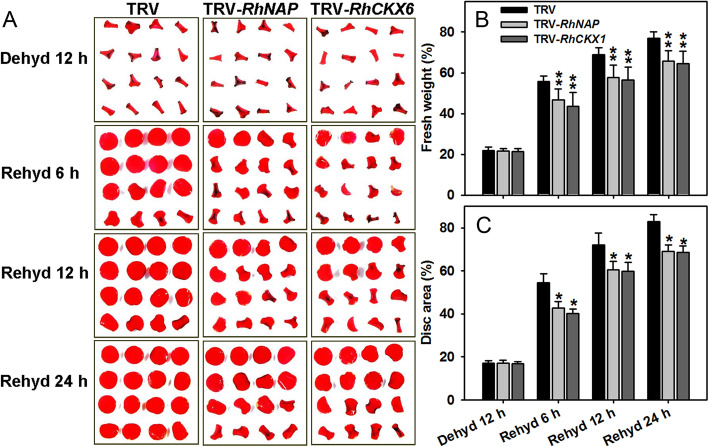
The dehydration tolerance of rose petals can be assessed by evaluating the expansion of intact petals or petal discs after rehydration, as described by Dai et al. (2012). In this current study, we similarly used petal disc fresh weight and expansion area to determine the potential roles of RhNAP and RhCKX6 in rose petal dehydration, after suppressing the expression of each gene in petal discs using virus-induced gene silencing (VIGS). The RhNAP- or RhCKX6-specific 3′ end regions were used to construct tobacco rattle virus vectors (TRV-RhNAP and TRV-RhCKX6, respectively) to enable specific gene silencing. Petal discs of rose flowers (stage 2) were dehydrated for 12 h and then rehydrated for 24 h (Fig. 2A). After 6 h of rehydration, the fresh weight of 56% of the discs derived from the TRV control petals had recovered, compared with only 47 and 44% of those from RhNAP- and RhCKX6-silenced petals, respectively (Fig. 2B). The differences between TRV control and gene silenced discs were still significant after 24 h of rehydration. Additionally, the areas of the expanded discs were significant decreased in RhNAP- and RhCKX6-silenced discs compared with those of the TRV treated control (Fig. 2C). These results suggest that RhNAP and RhCKX6 are involved in dehydration tolerance in young rose petals.
Fig. 2.
RhNAP- or RhCKX6-silencing reduced dehydration tolerance in rose petal discs. Expression of RhNAP and RhCKX6 was silenced in petal discs by virus induced gene silencing (VIGS). Petal discs were dehydrated for 12 h and examined at intervals during 24 h of rehydration. A The phenotypes of the petal discs were recorded and photographed at different time points. The fresh weight (B) and recovery area (C) of the petal discs were determined. n = 5 ± standard deviation values are shown in (B) and (C); Student’s t-test, *P < 0.05, **P < 0.01. Dehyd, dehydration; Rehyd, rehydration
To further examine the function of RhNAP and RhCKX6 in dehydration tolerance, we overexpressed RhNAP and RhCKX6 separately in A. thaliana and subjected the two-week old seedlings grown in soil to drought stress. After 15 d of drought treatment, the plants were allowed to recover under normal growth conditions for 3 d. More than 60% of the RhNAP-ox and RhCKX6-ox Arabidopsis plants survived compared with only 32% of the controls (Fig. S5). Thus, RhNAP and RhCKX6 can confer drought stress tolerance when expressed heterologously in A. thaliana seedlings.
Functional association of RhNAP and RhCKX6 with rose petal senescence
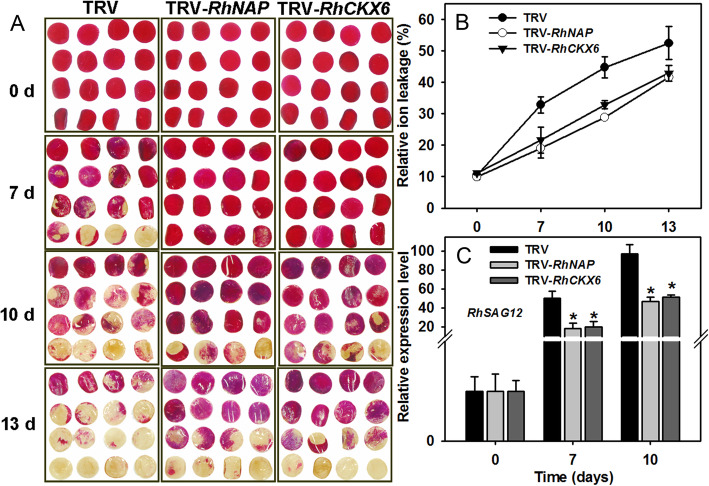
Both NAP TFs and CKs have previously been associated with petal senescence: AtNAP was reported to be upregulated in senescing A. thaliana petals (Wagstaff et al. 2009), as was also the case with a gene homolog in senescing morning glory petals (Shinozaki et al. 2014), while CKs have been reported to delay rose flower senescence (Mayak and Halevy 1970, 1974). We first investigated the effects of different types of CKs on the rose petal senescence and determined that the application of all those tested (6-BA, tZ and iP) delayed the senescence of petal discs, while the CK inhibitor lovastatin promoted their senescence (Fig. S6). We hypothesized that the senescence-induced RhNAP and RhCKX6 genes may be involved in rose petal senescence and tested this using RhNAP- and RhCKX6-silenced petal discs. We found that suppressing RhNAP and RhCKX6 expression indeed delayed petal disc senescence compared with TRV-treated controls (Fig. 3A), and that ion leakage was also significantly reduced compared to the control discs (Fig. 3B). Additionally, RhSAG12 transcript levels were significantly down-regulated in RhNAP- and RhCKX6-silenced discs compared with those of the TRV-treated control (Fig. 3C).
Fig. 3.
RhNAP- or RhCKX6-silencing delays senescence in rose petal discs. RhNAP and RhCKX6 expression was silenced in petal discs by virus induced gene silencing (VIGS). A The phenotypes of the petal discs were recorded and photographed at various time points (days) until necrosis was observed. The ion leakage (B) and relative expression of RhSAG12 by qRT-PCR (C) were determined. n = 3 ± standard deviation values are shown in (B) and (C); Student’s t-test, *P < 0.05
To further test the putative functional association between RhNAP and RhCKX6 and senescence, we expressed each gene separately in A. thaliana, generating RhNAP-ox and RhCKX6-ox transgenic lines, respectively. As shown in Fig. S7, plants from both genotypes had smaller leaves and age equivalent transgenic plants (approximately 35 d after germination, or DAG) exhibited precocious leaf senescence phenotypes compared with the controls.
Expression of RhCKX6 is predominantly dependent on RhNAP
Our results showed that RhNAP and RhCKX6 exhibit similar expression patterns under dehydration conditions and during senescence, yield similar phenotypes when silenced in petal discs, and confer similar degrees of enhanced drought tolerance and leaf senescence when overexpressed in A. thaliana. To test the hypothesis that RhCKX6 operates downstream of RhNAP action in rose petals, we examined the effect of silencing of RhNAP on the expression of six rose CKX (RhCKX) genes. We observed that the expression level of RhCKX6 was reduced to 20% of control levels in RhNAP-silenced petals (Fig. 4). In addition, RhCKX1 and RhCKX7, two close homologs of RhCKX6, were also clearly down-regulated in the RhNAP-silenced petals compared with TRV controls (Fig. 4). However, RhCKX1 and RhCKX7 were not induced by ABA (Fig. S4) or dehydration treatments, or as a result of aging (Fig. S8A and B), and so are likely not regulated by RhNAP during petal dehydration and senescence. Taken together these data suggest that the dehydration- and senescence-upregulated RhCKX6 gene is likely regulated by the RhNAP TF.
Fig. 4.
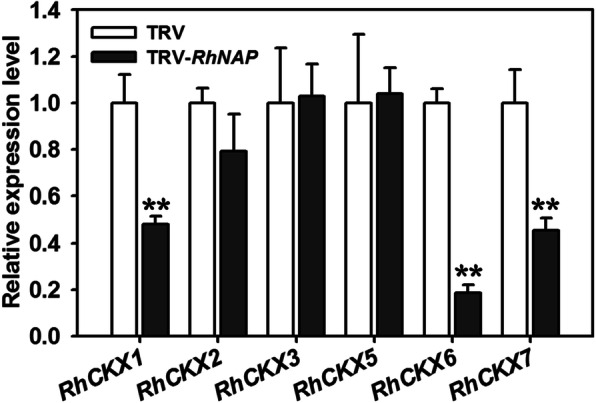
Transcript levels of RhCKX genes in RhNAP-silenced petals. Expression of RhNAP in petals was silenced by virus induced gene silencing (VIGS). Petals were sampled for qRT-PCR analysis of RhCKX gene expression and RhUBI1 was used as an internal control. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01
RhNAP binds to the RhCKX6 promoter
To test the hypothesis that RhNAP directly regulates RhCKX6 expression in rose petals, we performed a gel-shift assay to determine whether the RhNAP protein binds to the RhCKX6 promoter. A 1308-bp putative promoter region immediately upstream of the RhCKX6 coding sequence was amplified and a 31-bp fragment spanning positions − 558 to − 528 of the RhCKX6 promoter was used as probe (Fig. 5A). The probe contains the 9-bp sequence 5’ATTCACGTG3’, which contains a predicted NAC recognition site CGT[G/A] (Tran et al. 2004; Franco-Zorrilla et al. 2014), and the reverse complementary sequence of this segment (5’CACGTGAAT3’) is very similar to AtNAP core binding sequence (5’CACGTAAGT3’) (Zhang and Gan 2012). A recombinant form of the RhNAP protein fused to the C terminus of glutathione S-transferase, (GST)-RhNAP, was expressed in and purified from E. coli, then co-incubated and electrophoresed with the biotin-labeled and/or non-labeled probe. As shown in Fig. 5A, a shifted DNA-binding band was detected with addition of GST-RhNAP and labeled DNA probes, but no band was detected in the GST control. When unlabeled DNA probe concentrations were gradually increased in the reaction mixture, the DNA-binding signal gradually weakened.
Fig. 5.
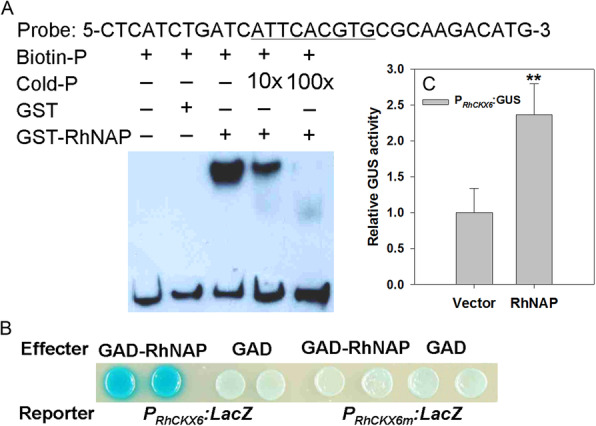
RhNAP binding to cis-elements in the promoter of RhCKX6. A Wild-type and mutant probes derived from the RhCKX6 promoter. The wild-type cis-element and its nucleotide substitutions in the mutants are underlined. Interaction between GST-RhNAP and the biotin-labeled probe on a native PAGE gel. Purified protein (3 μg) was incubated with 25 pM of the biotin-labeled wild-type probe. Non-labeled probe with different concentrations (from 10 to 100 x) was added for the competition test. B Transactivation activity of RhNAP with the RhCKX6 promoter in yeast. GAD-RhNAP, but not GAD itself, activates expression of the LacZ reporter gene driven by the wild-type 31-bp fragment of the RhCKX6 promoter. The mutated fragment abolishes activation of the LacZ reporter gene expression. C Regulation of the RhCKX6 promoter activity by RhNAP in A. thaliana mesophyll protoplasts. The effector constructs contained GFP-RhNAP or GFP alone, driven by the super1300 promoter. The reporter constructs contained the RhCKX6 promoter (− 1308 bp to − 1 bp upstream of ATG). Protoplasts were co-transformed with different combinations of effector and reporter constructs and the relative GUS activity indicated the promoter activity. Normalized GUS activities are presented as the means ± standard deviation (n = 6). The difference was statistically significant (Student’s t-test, P < 0.01) as denoted by asterisks
To test the interaction of RhNAP with the RhCKX6 promoter in vitro, we performed a yeast one-hybrid assay (Fig. 5B). The RhCKX6 cis-element promoter fragment and its corresponding mutant, 5’CGGACATGT3’, were each used to drive the LacZ reporter gene (Fig. 5B). The RhNAP open reading frame (ORF) was fused to the yeast GAL4 activation domain (GAD) to generate the effector construct GAD-RhNAP. The yeast one-hybrid experiment confirmed that RhNAP can indeed bind to the RhCKX6 promoter baits, but not to the GAD promoter or the RhCKX6 mutant fragment. These results indicate that RhNAP is capable of directly promoting RhCKX6 expression through binding to a 9-bp cis-element sequence, 5’ATTCACGTG3′ in the RhCKX6 promoter.
We also tested the effects of RhNAP action on RhCKX6 expression in A. thaliana protoplasts. When both the Super1300:RhNAP effector construct and the PRhCKX6:GUS reporter construct were introduced into the protoplasts, GUS activity was nearly 2.5-fold greater than that of the controls (Fig. 5C).
Cytokinin contents of RhNAP-silenced rose petals
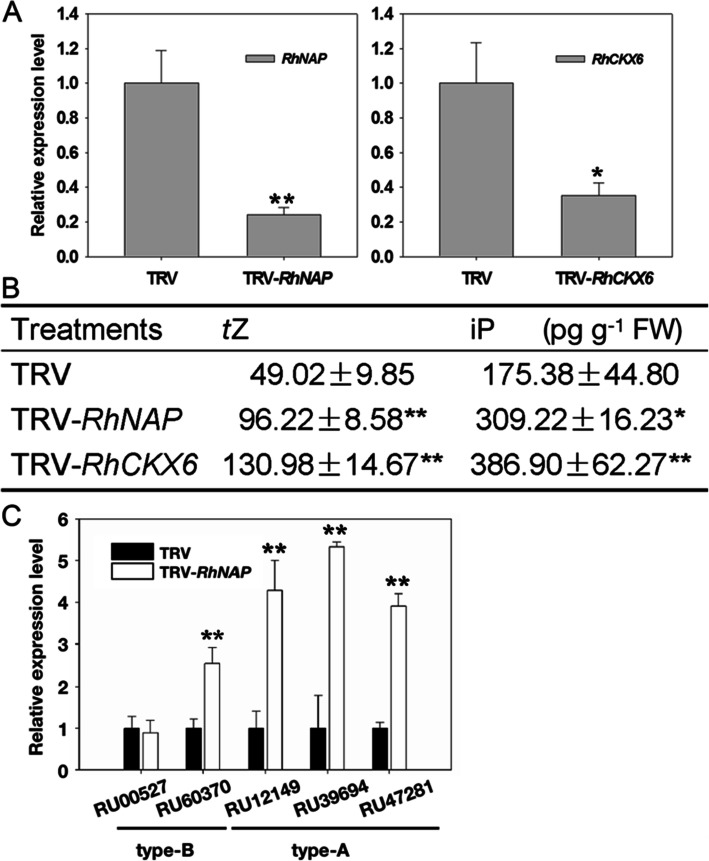
It is well established that CKX enzymes catalyze the breakdown of CKs (Frébort et al. 2011), so we analyzed of RhNAP or RhCKX6 expression in silenced petal discs, respectively (Fig. 6A), and then measured endogenous CK levels in TRV, TRV-RhNAP and TRV-RhCKX6 treated petal discs. Levels of the CKs trans-zeatin (tZ) and N6-(Δ2-isopentenyl) adenine (iP) in TRV-RhNAP were 196, and 176%, respectively, of those detected in TRV control discs, while the abundance of tZ and iP in TRV-RhCKX6 treated discs were 267 and 221%, respectively, those of the controls (Fig. 6B). Thus, silencing of either RhCKX6 or RhNAP resulted in increased levels of CKs, further suggesting the regulation of RhCKX6 activity by RhNAP.
Fig. 6.
Endogenous cytokinins levels in TRV, TRV-RhNAP and TRV-RhCKX6 treated petals. A The relative expression levels of RhNAP and RhCKX6 in silenced rose petal discs by qRT-PCR. RhUBI1 was used as the internal control. B The cytokinin quantification analysis. FW, fresh weight. C Expression analysis of type-A (RU12149, RU39694, RU47281) and type-B (RU00527 and RU60370) response regulator (RR) genes in RhNAP-silenced petals by qRT-PCR. RhUBI1 was used as an internal control. n = 3 ± standard deviation values are shown; Student’s t-test, *P < 0.05, **P < 0.01
Two types (type A and B) of functional response regulators (RRs) are involved in CK signaling in order to activate the transcription of CK responsive genes (Kieber and Schaller 2018). To gain further insights into the molecular processes associated with the elevated levels of CKs in RhNAP-silenced petals, we investigated the transcript levels of RR genes in the petals. Five RR genes were selected, comprising three type-A (RU12149, RU39694, RU47281) and two type-B (RU00527 and RU60370). Other than RU00527, the expression of the RR genes was significantly up-regulated in the RhNAP-silenced petals (Fig. 6C). These results suggest that RhNAP-RhCKX6 interaction controls CK steady state levels and subsequent downstream CK signaling during petal dehydration tolerance and senescence.
RhNAP activates the ABA signaling cascade in rose petals
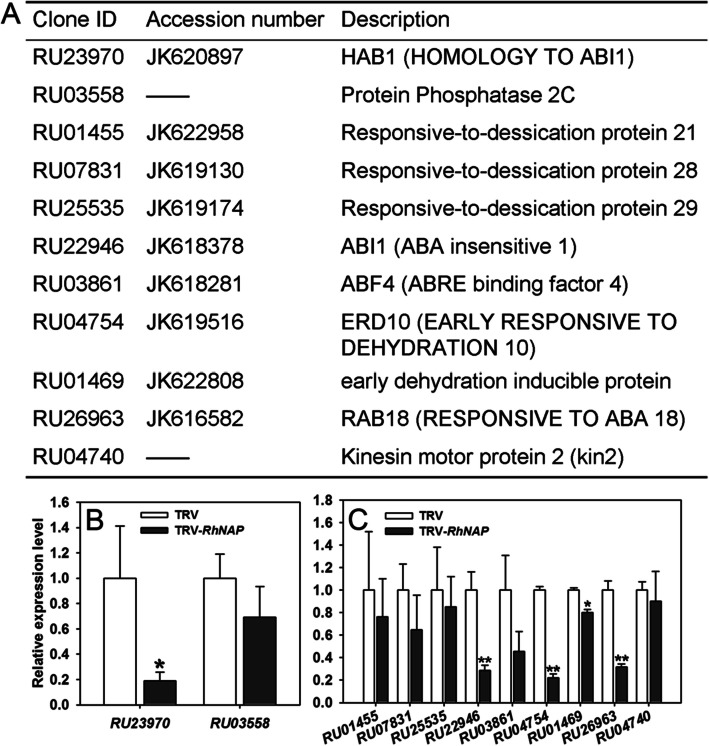
AtNAP has been reported to be involved in regulating leaf senescence and stomatal movement by influencing the action of SAG113, a PP2C family PP through an ABA-RhNAP-SAG113 regulatory chain (Zhang and Gan 2012). Since we determined that RhNAP is also induced by ABA (Fig. S4), we reasoned that RhNAP might regulate the expression of PP2C family genes in rose petals. We therefore assessed the expression of two PP2C genes that we had previously identified from our rose transcriptome databases (Dai et al. 2012; Pei et al. 2013), a rose homolog of SAG113 (RU03558) and a dehydration-induced PP2C gene (RU23970) (Fig. 7A), in RhNAP-silenced rose petals. We found that both genes were down-regulated in RhNAP-silenced petals, and that RU23970 showed a particularly low expression level of (Fig. 7B). This suggested that the ABA signaling pathway is also involved in RhNAP-mediated functions during petal dehydration tolerance. We therefore evaluated the expression of 9 additional rose genes putatively involved in ABA-signaling pathways (Fig. 7A). qRT-PCR analysis revealed that the expression levels of 6 of these genes, including the rose homologs of RD21, RD28, ABI1, ABF4, ERD10 and RAB18, were clearly repressed in RhNAP-silenced rose petals (with a fold change < 0.8) (Fig. 7C). These data suggest RhNAP activates ABA-responsive gene expression via the ABA signaling pathway, thereby enhancing the dehydration tolerance of rose petals.
Fig. 7.
Expression of PP2C genes and other ABA-related genes in RhNAP-silenced rose petals. A The putative ABA signaling and downstream rose genes from the ABA-signaling pathway. The clone IDs correspond to the rose transcriptome database (Dai et al. 2012). The description of the A. thaliana homolog is as given by The Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org). B qRT-PCR analysis of two PP2C genes in RhNAP-silenced rose petals. C The expression patterns of 9 ABA responsive genes in RhNAP-silenced rose petals were analyzed by qPCR. The internal control used was RhUBI1. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01
Discussion
RhNAP integrates the signals of dehydration- and aging-induced petal senescence in rose flower
Plant-specific NAC (NAM/ATAF1,2/CUC2) TFs play important roles in regulating diverse biological processes, including development, senescence, growth, cell division and responses to environmental stresses (Olsen et al. 2005). Comparative analysis of A. thaliana gene expression has shown that some NAC TFs are up-regulated in senescent petals (Wagstaff et al. 2009). NAP (NAC-LIKE, ACTIVATED BY AP3/PI), a member of the NAC subfamily, was reported to function in the transition between growth by cell division and by cell expansion in stamens and petals (Sablowski and Meyerowitz 1998). Later reports showed that the NAP genes are mainly involved in organ senescence, such as AtNAP in leaves, siliques and flowers (Guo and Gan 2006; Zhang and Gan 2012), OsNAP in leaves of rice (Liang et al. 2014), and the morning glory gene InNAP in flowers (Shinozaki et al. 2014). In this current study, we identified a dehydration-induced rose NAC gene, RhNAP, whose expression is also induced during petal aging (Fig. 1C) and by ABA treatments (Fig. S4). Furthermore, silencing of RhNAP expression decreased the tolerance to dehydration and delayed the senescence of petal discs (Figs. 2 and 3).
As well as being developmentally controlled, the onset of senescence can also be induced by unfavorable environmental conditions, such as water deficit (Sade et al. 2018; Patharkar and Walker 2019; Guo and Gan 2012). In the context of senescence, expression profiling of A. thaliana TFs has revealed that a considerable number are induced during senescence and are also upregulated by various stresses, suggesting extensive overlap between senescence and stress responses (Chen et al. 2002; Li et al. 2018). Here we found that RhNAP both enhances dehydration tolerance in young rose petals and promotes petal senescence. Moreover, RhNAP-overexpressing A. thaliana seedlings showed improved drought tolerance while the mature plants exhibited precocious senescence (Fig. S5 and S7). We interpret these results to suggest that RhNAP integrates the signals of developmental senescence and dehydration-induced senescence in rose petals at juvenile stages.
RhNAP is involved in petal dehydration tolerance and senescence through promotion of RhCKX6 expression
CKs regulate numerous biological processes, including organ senescence and responses to environmental stresses via complex metabolic and signaling networks (Frébort et al. 2011; Ha et al. 2012; Hwang et al. 2012; Kieber and Schaller 2018; Hönig et al. 2018). It is well established that CKX enzymes degrade active CKs into adenine or adenosine and side chains; however, less is known about the regulation of the corresponding genes. In rose petals, we found that the CKX gene, RhCKX6 was induced by both dehydration treatments and aging (Fig. 1B and D). Silencing of RhCKX6 expression decreased petal disc dehydration tolerance and delayed their senescence, as was observed with silencing of RhNAP (Figs. 2 and 3). Moreover, we found that the expression of RhCKX6 was reduced in RhNAP-silenced petals (Fig. 4). We observed that RhNAP can bind to a 9 bp ATTCACGTG segment of the RhCKX6 promoter, as revealed using EMSA method (Fig. 5A), and that it can activate the RhCKX6 promoter in yeast and A. thaliana protoplasts (Fig. 5B and C). Furthermore, the CK contents of both RhNAP-silenced and RhCKX6-silenced petals were shown to be higher than those of the TRV control (Fig. 6B). Interestingly, we also found that RhCKX1 and RhCKX7 expression was reduced in RhNAP-silenced petals and 6-BA treated petals. However, these two genes were not induced by ABA (Fig. S4) or dehydration treatments, or as a consequence of aging (Fig. S8A and B). We suggest that RhCKX1 and RhCKX7 are not directly regulated by RhNAP, but that their reduced expression resulted from the high levels of CKs in RhNAP-silenced petals (Fig. 6B), and we note that CKs have been reported to upregulate the expression of multiple CKX genes in A. thaliana (Nishiyama et al. 2011) and rice (Raines et al. 2016). Based on these results, the RhNAP/RhCKX6 interaction is proposed as a key step in the regulation of dehydration tolerance and senescence in rose petals.
RhNAP functions in dehydration tolerance and senescence via stomata-independent pathways in rose petals
Petals and leaves share common evolutionary origins but perform very different functions and, accordingly, their physiologies and gene expression profiles have common but distinct patterns during their senescence (Price et al. 2008; Wagstaff et al. 2009). An ABA-AtNAP-SAG113 PP2C regulatory chain has been proposed to regulate leaf senescence by controlling stomatal movement and water loss in A. thaliana (Zhang and Gan 2012). In rice leaves, OsNAP is induced by ABA, and its expression is reduced in the ABA biosynthetic mutants aba1 and aba2 (Liang et al. 2014). We determined in this current study that RhNAP expression is induced by dehydration and ABA in astomatous rose petals (Fig. 1A, S4 and S9), and that its silencing results in reduced expression of two predicted PP2C genes (RU03558 and RU23970) (Fig. 7B). In addition, several putative ABA-signaling pathway genes were also down-regulated (Fig. 7C), which we propose contributed to the observed decreased dehydration tolerance of RhNAP-silenced young petals. These observations are congruent with several reports that variation in plant drought phenotypes is not always related to stomatal function (Bartels and Sunkar 2005; Fujita et al. 2005; Hirayama and Shinozaki 2010).
Changes in endogenous CK levels have been reported to alter the stress tolerance of plants (Rivero et al. 2007; Havlova et al. 2008; Nishiyama et al. 2011) and prolonged drought has been associated with a reduction in active CK levels (Nishiyama et al. 2011), growth reduction and reallocation of limited energy resources towards defense against environmental stresses. Here, elevated CKs concentrations that resulted from silencing RhNAP or RhCKX6 expression were also correlated with reduced dehydration tolerance in young rose petal discs (flower opening stage 2) (Figs. 2 and 6B). Conversely, decreased CKs levels in RhNAP- or RhCKX6-overexpressing A. thaliana transgenic plants were associated with enhanced drought stress tolerance of the young seedlings (Fig. S5). Interestingly, while microarray and RT-PCR experiments have demonstrated that A. thaliana CKX1, CKX3, CKX4 and CKX6 are down-regulated by ABA (Werner et al. 2006), we observed that ABA treatment increased the expression of RhCKX genes in rose petals (Fig. S4), as did dehydration (Dai et al. 2012). We hypothesize that this may be due to the stressed young rose petals having high CK activity (Mayak and Halevy 1970).
CKs are known to delay the senescence of vegetative and floral organs (Van Staden et al. 1988). For example, elevated CK levels delayed flower petal senescence in several ornamental plants, including carnations (Taverner et al. 2000; Hoeberichts et al. 2007), petunia (Taverner et al. 1999; Chang et al. 2003), and roses (Mayak and Halevy 1970, 1974). In our study, the concentration of CKs increased and the expression levels of CK signaling and responsive genes were upregulated, which we propose contributed to the observed delayed senescence of RhNAP- or RhCKX6-silenced petal discs (Figs. 3 and 6). This is consistent with the observation that leaf senescence is associated with a decrease in CK content and CK signaling suppression (Gan and Amasino 1995; Kim et al. 2006; Hu et al. 2021).
In summary, we have shown that dehydration- and aging-induced RhNAP expression modulates dehydration tolerance and senescence in rose petals and that RhCKX6 functions as a direct downstream target of RhNAP. The RhNAP integrates the signals of developmental senescence and dehydration-induced senescence in rose petals at juvenile stages. We conclude that the RhNAP/RhCKX6 regulatory interaction promotes dehydration tolerance in young rose petals and accelerates petal senescence via stomata-independent mechanisms (Fig. 8).
Fig. 8.
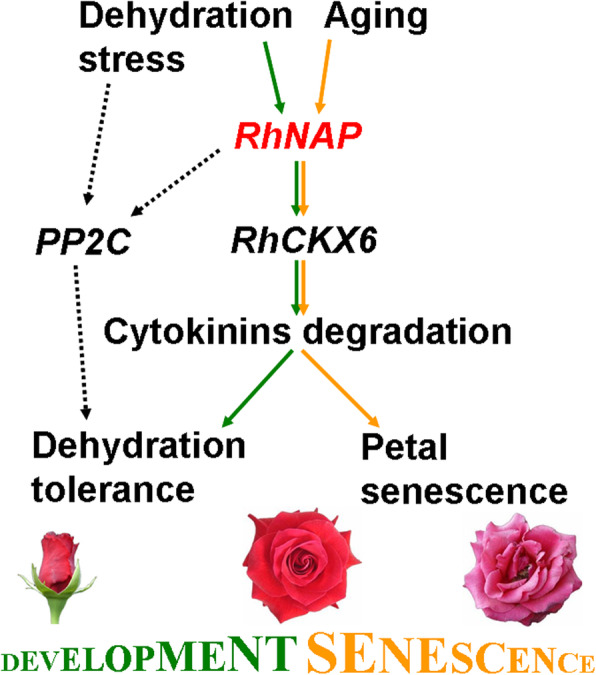
RhNAP regulating rose petal dehydration tolerance and senescence by modulating the degradation of CKs. Dehydration stress and aging induce the expression of RhNAP, which directly activates the cytokinin oxidase/dehydrogenase gene 6 (RhCKX6), resulting in the degradation of endogenous CKs, thereby enhancing dehydration tolerance in young petals and promoting petal senescence. The PP2C genes also participate in the up-regulation of petal dehydration tolerance via RhNAP action. Green lines indicate the signaling pathway for dehydration stress via the RhNAP/RhCKX6 regulatory point in young rose petals, while yellow lines indicate aging through the RhNAP/RhCKX6 regulatory step in mature rose petals. Dashed lines indicate steps operating via the ABA signaling pathway or possible unidentified components
Methods
Plant material and growth conditions
Flowers of Rosa hybrida (cv. ‘Samantha’) were harvested at the different opening stages (Ma et al. 2005), placed in water and delivered to the laboratory within 1 h. Stems were re-cut under water to ~ 25 cm length and uniform flowers were selected and kept in deionized water until further processing. Petal discs were taken from the same whorl of petals from flowers at opening stage 2 and were immersed in solutions containing different CKs, including 6-benzylaminopurine (6-BA, 100 μM), trans-zeatin (tZ, 10 μM), isopentenyladenine (iP, 10 μM), or the CK biosynthesis inhibitor lovastatin (20 μM). Control samples were treated with 0.05% EtOH without any phytohormones.
A. thaliana ecotype Col-0 seeds were sterilized and sown on Murashige and Skoog (MS) salts medium and appropriate antibiotics, then kept at 4 °C for 3 d to allow germination. Approximately 7-day old seedlings were transplanted into pots containing a 1:1 mixture of vermiculite and peat moss. Seedlings were grown at 22 °C and 50% relative humidity under a 16/8 h light/dark photoperiod.
Cloning, plasmid construction and plant transformation
The ORFs and promoter sequences of RhNAP and RhCKX6 were amplified using SMART™ RACE cDNA Amplification kit (Clontech, Palo Alto, CA, USA). All PCR products were subcloned into the pGEM T-Easy Vector (Promega, Madison, WI, USA) and then transformed into E. coli DH5a cells and sequenced. All primer sequences used in this study are listed in Table S1.
For construction of the RhNAP VIGS vector, a 347-bp fragment at the 3′ end of RhNAP was amplified. The PCR products were digested with BamHI and XhoI and ligated into the corresponding sites of the pTRV2 vector (Dai et al. 2012) to generate the pTRV2-RhNAP construct. For the construction of the RhCKX6 VIGS vector, a 228-bp fragment from the 3’ end of the gene was used.
In order to express the RhNAP recombinant protein in E. coli for EMSA assays, the RhNAP ORF was amplified by PCR and subcloned into the BamHI and EcoRI sites of the pGEX-2 T vector (GE Healthcare, Piscataway, NJ, USA), allowing the production of the GST-RhNAP fusion protein. The GST tag in pGEX-2 T was used to facilitate the purification of the fusion protein.
For the yeast one-hybrid assay, the RhNAP ORF was cloned into the EcoRI and XhoI sites of the pJG4–5 vector (Clontech) to produce the GAD-RhNAP construct. To generate the LacZ reporter genes driven by the RhCKX6 promoter with a wild-type or mutant motif, 35-bp oligonucleotides were synthesized. The annealed oligonucleotides were ligated into the EcoRI and XhoI sites of pLacZi2μ (Lin et al. 2007), generating the constructs PRhCKX6:LacZ and PRhCKX6m:LacZ, respectively.
To generate the GUS reporter gene driven by the RhCKX6 promoter (PRhCKX6:GUS), a 1308-bp fragment upstream of RhCKX6 ATG was PCR amplified and cloned into the binary pBI121 vector (Clontech) to replace the 35S CaMV promoter.
To generate the RhNAP and RhCKX6 overexpression binary vectors, The ORFs of RhNAP and RhCKX6 were amplified by PCR and the resulting fragments were inserted into a modified binary pCAMBIA 1300 vector harboring a super promoter (Super1300) and a green fluorescent protein (GFP) encoding sequence (Gong et al. 2002). The resulting constructs (Super1300:RhNAP and Super1300:RhCKX6) were transformed into Agrobacterium strain GV3101 and then introduced into A. thaliana plants via the floral dip method (Clough and Bent 1998).
The Arabidopsis mesophyll protoplasts were isolated as described in Yoo et al. (2007). The plasmids were extracted from transformed E. coli DH5α cells using the MACHEREY-NAGEL nucleic acid purification kit (MACHEREY-NAGEL, Düren, Germany), then 10 μg effector plasmid (Super1300:RhNAP) and 10 μg reporter plasmid (PRhCKX6:GUS) were transformed into 100 μl protoplasts containing ~ 2 × 104 protoplasts by polyethylene glycol mediated transformation.
For the transactivation assay in yeast (S. cerevisiae), different portions of RhNAP to be examined were PCR amplified using forward primers with the SalI site at the 5′ end and reverse primers with the PstI site at the 5′ end. The amplified fragments were digested with SalI and PstI and inserted in frame into the SalI and PstI sites of the pBD vector (Clontech) to make expression vectors. The proteins fused with pBD-GAL4 are as follows: pBD-RhNAPF (1–282 of RhNAP), pBD-RhNAPN (1–160 of RhNAP), pBD-RhNAPC (161–282 of RhNAP).
Silencing of RhNAP and RhCKX6 in rose petals by VIGS
Silencing of RhNAP and RhCKX6 expression by VIGS was performed as described by Dai et al. (2012), with some minor modifications. The pTRV1, pTRV2, pTRV2-RhNAP and pTRV2-RhCKX6 vectors were transformed into the A. tumefaciens strain GV3101, and the transformed A. tumefaciens lines were cultured for 24 h in Luria-Bertani (LB) medium supplemented with 20 mM acetosyringone, 50 μg ml− 1 kanamycin and 50 μg ml− 1 gentamycin sulfate. The cultures were harvested and suspended in infiltration buffer (10 mM MgCl2, 200 mM acetosyringone and 10 mM MES, pH 5.6) to a final OD600 of approximately 1.8. A mixture of cultures containing an equal ratio (v/v) of pTRV1 and pTRV2, pTRV1 and pTRV2-RhNAP or pTRV1 and pTRV2-RhCKX6, were used as TRV control, TRV-RhNAP and TRV2-RhCKX6, respectively. The mixtures were placed at room temperature in the dark for 4 h before vacuum infiltration of rose petals. Petals from the middle whorl at flower stage 2 were collected and 1 cm diameter discs were excised from the center of the petals with a hole punch. Vacuum infiltration was performed by immersing rose petals or discs in the bacterial suspension solution and infiltrating under a vacuum at 0.7 MPa. After release of the vacuum, petals and discs were washed in deionized water and kept in deionized water for 3 d at 8 °C, followed by an equilibrium step at 23 °C for 1 d. For RNA extraction, petals were kept in deionized water at 23 °C until sampling. For determination of dehydration tolerance, petal discs were dehydrated for 12 h and examined at intervals during 24 h of rehydration. The senescing phenotypes of petal discs were observed daily until necrosis was observed.
Extraction and quantification of endogenous cytokinins
Endogenous cytokinins (CKs) were extracted from rose petals and quantified as described in Pan et al. (2010). Petal disc material (approximately 100 mg) was frozen in liquid nitrogen, ground to fine powder and extracted with extraction solvent (2-propanol:H2O:concentrated HCl [2:1:0.002 v/v/v]; sample:solvent = 1:10 mg μl− 1) on a shaker (100 rpm) at 4 °C for 30 min. One milliliter of dichloromethane was added to each sample, and the samples were shaken (100 rpm) for 30 min at 4 °C. After centrifugation (13,000 g, 4 °C, 5 min), two phases were present and the lower phase (~ 1.5 ml) was collected. The solvent mixture was concentrated to near dryness using a concentrator (Eppendorf, Hamburg, Germany) and then redissolved in 0.1 ml methanol. The sample solution was centrifuged at 12,000 g for 5 min and then analyzed HPLC-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS). The extracts were analyzed by multiple reaction monitoring (MRM) on an Agilent 1260 Infinity HPLC System (Agilent, Santa Clara, CA, USA) coupled via ESI source to a QTrap 5500 System (AB Sciex, Foster City, CA, USA). A 10 μl aliquot of solution was injected, and analyzed on an Agilent SB-C18 (4.6 mm id, 50 mm length, 1.8 μm C18 resin, Agilent) at 30 °C. Eluent A was acetonitrile, and eluent B consisted of a 0.1% acetic acid aqueous solution. A gradient elution with the following composition was used: 10% A at 0 min, 90% A at 5 min. The flow rate was 0.8 ml min− 1. Data acquisition and processing were performed with AB Analyze software (AB Sciex).
Ion leakage quantification
To measure relative electrolyte leakage, petal samples at each time point were placed in a 50-ml tube containing 20 ml of deionized water and incubated at 25 °C for 30 min on an orbital shaker (200 rpm). The initial conductivity of the fluid was measured with a conductivity detector (Shanghai INESA, Shanghai, China). The samples were then boiled for 10 min in deionized water and cooled to room temperature. The total conductivity was then determined as before, and the relative electrolyte leakage was expressed as the percentage of the initial conductivity versus total conductivity.
Quantitative RT-PCR
For qRT-PCR analysis, 1 μg DNase treated total RNA was used to synthesize cDNA according to the manufacturer’s instructions using a reverse transcription system A3500 kit (Promega), with a 20 μl reaction volume. A 2 μl aliquot of cDNA was used as the template in a 20 μl qRT-PCR reaction using the Applied Biosystems StepOnePlus™ real-time PCR system (Applied Biosystems, CA, USA) with KAPA™ SYBR® FAST qPCR kits (Kapa biosystems, Boston, MA, USA). All reactions were performed with three biological replicates. Relative gene expression values were calculated according to the 2−ΔΔCT method, in which RhUBI1 (GenBank accession JK622648) was used as an internal control (Meng et al. 2013).
Purification of recombinant protein and EMSA
EMSA assays were performed as previously described (Lü et al. 2014). Briefly, the GST-RhNAP fusion protein was induced in 100 ml cultures of the transformed E. coli BL21 cells by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.2 mM and the cultures were incubated at 28 °C for 6 h. The recombinant protein was purified using Glutathione Sepharose 4B beads (GE Healthcare, Piscataway, NJ, USA) according to the manufacturer’s instructions. EMSA was performed using the LightShift chemiluminescent EMSA kit (Pierce, IL, USA), according to the manufacturer’s instructions. Briefly, the biotin-labeled DNA fragments (5′-CTCATCTGATCATTCACGTGCGCAAGACATG-3′) were synthesized, annealed and used as probes, with unlabeled DNA of the same sequence used as a competitor.
Yeast one-hybrid assay
Yeast one-hybrid assays were performed as described by Lin et al. (2007). Briefly, a plasmid containing the GAD-RhNAP fusion sequence was co-transformed with different LacZ reporter gene constructs into the yeast strain EGY48 as described in the Yeast Protocols Handbook (Clontech). Transformants were grown on SD/−Trp-Ura dropout plates containing 80 mg L− 1 X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) and the color development of yeast colonies was observed.
Supplementary Information
Additional file 1: Figure S1. Analysis of the RhNAP protein sequence. (A) Phylogenetic analysis of RhNAP together with known NAC family proteins. The phylogenetic tree file was produced by MEGA 5.2. Bootstrap values indicate the divergence of each branch and the scale indicates branch length. Accession numbers are as follows: ATAF1 (AT1G01720), ATAF2 (AT5G08790), ANAC019 (AT1G52890), ANAC032 (AT1G77450), ANAC055 (AT3G15500), ANAC072 (AT4G27410), ANAC102 (AT5G63790), OsNAC6 (BAA89800), TaNAC69 (AAY44098.1), GmNAC11 (ACC66315.1), GmNAC20 (ACC66314), AtNAP (AT1G69490), PhNAP (AAM34773), InNAP (AB639146), OsNAP (LOC_Os03g21060), RhNAP (JK619941), RhNAC2 (JK619963), RhNAC3 (JK617768), RhNAC100 (AFS95065.1), CUC1 (AT3G15170), CUC2 (AT5G53950), CUC3 (AT1G76420), VND1 (AT2G18060), VND2 (AT4G36160), VND3 (AT5G66300), VND4 (AT1G12260), VND5 (AT1G62700). (B) Alignment of the deduced amino acid sequence of RhNAP with those of NAP proteins from other plant species.
Additional file 2: Figure S2. Transcriptional activation of RhNAP. Transcriptional regulation activity assays in protoplasts. GAL4-BD, vector control; GAL4-BD-RhNAPF, GAL4-BD-RhNAPN and RhNAPC represent full length, N-terminal and C-terminal of RhNAP fused to the GAL4-BD, respectively. Error bars indicate SE (n = 6); Student’s t-test, *P < 0.05, **P < 0.01.
Additional file 3: Figure S3. Analysis of the RhCKX6 protein sequence. Phylogenetic analysis of RhCKX6 together with known CKX family proteins. The phylogenetic tree file was produced by MEGA 5.2. Bootstrap values indicate the divergence of each branch and the scale indicates branch length. Accession numbers are as follows: RhCKX6 (JK618028), AtCKX1 (At2g41510), AtCKX2 (At2g19500), AtCKX3 (At5g56970), AtCKX4 (At4g29740), AtCKX5 (At1g75450), AtCKX6 (At3g63440), AtCKX7 (At5g21482), OsCKX1 (LOC_Os01g09260), OsCKX6 (LOC_Os02g12770), OsCKX7 (LOC_Os02g12780), ZmCKX1 (NP_001105591.1), ZmCKX6 (ADP38082.1), TaCKX1 (ABH07114.1), FvCKX1 (XP_004305707.1), FvCKX6 (XP_004303072.1), VvCKX1 (XP_002284560.1), VvCKX6 (XP_002270841.1), SlCKX2 (NP_001244909.1), SlCKX5 (NP_001244907.1), SlCKX7 (NP_001244908.1), MtCKX (XP_003599606.1) PhCKX (BAK52671.1), NtCKX7 (AII20187.1).
Additional file 4: Figure S4. qRT-PCR analysis of the expression of RhNAP and RhCKX genes in rose petals in response to exogenous ABA. Rose flowers at opening stage 2 were analyzed after 24 h of 100 μM ABA treatment. Control samples were treated with 0.05% EtOH without phytohormones for 24 h. RhUBI1 was used as an internal control. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01.
Additional file 5: Figure S5. Drought tolerance and gene expression of RhNAP and RhCKX6 overexpressing A. thaliana lines. (A) RT-PCR was conducted with fully expanded leaves of transgenic A. thaliana plants. ACTIN2 was used as an internal control. (B) Tolerance of RhNAP- or RhCKX6-overexpressing A. thaliana plants to drought. T3 homozygous transformants were used in this experiment. 0 day drought, 14-day old well-watered plants; 15 days drought, 15 days after withholding water; 3 days rewatering, 3 days after rewatering; Survival rate was calculated from three independent experiments (~ 40 plants per line in one experiment).
Additional file 6: Figure S6. Effects of exogenous 6-benzylaminopurine (6-BA), trans-zeatin (tZ), isopentenyladenine (iP) and lovastatin on the senescence of rose petal discs. After 24 h pre-treatment with combinations of 100 μM 6-BA, 10 μM tZ, 10 μM iP, and 20 μM lovastatin, the petal discs were kept in water and photographed on days 7 and 13.
Additional file 7: Figure S7. Phenotypic analysis of RhNAP and RhCKX6 overexpression lines. (A) Phenotypes of age-matched plants (approximately 35 days after germination, DAG) of wild type (WT), RhNAP and RkCKX6. (B) Phenotypes of leaves detached from the age-matched 40 DAG plants in A.
Additional file 8: Figure S8. Analysis of RhCKX gene expression. Expression of RhCKX1 and RhCKX7 in rose petals in response to dehydration treatment (A) and various opening stages (B). The internal control used was RhUBI1. (C) qRT-PCR analysis of RhCKX gene expression in rose petals in response to exogenous 6-BA. Rose flowers at opening stage 2 were analyzed after 24 h of 100 μM 6-BA treatment. Control samples were treated with 0.1 M NaOH without phytohormones for 24 h. RhUBI1 was used as an internal control. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01.
Additional file 9: Figure S9. Anatomical structure of a petal viewed by scanning electron microscopy at flower full opening stage. (A, B) Adaxial epidermis; (C, D) Abaxial epidermis. Scale bar: 200 μm in A and C, magnification 200 x; 20 μm in B and D, magnification 2000 x.
Additional file 10: Table S1. Primers used in this study.
Acknowledgements
We thank Dr. Susheng Gan of Cornell University for his helps in this study. We also thank PlantScribe (www.plantscribe.com) for editing our manuscript.
Abbreviations
- ABA
Abscisic acid
- CKs
Cytokinins
- CKX
Cytokinin oxidase/dehydrogenase
- DAG
Days after germination
- EMSA
Electrophoresis Mobility Shift Assay
- GAD
GAL4 activation domain
- GFP
Green fluorescent protein
- GST
Glutathione S-transferase
- NAP
NAC-LIKE, ACTIVATED BY AP3/PI
- ORF
Open reading frame
- PCD
Programmed cell death
- PP2C
Protein phosphatase 2C
- SAGs
Senescence associated genes
- TFs
Transcription factors
- TRV
Tobacco rattle virus
- VIGS
Virus-induced gene silencing
Authors’ contributions
J.Z. and P.L. performed most of the experimental work. L.J. and K.L. performed the gene silencing. T.Z. and J.C. performed the EMSA and Y1H. Y.Y.and Y.C. transformed the Arabidopsis. J.G. co-supervised the work. C.Z. supervised all the work. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2019YFD1000404) and the Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF-PXM2019_014207_000032).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zou and Peitao Lü contributed equally to this work.
References
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;24(1):23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell. 2011;23(1):69–80. doi: 10.1105/tpc.110.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Jones ML, Banowetz GM, Clark DG. Overproduction of cytokinins in petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol. 2003;132(4):2174–2183. doi: 10.1104/pp.103.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WQ, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou GZ, Whitham SA, Budworth PR, Tao Y, Xie ZY, Chen X, Lam S, Kreps JA, Harper JF, Si-Ammour A, Mauch-Mani B, Heinlein M, Kobayashi K, Hohn T, Dangl JL, Wang X, Zhu T. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell. 2002;14(3):559–574. doi: 10.1105/tpc.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dai FW, Zhang CQ, Jiang XQ, Kang M, Yin X, Lu PT, Zhang X, Zheng Y, Gao JP. RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 2012;160(4):2064–2082. doi: 10.1104/pp.112.207720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger W. Role of cytokinins in carnation flower senescence. Plant Physiol. 1977;59(4):707–709. doi: 10.1104/pp.59.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Lopez-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci U S A. 2014;111(6):2367–2372. doi: 10.1073/pnas.1316278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P. Evolution of cytokinin biosynthesis and degradation. J Exp Bot. 2011;62(8):2431–2452. doi: 10.1093/jxb/err004. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17(12):3470–3488. doi: 10.1105/tpc.105.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270(5244):1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Gao S, Gao J, Zhu XY, Song Y, Li ZP, Ren GD, Zhou X, Kuai BK. ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol Plant. 2016;9(9):1272–1285. doi: 10.1016/j.molp.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Gong ZZ, Lee H, Xiong LM, Jagendorf A, Stevenson B, Zhu JK. RNA helicase-like protein as an early regulator of transcription factors for plant chilling and freezing tolerance. Proc Natl Acad Sci U S A. 2002;99(17):11507–11512. doi: 10.1073/pnas.172399299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gan S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006;46(4):601–612. doi: 10.1111/j.1365-313X.2006.02723.x. [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2012;35(3):644–655. doi: 10.1111/j.1365-3040.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012;17(3):172–179. doi: 10.1016/j.tplants.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Havlova M, Dobrev PI, Motyka V, Storchova H, Libus J, Dobra J, Malbeck J, Gaudinova A, Vankova R. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008;31(3):341–353. doi: 10.1111/j.1365-3040.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010;61(6):1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- Hoeberichts FA, van Doorn WG, Vorst O, Hall RD, van Wordragen MF. Sucrose prevents up-regulation of senescence-associated genes in carnation petals. J Exp Bot. 2007;58(11):2873–2885. doi: 10.1093/jxb/erm076. [DOI] [PubMed] [Google Scholar]
- Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int J Mol Sci. 2018;19(12):4045. 10.3390/ijms19124045. [DOI] [PMC free article] [PubMed]
- Hu YZ, Liu B, Ren HZ, Chen LP, Watkins C, Gan S. The leaf senescence-promoting transcription factor AtNAP activates its direct target gene cytokinin oxidase 3 to facilitate senescence by degrading cytokinins. Mol Horticulture 1. 2021. 10.1186/s43897-021-00017-6. [DOI] [PMC free article] [PubMed]
- Hwang I, Sheen J, Müller B. Cytokinin signaling networks. Annu Rev Plant Biol. 2012;63(1):353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- Jiang GX, Yan HL, Wu FW, Zhang DD, Zeng W, Qu HX, Chen F, Tan L, Duan XW, Jiang YM. Litchi fruit LcNAC1 is a target of LcMYC2 and regulator of fruit senescence through its interaction with LcWRKY1. Plant Cell Physiol. 2017;58(6):1075–1089. doi: 10.1093/pcp/pcx054. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liang G, Yang S, Yu D. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell. 2014;26(1):230–245. doi: 10.1105/tpc.113.117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbage M, Kessens R, Bartholomay LC, Williams B. The life and death of a plant cell. Annu Rev Plant Biol. 2017;68:375–404. doi: 10.1146/annurev-arplant-043015-111655. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE. Cytokinin signaling in plant development. Development. 2018;145(4). 10.1242/dev.149344. [DOI] [PubMed]
- Kim HJ, Ryu H, Hong SH, Woo HR, Lim PO, Lee IC, Sheen J, Nam HG, Hwang I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103(3):814–819. doi: 10.1073/pnas.0505150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page-Degivry MT, Orlandini M, Garello G, Barthe P, Gudin S. Regulation of ABA levels in senescing petals of rose flowers. J Plant Growth Regul. 1991;10(1-4):67–72. doi: 10.1007/BF02279314. [DOI] [Google Scholar]
- Li Z, Woo HR, Guo H. Genetic redundancy of senescence-associated transcription factors in Arabidopsis. J Exp Bot. 2018;69(4):811–823. doi: 10.1093/jxb/erx345. [DOI] [PubMed] [Google Scholar]
- Liang CZ, Wang YQ, Zhu YN, Tang JY, Hu B, Liu LC, Ou SJ, Wu HK, Sun XH, Chu JF, Chu CC. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci U S A. 2014;111(27):10013–10018. doi: 10.1073/pnas.1321568111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, Ding L, Casola C, Ripoll DR, Feschotte C, Wang HY. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science. 2007;318(5854):1302–1305. doi: 10.1126/science.1146281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü PT, Zhang CQ, Liu JT, Liu XW, Jiang GM, Jiang XQ, Khan MA, Wang LS, Hong B, Gao JP. RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J. 2014;78(4):578–590. doi: 10.1111/tpj.12494. [DOI] [PubMed] [Google Scholar]
- Ma N, Cai L, Lu WJ, Tan H, Gao JP. Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Sci China C Life Sci. 2005;48(5):434–444. doi: 10.1360/062004-37. [DOI] [PubMed] [Google Scholar]
- Ma N, Ma C, Liu Y, Shahid MO, Wang C, Gao J. Petal senescence: a hormone view. J Exp Bot. 2018;69(4):719–732. doi: 10.1093/jxb/ery009. [DOI] [PubMed] [Google Scholar]
- Mayak S, Halevy AH. Cytokinin activity in rose petals and its relation to senescence. Plant Physiol. 1970;46(4):497–499. doi: 10.1104/pp.46.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S, Halevy AH. The action of kinetin in improving the water balance and delaying senescence processes of cut rose flowers. Physiol Plant. 1974;32(4):330–336. doi: 10.1111/j.1399-3054.1974.tb03146.x. [DOI] [Google Scholar]
- Meng YL, Li N, Tian J, Gao JP, Zhang CQ. Identification and validation of reference genes for gene expression studies in postharvest rose flower (Rosa hybrida) Sci Horticulturae. 2013;158:16–21. doi: 10.1016/j.scienta.2013.04.019. [DOI] [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmuelling T, Tran LSP. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 2011;23(6):2169–2183. doi: 10.1105/tpc.111.087395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AN, Ernst HA, Leggio LL, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005;10(2):79–87. doi: 10.1016/j.tplants.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Pan X, Welti R, Wang X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat Protoc. 2010;5(6):986–992. doi: 10.1038/nprot.2010.37. [DOI] [PubMed] [Google Scholar]
- Patharkar OR, Walker JC. Connections between abscission, dehiscence, pathogen defense, drought tolerance, and senescence. Plant Sci. 2019;284:25–29. doi: 10.1016/j.plantsci.2019.03.016. [DOI] [PubMed] [Google Scholar]
- Pei HX, Ma N, Tian J, Luo J, Chen JW, Li J, Zheng Y, Chen X, Fei ZJ, Gao JP. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol. 2013;163(2):775–791. doi: 10.1104/pp.113.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilova J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol Plant. 2003;46(4):491–506. doi: 10.1023/A:1024894923865. [DOI] [Google Scholar]
- Pospisilova J, Vagner M, Malbeck J, Travnickova A, Batkova P. Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biol Plant. 2005;49(4):533–540. doi: 10.1007/s10535-005-0047-0. [DOI] [Google Scholar]
- Price AM, Orellana DFA, Salleh FM, Stevens R, Acock R, Buchanan-Wollaston V, Stead AD, Rogers HJ. A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol. 2008;147(4):1898–1912. doi: 10.1104/pp.108.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines T, Blakley IC, Tsai YC, Worthen JM, Franco-Zorrilla JM, Solano R, Schaller GE, Loraine AE, Kieber JJ. Characterization of the cytokinin-responsive transcriptome in rice. BMC Plant Biol. 2016;16(1):260. doi: 10.1186/s12870-016-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci U S A. 2007;104(49):19631–19636. doi: 10.1073/pnas.0709453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski RW, Meyerowitz EM. A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell. 1998;92(1):93–103. doi: 10.1016/S0092-8674(00)80902-2. [DOI] [PubMed] [Google Scholar]
- Sade N, Del Mar Rubio-Wihelmi M, Umnajkitikorn K, Blumwald E. Stress-induced senescence and plant tolerance to abiotic stress. J Exp Bot. 2018;69(4):845–853. doi: 10.1093/jxb/erx235. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Jeong J, Kang MY, Kim J, Paek NC, Choi G. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat Commun. 2014;5(1):4636. doi: 10.1038/ncomms5636. [DOI] [PubMed] [Google Scholar]
- Shahri W, Tahir I. Flower senescence: some molecular aspects. Planta. 2014;239(2):277–297. doi: 10.1007/s00425-013-1984-z. [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Tanaka T, Ogiwara I, Kanekatsu M, Van Doorn WG, Yamada T. Expression of an AtNAP gene homolog in senescing morning glory (Ipomoea nil) petals of two cultivars with a different flower life span. J Plant Physiol. 2014;171(8):633–638. doi: 10.1016/j.jplph.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Sun XM, Qin MZ, Yu Q, Huang ZW, Xiao Y, Li Y, et al. Molecular understanding of postharvest flower opening and senescence. Mol Horticult. 2021. 10.1186/s43897-021-00015-8. [DOI] [PMC free article] [PubMed]
- Taverner E, Letham D, Wang J, Cornish E. Inhibition of carnation petal inrolling by growth retardants and cytokinins. Aus J Plant Physiol. 2000;27:357–362. [Google Scholar]
- Taverner E, Letham D, Wang J, Cornish E, Willcocks D. Influence of ethylene on cytokinin metabolism in relation to Petunia corolla senescence. Phytochemistry. 1999;51(3):341–347. doi: 10.1016/S0031-9422(98)00757-2. [DOI] [Google Scholar]
- Tran LSP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell. 2004;16(9):2481–2498. doi: 10.1105/tpc.104.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG. Water relations of cut flower. Hortic Rev. 1997;18:1–85. [Google Scholar]
- van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence. J Exp Bot. 2008;59(3):453–480. doi: 10.1093/jxb/erm356. [DOI] [PubMed] [Google Scholar]
- Van Staden J, Cook E, Nooden LD. Cytokinins and senescence. In: Nooden LD, Leopold AC, editors. Senescence and aging in plants. San Diego: Academic Press; 1988. pp. 281–328. [Google Scholar]
- Verma V, Ravindran P, Kumar PP. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016;16:86. doi: 10.1186/s12870-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE. ABA and cytokinins: challenge and opportunity for plant stress research. Plant Mol Biol. 2016;91:629–640. doi: 10.1007/s11103-016-0458-7. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Yang TJ, Stead AD, Buchanan-Wollaston V, Roberts JA. A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J. 2009;57(4):690–705. doi: 10.1111/j.1365-313X.2008.03722.x. [DOI] [PubMed] [Google Scholar]
- Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T. New insights into the biology of cytokinin degradation. Plant Biol (Stuttg) 2006;8(3):371–381. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- Woo HR, Masclaux-Daubresse C, Lim PO. Plant senescence: how plants know when and how to die. J Exp Bot. 2018;69(4):715–718. doi: 10.1093/jxb/ery011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Ma N, Jia YC, Zhang Y, Feng M, Jiang CZ, Ma C, Gao JP. An ethylene-induced regulatory module delays flower senescence by regulating Cytokinin content. Plant Physiol. 2017;173(1):853–862. doi: 10.1104/pp.16.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gan S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2012;158(2):961–969. doi: 10.1104/pp.111.190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Analysis of the RhNAP protein sequence. (A) Phylogenetic analysis of RhNAP together with known NAC family proteins. The phylogenetic tree file was produced by MEGA 5.2. Bootstrap values indicate the divergence of each branch and the scale indicates branch length. Accession numbers are as follows: ATAF1 (AT1G01720), ATAF2 (AT5G08790), ANAC019 (AT1G52890), ANAC032 (AT1G77450), ANAC055 (AT3G15500), ANAC072 (AT4G27410), ANAC102 (AT5G63790), OsNAC6 (BAA89800), TaNAC69 (AAY44098.1), GmNAC11 (ACC66315.1), GmNAC20 (ACC66314), AtNAP (AT1G69490), PhNAP (AAM34773), InNAP (AB639146), OsNAP (LOC_Os03g21060), RhNAP (JK619941), RhNAC2 (JK619963), RhNAC3 (JK617768), RhNAC100 (AFS95065.1), CUC1 (AT3G15170), CUC2 (AT5G53950), CUC3 (AT1G76420), VND1 (AT2G18060), VND2 (AT4G36160), VND3 (AT5G66300), VND4 (AT1G12260), VND5 (AT1G62700). (B) Alignment of the deduced amino acid sequence of RhNAP with those of NAP proteins from other plant species.
Additional file 2: Figure S2. Transcriptional activation of RhNAP. Transcriptional regulation activity assays in protoplasts. GAL4-BD, vector control; GAL4-BD-RhNAPF, GAL4-BD-RhNAPN and RhNAPC represent full length, N-terminal and C-terminal of RhNAP fused to the GAL4-BD, respectively. Error bars indicate SE (n = 6); Student’s t-test, *P < 0.05, **P < 0.01.
Additional file 3: Figure S3. Analysis of the RhCKX6 protein sequence. Phylogenetic analysis of RhCKX6 together with known CKX family proteins. The phylogenetic tree file was produced by MEGA 5.2. Bootstrap values indicate the divergence of each branch and the scale indicates branch length. Accession numbers are as follows: RhCKX6 (JK618028), AtCKX1 (At2g41510), AtCKX2 (At2g19500), AtCKX3 (At5g56970), AtCKX4 (At4g29740), AtCKX5 (At1g75450), AtCKX6 (At3g63440), AtCKX7 (At5g21482), OsCKX1 (LOC_Os01g09260), OsCKX6 (LOC_Os02g12770), OsCKX7 (LOC_Os02g12780), ZmCKX1 (NP_001105591.1), ZmCKX6 (ADP38082.1), TaCKX1 (ABH07114.1), FvCKX1 (XP_004305707.1), FvCKX6 (XP_004303072.1), VvCKX1 (XP_002284560.1), VvCKX6 (XP_002270841.1), SlCKX2 (NP_001244909.1), SlCKX5 (NP_001244907.1), SlCKX7 (NP_001244908.1), MtCKX (XP_003599606.1) PhCKX (BAK52671.1), NtCKX7 (AII20187.1).
Additional file 4: Figure S4. qRT-PCR analysis of the expression of RhNAP and RhCKX genes in rose petals in response to exogenous ABA. Rose flowers at opening stage 2 were analyzed after 24 h of 100 μM ABA treatment. Control samples were treated with 0.05% EtOH without phytohormones for 24 h. RhUBI1 was used as an internal control. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01.
Additional file 5: Figure S5. Drought tolerance and gene expression of RhNAP and RhCKX6 overexpressing A. thaliana lines. (A) RT-PCR was conducted with fully expanded leaves of transgenic A. thaliana plants. ACTIN2 was used as an internal control. (B) Tolerance of RhNAP- or RhCKX6-overexpressing A. thaliana plants to drought. T3 homozygous transformants were used in this experiment. 0 day drought, 14-day old well-watered plants; 15 days drought, 15 days after withholding water; 3 days rewatering, 3 days after rewatering; Survival rate was calculated from three independent experiments (~ 40 plants per line in one experiment).
Additional file 6: Figure S6. Effects of exogenous 6-benzylaminopurine (6-BA), trans-zeatin (tZ), isopentenyladenine (iP) and lovastatin on the senescence of rose petal discs. After 24 h pre-treatment with combinations of 100 μM 6-BA, 10 μM tZ, 10 μM iP, and 20 μM lovastatin, the petal discs were kept in water and photographed on days 7 and 13.
Additional file 7: Figure S7. Phenotypic analysis of RhNAP and RhCKX6 overexpression lines. (A) Phenotypes of age-matched plants (approximately 35 days after germination, DAG) of wild type (WT), RhNAP and RkCKX6. (B) Phenotypes of leaves detached from the age-matched 40 DAG plants in A.
Additional file 8: Figure S8. Analysis of RhCKX gene expression. Expression of RhCKX1 and RhCKX7 in rose petals in response to dehydration treatment (A) and various opening stages (B). The internal control used was RhUBI1. (C) qRT-PCR analysis of RhCKX gene expression in rose petals in response to exogenous 6-BA. Rose flowers at opening stage 2 were analyzed after 24 h of 100 μM 6-BA treatment. Control samples were treated with 0.1 M NaOH without phytohormones for 24 h. RhUBI1 was used as an internal control. All data shown are means ± standard deviation (n = 3); Student’s t-test, *P < 0.05, **P < 0.01.
Additional file 9: Figure S9. Anatomical structure of a petal viewed by scanning electron microscopy at flower full opening stage. (A, B) Adaxial epidermis; (C, D) Abaxial epidermis. Scale bar: 200 μm in A and C, magnification 200 x; 20 μm in B and D, magnification 2000 x.
Additional file 10: Table S1. Primers used in this study.
Data Availability Statement
Not applicable.