Abstract
Background:
Variability in the FADS2 gene, which codifies the Delta-6 Desaturases and modulates the conversion of essential n-3 and n-6 fatty acids into long-chain polyunsaturated fatty acids, might modify the impact of prenatal supplementation with n-3 docosahexaenoic acid (DHA) on neurodevelopment.
Objective:
To assess if maternal FADS2 single nucleotide polymorphisms (SNPs) modified the effect of prenatal DHA on offspring development at 5 years.
Design:
We conducted a post-hoc interaction analysis of the POSGRAD randomized controlled trial (NCT00646360) of prenatal supplementation with algal-DHA where 1,094 pregnant women originally randomized to 400 mg/day of preformed algal DHA or a placebo from gestation week 18-22 through delivery. In this analysis, we included offspring with information on maternal genotype and neurodevelopment at 5 years (DHA=316; Control=306) and used generalized linear models to assess interactions between FADS2 SNPs rs174602 or rs174575 and prenatal DHA on neurodevelopment at 5 years measured with McCarthy Scales of Children’s Abilities (MSCA).
Results:
Maternal and offspring characteristics were similar between groups. At baseline, mean (± standard deviation) maternal age was 26 ± 5 years and schooling was 12 ± 4 years. Forty-six percent (46%) of the children were female. Maternal minor allele frequencies were 0.37 and 0.33 for SNPs rs174602 and rs174575, respectively. There were significant variations by SNP rs174602 and intervention group (p for interactions <0.05) where children in the intervention group had higher MSCA scores on the quantitative (DHA: mean ± SEM =22.6 ± 0.9 vs. Control= 19.1 ± 0.9, mean difference (Δ)= 3.45; p=0.01) and memory (DHA= 27.9 ±1.1 vs. Control= 23.7 ± 1.1, Δ=4.26; p=0.02) scales only among offspring of TT (minor allele homozygotes).
Conclusions:
Maternal FADS2 SNP rs174602 modified the effect of prenatal DHA on cognitive development at 5 years. Variations in the genetic make-up of target populations could be an important factor to consider for prenatal DHA supplementation interventions.
Keywords: essential fatty acids, fatty acid desaturases, child development, prenatal supplementation, docosahexaenoic acid, arachidonic acid, gene-nutrient interactions
Introduction
Long-chain polyunsaturated fatty acids (LCPFAs) are conditionally-essential nutrients and during the prenatal period are obtained from the mother through placental transfer. Both docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (ARA, 20:4n-6) accrete in the fetal brain, where they have important membrane structural, cell signaling, and gene expression regulatory functions. (1, 2) In particular, DHA is important for the process of myelination, for visual functioning, and brain development in general.(3)
In observational studies, self-reported maternal DHA intake and plasma concentrations of DHA during pregnancy have been associated with heavier birth weights, extended gestational age, lower odds of preterm birth, and higher scores in mental development tests during infancy and early childhood.(4) However, except for preterm or high-risk populations, human experimental studies have failed to show consistent benefits of prenatal supplementation with DHA on a range of birth outcomes or on global cognitive development during childhood.(5, 6)
Recent evidence suggests that variants in the FADS2 gene, which encodes for the fatty acid delta-6-desaturase enzyme (D6D) responsible for converting n-3 and n-6 essential fatty acids into LCPUFAs, may modify dietary requirements.(7) Two distinct haplotypes have been identified to date: one, more prevalent in European populations, that has been associated with more efficient conversion of n-6 dietary precursor linoleic acid (LA, 18:2n-6) into ARA; and the other, more prevalent in Native Americans and Mexican American populations, that has been associated with less efficient conversion.(8, 9) This variation in the geographic distribution of single nucleotide polymorphisms (SNPs) in the FADS genes has been attributed to high selective pressure based on climate and fatty acid composition of the diet.(10)
Two FADS2 SNPs (rs174575 and rs174602) have been identified as potential modulators of the impact of dietary intake on child growth and development. (11-13) We have previously reported that in a randomized controlled trial of prenatal supplementation with DHA in Mexico, the intervention improved birth weight only among offspring of carriers of the FADS2 SNP rs174602 minor allele for Mexican populations (T).(11) In observational studies from high-income countries (New Zealand and Britain), rs174575 modified the association between early feeding practices and cognitive development at 8 years.(12, 13) The potential role of FADS2 genotype modifying the impact of prenatal supplementation with DHA on child development has not been studied in randomized controlled trials. Hence, the objective of this study was to assess if maternal FADS2 SNPs rs174575 and rs174602 modified the impact of prenatal DHA supplementation on global cognitive development at 5 years among Mexican children whose mothers participated in a DHA-supplementation trial during their pregnancy.
Methods
Parent study and sample selection
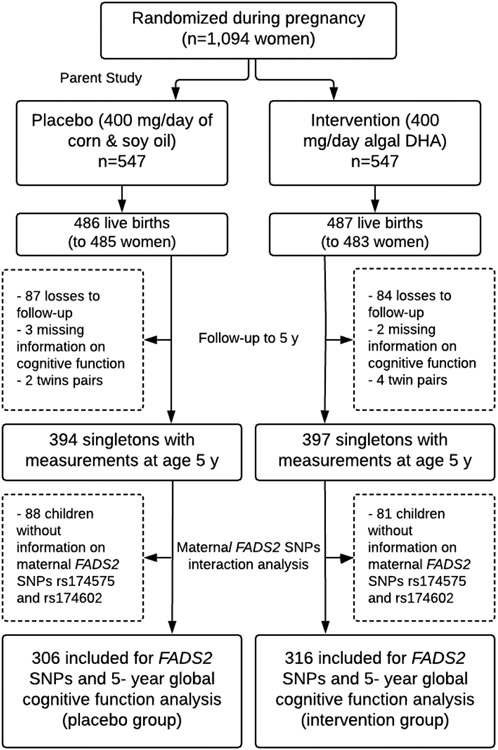
Data from this study came from POSGRAD (Prenatal Omega-3 Supplementation on Child Growth and Development), a double-blind randomized controlled trial (NCT00646360) conducted in Mexico. The original trial methodology has been described elsewhere; (11) briefly, between 2002 and 2006, 1,094 pregnant women in their 18-22 week of gestation were randomized to receive 400 mg/day of algal DHA or a placebo mixture of corn and soybean oil through delivery; on average women consumed 88% of the capsules provided. (11) Among the 968 women who completed the study, there were 973 live births (including 5 pairs of twins). For the purpose of this analysis, we included 622 mother-child pairs in which the women consented to genetic testing and singleton children had valid measures of global cognitive development at the 5-year data collection time point (Figure 1).
Figure 1: Flowchart for the maternal FADS2 single nucleotide polymorphism effect modification analysis of the POSGRAD supplementation trial.
The Emory University Institutional Review Board and the Ethics Board of the Mexican National Institute of Public Health reviewed and approved this study. Written informed consent was obtained from the mothers at enrollment and again on behalf of the child at the 5-year follow-up.
Cognitive Development Assessment
We used the Spanish version of the McCarthy Scales of Children’s Abilities (MSCA) to assess cognitive development at 5 years of age. The MSCA is designed to assess development in children 2.5 to 8.5 years and includes six different scales: Verbal, Perceptual-Performance, Quantitative, Memory, Motor, and General Cognitive (which is derived from the Verbal, Perceptual-Performance, and Quantitative Scales). These scales are assessed through 18 subtests.(14) The Verbal scale includes measurements of word knowledge, verbal memory, verbal fluency and opposite analogies; The Perceptual Performance scale includes block building, puzzle solving, tapping sequences, left-right orientation, and drawing; The Quantitative scale covers number and arithmetic questions, numerical memory, and counting and sorting; The Memory scale includes questions on pictorial memory, tapping sequences, verbal and numerical memory; and the Motor scale includes leg and arm coordination, imitative action, and drawing. (14)
The MSCA was applied by three trained psychologists in a quiet setting within a hospital; application of the entire battery took on average 1 hour. Administration of the test was supervised by the study lead psychologist through random observations and a full review of all tests was performed on site before data were entered.(15) Raw scores were computed by adding the results of the individual tests and were used for this analysis. The MSCA has been validated in Spain(16) and used by others to assess the impact of dietary and environmental exposures in Mexican children.(17, 18)
Genotyping and Tag SNP selection
Stored blood samples that had been collected from the mothers at baseline were shipped from the Mexican National Institute of Public Health to the Hemholtz Center (Munich, Germany) for genetic analysis. Polymerase chain reaction amplification and genotyping procedures were carried out on extracted DNA (total of 5μL) using the MassARRAY system and iPLEX chemistry . Fifteen candidate SNPs were assessed based on evidence suggesting that they might play a role in LCPUFA metabolism (7, 11, 19) and to represent the FADS1 (rs174556, rs174561, rs174558), FADS2 (rs174570, rs174575, rs2727271, rs174576, rs174578, rs174579, rs498793, rs174602), and FADS3 (rs174455, rs174448) genes. Genotype data were sent to Emory University in encrypted files for statistical analysis. The two SNPs for this analysis (rs174575, rs174602) were selected based on evidence of clinical significance, location in the FADS2 cluster, Hardy-Weinberg Equilibrium in the sample(11) and frequency of minor allele.
Other covariates
Measurement of potential maternal and child confounders has been described elsewhere; (20) briefly, sociodemographic predictors were measured at baseline and follow-ups using surveys. Maternal non-verbal intelligence was measured using the Raven Progressive Matrices questionnaire. (21) Maternal height and weight were measured at baseline using standard procedures. Maternal dietary intake was measured using a food frequency questionnaire, and plasma fatty acid composition was measured in a subsample at baseline and delivery. (20) Child anthropometric measurements at birth were collected from hospital records within 24 hours of delivery. The Home Observation Measurement of the Environment (HOME) (22) was conducted by trained psychologists when children were between 12 and 24 months.(23) During the 4- and 5- year follow-up, respectively, children’s dietary intake was measured using a food frequency questionnaire completed by the mothers, and anthropometric measurements were collected by trained personnel following standard procedures.(24)
Statistical analysis
The analysis included all children with maternal genetic information and MSCA measurement at 5 years. Maternal baseline characteristics, child characteristics, and cognitive development measurements were compared between the analytic sample and those with missing information using chi-square, t-tests, or ANOVA as needed.
Generalized linear models (MANOVA) were used to assess differences in MSCA cognitive development scores by FADS SNPs (Categorized into major allele homozygotes, heterozygotes, and minor allele homozygotes) and to test for interactions between each of the two FADS SNPs and DHA supplementation groups with each of the MSCA as an outcome. Additionally, we conducted an exploratory analysis testing for heterogeneity by sex. For SNP*intervention interactions significant at p<0.05, we conducted stratified analysis and tested pairwise comparisons between the DHA and placebo groups by allele combinations. We adjusted all estimates by age at measurement and sex and by potential maternal and child confounders (Supplemental table 1) selected using PROC GLMSELECT with backwards stepwise elimination. Multiple imputation was used to account for missing covariates. PROC MI was used to generate twenty imputed datasets using fully conditional specification. Generalized linear models were then conducted for each of the twenty imputed datasets and the estimates were pooled by PROC MIANALYZE.
The sample had 80% power to detect minimum SNP*diet interaction beta coefficients of 0.45 with a conservative minor allele frequency of 0.3 at an alpha-level of 0.05. For the three-way interaction with sex, we had 70% power to detect a minimum interaction beta coefficient of 1.2.
We conducted a sensitivity analysis with the subsample that had maternal baseline and delivery plasma DHA concentration and genotype information (n=123). We conducted multivariate linear regression to test associations between number of FADS SNP rs174602 minor alleles and plasma concentrations by supplementation group at baseline and delivery after adjusting for other FADS SNPs (rs174455, rs174556, rs498793), and tested a three- way interaction among intervention group, time point, and rs172602 genotype using PROC GLM to conduct a double difference analysis in SAS.
Power calculations were conducted using Quanto 1.2.4 (Los Angeles, CA)(25) and statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Significance was set at p<0.05. Results are presented as marginal means with standard error of the mean.
Results
The final sample for this study included 622 children (306 in the control group and 316 in the intervention group) with maternal genetic information and measures of cognitive development at 5 years (Figure 1).
At randomization, mothers were on average 27 years (26.5 ± 4.8), had approximately 12 years of schooling, and approximately a third were primigravid (34%). Mean offspring birth weight (SD) was 3.2 (0.5) kg and gestational age was 39 weeks (1.7). Exclusive breastfeeding at 3 months was 24%, with most children receiving mixed breastfeeding (58% combining breastmilk and formula). The intervention and control groups were well-balanced on maternal and child characteristics (Supplemental Table 1).
There were no significant differences in offspring MSCA scores at 5 years by intervention group (Table 1), and maternal FADS2 SNPs rs174602 or rs174575 were not associated with children’s MSCA scores at 5 years (Table 2).
Table 1:
McCarthy Scales of Child Abilities scores at 5 years group among Mexican children whose mothers participated in the POSGRAD randomized controlled trial of prenatal Docosahexaenoic n-3 fatty acid (DHA) supplementation, by intervention group.
| Placebo (n= 306) | Intervention (n= 316) b | p-value a | |
|---|---|---|---|
| McCarthy Scales of Child Ability, 5 years | |||
| Composite Score c | 121.0 (1.3) | 121.4 (1.3) | 0.86 |
| Quantitative Score | 20.0 (0.4) | 20.0 (0.4) | 0.95 |
| Verbal Score | 54.3 (0.7) | 53.6 (0.7) | 0.49 |
| Perceptual Score | 46.5 (0.5) | 47.3 (0.5) | 0.28 |
| Memory Score | 25.2 (0.4) | 25.3 (0.4) | 0.95 |
Values are raw score means (standard error of the mean) and are result of generalized linear models testing mean differences by supplementation group adjusted for age at measurement and child sex.
The intervention was 400mg/day of algal n-3 docosahexaenoic acid and the placebo was 400 mg/day of soy and corn oil from week 18-22 of pregnancy through delivery.
The composite score is the sum of verbal, perceptual and quantitative scores.
Table 2:
Child cognitive function at 5 years by FADS2 SNPs among Mexican children whose mothers participated in the POSGRAD randomized controlled trial of prenatal n-3 Docosahexaenoic acid (DHA) supplementation.
| SNP rs174602 | SNP rs174575 | |||||||
|---|---|---|---|---|---|---|---|---|
| CC (n=259) |
CT (n=273) |
TT (n=90) |
p- value |
CC (n=213) |
GC (n=322) |
GG (n=87) |
p- value |
|
| McCarthy Scales of Child Ability, raw scoresa | ||||||||
| Composite Scoreb | 120.9 (1.3) | 121.1 (1.3) | 120.6 (2.2) | 0.81 | 119.9 (1.5) | 121.2 (1.2) | 123.3 (2.3) | 0.43 |
| Quantitative Score | 20.0 (0.4) | 19.9 (0.4) | 20.8 (0.6) | 0.40 | 19.9 (0.4) | 20.0 (0.3) | 20.6 (0.6) | 0.62 |
| Verbal Score | 53.9 (0.7) | 53.9 (0.7) | 53.9 (1.2) | 0.58 | 53.2 (0.8) | 54.2 (0.6) | 55.3 (1.2) | 0.32 |
| Perceptual Score | 47.1 (0.6) | 47.3 (0.5) | 45.8 (0.9) | 0.36 | 46.8 (0.6) | 47.1 (0.5) | 47.4 (1.0) | 0.54 |
| Memory Score | 25.3 (0.4) | 25.1 (0.4) | 25.8 (0.8) | 0.75 | 25.0 (0.5) | 25.4 (0.4) | 25.9 (0.8) | 0.54 |
Values are raw score means (standard error of the mean) and are result of generalized linear models testing mean differences by supplementation group adjusted for child sex and age at measurement and maternal SES, Raven Progressive Matrices score and years of schooling.
The composite score is the sum of verbal, perceptual and quantitative scores.
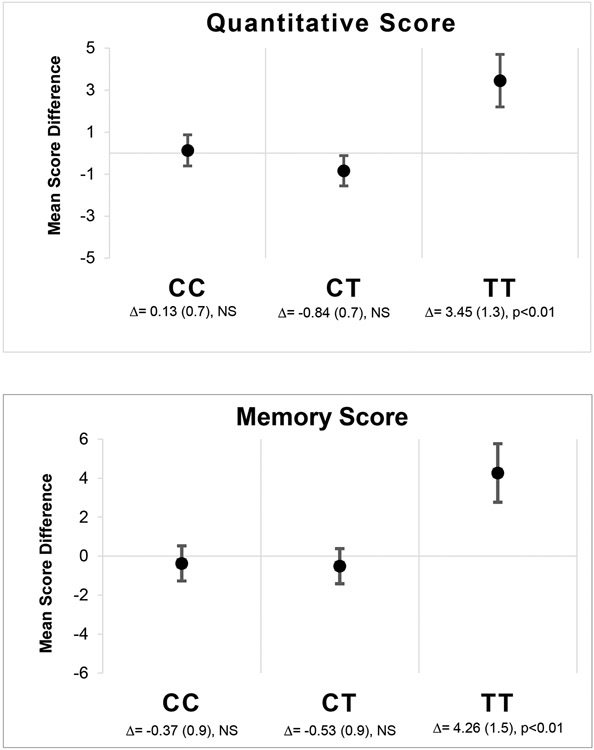
We found evidence of an interaction between the intervention and maternal SNP rs174602 in the Quantitative (p=0.02) and Memory scales (p=0.01) of the MSCA test (Table 3). After adjustment for baseline socioeconomic status score, maternal intelligence and schooling, and child sex and age at measurement, offspring of women who were homozygous for the minor allele TT for SNP rs174602 and received prenatal DHA had higher Quantitative and Memory scores on the MSCA when compared to those born to homozygous women in the control group. For Quantitative scores, among children whose mothers were homozygotes for the minor allele T, those in the intervention group had on average 3.5 higher scores than those in the control, which is equivalent to a difference of 0.6 SD. Similarly, for Memory scores, among offspring of T homozygotes, those who received the intervention had scored on average 4.3 points higher, which also represents a difference of 0.6 SD (Figure 2).
Table 3:
McCarthy Scales of Child Abilities scores at 5 years by maternal FADS2 SNPs and supplementation group among Mexican children whose mothers participated in the POSGRAD randomized controlled trial of prenatal n-3 Docosahexaenoic acid (DHA) supplementation.
| Placebob (n= 306) |
Interventionb (n= 316) |
||||||
|---|---|---|---|---|---|---|---|
| FADS SNP rs174602 a |
CC (n=132) |
CT (n=129) |
TT (n=45) |
CC (n=127) |
CT (n=144) |
TT (n=45) |
p- valued |
| Composite Score c | 121.0 (1.8) | 121.7 (1.9) | 115.4 (3.1) | 120.9 (1.9) | 120.6 (1.8) | 125.8 (3.1) | 0.11 |
| Quantitative Score | 19.9 (0.5) | 20.3 (0.5) | 19.1 (0.9) | 20.0 (0.5) | 19.4 (0.5) | 22.6 (0.9) | 0.01 |
| Verbal Score | 54.2 (1.0) | 54.7 (1.0) | 52.0 (1.7) | 53.7 (1.0) | 53.2 (1.0) | 55.7 (1.7) | 0.16 |
| Perceptual Score | 46.9 (0.8) | 46.7 (0.8) | 44.3 (1.3) | 47.2 (0.8) | 48.0 (0.8) | 47.3 (1.3) | 0.41 |
| Memory Score | 25.5 (0.6) | 25.4 (0.6) | 23.7 (1.1) | 25.1 (0.6) | 24.9 (0.6) | 27.9 (1.1) | 0.02 |
| FADS SNP rs174575 a |
CC (n=119) |
CG (n=149) |
GG (n=38) |
CC (n=94) |
CG (n=173) |
GG (n=49) |
p- valued |
| Composite Score c | 118.2 (1.9) | 121.6 (1.7) | 122.8 (3.4) | 121.5 (2.2) | 120.8 (1.6) | 123.8 (3.0) | 0.55 |
| Quantitative Score | 19.6 (0.5) | 20.0 (0.5) | 20.8 (1.0) | 20.3 (0.6) | 20.0 (0.5) | 20.4 (0.9) | 0.69 |
| Verbal Score | 52.7 (1.0) | 55.0 (0.9) | 54.9 (1.8) | 53.7 (1.2) | 53.3 (0.9) | 55.6 (1.6) | 0.34 |
| Perceptual Score | 46.1 (0.8) | 46.6 (0.7) | 47.1 (1.5) | 47.5 (0.9) | 47.6 (0.7) | 47.8 (1.3) | 0.39 |
| Memory Score | 24.5 (0.7) | 25.5 (0.6) | 26.2 (1.2) | 25.5 (0.7) | 25.3 (0.5) | 25.6 (1.0) | 0.60 |
Values are raw score means (standard error of the mean) and are result of generalized linear models testing the interaction between FADS2 single nucleotide polymorphism and supplementation group on cognitive development scores measured using the McCarthy Scales of Infant Abilities at 5 years adjusted for child sex and age at measurement (months), and maternal SES, Raven Score and years of schooling.
The intervention was 400mg/day of algal n-3 docosahexaenoic acid and the placebo was 400 mg/day of soy and corn oil from week 18-22 of pregnancy through delivery.
The composite score is the sum of verbal, perceptual and quantitative scores.
P-values refer to the interaction term (intervention*FADS2 SNP, error=615 df)
Figure 2-. Quantitative and memory scores contrast-specific mean differences (Δ) between intervention and placebo by FADS2 SNP rs174602.
Models were adjusted for child sex and age at measurement, and maternal SES, Ravens Progressive Matrices score, and years of schooling.
In the sensitivity analysis conducted in the subsample with plasma fatty acid information, there was no significant three-way interaction between time, genotype, and the intervention on plasma DHA (p=0.6) (Supplemental table 2); DHA plasma concentrations increased in all rs174602 genotypes. The inverse association between SNP rs174602 minor allele and plasma concentration at baseline that was previously observed in the entire subsample, remained significant in the control group at baseline and delivery; however, it was not significant in the stratified analysis for the group that received prenatal DHA either at baseline or at follow-up (p>0.05) (Supplemental table 3).
There was no interaction between the intervention and SNP rs174575 for any outcome measured (Table 3). There was no observed heterogeneity by sex (data not shown).
Discussion
In this analysis, we assessed if two maternal FADS2 SNPs modified the impact of prenatal supplementation with n-3 DHA on measures of global cognition at age 5 years and found that the intervention selectively improved quantitative and memory scores among offspring of homozygotes for FADS2 SNP rs174602 minor allele TT. This is consistent with previous results from this trial where we observed an impact of the intervention on birthweight only among carriers of the minor allele for this same SNP.(11)
The importance of DHA for neurodevelopment is well-established,(1, 26) however the positive impact of supplementation on child development during the preschool years and beyond remains controversial.(4, 27) Clarification of this important question is further complicated by different doses and composition of the supplements that have been tested in clinical trials, as well as by the diversity of tools to assess child cognition and the different brain regions and functions that they target. Previous trials that have reported effects of DHA supplementation on visual acuity have had mixed results for different domains of childhood cognitive functioning.(27) For example, there is evidence of a potential negative impact of DHA and other n-3 fatty acid supplementation on verbal development, especially in girls.(28, 29)
In this trial, we had previously showed an impact of prenatal supplementation on attention at 5 years measured by the Conners Kiddie Continuous Performance Test, where offspring of women who received the intervention committed fewer omissions, which is consistent with an impact on visual acuity and attention.(15) There was however no overall impact on measures of mental or motor functioning at 18 months(23) or 5 years.(15) In this analysis, we found a post-hoc gene-supplement interaction for the Memory and Quantitative scales, which are processes related to the parietal, pre-frontal, and frontal cortices(30, 31) where there is evidence of DHA accretion in early life and of attention-related activation.(32, 33)
Our results highlight the role of genetic variations as another factor modifying the impact of prenatal supplementation with DHA on development. We found an interaction of prenatal DHA supplementation with FADS2 SNP rs174602, a functional intron variant in the region encoding for D6D, which is responsible for a step in the synthesis of n-6 AA and n-3 EPA, and two steps in the synthesis of n-3 DHA. While other SNPs in the FADS genes have been associated with delta desaturases activity using plasma or erythrocyte concentrations of n-6 LA, AA or their ratio as proxies,(34, 35) FADS2 SNP rs174602 does not seem to be associated with n-6 concentrations or with LA to AA ratios.(36) In contrast, it has been identified as showing strong signatures of adaptation to a diet high in n-3 in Greenlandic Inuit populations.(10) We have previously shown that the presence of the rs174602 minor allele T predicts lower plasma concentrations of DHA in our study sample at baseline after adjusting for other FADS SNPs, (11) supporting that the subgroup carrying this minor allele has higher requirements for pre-formed DHA, which can explain the selective positive impact of this prenatal intervention on child development observed only among homozygotes for the minor allele T. The additional sensitivity analysis conducted in this study showed a similar increase in DHA plasma concentrations in the intervention group compared to the placebo across rs174602 genotypes, but we had limited power to test associations with the minor allele within the DHA group.
Another possible mechanism that could explain this association is the effect that rs174602 (or other FADS SNPs in high linkage disequilibrium) could have on n-6, which competes with n-3 for conversion by desaturases; it is possible that, in a context where n-6 fatty acids are abundant, as demonstrated by the extremely high n-6/n-3 intake ratio in our study population, genotypes that result less efficient conversion of n-6 fatty acids may be advantageous in supporting preferential tissue incorporation of n-3 fatty acid metabolites such as DHA. In this study conducted in a context where n-6 fatty acids have traditionally been abundant in the diet and n-3 is scarce, only a minority of the study population saw improvements on cognitive development after an intervention providing pre-formed n-3 long- chain polyunsaturated fatty acid DHA.
In contrast, we found no effect modification by FADS2 SNP rs174575, another intron variant that had been identified as a potential effect modifier of the impact of infant feeding practices (breastfeeding or formula) on childhood IQ. The results on gene-diet interactions for this SNP have not been consistent: Caspi et al. found that the impact of breastfeeding on IQ was only present among carriers of the major allele (C),(13) while Steer et al found that breastfeeding was particularly important for minor allele homozygotes (GG)(12). A smaller study from the United States found associations between maternal rs174575 genotype and declarative memory ability at 16 months, where toddlers whose mothers carried the allele C performed better than those whose mothers were GG homozygotes, and these associations were mediated by methylation in the child DNA supporting the role of programming.(37) We did not find any effect modification of prenatal supplementation with DHA by maternal FADS2 SNP rs174575; it is possible that the child genotype and diet, including breastfeeding or the intake of other polyunsaturatedfatty acids, are more relevant to study the role of this SNP on child development.
The impact of prenatal DHA supplementation on childhood cognitive function can be explained by a metabolic impact on prenatal brain development because the intervention addressed the requirements for this fatty acid involved in myelination, gene expression and signaling during a critical period of brain development.(26) However, a potential continued effect during the continuing rapid brain development after birth cannot be excluded because we expect maternal prenatal DHA status to modulate neonatal body DHA stores, given that maternal DHA serum concentrations in pregnancy predict neonatal cord blood DHA levels. (38)
Even though the minor allele frequencies of 0.38 for SNP rs174602 and 0.33 for SNP rs174575 allowed us sufficient power to detect gene-diet interactions,(39) we had limited power to examine these relationships stratified by sex. In this sense, missing data is another limitation: attrition in the cohort was only 15% through five years but not every woman consented to the genetic analysis. The availability of plasma lipid concentrations in a small subsample of study participants is another limitation that impeded the detection of associations between plasma DHA concentrations and child cognitive function in relationship with genotype and the intervention. Similarly, lack of information on offspring genotypes is another potential limitation of this study, although we do not expect it to differ by intervention group, and previous studies have found the role of the maternal genotype more important for young children’s cognitive functioning.(38) We were also able to determine that breastfeeding practices and offspring fatty acid intake at 4 y did not differ by maternal FADS2 SNPs and intervention subgroups. Further studies with larger sample sizes and including the offspring genotype will be important to fully elucidate the role of FADS2 genotype moderating the impact of polyunsaturated fatty acids on child development before this can be translated into precision nutrition applications.
An important strength of this study is that were able to assess effect modification by maternal genotype within the design of a randomized controlled trial. Also, we had a sufficient sample size to assess tag SNP interactions with the intervention, a follow up of the birth cohort through the preschool years, standardized protocols for data collection and information on several maternal and child characteristics. To our knowledge, this is the first study to report a role of the FADS2 genotype moderating the impact of prenatal DHA on childhood cognitive development as part of the follow-up of a randomized controlled trial.
In summary, we found prenatal DHA supplementation benefits only for children whose mothers were homozygotes for the FADS2 SNP rs174602 minor allele T. These results need to be confirmed through additional studies with larger sample sizes, in diverse populations, and with different dosage of DHA and other essential fatty acids. If results are consistent, that would mean that the beneficial impact of prenatal DHA supplementation at a population level depends on the prevalence of FADS2 SNP rs174602 T homozygotes. This could have important implications for the design of n-3 and n-6 prenatal supplementation or dietary interventions, where genetic screening could help target groups at risk of deficiency. Further, there is evidence that excessive amounts of dietary DHA can have negative effects on early development especially in the absence of sufficient n-6 fatty acids,(19) which would make genetic targeting of essential fatty acid supplementation interventions even more relevant.
Supplementary Material
Acknowledgements:
the authors’ contributed to the original idea and design of the study (IGC, ADS, AMD, LS IR, JAR, BK, UR), data collection and analysis (IGC, MS, ST, ADS, ABV, AD, HD, IRS, RGF, PR, IS, MS, LS), funding acquisition (IGC, ABV, JAR, BK, UR), initial writing (IGC), and critical review and approval of the final manuscript (all authors). The authors acknowledge the contributions of Reynaldo Martorell, PhD to the POSGRAD study.
Funding:
This research was supported by NIH (HD043099, HD058818, HD087606), March of Dimes, Nutricia Foundation, and CONACYT Mexico (87121, 202062). The work of IGC was financially supported by the Thrasher Research Fund and the National Heart, Blood and Lung Institute (HL137338-03S1 and K12HL13804). The work of HD, BK and PR was financially supported in part by the European Research Council Advanced Grant META-GROWTH ERC-2012-AdG–no.322605, the European Joint Programming Initiative Projects NutriPROGRAM and EndObesity, the German Ministry of Education and Research, Berlin (Grant Nr. 01 GI 0825), and the German Research Council (INST 409/224-1 FUGG). BK is the Else Kröner-Seniorprofessor of Paediatrics at LMU co-funded by the Else Kröner-Fresenius Foundation, LMU Medical Faculty and LMU University Hospitals.
Abbreviations:
- ARA
Arachidonic Acid
- DHA
Docosahexaenoic Acid
- MSCA
McCarthy Scales of Child Abilities
- SNP
Single Nucleotide Polymorphism
Footnotes
Data described in the manuscript, code book, and analytic code will be made available upon request to the corresponding author pending application and IRB approval.
Trial Registration: https://clinicaltrials.gov/ct2/show/NCT00646360
References
- 1.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. The Journal of Pediatrics. 2003;143(4, Supplement):1–8. [DOI] [PubMed] [Google Scholar]
- 2.Kuratko CN, Barrett EC, Nelson EB, Salem N Jr. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients. 2013;5(7):2777–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calder PC. Docosahexaenoic Acid. Annals of Nutrition and Metabolism. 2016;69(suppl 1)(Suppl. 1):8–21. [DOI] [PubMed] [Google Scholar]
- 4.Campoy C, Escolano-Margarit MV, Anjos T, Szajewska H, Uauy R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. British Journal of Nutrition. 2012;107(S2):S85–S106. [DOI] [PubMed] [Google Scholar]
- 5.Uauy R, Hoffman DR, Mena P, Llanos A, Birch EE. Term infant studies of DHA and ARA supplementation on neurodevelopment: results of randomized controlled trials. The Journal of Pediatrics. 2003;143(4, Supplement):17–25. [DOI] [PubMed] [Google Scholar]
- 6.Simmer K. Fish-oil supplementation: the controversy continues. The American Journal of Clinical Nutrition. 2015;103(1):1–2. [DOI] [PubMed] [Google Scholar]
- 7.Koletzko B, Reischl E, Tanjung C, Gonzalez-Casanova I, Ramakrishnan U, Meldrum S, et al. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. 2019;39(1):21–44. [DOI] [PubMed] [Google Scholar]
- 8.Ameur A, Enroth S, Johansson A, Zaboli G, Igl W, Johansson AC, et al. Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. American journal of human genetics. 2012;90(5):809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathias RA, Pani V, Chilton FH. Genetic Variants in the FADS Gene: Implications for Dietary Recommendations for Fatty Acid Intake. Curr Nutr Rep. 2014;3(2):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jørgensen ME, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349(6254):1343. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Casanova I, Rzehak P, Stein AD, Garcia Feregrino R, Rivera Dommarco JA, Barraza-Villarreal A, et al. Maternal single nucleotide polymorphisms in the fatty acid desaturase 1 and 2 coding regions modify the impact of prenatal supplementation with DHA on birth weight. Am J Clin Nutr. 2016;103(4):1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steer CD, Davey Smith G, Emmett PM, Hibbeln JR, Golding J. FADS2 polymorphisms modify the effect of breastfeeding on child IQ. PloS one. 2010;5(7):e11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caspi A, Williams B, Kim-Cohen J, Craig IW, Milne BJ, Poulton R, et al. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc Natl Acad Sci U S A. 2007;104(47):18860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrader A, D’Amato RC. McCarthy Scales of Children’s Abilities. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. p. 1531–2. [Google Scholar]
- 15.Ramakrishnan U, Gonzalez-Casanova I, Schnaas L, DiGirolamo A, Quezada AD, Pallo BC, et al. Prenatal supplementation with DHA improves attention at 5 y of age: a randomized controlled trial. The American Journal of Clinical Nutrition. 2016;104(4):1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D. M. Escalas de McCarthy de Aptitudes y Psicomotricidad para Niños. EA Ediciones SA, editor. Madrid, España: 2006 [Google Scholar]
- 17.Torres-Sánchez L, Schnaas L, Rothenberg SJ, Cebrián ME, Osorio-Valencia E, Hernández MdC, et al. Prenatal p,p´-DDE exposure and neurodevelopment among children 3.5-5 years of age. Environ Health Perspect. 2013;121(2):263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malin AJ, Busgang SA, Cantoral AJ, Svensson K, Orjuela MA, Pantic I, et al. Quality of Prenatal and Childhood Diet Predicts Neurodevelopmental Outcomes among Children in Mexico City. 2018;10(8):1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koletzko B, Bergmann K, Brenna JT, Calder PC, Campoy C, Clandinin MT, et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. The American Journal of Clinical Nutrition. 2020;111(1):10–6. [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan U, Stein AD, Parra-Cabrera S, Wang M, Imhoff-Kunsch B, Juárez-Márquez S, et al. Effects of Docosahexaenoic Acid Supplementation During Pregnancy on Gestational Age and Size at Birth: Randomized, Double-Blind, Placebo-Controlled Trial in Mexico. Food and Nutrition Bulletin. 2010;31(2_suppl2):S108–S16. [DOI] [PubMed] [Google Scholar]
- 21.Raven JC. Manual for Raven's progressive matrices and vocabulary scales: Revised. London : H. K. Lewis ; Los Angeles, Calif. : Western Psychological Services, 1978, c1977.; 1978. [Google Scholar]
- 22.Bradley RH, Caldwell BM. Home observation for measurement of the environment: A revision of the preschool scale. US: American Assn on Mental Retardation; 1979. p. 235–44. [PubMed] [Google Scholar]
- 23.Ramakrishnan U, Stinger A, DiGirolamo AM, Martorell R, Neufeld LM, Rivera JA, et al. Prenatal Docosahexaenoic Acid Supplementation and Offspring Development at 18 Months: Randomized Controlled Trial. PloS one. 2015;10(8):e0120065–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Casanova I, Stein AD, Hao W, Garcia-Feregrino R, Barraza-Villarreal A, Romieu I, et al. Prenatal Supplementation with Docosahexaenoic Acid Has No Effect on Growth through 60 Months of Age. J Nutr. 2015;145(6):1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauderman WJ. Sample Size Requirements for Association Studies of Gene-Gene Interaction. American Journal of Epidemiology. 2002;155(5):478–84. [DOI] [PubMed] [Google Scholar]
- 26.Gow RV, Hibbeln JR. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc Psychiatr Clin N Am. 2014;23(3):555–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LBS, Ciappolino V, Agostoni C. DHA Effects in Brain Development and Function. Nutrients. 2016;8(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauritzen L, Jørgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reproduction, nutrition, development. 2005;45(5):535–47. [DOI] [PubMed] [Google Scholar]
- 29.Gawlik NR, Anderson AJ, Makrides M, Kettler L, Gould JF. The Influence of DHA on Language Development: A Review of Randomized Controlled Trials of DHA Supplementation in Pregnancy, the Neonatal Period, and Infancy. Nutrients. 2020;12(10):3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arsalidou M, Pawliw-Levac M, Sadeghi M, Pascual-Leone J. Brain areas associated with numbers and calculations in children: Meta-analyses of fMRI studies. Developmental Cognitive Neuroscience. 2018;30:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Current Opinion in Neurobiology. 2004;14(2):163–8. [DOI] [PubMed] [Google Scholar]
- 32.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. The American journal of clinical nutrition. 2010;91(4):1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNamara RK. DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J Nutr. 2010;140(4):864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lankinen MA, de Mello VD, Meuronen T, Sallinen T, Ågren J, Virtanen KA, et al. The FADS1 Genotype Modifies Metabolic Responses to the Linoleic Acid and Alpha-linolenic Acid Containing Plant Oils–Genotype Based Randomized Trial FADSDIET2. Molecular Nutrition & Food Research. 2021;65(7):2001004. [DOI] [PubMed] [Google Scholar]
- 35.Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Human molecular genetics. 2006;15(11):1745–56. [DOI] [PubMed] [Google Scholar]
- 36.Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, Widhalm K, et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res. 2010;51(8):2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheatham CL, Lupu DS, Niculescu MD. Genetic and epigenetic transgenerational implications related to omega-3 fatty acids. Part II: maternal FADS2 rs174575 genotype and DNA methylation predict toddler cognitive performance. Nutrition Research. 2015;35(11):948–55. [DOI] [PubMed] [Google Scholar]
- 38.Krauss-Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Del Carmen Ramirez-Tortosa M, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol. 2008;121(2):464–70 e6. [DOI] [PubMed] [Google Scholar]
- 39.Park J-H, Gail MH, Weinberg CR, Carroll RJ, Chung CC, Wang Z, et al. Distribution of allele frequencies and effect sizes and their interrelationships for common genetic susceptibility variants. Proc Natl Acad Sci U S A. 2011;108(44):18026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.