Abstract
Background
Combinations of avelumab [anti-programmed death-ligand 1 (anti-PD-L1)] or talazoparib [poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor] with binimetinib (MEK inhibitor) were expected to result in additive or synergistic antitumor activity relative to each drug administered alone. Here, we report phase Ib results from JAVELIN PARP MEKi, which investigated avelumab or talazoparib combined with binimetinib in metastatic pancreatic ductal adenocarcinoma (mPDAC).
Patients and Methods
Patients with mPDAC that had progressed with prior treatment received avelumab 800 mg every 2 weeks plus binimetinib 45 mg or 30 mg two times daily (continuous), or talazoparib 0.75 mg daily plus binimetinib 45 mg or 30 mg two times daily (7 days on/7 days off). The primary endpoint was dose-limiting toxicity (DLT).
Results
A total of 22 patients received avelumab plus binimetinib 45 mg (n = 12) or 30 mg (n = 10). Among DLT-evaluable patients, DLT occurred in five of 11 patients (45.5%) at the 45-mg dose, necessitating de-escalation to 30 mg; DLT occurred in three of 10 patients (30.0%) at the 30-mg dose. Among patients treated at the 45-mg dose, one (8.3%) had a best overall response of partial response. Thirteen patients received talazoparib plus binimetinib 45 mg (n = 6) or 30 mg (n = 7). Among DLT-evaluable patients, DLT occurred in two of five patients (40.0%) at the 45-mg dose, necessitating de-escalation to 30 mg; DLT occurred in two of six patients (33.3%) at the 30-mg dose. No objective responses were observed.
Conclusions
Combinations of avelumab or talazoparib plus binimetinib resulted in higher-than-expected DLT rates. However, most DLTs were single occurrences, and the overall safety profiles were generally consistent with those reported for the single agents.
Clinical trial registration
ClinicalTrials.govNCT03637491; https://clinicaltrials.gov/ct2/show/NCT03637491
Key words: immune checkpoint inhibitor, PD-L1, PARP inhibitor, MEK inhibitor, metastatic pancreatic ductal adenocarcinoma
Highlights
-
•
Combining avelumab or talazoparib plus binimetinib was investigated in mPDAC.
-
•
Both combinations had higher-than-expected rates of DLT and limited clinical activity.
-
•
However, overall safety profiles were generally consistent with prior monotherapy studies.
-
•
One patient treated with avelumab plus binimetinib had a partial response.
-
•
The trial was terminated before a recommended phase II dose was established, and a triplet combination was not explored.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer associated with a poor prognosis and limited efficacious treatment options. PDAC is typically diagnosed at an advanced stage, once the disease has progressed and metastasized1,2; the 5-year survival rate in the United States for patients diagnosed with metastatic pancreatic cancer is 3.1%.3 Mutations in RAS have been reported in ≈90% of pancreatic cancers.4 RAS is a GTPase that acts as a molecular switch in the control of pro-proliferation signaling pathways.5,6 Aberrant activation of RAS by mutation results in prolonged signaling through kinase cascades, such as the RAF–MEK–ERK pathway, resulting in increased cell proliferation and survival.5,6 Increased signaling through the RAF–MEK–ERK pathway has also been shown to upregulate the expression of genes such as CD274, which encodes programmed death-ligand 1 (PD-L1).7
Binimetinib is an oral, potent, small-molecule MEK1/2 inhibitor that, in vivo, has been shown to inhibit ERK phosphorylation by MEK, thereby inhibiting cell proliferation, particularly those harboring RAS mutations.8 Furthermore, in preclinical studies, binimetinib inhibited the growth of tumor xenograft models, including pancreatic cancer.8 Binimetinib is approved in combination with encorafenib (a BRAF inhibitor) for the treatment of patients with BRAF-mutant (V600E or V600K) unresectable or metastatic melanoma.9 However, despite promising preclinical activity of various MEK inhibitors, early-phase trials of the MEK inhibitors trametinib and pimasertib have failed to show meaningful activity in combination with gemcitabine in patients with PDAC.10,11
Avelumab, an anti-PD-L1 monoclonal antibody, selectively binds PD-L1 and competitively blocks its interaction with programmed cell death protein 1 (PD-1), interfering with a key immune checkpoint inhibition pathway.12 Avelumab is approved in various countries as first-line maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma that has not progressed with first-line platinum-based chemotherapy and for patients with disease progression after platinum-based chemotherapy; as monotherapy for patients with metastatic Merkel cell carcinoma; and in combination with axitinib as first-line treatment for patients with advanced renal cell carcinoma.13,14
Talazoparib is a potent, orally bioavailable poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor that is cytotoxic to cancer cell lines harboring gene mutations that compromise DNA repair.15,16 Talazoparib is approved for treatment of patients with germline BRCA-mutated, human epidermal growth factor receptor 2-negative, locally advanced or metastatic breast cancer.17
Combinations of avelumab, talazoparib, and binimetinib were expected to result in additive or synergistic antitumor activity relative to each drug alone. Studies suggest that inhibition of MEK combined with immune checkpoint blockade promotes T-cell and antitumor activity.18 In a KRAS-mutant tumor model, the combination of a MEK inhibitor with an anti-PD-L1 antibody showed greater antitumor activity than either agent alone.18 Furthermore, in multiple RAS-mutant tumor models, combining talazoparib with the MEK inhibitor trametinib induced synergistic cytotoxic effects.19 Finally, in KRAS-mutant tumor models, observed resistance to PARP inhibition and anti-PD-L1 therapy, attributed to KRAS mutation, was reversed by the inclusion of an MEK inhibitor, with a triplet combination resulting in substantial antitumor activity.20
We report safety, pharmacokinetics, pharmacodynamics, and efficacy results from phase Ib of the JAVELIN PARP MEKi trial, which evaluated avelumab or talazoparib in combination with binimetinib in patients with metastatic PDAC (mPDAC). We also report exploratory biomarker analyses from patients who received avelumab plus binimetinib.
Patients and methods
Study design
JAVELIN PARP MEKi (NCT03637491) was a phase Ib/II, open-label, multicenter trial designed to investigate combinations of avelumab, talazoparib, and binimetinib in patients with mPDAC and other locally advanced or metastatic KRAS- or NRAS-mutant solid tumors. Initially it was planned that the combination of avelumab plus binimetinib would be investigated to identify a recommended phase II dose (RP2D) before assessing the RP2D of a triplet combination (avelumab + talazoparib + binimetinib), followed by phase II. After a protocol amendment, an additional dose-finding part of phase Ib was added for the doublet combination of talazoparib plus binimetinib to identify the RP2D, followed by the triplet combination and then phase II. Data reported are from phase Ib in patients with mPDAC who received avelumab or talazoparib plus binimetinib.
In phase Ib, eligible patients were aged ≥18 years and had histologically confirmed mPDAC that had progressed during or following one or two prior lines of treatment for advanced or metastatic disease. Other eligibility criteria were measurable disease per RECIST version 1.1; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; adequate bone marrow, renal, liver, and cardiac function; and negative pregnancy test (in all females of childbearing potential). Patients were excluded if they had been previously treated with avelumab, a PARP inhibitor, or an MEK inhibitor. Other key exclusion criteria were known hypersensitivity to any of the study drugs; known history of immune-mediated colitis, inflammatory bowel disease, pneumonitis, pulmonary fibrosis, uveitis, or iritis; active autoimmune disease that might deteriorate when receiving an immunostimulatory agent (excluding type 1 diabetes, vitiligo, psoriasis, or hypothyroid/hyperthyroid disease not requiring immunosuppressive treatment); known symptomatic brain metastases requiring steroids; clinically significant cardiovascular, cerebrovascular, or thromboembolic disease in the last 6 months; uncontrolled hypertension; and retinal degenerative disease, retinal vascular occlusion, or risk factors for retinal vascular occlusion. The trial was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation Guideline for Good Clinical Practice, and the International Ethical Guidelines for Biomedical Research Involving Human Subjects. All patients provided written informed consent before enrollment. The protocol, amendments, and informed consent forms were approved by the institutional review board or independent ethics committee at each center.
Study treatment
Patients received avelumab or talazoparib plus binimetinib. For each combination, patients were enrolled in cohorts of three to six patients. The starting dose for the avelumab plus binimetinib combination was avelumab 800 mg by 1-hour intravenous infusion every 2 weeks plus binimetinib 45 mg orally two times daily (continuous). To mitigate the potential for infusion-related reactions (IRRs), premedication with an antihistamine and paracetamol (acetaminophen) was mandatory before the first four infusions of avelumab. The starting dose for the talazoparib plus binimetinib combination was talazoparib 0.75 mg orally one time daily (continuous) plus binimetinib 45 mg orally two times daily in a 7 days on/7 days off schedule. For both combinations, dose-level selection was guided by the escalation with overdose control (EWOC) criterion21 that required that a dose level could only be used for newly enrolled patients if the risk of excessive toxicity [i.e. dose-limiting toxicity (DLT) rate ≥33%] was <25% [a posterior probability of overdose (PrOD) of <0.25]. Patients received study treatment until objective disease progression as assessed by investigator, patient withdrawal, unacceptable toxicity, loss to follow-up, or other criteria for discontinuation occurred.
Endpoints and assessments
The primary endpoint for phase Ib was DLT during the DLT evaluation period [cycle 1 (28 days)]. Secondary endpoints were safety, confirmed objective response according to RECIST version 1.1 per investigator, pharmacokinetics, immunogenicity of avelumab [antidrug antibody (ADA) levels], and biomarker analyses (including PD-L1 expression and gene alterations). Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, and coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 23.1. DLT was defined as any of the following events occurring in the DLT observation period [cycle 1 (28 days)] if considered related to either or both study drugs: grade 4 neutropenia (>5 days), febrile neutropenia, neutropenic infection (absolute neutrophil count <1000/mm3 or <1.0 × 109/L, and grade >3 infection), grade ≥3 thrombocytopenia with bleeding, grade 4 thrombocytopenia, grade 4 anemia, grade ≥3 creatine phosphokinase (CPK) increase that was associated with an increase in creatinine ≥1.5 × the patient’s baseline level, grade 3 troponin increase associated with any sign of cardiac toxicity, and any potential Hy’s law cases. Other grade ≥3 nonhematologic toxicities of any duration were also defined as DLTs, except for certain AEs that were clinically manageable and/or of short duration, and laboratory anomalies without symptoms or clinical correlate. Additional DLTs included eye disorders (e.g. retinal vascular disorder), cardiac disorders (e.g. absolute left ventricular ejection fraction decrease >10% to below the lower limit of normal), respiratory disorders (e.g. grade ≥2 interstitial lung disease/pneumonitis), and skin disorders. Any treatment-related AE (TRAE) resulting in a dose reduction of talazoparib or binimetinib during the DLT observation period or the inability to complete 75% of the planned doses of any study drug during the DLT observation period was also classified as a DLT. IRRs were identified using prespecified lists of MedDRA terms in association with time of onset and resolution. Objective response was defined as a complete response (CR) or partial response (PR) per RECIST version 1.1. Both CRs and PRs must have been confirmed by repeat assessments carried out ≥4 weeks after the criteria for response were first met. Tumor assessments were carried out at baseline, every 8 weeks for 52 weeks, and every 16 weeks thereafter.
Blood samples for pharmacokinetic analyses were collected before and after dose on days 1 and 15 of cycles 1 and 2, after dose on day 7 of cycle 1, and before dose on day 1 of cycles 3, 5, 9, and 12 for avelumab; before dose on days 1, 8 (talazoparib + binimetinib combination only), and 15 of cycle 1 and on day 1 of cycles 2 and 3 for talazoparib and binimetinib; and 1, 2, and 3 hours post-binimetinib dosing on days 1 and 8 of cycle 1 in patients receiving the talazoparib plus binimetinib combination for binimetinib and metabolites. Analyses were quantified using validated bioanalytical methods. Pharmacokinetic parameters of interest were directly observed from data.
For the avelumab plus binimetinib combination, blood samples for avelumab immunogenicity (ADA) testing were collected prior to dosing on days 1 and 15 of cycles 1 and 2 and day 1 of cycles 3, 5, 9, and 12. ADA detection was conducted using an avelumab homogenous bridging electrochemiluminescence ADA assay (QPS; Newark, DE, USA).
Biomarker analyses
For biomarker assessments in the avelumab plus binimetinib groups, tumor biopsies were taken prior to study treatment, either during screening or within 1 year prior to enrollment (if the patient had not received any subsequent systemic anticancer treatment), and blood samples were collected prior to dosing on days 1, 8, and 15 of cycle 1, day 1 of cycles 2-5, and at end of treatment. CD8+ tumor-infiltrating lymphocytes (TILs) in baseline tumor samples were assessed by immunohistochemistry using the SP239 (Abcam) antibody. PD-L1 expression was analyzed by immunohistochemistry (Ventana SP263 assay) using a combined positivity score (number of PD-L1-stained immune and tumor cells divided by the total number of viable tumor cells, multiplied by 100). Molecular alterations were assessed by next-generation sequencing of plasma circulating tumor DNA using the Guardant Health OMNI assay (500-gene panel; 2.1 MB).
Statistical analysis
Safety and antitumor activity were assessed in all patients who received one or more dose of study treatment. DLTs were evaluated in all patients who received one or more doses of combination treatment and either experienced DLT during the DLT observation period (cycle 1) or completed this period without DLT. Patients without DLTs who withdrew from study treatment before receiving ≥75% of the planned dose in cycle 1 for reasons other than toxicity attributable to study treatment were not evaluable for DLT. Planned enrollment and dose assignment for each doublet combination in phase Ib were guided by a Bayesian logistic regression model. The posterior distribution for the risk of DLT was evaluated and summarized to provide the posterior probability that the risk of DLT lay within the following intervals: underdosing (0-0.16), target toxicity (0.16-0.33), and excessive toxicity (0.33-1). Objective response rate (ORR) was defined as the proportion of patients with a confirmed CR or PR among all treated patients per investigator assessment according to RECIST version 1.1; patients with inadequate data for tumor assessment (e.g. no baseline assessment or follow-up assessments) were considered nonresponders. Two-sided 95% confidence intervals (95% CIs) for ORRs were calculated using the Clopper–Pearson method.
Results
The initial cohort of patients with mPDAC received avelumab plus binimetinib 45 mg; however, because the level of DLTs exceeded prespecified criteria, the binimetinib dose was de-escalated to 30 mg for subsequent patients. The protocol was amended, and an additional dose-finding part of phase Ib was planned to investigate the doublet combination of talazoparib plus binimetinib to identify an RP2D before proceeding to assessment of the RP2D of the triplet combination to continue to phase II. In addition, because of observed DLT associated with continuous dosing of binimetinib in the initial avelumab plus binimetinib combination, the dosing schedule for binimetinib in the talazoparib plus binimetinib combination was modified to a 7 days on/7 days off intermittent dosing schedule in an attempt to mitigate potential toxicities. However, an excess of DLTs was also observed at the talazoparib plus binimetinib 45-mg dose level, leading to the binimetinib dose being de-escalated to 30 mg (7 days on/7 days off). Subsequently, the JAVELIN PARP MEKi trial was terminated before an RP2D was established, the triplet combination was not explored, and phase II was not initiated. Therefore, only phase Ib results for the two doublet combinations are reported within this article.
Avelumab plus binimetinib
Patients
A total of 22 patients with mPDAC were enrolled and treated with avelumab 800 mg plus binimetinib 45 mg (n = 12) or 30 mg (n = 10). Most patients (95.5%) had received two or more prior regimens of anticancer therapy (Table 1). The majority were male (68.2%) and had an ECOG PS of 1 (86.4%). All patients discontinued study treatment (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101584). The most common reason for discontinuation of either drug was disease progression at both the avelumab plus binimetinib 45-mg dose [avelumab, n = 8 (66.7%); binimetinib, n = 7 (58.3%)] and the avelumab plus binimetinib 30-mg dose [avelumab, n = 6 (60.0%); binimetinib, n = 4 (40.0%)]. The median duration of treatment was 13.0 weeks (range, 2.0-80.1) for avelumab and 9.0 weeks (range, 2.0-30.7) for binimetinib at the avelumab plus binimetinib 45-mg dose and 7.9 weeks (range, 2.0-32.1) for avelumab and 5.9 weeks (range, 2.0-31.6) for binimetinib at the avelumab plus binimetinib 30-mg dose (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101584).
Table 1.
Baseline characteristics of patients in the avelumab plus binimetinib and talazoparib plus binimetinib combination groups
| Avelumab + binimetinib 45 mga (n = 12) | Avelumab + binimetinib 30 mga (n = 10) | Avelumab + binimetinib pooleda (n = 22) | Talazoparib + binimetinib 45 mgb (n = 6) | Talazoparib + binimetinib 30 mgb (n = 7) | Talazoparib + binimetinib pooledb (n = 13) | |
|---|---|---|---|---|---|---|
| Age, median (range), years | 66.0 (53.0-80.0) | 66.0 (42.0-83.0) | 66.0 (42.0-83.0) | 67.5 (51.0-78.0) | 69.0 (51.0-81.0) | 68.0 (51.0-81.0) |
| Sex, n (%) | ||||||
| Male | 9 (75.0) | 6 (60.0) | 15 (68.2) | 3 (50.0) | 1 (14.3) | 4 (30.8) |
| Female | 3 (25.0) | 4 (40.0) | 7 (31.8) | 3 (50.0) | 6 (85.7) | 9 (69.2) |
| Race, n (%) | ||||||
| White | 12 (100) | 9 (90.0) | 21 (95.5) | 5 (83.3) | 6 (85.7) | 11 (84.6) |
| Asian | 0 (0) | 1 (10.0) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) |
| Not reported | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (14.3) | 2 (15.4) |
| Pooled geographic region, n (%) | ||||||
| North America | 12 (100) | 8 (80.0) | 20 (90.9) | 4 (66.7) | 7 (100) | 11 (84.6) |
| Europe | 0 (0) | 1 (10.0) | 1 (4.5) | 2 (33.3) | 0 (0) | 2 (15.4) |
| Asia | 0 (0) | 1 (10.0) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) |
| ECOG PS, n (%) | ||||||
| 0 | 0 (0) | 3 (30.0) | 3 (13.6) | 4 (66.7) | 1 (14.3) | 5 (38.5) |
| 1 | 12 (100) | 7 (70.0) | 19 (86.4) | 2 (33.3) | 6 (85.7) | 8 (61.5) |
| Primary diagnosis, n (%) | ||||||
| Pancreatic ductal adenocarcinoma | 12 (100) | 10 (100) | 22 (100) | 6 (100) | 7 (100) | 13 (100) |
| TNM stage,22n (%) | ||||||
| IV | 10 (83.3) | 10 (100) | 20 (90.9) | 6 (100) | 7 (100) | 13 (100) |
| Not reported | 2 (16.7) | 0 (0) | 2 (9.1) | 0 (0) | 0 (0) | 0 (0) |
| Prior regimens of anticancer therapy, n (%)c | ||||||
| 1 | 0 (0) | 1 (10.0) | 1 (4.5) | 1 (16.7) | 3 (42.9) | 4 (30.8) |
| 2 | 9 (75.0) | 4 (40.0) | 13 (59.1) | 2 (33.3) | 1 (14.3) | 3 (23.1) |
| 3 | 2 (16.7) | 4 (40.0) | 6 (27.3) | 2 (33.3) | 0 (0) | 2 (15.4) |
| ≥4 | 1 (8.3) | 1 (10.0) | 2 (9.1) | 1 (16.7) | 3 (42.9) | 4 (30.8) |
| Prior radiotherapy, n (%) | ||||||
| Yes | 2 (16.7) | 4 (40.0) | 6 (27.3) | 2 (33.3) | 2 (28.6) | 4 (30.8) |
| No/not reported | 10 (83.3) | 6 (60.0) | 16 (72.7) | 4 (66.7) | 5 (71.4) | 9 (69.2) |
ECOG PS, Eastern Cooperative Oncology Group performance status; TNM, tumour–node–metastasis.
Dosing schedule: avelumab every 2 weeks plus binimetinib two times daily (continuous).
Dosing schedule: talazoparib once daily (continuous) plus binimetinib two times daily (7 days on/7 days off).
Including regimens of neoadjuvant, adjuvant, advanced/metastatic, or locoregional disease/recurrence drug therapies.
Safety
Of 11 DLT-evaluable patients, DLT occurred in five patients (45.5%) at the avelumab plus binimetinib 45-mg dose (Table 2); the DLTs in individual patients were CPK increased (grade 4); pustular rash (grade 3); hypertension (grade 3); retinal pigment epithelium detachment (grade 3); and abdominal pain, nausea, vomiting (all grade 2), and diarrhea (grade 1). The PrOD at the binimetinib 45-mg dose was 0.535, exceeding the EWOC criterion of PrOD <0.25; therefore the dose was de-escalated to 30 mg for subsequent patients. DLT occurred in three of 10 patients (30.0%) at the avelumab plus binimetinib 30-mg dose and included mucosal inflammation (mucositis), acneiform dermatitis, and CPK increase (all grade 3 and n = 1). The PrOD at the 30-mg dose was 0.238.
Table 2.
Summary of DLTs in DLT-evaluable patients in the avelumab plus binimetinib or talazoparib plus binimetinib combination groups
| Avelumab + binimetinib 45 mga (n = 11) | Avelumab + binimetinib 30 mga (n = 10) | Avelumab + binimetinib pooleda (n = 21) | Talazoparib + binimetinib 45 mgb (n = 5) | Talazoparib + binimetinib 30 mgb (n = 6) | Talazoparib + binimetinib pooledb (n = 11) | |
|---|---|---|---|---|---|---|
| Patients with DLT, n (%) | 5 (45.5) | 3 (30.0) | 8 (38.1) | 2 (40.0) | 2 (33.3) | 4 (36.4) |
| Anemia | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (9.1) |
| Detachment of retinal pigment epithelium | 1 (9.1) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 1 (9.1) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 1 (9.1) | 0 (0) | 1 (4.8) | 1 (20.0) | 0 (0) | 1 (9.1) |
| Nausea | 1 (9.1) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 1 (9.1) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (9.1) |
| Mucosal inflammation | 0 (0) | 1 (10.0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Rash pustular | 1 (9.1) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Blood creatine phosphokinase increased | 1 (9.1) | 1 (10.0) | 2 (9.5) | 0 (0) | 1 (16.7) | 1 (9.1) |
| Neutrophil count decreased | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (9.1) |
| Platelet count decreased | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (9.1) |
| Myalgia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (9.1) |
| Pulmonary embolism | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (9.1) |
| Dermatitis acneiform | 0 (0) | 1 (10.0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Deep vein thrombosis | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (9.1) |
| Hypertension | 1 (9.1) | 0 (0) | 1 (4.8) | 0 (0) | 0 (0) | 0 (0) |
DLT, dose-limiting toxicity.
Dosing schedule: avelumab every 2 weeks plus binimetinib two times daily (continuous).
Dosing schedule: talazoparib once daily (continuous) plus binimetinib two times daily (7 days on/7 days off).
Across both doses, any-grade treatment-emergent AEs (any causality) occurred in all 22 patients. Grade ≥3 AEs occurred in nine of 12 patients (75.0%) at the avelumab plus binimetinib 45-mg dose and 10 (100%) at the avelumab plus binimetinib 30-mg dose. Any-grade TRAEs occurred in all 22 patients (Table 3). Grade ≥3 TRAEs occurred in four patients (33.3%) at the avelumab plus binimetinib 45-mg dose and eight (80.0%) at the avelumab plus binimetinib 30-mg dose. The most common grade ≥3 TRAE at both doses was CPK increased [45 mg, n = 2 (16.7%); 30 mg, n = 3 (30.0%)]. TRAEs led to discontinuation of avelumab in one patient (8.3%) at the avelumab plus binimetinib 45-mg dose (pneumonitis) and one (10.0%) at the avelumab plus binimetinib 30-mg dose (mucosal inflammation). TRAEs led to discontinuation of binimetinib in three patients at the avelumab plus binimetinib 45-mg dose [25.0%; CPK increased, detachment of retinal pigment epithelium, abdominal pain, and diarrhea (n = 1 each)] and three patients at the avelumab plus binimetinib 30-mg dose [30.0%; CPK increased (n = 2) and mucosal inflammation (n = 1)]. One patient at the avelumab plus binimetinib 30-mg dose discontinued both study drugs (mucosal inflammation). No treatment-related deaths occurred. IRRs occurred in one patient (8.3%) at the avelumab plus binimetinib 45-mg dose and in three patients (30.0%) at the avelumab plus binimetinib 30-mg dose; all were grade 2, and none led to discontinuation of avelumab.
Table 3.
Most common TRAEs in the pooled avelumab plus binimetinib or talazoparib plus binimetinib combination groups
| Avelumab + binimetinib 45 mga (n = 12) |
Avelumab + binimetinib 30 mga (n = 10) |
Avelumab + binimetinib pooleda (n = 22) |
Talazoparib + binimetinib 45 mgb (n = 6) |
Talazoparib + binimetinib 30 mgb (n = 7) |
Talazoparib + binimetinib pooledb (n = 13) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any TRAE, n (%) | 12 (100) | 4 (33.3) | 10 (100) | 8 (80.0) | 22 (100) | 12 (54.5) | 6 (100) | 3 (50.0) | 7 (100) | 0 (0) | 13 (100) | 3 (23.1) |
| Rash | 7 (58.3) | 0 (0) | 4 (40.0) | 0 (0) | 11 (50.0) | 0 (0) | 1 (16.7) | 0 (0) | 2 (28.6) | 0 (0) | 3 (23.1) | 0 (0) |
| Blood creatine phosphokinase increased | 4 (33.3) | 2 (16.7) | 3 (30.0) | 3 (30.0) | 7 (31.8) | 5 (22.7) | 0 (0) | 0 (0) | 3 (42.9) | 0 (0) | 3 (23.1) | 0 (0) |
| Dermatitis acneiform | 3 (25.0) | 0 (0) | 4 (40.0) | 1 (10.0) | 7 (31.8) | 1 (4.5) | 3 (50.0) | 1 (16.7) | 2 (28.6) | 0 (0) | 5 (38.5) | 1 (7.7) |
| Aspartate aminotransferase increased | 2 (16.7) | 0 (0) | 4 (40.0) | 1 (10.0) | 6 (27.3) | 1 (4.5) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0 (0) |
| Nausea | 4 (33.3) | 0 (0) | 1 (10.0) | 0 (0) | 5 (22.7) | 0 (0) | 4 (66.7) | 0 (0) | 2 (28.6) | 0 (0) | 6 (46.2) | 0 (0) |
| Vomiting | 3 (25.0) | 0 (0) | 1 (10.0) | 0 (0) | 4 (18.2) | 0 (0) | 2 (33.3) | 1 (16.7) | 1 (14.3) | 0 (0) | 3 (23.1) | 1 (7.7) |
| Diarrhea | 3 (25.0) | 0 (0) | 1 (10.0) | 0 (0) | 4 (18.2) | 0 (0) | 3 (50.0) | 1 (16.7) | 1 (14.3) | 0 (0) | 4 (30.8) | 1 (7.7) |
| Pruritus | 2 (16.7) | 0 (0) | 2 (20.0) | 1 (10.0) | 4 (18.2) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Alanine aminotransferase increased | 1 (8.3) | 0 (0) | 3 (30.0) | 1 (10.0) | 4 (18.2) | 1 (4.5) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0 (0) |
| Infusion-related reaction | 1 (8.3) | 0 (0) | 3 (30.0) | 0 (0) | 4 (18.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Edema peripheral | 2 (16.7) | 0 (0) | 1 (10.0) | 0 (0) | 3 (13.6) | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 1 (7.7) | 0 (0) |
| Retinopathy | 3 (25.0) | 0 (0) | 0 (0) | 0 (0) | 3 (13.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 1 (8.3) | 0 (0) | 2 (20.0) | 1 (10.0) | 3 (13.6) | 1 (4.5) | 1 (16.7) | 0 (0) | 4 (57.1) | 0 (0) | 5 (38.5) | 0 (0) |
| Hypertension | 1 (8.3) | 1 (8.3) | 1 (10.0) | 1 (10.0) | 2 (9.1) | 2 (9.1) | 1 (16.7) | 1 (16.7) | 0 (0) | 0 (0) | 1 (7.7) | 1 (7.7) |
| Mucosal inflammation | 1 (8.3) | 0 (0) | 1 (10.0) | 1 (10.0) | 2 (9.1) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Myalgia | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 2 (28.6) | 0 (0) | 2 (15.4) | 0 (0) |
| Anemia | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 1 (4.5) | 0 (0) | 2 (33.3) | 1 (16.7) | 1 (14.3) | 0 (0) | 3 (23.1) | 1 (7.7) |
| Pneumonitis | 1 (8.3) | 1 (8.3) | 0 (0) | 0 (0) | 1 (4.5) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Detachment of retinal pigment epithelium | 1 (8.3) | 1 (8.3) | 0 (0) | 0 (0) | 1 (4.5) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rash pustular | 1 (8.3) | 1 (8.3) | 0 (0) | 0 (0) | 1 (4.5) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lipase increased | 0 (0) | 0 (0) | 1 (10.0) | 1 (10.0) | 1 (4.5) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Platelet count decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 1 (16.7) | 1 (14.3) | 0 (0) | 3 (23.1) | 1 (7.7) |
| Decreased appetite | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) | 2 (15.4) | 0 (0) |
| Deep vein thrombosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 1 (14.3) | 0 (0) | 2 (15.4) | 0 (0) |
| Vision blurred | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) | 2 (15.4) | 0 (0) |
| Pulmonary embolism | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (16.7) | 0 (0) | 0 (0) | 1 (7.7) | 1 (7.7) |
| Neutrophil count decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (16.7) | 0 (0) | 0 (0) | 1 (7.7) | 1 (7.7) |
Any-grade TRAEs occurring in ≥10% of patients and all grade ≥3 TRAEs in the pooled avelumab plus binimetinib or talazoparib plus binimetinib combinations.
TRAE, treatment-related adverse event.
Dosing schedule: avelumab every 2 weeks plus binimetinib two times daily (continuous).
Dosing schedule: talazoparib once daily (continuous) + binimetinib two times daily (7 days on/7 days off).
Efficacy
At the avelumab plus binimetinib 45-mg dose, one patient had a confirmed PR [ORR, 8.3% (95% CI 0.2-38.5)] and six (50.0%) had a best overall response of stable disease (SD; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101584). No objective responses were observed at the avelumab plus binimetinib 30-mg dose; one patient (10.0%) had SD.
Pharmacokinetics
Avelumab concentrations at the end of the dosing interval (Ctrough) and maximum concentrations (Cmax) were consistent with expected exposures (Supplementary Figure S1A, available at https://doi.org/10.1016/j.esmoop.2023.101584); steady state was achieved by cycle 2 day 1. Binimetinib Ctrough concentrations were within the range of predicted concentrations (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2023.101584); steady-state exposures were achieved by the first pharmacokinetic assessment after day 1.
Immunogenicity
Of the 22 patients who received the avelumab plus binimetinib combination, three (13.6%) developed treatment-induced ADAs. Of these, two patients (9.1%; both at the 30-mg dose) had a persistent ADA response. Because of the low incidence of ADA, analysis of neutralizing antibodies against avelumab was not conducted.
Biomarker analyses
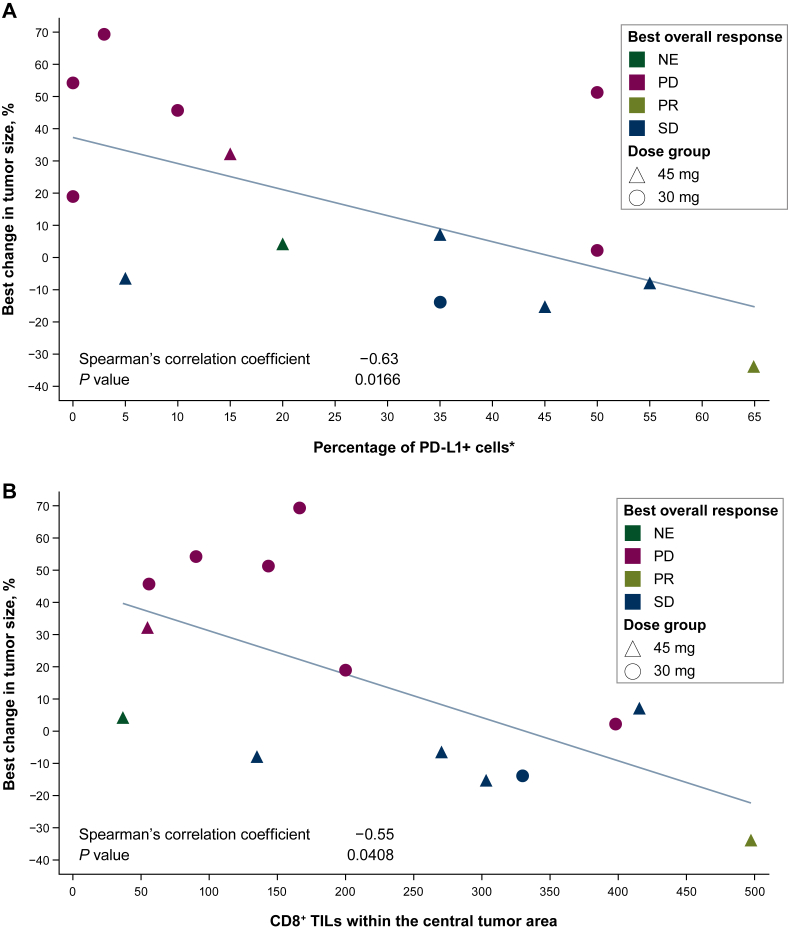
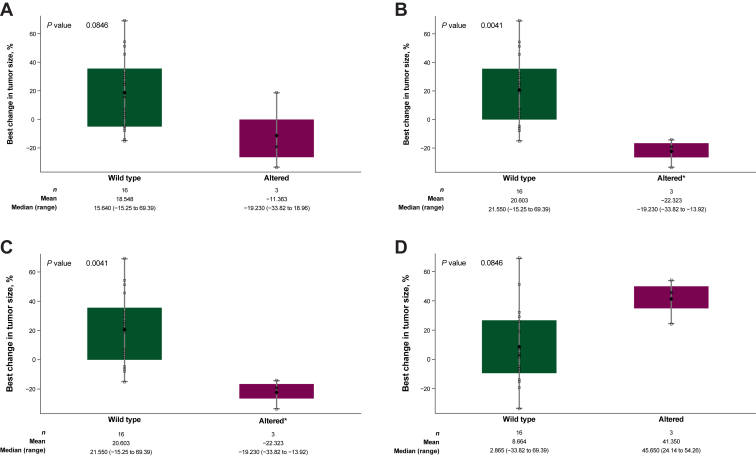
In 14 biomarker-evaluable patients across both doses, tumor shrinkage correlated with higher baseline PD-L1 expression (using a combined positivity score including expression on immune and tumor cells) and a higher number of CD8+ TILs (Figure 1). Plasma circulating tumor DNA analysis of baseline samples from both doses (n = 19) identified different genetic alterations that appeared to correlate with changes in tumor size (Figure 2). Alterations in MEK1/2, PI3KCA, and RNF43 were associated with tumor shrinkage, whereas alterations in ERBB4 correlated inversely with changes in tumor size. Of note, the patient who had a PR had high tumor PD-L1 expression; a high number of CD8+ TILs; mutations in ARID1A, CDKN2A, KRAS, LRP1B, MEK2, PCDH15, PIK3CA, TP53, and TRAF3; and also an amplification in RNF43.
Figure 1.
Best percentage change in tumor size in patients receiving the avelumab plus binimetinib combination by (A) PD-L1 expression and (B) number of CD8+ TILs.
∗Number of PD-L1+ cells (using a combined positivity score including expression on immune and tumor cells) divided by total number of viable tumor cells, multiplied by 100.
NE, not evaluable; PD, progressive disease; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease; TIL, tumor-infiltrating lymphocyte.
Figure 2.
Best percentage change in tumor size in patients receiving the avelumab plus binimetinib combination by alterations in (A) MEK1/2, (B) PIK3CA, (C) RNF43, and (D) ERBB4.
Note: P values are based on the Wilcoxon rank sum test. Diamonds represent the mean, and X symbols represent the median. ∗The same three patients had alterations in both PIK3CA and RNF43 (B and C).
Talazoparib plus binimetinib
Patients
A total of 13 patients with mPDAC were enrolled and treated with talazoparib 0.75 mg plus binimetinib 45 mg (n = 6) or 30 mg (n = 7). Most patients (69.2%) had received two or more prior regimens of anticancer therapy (Table 1). The majority were female (69.2%) and had an ECOG PS of 1 (61.5%); the median age was 68.0 years. Because of observed DLTs with continuous binimetinib dosing in the avelumab plus binimetinib combination, the dosing schedule for binimetinib for this combination was modified to a 7 days on/7 days off intermittent dosing schedule in an attempt to mitigate potential toxicities. All patients discontinued study treatment (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101584). The most common reason for discontinuation of either drug was disease progression at both the talazoparib plus binimetinib 45-mg dose [talazoparib, n = 4 (66.7%); binimetinib, n = 4 (66.7%)] and the talazoparib plus binimetinib 30-mg dose [talazoparib, n = 4 (57.1%); binimetinib, n = 4 (57.1%)]. The median duration of treatment was 6.9 weeks (range, 2.0-20.0) for talazoparib and 6.6 weeks (range, 1.1-19.0 weeks) for binimetinib at the talazoparib plus binimetinib 45-mg dose and 5.1 weeks (range, 2.3-9.6 weeks) for talazoparib and 5.1 weeks (range, 2.3-9.1 weeks) for binimetinib at the talazoparib plus binimetinib 30-mg dose (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101584).
Safety
Among five DLT-evaluable patients, DLT occurred in two patients (40.0%) at the talazoparib plus binimetinib 45-mg dose (Table 2); the DLTs in individual patients were pulmonary embolism (grade 3) and deep vein thrombosis (grade 2); and anemia, neutrophil count decrease, platelet count decrease, and diarrhea (all grade 3). The PrOD at the 45-mg dose was 0.359, exceeding the EWOC criterion of PrOD <0.25, leading to de-escalation of the binimetinib dose to 30 mg for subsequent patients. At the talazoparib plus binimetinib 30-mg dose, DLT occurred in two of six evaluable patients (33.3%; Table 2). The DLTs in individual patients were myalgia (grade 2), CPK increase (grade 1), and fatigue (grade 2). The PrOD at the 30-mg dose was 0.219.
Any-grade treatment-emergent AEs (any causality) occurred in all 13 patients across both doses. Grade ≥3 AEs occurred in five of six patients (83.3%) at the talazoparib plus binimetinib 45-mg dose and in four of seven patients (57.1%) at the talazoparib plus binimetinib 30-mg dose. Any-grade TRAEs occurred in all 13 patients receiving the talazoparib plus binimetinib combination (Table 3). At the talazoparib plus binimetinib 45-mg dose, grade ≥3 TRAEs occurred in three patients (50.0%); these included anemia, dermatitis acneiform, diarrhea, hypertension, neutrophil count decrease, platelet count decrease, pulmonary embolism, and vomiting [n = 1 each (16.7%)]. No grade ≥3 TRAEs occurred at the talazoparib plus binimetinib 30-mg dose. At the talazoparib plus binimetinib 45-mg dose, TRAEs led to discontinuation of both talazoparib and binimetinib for one patient (16.7%; pulmonary embolism and deep vein thrombosis). No TRAEs led to discontinuation of either study drug at the talazoparib plus binimetinib 30-mg dose. No treatment-related deaths occurred.
Efficacy
No objective responses were observed at either talazoparib plus binimetinib dose (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101584). At the talazoparib plus binimetinib 45- and 30-mg doses, two patients (33.3%) and one patient (14.3%), respectively, had a best overall response of SD.
Pharmacokinetics
Talazoparib Ctrough concentrations were at expected levels, although data were limited (Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2023.101584). Binimetinib concentrations were similar to those observed with the avelumab plus binimetinib combination (Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2023.101584). The concentration of binimetinib and the metabolite AR00426032 when binimetinib was given in a 7 days on/7 days off schedule was assessed at 1, 2, and 3 hours post dose on days 1 and 8 of cycle 1. The median plasma concentrations of binimetinib were characterized by a rapid absorption phase, typically reaching peak plasma levels within 1-2 hours post dose, and was quickly converted to AR00426032, with peak AR00426032 plasma levels occurring within 2 hours post dose. One patient at the talazoparib plus binimetinib 30-mg dose had low exposures of binimetinib on day 8, which were not explained by dosing records or other factors. Following the 7-day off-treatment period, most binimetinib and metabolite concentrations were below the limit of quantitation.
Discussion
The JAVELIN PARP MEKi trial was terminated before an RP2D was established. In patients with mPDAC treated with combinations of avelumab plus binimetinib or talazoparib plus binimetinib, the starting dose of binimetinib 45 mg was associated with DLT rates (PrODs of 0.535 and 0.359, respectively) that necessitated investigation of a lower dose (30 mg). These PrODs suggested that the combination of either avelumab or talazoparib with binimetinib 45 mg may have exceeded the maximum tolerated dose. Although the EWOC criterion was not exceeded with the binimetinib 30-mg dose in either combination, proceeding with a triplet combination did not appear to be feasible, even with intermittent binimetinib dosing.
Most DLTs were single occurrences, except for diarrhea and CPK increase, which were reported as DLTs for two and three patients, respectively, across the different doses. Despite the higher-than-expected rates of DLT at both binimetinib doses, the overall safety profiles of the combinations were generally consistent with those reported for the agents when given as monotherapy,15,23,24 and no new safety concerns were observed for any drug. Antitumor activity for both combinations was limited. As a result, a high proportion of patients discontinued study treatment due to disease progression, and the median duration of treatment for all drugs was short (5.1-13.0 weeks). With the caveat of small numbers, for the avelumab plus binimetinib combination, a higher dose of binimetinib (45 mg) had a higher number of patients with SD and one confirmed PR compared with the lower dose (30 mg). Pharmacokinetic data, although limited, indicated that avelumab, talazoparib, and binimetinib exposures were within the range of those in previous monotherapy studies.12,25,26 There was no evidence of a pharmacokinetic-mediated drug interaction between avelumab and binimetinib, and, despite limited trial data, a pharmacokinetic-mediated drug interaction was neither observed nor expected for talazoparib plus binimetinib based on their metabolic profiles. A low incidence of ADA in patients receiving the avelumab plus binimetinib combination was observed that did not appear to impact the pharmacokinetics of avelumab or the safety of the combination treatment.
Results from biomarker analyses of the avelumab plus binimetinib combination provide insights into potential mechanisms of treatment response and resistance. Tumor shrinkage correlated with higher baseline PD-L1 expression; higher number of CD8+ TILs; and alterations in MEK1/2, PI3KCA, and RNF43. However, patient numbers were small, hindering definitive conclusions. Biomarker analyses were not conducted for the talazoparib plus binimetinib combination due to the limited clinical benefit observed.
Based on results from the phase Ib part of this trial, phase II was not initiated due to the low probability of achieving target doses with a triplet combination while maintaining acceptable tolerability and the limited antitumor activity observed in patients with mPDAC.
Acknowledgements
The authors thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers.
Funding
This trial was sponsored by Pfizer as part of an alliance between Pfizer and Merck (CrossRef Funder ID: 10.13039/100009945; no grant number). Medical writing support was provided by Sophie Saunders of Clinical Thinking and funded by Pfizer and Merck.
Disclosure
JRA reports nonfinancial support and reasonable reimbursement for travel from the European Society for Medical Oncology; has received travel and accommodation expenses from Ellipses Pharma, IONCTURA, Kelun Pharmaceuticals/KLUS Pharma, Molecular Partners, and Peptomyc; has served in consulting or advisory roles for Boxer Capital, Chinese University of Hong Kong, Ellipses Pharma, IONCTURA (including serving on a scientific advisory board), Kelun Pharmaceuticals/KLUS Pharma, Molecular Partners, Peptomyc, Vall d’Hebron Institute of Oncology/Ministerio De Empleo Y Seguridad Social, and Tang Advisors; has received research funding from Black Diamond Therapeutics, Blueprint Medicines, Hummingbird, MSD, Vall d’Hebron Institute of Oncology/Cancer Core Europe, and Yingli; and has served as investigator in clinical trials with Aadi Bioscience, Amgen, Bayer, BioAtla, BioMed Valley Discoveries, Bicycle Therapeutics, Cancer Core Europe, Cellestia, Curis, CytomX, Deciphera, Fore Biotherapeutics, Genmab, GSK, Hummingbird, Hutchison MediPharma, IDEAYA, Kelun-Biotech, Loxo Oncology, Merus, Mirati Linnaeus Therapeutics, Novartis, Nuvation, Pfizer, Roche Pharmaceuticals, Spectrum Pharmaceuticals, Symphogen, Taiho, Takeda-Millennium, Tango Therapeutics, and Yingli. DSWT has received institutional research funding from AstraZeneca, GSK, and Novartis; has received honoraria from BMS, Novartis, Pfizer, Roche, and Takeda; has served in consulting or advisory roles for AstraZeneca, Loxo, MSD, Novartis, Pfizer, and Roche; and has received travel and accommodation expenses from Boehringer Ingelheim, Pfizer, and Roche. IGL has served in consulting or advisory roles for Array BioPharma, Eisai, Jazz Pharmaceuticals, Kanaph, OncXerna, SOTIO, and Sumitomo; and has received institutional research funding from ARMO BioSciences, Bayer, BMS, BridgeBio, GSK, Glenmark, Halozyme, Ignyta, Incyte, Lilly, MedImmune, NewLink Genetics, Novartis, OncoMed, and Pfizer. WH has received research funding from AI Therapeutics. JTB has received research funding from Novartis and Pfizer. NB has served in consulting or advisory roles for AstraZeneca, BMS, Celgene, and Exelixis. ZZ reports stock and other ownership interests in and was employed by Pfizer at the time of study. SD, KK, CW, and RJL report employment by and stock and other ownership interests in Pfizer. NP reports stock and other ownership interests in and was employed by Pfizer at the time of study. WAM has served in consulting or advisory roles for Five Prime Therapeutics (DMSC), Zymeworks (DMSC), and QED Therapeutics (DMSC); and has received institutional research funding from ALX Oncology, AstraZeneca, BeiGene, EDDC/D3, Exelixis, Fate Therapeutics, Pfizer, Roche/Genentech, and Takeda. AB, SR, NK have declared no conflicts of interest.
Data sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics approval and consent to participate
The trial was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation Guideline for Good Clinical Practice, and the International Ethical Guidelines for Biomedical Research Involving Human Subjects. All patients provided written informed consent before enrollment. The protocol, amendments, and informed consent forms were approved by the institutional review board or independent ethics committee at each center.
Supplementary data
References
- 1.Sarantis P., Koustas E., Papadimitropoulou A., Papavassiliou A.G., Karamouzis M.V. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12:173–181. doi: 10.4251/wjgo.v12.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberstein P.E., Olive K.P. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321–337. doi: 10.1177/1756283X13478680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute. SEER Cancer Stat Facts: Pancreatic Cancer. https://seer.cancer.gov/statfacts/html/pancreas.html Available at.
- 4.Hofmann M.H., Gerlach D., Misale S., Petronczki M., Kraut N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 2022;12:924–937. doi: 10.1158/2159-8290.CD-21-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pylayeva-Gupta Y., Grabocka E., Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waters A.M., Der C.J. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect Med. 2018;8:a031435. doi: 10.1101/cshperspect.a031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coelho M.A., de Carné Trécesson S., Rana S., et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity. 2017;47:1083–1099. doi: 10.1016/j.immuni.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee P.A., Wallace E., Marlow A., et al. Abstract 2515: Preclinical development of ARRY-162, a potent and selective MEK 1/2 inhibitor. Cancer Res. 2010;70(suppl 8):2515. [Google Scholar]
- 9.Mektovi (binimetinib) Array BioPharma Inc; Boulder, CO: 2020. Prescribing information. [Google Scholar]
- 10.Infante J.R., Somer B.G., Park J.O., et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072–2081. doi: 10.1016/j.ejca.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E., Hidalgo M., Canon J.-L., et al. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int J Cancer. 2018;143:2053–2064. doi: 10.1002/ijc.31603. [DOI] [PubMed] [Google Scholar]
- 12.Heery C.R., O’Sullivan-Coyne G., Madan R.A., et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587–598. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bavencio (avelumab) EMD Serono, Inc., an affiliate of Merck KGaA; Rockland, MA: 2022. Prescribing information. [Google Scholar]
- 14.Bavencio (avelumab) Merck Europe B.V., an affiliate of Merck KGaA; Amsterdam, Netherlands: 2022. Summary of product characteristics. [Google Scholar]
- 15.Litton J.K., Rugo H.S., Ettl J., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Bono J.S., Mehra N., Scagliotti G.V., et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22:1250–1264. doi: 10.1016/S1470-2045(21)00376-4. [DOI] [PubMed] [Google Scholar]
- 17.Talzenna (talazoparib) Pfizer Inc; New York, NY: 2021. Prescribing information. [Google Scholar]
- 18.Ebert P.J.R., Cheung J., Yang Y., et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44:609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Sun C., Fang Y., Yin J., et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang B., Li X., Fu Y., et al. MEK inhibition remodels the immune landscape of mutant KRAS tumors to overcome resistance to PARP and immune checkpoint inhibitors. Cancer Res. 2021;81:2714–2729. doi: 10.1158/0008-5472.CAN-20-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babb J., Rogatko A., Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17:1103–1120. doi: 10.1002/(sici)1097-0258(19980530)17:10<1103::aid-sim793>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Amin M.B., Greene F.L., Edge S.B., et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 23.Kelly K., Infante J.R., Taylor M.H., et al. Safety profile of avelumab in patients with advanced solid tumors: a pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018;124:2010–2017. doi: 10.1002/cncr.31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dummer R., Schadendorf D., Ascierto P.A., et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435–445. doi: 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- 25.Bendell J.C., Javle M., Bekaii-Saab T.S., et al. A phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br J Cancer. 2017;116:575–583. doi: 10.1038/bjc.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y., Durairaj C., Shi H., Wang D.D. Population pharmacokinetics of talazoparib in patients with advanced cancer. J Clin Pharmacol. 2020;60:218–228. doi: 10.1002/jcph.1520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.