Abstract
Background
Approximately 80% of all breast cancers (BCs) are currently categorized as human epidermal growth factor receptor 2 (HER2)-negative [immunohistochemistry (IHC) 0, 1+, or 2+/in situ hybridization (ISH) negative]; approximately 60% of BCs traditionally categorized as HER2-negative express low levels of HER2. HER2-low (IHC 1+ or IHC 2+/ISH−) status became clinically actionable with approval of trastuzumab deruxtecan to treat unresectable/metastatic HER2-low BC. Greater understanding of patients with HER2-low disease is urgently needed.
Patients and methods
This global, multicenter, retrospective study (NCT04807595) included tissue samples from patients with confirmed HER2-negative unresectable/metastatic BC [any hormone receptor (HR) status] diagnosed from 2014 to 2017. Pathologists rescored HER2 IHC-stained slides as HER2-low (IHC 1+ or IHC 2+/ISH−) or HER2 IHC 0 after training on low-end expression scoring using Ventana 4B5 and other assays at local laboratories (13 sites; 10 countries) blinded to historical scores. HER2-low prevalence and concordance between historical scores and rescores were assessed. Demographics, clinicopathological characteristics, treatments, and outcomes were examined.
Results
In rescored samples from 789 patients with HER2-negative unresectable/metastatic BC, the overall HER2-low prevalence was 67.2% (HR positive, 71.1%; HR negative, 52.8%). Concordance was moderate between historical and rescored HER2 statuses (81.3%; κ = 0.583); positive agreement was numerically higher for HER2-low (87.5%) than HER2 IHC 0 (69.9%). More than 30% of historical IHC 0 cases were rescored as HER2-low overall (all assays) and using Ventana 4B5. There were no notable differences between HER2-low and HER2 IHC 0 in patient characteristics, treatments received, or clinical outcomes.
Conclusions
Approximately two-thirds of patients with historically HER2-negative unresectable/metastatic BC may benefit from HER2-low-directed treatments. Our data suggest that HER2 reassessment in patients with historical IHC 0 scores may be considered to help optimize selection of patients for treatment. Further, accurate identification of patients with HER2-low BC may be achieved with standardized pathologist training.
Key words: breast cancer, human epidermal growth factor receptor 2, HER2-low, immunohistochemistry, prevalence, retrospective study
Highlights
-
•
Prevalence of HER2-low (IHC 1+ or IHC 2+/ISH–) was 67.2% in 789 rescored historical HER2-negative BC samples.
-
•
Concordance between historical and rescored HER2 statuses was 81.3% (κ = 0.583).
-
•
The lower positive agreement for HER2 IHC 0 (69.9%) versus HER2-low (87.5%) supports reassessment of historical IHC 0.
-
•
No notable differences were seen between HER2-low and HER2 IHC 0 in clinicopathological characteristics/clinical outcomes.
-
•
Two-thirds of patients with HER2-negative unresectable/metastatic BC may benefit from HER2-low-directed treatments.
Introduction
Historically, breast cancers (BCs) were classified as either human epidermal growth factor receptor 2 (HER2) positive or HER2 negative based on results from immunohistochemistry (IHC) and in situ hybridization (ISH) testing. Per the 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) and National Comprehensive Cancer Network® (NCCN®) scoring guidelines,1,2 HER2 classification is binary: positive (defined as IHC 3+ or IHC 2+/ISH+) or negative (defined as IHC 0, IHC 1+, or IHC 2+/ISH−). Traditionally, HER2-positive or HER2-negative status was defined based on the clinical benefit observed with HER2-directed therapies (e.g. trastuzumab) in patients with very high levels of HER2 expression (e.g. associated with HER2 amplification).2, 3, 4 Approximately 80% of all BCs are currently categorized as HER2-negative using these criteria,5 but previous studies have reported that nearly 60% of BCs categorized as HER2-negative meet the criteria for HER2-low (IHC 1+ or IHC 2+/ISH−).6,7
Until recently, patients with HER2-low tumors were not eligible for treatment with HER2-directed agents. The antibody–drug conjugate trastuzumab deruxtecan (T-DXd) received approval for the treatment of patients with unresectable/metastatic HER2-low BC who had received a prior chemotherapy in the metastatic setting from the US Food and Drug Administration (FDA) in August 2022 and from the European Commission in January 2023.8,9 T-DXd was thus the first HER2-directed agent approved to treat HER2-low tumors. These approvals were based on data from the phase III DESTINY-Breast04 trial (NCT03734029) in which treatment with T-DXd improved survival versus physician’s choice of chemotherapy in patients with HER2-low unresectable/metastatic BC who had received one prior line of chemotherapy.10 Based on DESTINY-Breast04 trial results, the ASCO11 and NCCN®2 treatment guidelines were modified to recommend T-DXd as a treatment option for patients with HER2-low (IHC 1+ or IHC 2+/ISH−) unresectable/metastatic BC, making HER2-low a clinically actionable subgroup. Previously, identification of HER2-low tumors, including the distinction between IHC 0 and IHC 1+ groups, was not clinically meaningful, and pathologists may not have differentiated these patients. With the approval of T-DXd for HER2-low tumors, differentiating HER2-low expression is now clinically relevant. Recently, the FDA granted premarket authorization for the Ventana 4B5 (Roche) IHC assay to identify HER2-low expression in metastatic BCs.12 A greater understanding of patients with HER2-low disease is needed, including the prevalence and proper identification of HER2-low in patients with unresectable/metastatic BC as determined using conventional IHC assays and historical IHC results.6,7,10
In this novel global retrospective study, we report the overall prevalence of HER2-low in patients previously diagnosed with HER2-negative unresectable/metastatic BC based on rescores of historical HER2 IHC slides after pathologists underwent standardized training on low-end HER2 expression rescoring. We also describe patient characteristics, treatments received, outcomes in patients with HER2-low and HER2 IHC 0 unresectable/metastatic BC, and the concordance between historical HER2 IHC scores and rescores.
Patients and methods
Study design
This was a worldwide, multicenter, noninterventional, retrospective study (NCT04807595) in patients with a confirmed diagnosis of unresectable and/or metastatic BC previously identified as HER2-negative (HER2 IHC 0, IHC 1+, or IHC 2+/ISH−). Local laboratories blinded to historical HER2 IHC scores rescored archival HER2 IHC-stained slides using Ventana 4B5 and other (i.e. non-Ventana) assays according to 2018 ASCO/CAP scoring guidelines1 after receiving training on low-end HER2 expression scoring. Non-Ventana assays included HercepTest (Agilent), Bond Oracle HER2 IHC System (Leica), and other unknown assays.
Pathologist training was completed using PathoTrainer software (CellCarta) to demonstrate scoring in the context of low HER2 expression. The pathologists completed a self-study review of the 2018 ASCO/CAP guidelines for HER2 IHC scoring, scored HER2-negative case collections, and received immediate feedback. Pathologists then completed a proficiency test of 30 cases with images from tumors stained with Ventana 4B5 or HercepTest (Agilent) assays; pathologists only scored cases tested with the assay used in their local laboratory.
Based on the rescores, slides were recategorized as HER2-low (IHC 1+ or IHC 2+/ISH−) or HER2 IHC 0, which was defined per 2018 ASCO/CAP scoring guidelines.1 In an exploratory analysis, samples categorized as HER2 IHC 0 were further subcategorized as having no perceivable staining (IHC 0 with no staining) or having faint or incomplete staining in <10% of cells [IHC 0 with staining (IHC > 0 < 1+); Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.101615].
Patient data were derived from electronic databases (electronic health/medical records and biobank registries) or extracted via patient chart review. The date of unresectable/metastatic BC diagnosis (between 1 January 2014 and 31 December 2017) was considered the index date. The data cutoff was 31 December 2020 (i.e. a minimum of 3 years of follow-up after unresectable/metastatic BC diagnosis).
Study population
Men or women aged ≥18 years (≥20 years in Japan) from 13 sites in 10 countries (Australia, Canada, France, Germany, Italy, Japan, Portugal, South Korea, United Kingdom, and United States) were eligible for study inclusion if they had a histologically or cytologically confirmed diagnosis of HER2-negative unresectable/metastatic BC (de novo or progression from early-stage BC; any hormone status) between 1 January 2014 and 31 December 2017 and experienced progression on any systemic anticancer therapy [e.g. endocrine therapy, chemotherapy, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, targeted therapies other than anti-HER2, or immunotherapy] in the advanced setting. Historical HER2 IHC-stained slides were required to be of acceptable quality for accurate rescoring by a trained pathologist. For patients who had primary BC that progressed to unresectable/metastatic status, primary-setting samples were accepted if they were the only slides available. Samples from patients with a history of other malignancies (other than basal cell carcinoma or squamous cell carcinoma of the skin), a historical HER2 status of IHC 2+/ISH+ or IHC 3+, or HER2 amplification were excluded. The patients identified for the current study were those in the relevant electronic health/medical record databases and biobanks who met the inclusion and exclusion criteria.
Objectives and outcomes
This study had two primary objectives. One was to determine the overall prevalence of HER2-low based on local laboratory rescoring of historical HER2 IHC-stained slides from samples previously scored as HER2-negative using the Ventana 4B5 assay. The other was to describe baseline patient characteristics (demographics and histopathological features), clinical presentation, treatments received, and clinical outcomes following unresectable/metastatic diagnosis in patients with HER2-low and to identify differences between these patients and those with HER2 IHC 0. The metastatic disease treatment outcomes examined were time to first subsequent treatment (TFST), defined as the length of time from the initiation of treatment to the initiation of the next systemic treatment; time to treatment failure (TTF), defined as the length of time from the initiation of treatment to premature discontinuation; and overall survival (OS), defined as the length of time from the initiation of treatment that patients remain alive. For all time-to-event treatment outcomes, the start date was the date of initiation of the first metastatic disease treatment. The end date was TFST, the earlier of the initiation of subsequent treatment or date of death; TTF, the earlier of the discontinuation of treatment line or date of death; and OS, date of death due to any cause during the study period. The secondary objectives included characterizing the concordance of historical HER2 IHC scores and local laboratory rescoring in samples previously scored as HER2-negative. Exploratory objectives included characterizing the prevalence of individual IHC categories (IHC 0 with no staining, IHC 0 with staining, IHC 1+, and IHC 2+/ISH−) and describing baseline patient characteristics, clinical presentation, treatments received, and clinical outcomes by IHC category.
Statistical methods
Standard summary statistics were used for all descriptive variables. Differences in characteristics and outcome measures of interest between HER2 groups were determined using the chi-square or Fisher’s exact test, with P < 0.05 considered statistically significant. Time-to-event outcomes were estimated using the Kaplan–Meier method and reported as median and 95% CI; comparisons between groups were evaluated using the log-rank test. Concordance was assessed using Cohen’s κ and the relative strength of agreement (i.e. κ > 0.8, almost perfect; 0.6 < κ ≤ 0.8, substantial; 0.4 < κ ≤ 0.6, moderate; 0.2 < κ ≤ 0.4, fair; 0 ≤ κ ≤ 0.2, slight; κ < 0, poor).13
Ethics
Patients provided written consent for future sample and clinical data use. For patients who were deceased, a waiver was accepted. This observational study was carried out in accordance with ethical principles consistent with the Declaration of Helsinki, ICH Good Clinical Practice, Good Guidance Practices, and applicable legislation on noninterventional/observational studies.
Results
Prevalence
The study included tissue samples from 789 patients previously categorized as having HER2-negative unresectable/metastatic BC; HER2 rescores were available for 787 patients. Overall, the prevalence of HER2-low based on rescored results was 67.2% (Table 1). HER2-low prevalence by country ranged from 54.7% to 76.1%. In samples rescored using the Ventana 4B5 assay (n = 556), HER2-low prevalence was similar to that in the overall findings (68.2%). Prevalence was also similar between the Ventana 4B5 and non-Ventana assays [68.2% (95% CI 64.3% to 72.0%) versus 63.8% (95% CI 57.3% to 70.3%), respectively; P = 0.253]. HER2-low prevalence was significantly greater in the hormone receptor (HR)-positive [71.1% (95% CI 67.3% to 74.9%)] versus HR-negative cohort [52.8% (95% CI 45.1% to 60.6%); P < 0.0001]. There was no obvious difference in the prevalence of HER2-low between tissue samples from the primary tumor site (140/206, 68.0%) and those from a metastatic tumor site (386/578, 66.8%).
Table 1.
Prevalence of HER2-lowa in patients previously diagnosed with HER2-negative unresectable/metastatic BC
| HR-positive, n/N (%) | HR-negative, n/N (%) | Total, n/N (%)b | |
|---|---|---|---|
| All | 394/554 (71.1)c,d | 84/159 (52.8)c | 529/787 (67.2)d |
| Breast cancer typee | |||
| Primary | 111/156 (71.2) | 16/32 (50.0) | 140/206 (68.0) |
| Metastatic | 280/395 (70.9) | 68/127 (53.5) | 386/578 (66.8) |
| Assayf | |||
| Ventana 4B5g | 278/386 (72.0) | 51/97 (52.6) | 379/556 (68.2)h |
| Non-Ventana 4B5i | 103/152 (67.8) | 31/58 (53.4) | 134/210 (63.8)h |
| Country | |||
| Australia | 14/22 (63.6) | 2/5 (40.0) | 17/28 (60.7) |
| Canada | 38/55 (69.1) | 13/24 (54.2) | 51/79 (64.6) |
| Germany | 70/85 (82.4) | 20/26 (76.9) | 137/180 (76.1) |
| France | 53/70 (75.7) | 1/2 (50.0) | 55/73 (75.3) |
| Italy | 59/88 (67.0) | 19/36 (52.8) | 78/124 (62.9) |
| Japan | 82/120 (68.3) | 13/35 (37.1) | 95/155 (61.3) |
| South Korea | 12/16 (75.0) | 4/9 (44.4) | 16/25 (64.0) |
| Portugal | 27/48 (56.3)d | 1/2 (50.0) | 29/51 (56.9)d |
| United Kingdom | 14/20 (70.0) | 5/9 (55.6) | 20/31 (64.5) |
| United States | 25/30 (83.3) | 6/11 (54.5) | 31/41 (75.6) |
Percentages were calculated based on reported/nonmissing data.
BC, breast cancer; CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; ISH, in situ hybridization.
IHC 1+ or IHC 2+/ISH−.
Includes all patients with unresectable/metastatic BC from 2014 to 2017, including those with missing HR status.
P < 0.0001 for HR-positive (71.1%; 95% CI 67.3% to 74.9%) versus HR-negative (52.8%; 95% CI 45.1% to 60.6%).
Two patients were missing HER2 rescore data.
This subcategory only includes patients with a sample with an available biopsy date. Primary BC was defined as a rescored biopsy sample dated 30 days before the unresectable/metastatic BC diagnosis date. Metastatic was defined as a rescored biopsy sample dated on or after 30 days, prior to the unresectable/metastatic BC diagnosis date.
Includes only known (nonmissing) IHC tests.
Primary endpoint.
P = 0.253 for Ventana 4B5 assay (68.2%; 95% CI 64.3% to 72.0%) versus non-Ventana 4B5 assays (63.8%; 95% CI 57.3% to 70.3%).
Non-Ventana 4B5 assays included HercepTest (Agilent), Bond Oracle HER2 IHC System (Leica), and unknown assays.
Patient characteristics
Nearly all patients were female (99.7%; Table 2). Most patients had HR-positive tumors and were ≥45 years of age, Asian or white, and postmenopausal. Visceral metastases were common among reported metastatic sites (55.6%); most patients (52.6%) had one reported metastatic site (Table 2). Overall, there were no remarkable differences in patient demographic or baseline disease characteristics between the HER2-low and HER2 IHC 0 groups within each HR subgroup.
Table 2.
Patient demographics and clinical characteristics
| HR-positive |
HR-negative |
Alla (N = 789) | |||
|---|---|---|---|---|---|
| HER2-lowb (n = 394) | HER2 IHC 0c (n = 160) | HER2-lowb (n = 84) | HER2 IHC 0c (n = 75) | ||
| Female, n (%) | 394 (100.0) | 159 (99.4) | 83 (98.8) | 75 (100.0) | 787 (99.7) |
| Age at index date, median (range), yearsd | 60 (31-97) | 59 (28-90) | 57 (31-80) | 52 (35-92) | 58 (28-97) |
| Age group, n (%) | |||||
| 18-44 years | 34 (8.6) | 19 (11.9) | 9 (10.7) | 19 (25.3) | 81 (10.3) |
| 45-64 years | 155 (39.3) | 68 (42.5) | 38 (45.2) | 30 (40.0) | 295 (37.4) |
| ≥65 years | 116 (29.4) | 48 (30.0) | 15 (17.9) | 15 (20.0) | 196 (24.8) |
| Age not reported/missing | 89 (22.6) | 25 (15.6) | 22 (26.2) | 11 (14.7) | 217 (27.5) |
| Race, n (%) | |||||
| American Indian or Alaska Native | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| Asian | 97 (24.6) | 42 (26.3) | 17 (20.2) | 28 (37.3) | 185 (23.4) |
| Black or African American | 5 (1.3) | 3 (1.9) | 1 (1.2) | 2 (2.7) | 11 (1.4) |
| White | 158 (40.1) | 59 (36.9) | 49 (58.3) | 35 (46.7) | 366 (46.4) |
| Other | 4 (1.0) | 0 (0) | 0 (0) | 0 (0) | 4 (0.5) |
| Not reported/missing | 129 (32.7) | 56 (35.0) | 17 (20.2) | 10 (13.3) | 222 (28.1) |
| Menopausal status, n (%)e | |||||
| Premenopausal | 89 (22.6) | 44 (27.5) | 25 (29.8) | 32 (42.7) | 205 (26.0) |
| Postmenopausal | 259 (65.7) | 99 (61.9) | 45 (53.6) | 35 (46.7) | 495 (62.7) |
| Not reported/missing | 46 (11.7) | 17 (10.6) | 14 (16.7) | 8 (10.7) | 89 (11.3) |
| Time from initial BC diagnosis to unresectable/metastatic BC diagnosisd | |||||
| <2 years | 153 (38.8) | 63 (39.4) | 28 (33.3) | 38 (50.7) | 284 (36.0) |
| 2-5 years | 53 (13.5) | 26 (16.3) | 19 (22.6) | 12 (16.0) | 111 (14.1) |
| >5 years | 98 (24.9) | 45 (28.1) | 15 (17.9) | 14 (18.7) | 173 (21.9) |
| Not reported/missing | 90 (22.8) | 26 (16.3) | 22 (26.2) | 11 (14.7) | 221 (28.0) |
| Metastatic locationf | |||||
| Bone only | 90 (22.8) | 31 (19.4) | 5 (6.0) | 4 (5.3) | 154 (19.5) |
| Brain | 14 (3.6) | 8 (5.0) | 9 (10.7) | 4 (5.3) | 37 (4.7) |
| Liver | 111 (28.2) | 51 (31.9) | 32 (38.1) | 17 (22.7) | 231 (29.3) |
| Lung | 94 (23.9) | 34 (21.3) | 24 (28.6) | 24 (32.0) | 192 (24.3) |
| Visceral | 214 (54.3) | 101 (63.1) | 53 (63.1) | 39 (52.0) | 439 (55.6) |
| Number of metastatic locationsf | |||||
| 1 | 222 (56.3) | 78 (48.8) | 41 (48.8) | 36 (48.0) | 415 (52.6) |
| 2 | 94 (23.9) | 40 (25.0) | 17 (20.2) | 21 (28.0) | 188 (23.8) |
| ≥3 | 75 (19.0) | 42 (26.3) | 24 (28.6) | 16 (21.3) | 162 (20.5) |
| Metastatic or locally advanced at index date | |||||
| Locally advanced | 7 (1.8) | 2 (1.3) | 0 (0) | 2 (2.7) | 11 (1.4) |
| Metastatic | 293 (74.4) | 129 (80.6) | 60 (71.4) | 62 (82.7) | 550 (69.7) |
| Both | 10 (2.5) | 6 (3.8) | 2 (2.4) | 2 (2.7) | 20 (2.5) |
| Not reported/missing | 84 (21.3) | 23 (14.4) | 22 (26.2) | 9 (12.0) | 208 (26.4) |
| Stage at initial BC diagnosis | |||||
| I | 18 (4.6) | 8 (5.0) | 8 (9.5) | 9 (12.0) | 43 (5.4) |
| II | 80 (20.3) | 36 (22.5) | 16 (19.0) | 21 (28.0) | 153 (19.4) |
| III | 47 (11.9) | 25 (15.6) | 18 (21.4) | 18 (24.0) | 108 (13.7) |
| IV | 53 (13.5) | 28 (17.5) | 2 (2.4) | 9 (12.0) | 93 (11.8) |
| Other/not reported/missing | 196 (49.7) | 63 (39.4) | 40 (47.6) | 18 (24.0) | 392 (49.7) |
ASCO, American Society of Clinical Oncology; BC, breast cancer; CAP, College of American Pathologists; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; ISH, in situ hybridization.
Includes patients with missing HR status.
IHC 1+ or IHC 2+/ISH−.
Classified according to ASCO/CAP 2018 guidelines (no staining observed or membrane staining that was incomplete, faint, or barely perceptible, and in <10% of the invasive tumor cells).
Index date was defined as the date of HER2-negative unresectable/metastatic BC diagnosis.
Status can be from records after the baseline period.
Reported over the course of disease.
Treatments and clinical outcomes
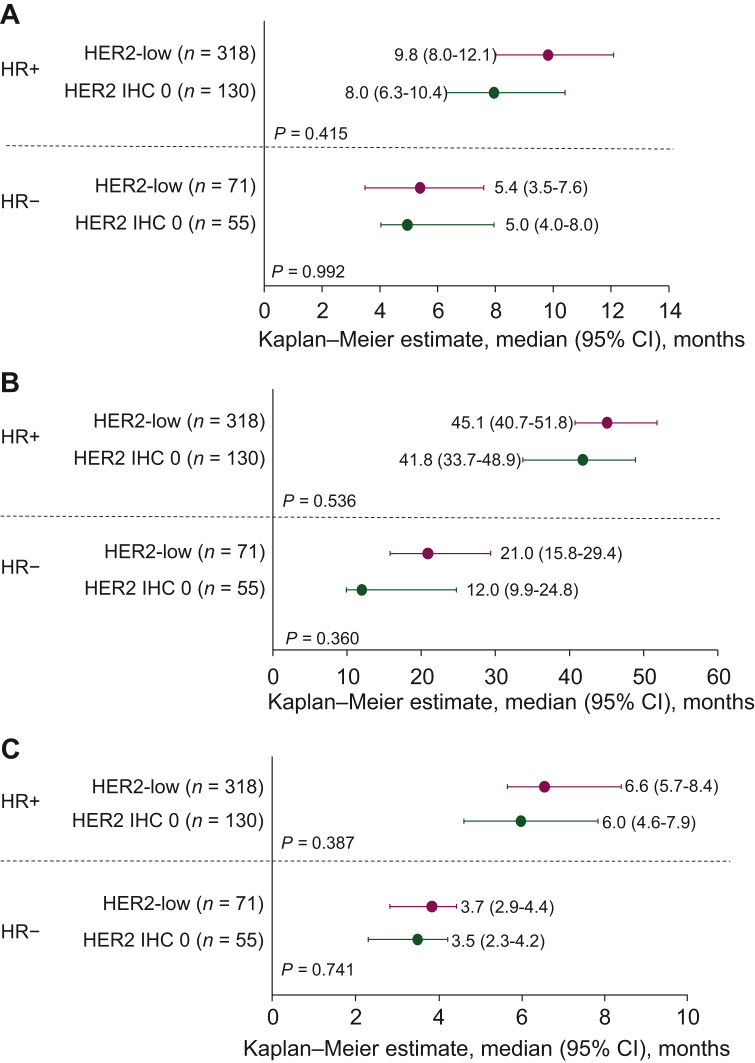
The most frequent therapies in the metastatic setting were endocrine therapy for patients with HR-positive BC and chemotherapy for patients with HR-negative BC (Table 3). Most (75.3%) patients with HR-positive and all patients with HR-negative disease received chemotherapy. The median TFST, OS, and TTF were not statistically different for HER2-low versus HER2 IHC 0 within both HR subgroups (Figure 1). For patients diagnosed with HR-positive disease, median OS (95% CI) from unresectable/metastatic BC diagnosis was 45.1 months (40.7-51.8 months; n = 318) in patients with HER2-low versus 41.8 months (33.7-48.9 months; n = 130) in patients with HER2 IHC 0 disease (P = 0.536). For those with HR-negative BC, median OS (95% CI) was 20.9 months (15.8-29.4 months; n = 71) in patients with HER2-low versus 12.0 months (9.9-24.8 months; n = 55) in patients with HER2 IHC 0 disease (P = 0.360). Overall, when examined by HR subgroup, no notable differences in treatments received or statistical differences in clinical outcomes were observed in patients with HER2-low versus HER2 IHC 0 tumors. In both the HER2-low and HER2 IHC 0 subgroups, median TFST, OS, and TTF were numerically greater in patients with HR-positive versus HR-negative disease.
Table 3.
Treatments received in the metastatic setting
| HR-positive, n/N (%) |
HR-negative, n/N (%) |
Alla (N = 635) | |||
|---|---|---|---|---|---|
| HER2-lowb (n = 318) | HER2 IHC 0c (n = 130) | HER2-lowb (n = 71) | HER2 IHC 0c (n = 55) | ||
| Overall | |||||
| Endocrine therapy | 287/318 (90.3) | 116/130 (89.2) | 17/71 (23.9)d | 6/55 (10.9)d | 474/635 (74.6) |
| Chemotherapy | 239/318 (75.2) | 98/130 (75.4) | 71/71 (100.0) | 55/55 (100.0) | 514/635 (80.9) |
| CDK4/6i | 117/318 (36.8) | 48/130 (36.9) | 3/71 (4.2) | 1/55 (1.8) | 172/635 (27.1) |
| Targeted therapiese | 89/318 (28.0) | 29/130 (22.3) | 12/71 (16.9) | 11/55 (20.0) | 175/635 (27.6) |
| First treatment | |||||
| Endocrine monotherapy | 137/318 (43.1) | 62/130 (47.7) | 2/71 (2.8) | 1/55 (1.8) | 231/635 (36.4) |
| Single-agent chemotherapy | 62/318 (19.5) | 23/130 (17.7) | 36/71 (50.7) | 23/55 (41.8) | 151/635 (23.8) |
| CDK4/6i | 1/318 (0.3) | 0/130 (0) | 0/71 (0) | 0/55 (0) | 1/635 (0.2) |
| Other targeted therapiese | 0/318 (0) | 0/130 (0) | 0/71 (0) | 0/55 (0) | 1/635 (0.2) |
| Immunotherapy | 0/318 (0) | 0/130 (0) | 2/71 (2.8) | 1/55 (1.8) | 3/635 (0.5) |
| Combination therapy (any) | 118/318 (37.1) | 45/130 (34.6) | 31/71 (43.7) | 30/55 (54.5) | 248/635 (39.1) |
Percentages were calculated based on reported/nonmissing data.
ADP, adenosine diphosphate; ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; ISH, in situ hybridization.
Includes patients with missing HR status.
IHC 1+ or IHC 2+/ISH−.
Classified according to ASCO/CAP 2018 guidelines (no staining observed or membrane staining that was incomplete, faint, or barely perceptible, and in ≤10% of the invasive tumor cells).1
The reason for endocrine therapy being given to patients who were HR negative is not available; plausible explanations include some patients switching from HR-positive to HR-negative status while on study and differing thresholds for HR-negative (e.g. <1% versus <10%) at some sites.
Includes anti-HER2 agents, antiangiogenesis agents, poly (ADP-ribose) polymerase inhibitors, phosphoinositide 3-kinase inhibitors, and mechanistic target of rapamycin inhibitors.
Figure 1.
Clinical outcomes in patients with HR-positive and HR-negative BC, stratified by HER2-low and HER2 IHC 0 status.
(A) TFST, (B) OS, (C) TTF.
BC, breast cancer; CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; OS, overall survival; TFST, time to first subsequent treatment; TTF, time to treatment failure.
Concordance
Concordance between HER2 classification of historical and rescored slides was 81.3% overall [κ (95% CI), 0.583 (0.523-0.643); Table 4]. The concordance between historical scores and rescores with Ventana 4B5 and non-Ventana assays was similar (81.8% and 80.0%, respectively). Concordance by country ranged from 67.9% to 94.1%. Concordance was numerically greater for HER2-low than for HER2 IHC 0 with all assays (87.5% and 69.9%, respectively) and with the Ventana 4B5 assay (90.3% and 67.9%, respectively).
Table 4.
Concordance between historical and rescored HER2 slides
| Agreement on HER2-low (positive percent agreementa), n/N (%) | Agreement on HER2 IHC 0 (positive percent agreementa), n/N (%) | Agreement on both HER2-low and HER2 IHC 0 (overall percent agreementb), n/N (%) | κ (95% CI) | |
|---|---|---|---|---|
| All | 446/510 (87.5) | 193/276 (69.9) | 639/786 (81.3) | 0.583 (0.52-0.64) |
| Assay | ||||
| Ventana 4B5 | 318/352 (90.3) | 146/215 (67.9) | 464/567 (81.8) | 0.602 (0.53-0.67) |
| Non-Ventana 4B5c | 121/150 (80.7) | 47/60 (78.3) | 168/210 (80.0) | 0.546 (0.43-0.67) |
| Country | ||||
| Australia | 11/14 (78.6) | 8/14 (57.1) | 19/28 (67.9) | 0.357 (0.02-0.70) |
| Canada | 50/63 (79.4) | 15/16 (93.8) | 65/79 (82.3) | 0.571 (0.38-0.76) |
| Germany | 100/108 (92.6) | 35/72 (48.6) | 135/180 (75.0) | 0.442 (0.31-0.57) |
| France | 52/56 (92.9) | 13/16 (81.3) | 65/72 (90.3) | 0.725 (0.53-0.92) |
| Italy | 66/81 (81.5) | 31/43 (72.1) | 97/124 (78.2) | 0.527 (0.37-0.68) |
| Japan | 80/92 (87.0) | 48/63 (76.2) | 128/155 (82.6) | 0.636 (0.51-0.76) |
| South Korea | 16/19 (84.2) | 6/6 (100.0) | 22/25 (88.0) | 0.719 (0.43-1.00) |
| Portugal | 27/28 (96.4) | 21/23 (91.3) | 48/51 (94.1) | 0.881 (0.75-1.00) |
| United Kingdom | 15/18 (83.3) | 8/13 (61.5) | 23/31 (74.2) | 0.459 (0.14-0.78) |
| United States | 29/31 (93.5) | 8/10 (80.0) | 37/41 (90.2) | 0.735 (0.49-0.98) |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
Positive percent agreement was the proportion of historical and rescored HER2 statuses that were in agreement on HER2-low. Percentages were calculated based on reported/nonmissing data.
Overall percent agreement was the proportion of historical and rescored HER2 statuses that were in agreement on either HER2 IHC 0 or HER2-low.
Non-Ventana 4B5 assays included HercepTest (Agilent), Bond Oracle HER2 IHC System (Leica), and unknown assays.
Analysis by IHC category
Overall, when rescored, most samples were categorized as IHC 1+ (411/789, 52.2%), with the rest fairly evenly distributed among the other IHC score categories [IHC 0 with no staining (144/789, 18.3%); IHC 0 with staining (114/789, 14.5%); IHC 2+/ISH− (118/789, 15.0%)]; prevalence by IHC score category and known HR status is shown in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101615. There were no notable differences in patient demographic or baseline disease characteristics across the IHC categories (Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.esmoop.2023.101615). In general, there were also no notable differences in treatments received or outcomes (median TFST, OS, or TTF) among IHC categories within the HR-positive and HR-negative subgroups (Supplementary Tables S4 and S5, available at https://doi.org/10.1016/j.esmoop.2023.101615), although low patient numbers in these subgroups limit interpretation. IHC rescores had an overall agreement of 66.8% [κ (95% CI), 0.484 (0.434-0.535)], indicating moderate agreement (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2023.101615). Historical IHC 2+ samples had the lowest proportion (100/207, 48.3%) rescored into the same category. Of the 107 historical IHC 2+ samples that were rescored into a different category, most (100/107) were rescored as IHC 1+; however, this change in IHC status did not affect HER2-low classification.
Discussion
In this study in patients with unresectable/metastatic BC previously diagnosed as HER2-negative, the prevalence of HER2-low was 67.2%. Our HER2-low prevalence findings were similar to those of other large studies in patients with any HER2-negative BC (2203/3689, 59.7%), HER2-negative metastatic BC (1811/3053, 59.3%), or HER2-negative de novo metastatic BC (20,636/30,929, 66.7%).6,14,15 Together, these data suggest that up to two-thirds of patients previously diagnosed with HER2-negative metastatic BC could benefit from new HER2-low-directed treatments such as T-DXd (US and European Union approvals).6,8, 9, 10 Similar to previous findings,6,14,15 we observed that HER2-low was more common in patients with HR-positive versus HR-negative disease (71% versus 53%). However, the rate of HER2-low identified among patients with HR-negative disease in our study (53%) was somewhat higher than that found in several previous studies (23%-38% in any BC or metastatic BC populations),6,16, 17, 18 but similar to the rate of 51% reported in a large cohort of patients with HR-negative de novo metastatic BC (n = 4997).15
This study provides important context regarding identification of patients with HER2-low. We found that the overall concordance between historical and rescored samples was 81.3%, with a κ value indicating moderate agreement; similar results were observed in the Ventana 4B5 subset. Previous analyses have suggested that between 8.4% and 34% of HER2 testing may be incorrect owing to testing variability.19, 20, 21, 22, 23, 24 However, some numerical differences were seen in positive agreement for historical scores and rescores between the HER2-low and HER2 IHC 0 categories. Using the Ventana 4B5 assay, >30% of patients with historically scored HER2 IHC 0 were reclassified as having HER2-low, while <10% of those who were historically classified as HER2-low were rescored as having HER2 IHC 0. These data suggest that when using the Ventana 4B5 assay, samples historically identified as HER2-low are more likely to keep the same categorization when rescored than those historically scored as HER2 IHC 0. Notably, the relatively high proportion (30%) of historical HER2 IHC 0 that was reclassified as HER2-low in this study indicates that reassessment in patients with this HER2 IHC status may be considered to determine whether these patients could benefit from HER2-low-directed therapy. In the non-Ventana subgroup, positive agreement for historical and rescored slides was similar for HER2-low and HER2 IHC 0 categorization; however, interpretation is limited by smaller sample sizes (n = 210 versus n = 567 for Ventana 4B5) and the inclusion of multiple assays in this subgroup.
Other studies have shown that the reproducibility of interpretation and scoring of lower levels of HER2 expression, particularly IHC 0 and IHC 1+, is often variable. A 2013 study found that central and local laboratory concordance for IHC 0 was 15%, with most (78/102) cases locally scored as IHC 0 scored centrally as IHC 1+.25 A more recent (2022) CAP survey of 1400 laboratories worldwide found poor agreement in the evaluation of IHC 0 and IHC 1+.24 Our study found somewhat higher concordance rates for IHC 0 and IHC 1+ (70% and 77%, respectively) than previous studies, possibly due to the standardized pathologist training used. Recently, another study showed that pathologists (n = 80) achieved acceptable accuracy in identifying HER2-low cases (n = 91), with an overall concordance of >80% after a 4-h training.26 In an analysis of the DESTINY-Breast04 study population, substantial concordance (78%) for HER2-low status was also seen between historical results and central testing using Ventana 4B5.27 A consistent benefit was observed with T-DXd across patient subgroups (e.g. IHC 1+ and IHC 2+/ISH−, HR-positive, and overall groups), which underscores the importance of accurate identification of those who may be eligible for this new treatment option.10,28 Given the limitations of human reproducibility in scoring, more quantitative measures of HER2 may be preferred in the future; alternative quantitative assays are in development but currently lack clinical validation.29,30
Our data support an understanding of prevalence and scoring agreements across individual historically HER2-negative IHC categories, including IHC 0 subgroups (with or without staining). Recent treatment guideline updates suggest the importance of differentiating HER2-low in identifying patients who may be eligible for T-DXd in the United States.2 In the phase II DAISY trial (NCT04132960), T-DXd showed clinically meaningful activity in patients with metastatic BC regardless of HER2 status [HER2 overexpression (IHC 3+ or IHC 2+/ISH+), low HER2 expression (IHC 1+ or IHC 2+/ISH−), and HER2 IHC 0] and biomarkers associated with drug response or resistance were identified.31 As T-DXd is being further evaluated in patients with BC with lower levels of HER2 expression (i.e. IHC 0 with staining) in the ongoing DESTINY-Breast06 trial (NCT04494425), a more distinct definition of very low HER2 expression to identify patients may be needed. The 2018 ASCO/CAP scoring guidelines do not include detailed guidance for differentiating HER2-low;1 however, the addition of the estrogen receptor low-positive (BC with 1%-10% of estrogen receptor expression) category in the guidelines supports the inclusion of intermediate-expression subgroups.32 Recently updated Groupe d’étude des facteurs pronostiques immunohistochimiques dans les Cancers du Sein recommendations for HER2 status assessment included a distinct HER2-low category and indicated that IHC scores 0 and 1+ must be included in reports for clinicians, demonstrating recognition of the need to facilitate identification of patients with HER2-low and determine eligibility for HER2-directed therapies.33
In general, the HER2-low and HER2 IHC 0 subgroups had no obvious differences in terms of patient demographics and clinical presentation, and no notable differences in treatments received and clinical outcomes were found within each HR subgroup. Other studies in populations with metastatic BC have also not identified a significant difference in OS or progression-free survival between patients with HER2-low and HER2 IHC 0.16,34 However, the prognostic significance of HER2-low is not yet clearly defined. A large (N = 2310) pooled study of patients with primary BC found that HER2-low was associated with significantly longer OS and disease-free survival (DFS) than HER2 IHC 0 in patients with HR-negative BC, but these outcomes were not significantly different in the HR-positive subgroup.35 In another study in early BC, outcomes such as OS and DFS with HER2-low and HER2 IHC 0 were related to genomic risk (as assessed by the Oncotype recurrence score).36 In patients with high genomic risk, HER2-low was associated with significantly longer OS and DFS than HER2 IHC 0, but there was no difference between HER2-low and HER2 IHC 0 in patients with low genomic risk.36 A recent National Cancer Database study of patients with de novo metastatic BC found a small but statistically significant difference in OS for those who had HER2-low tumors (n = 20,636) compared with those who had HER2 IHC 0 tumors (n = 10,293) in both HR-positive (median OS, 40.9 versus 39.2 months, respectively; P = 0.003) and HR-negative subgroups (median OS, 16.0 versus 14.1 months, respectively; P = 0.007).15 Further, a recent retrospective cohort analysis of the National Cancer Database, including 1,136,016 patients with invasive BC, found that HER2-low was associated with a slightly lower rate of pathological complete response than HER2 IHC 0 disease on multivariate analysis. HER2-low status was also associated with small improvements in OS in HER-low BC, especially in advanced triple-negative BC, although the extent of the difference was of questionable clinical relevance with overlapping survival curves when plotted by stage and receptor status.37
An important observation from this retrospective study is that patients previously diagnosed with HER2-negative unresectable/metastatic BC have unmet treatment needs. In our study cohort, we found poor survival (<4 years and <2 years in patients with HR-positive and HR-negative disease, respectively) after unresectable/metastatic BC diagnosis. Further, most of the study population received chemotherapy during the observation period. These findings suggest a need for more targeted therapies based on prognostic indicators to improve outcomes in patients with historically categorized HER2-negative BC.
This study comprised a large, globally representative sample and used standardized training on low-end HER2 expression rescoring; it represents one of the largest studies of this type. To our knowledge, this study is the first to examine the distinction between HER2 IHC 0 with no staining and HER2 IHC 0 with staining, thereby providing insights into further delineation of very low or no HER2 expression categories.
The study has inherent limitations associated with its retrospective nature, including missing or inaccurate data in health/medical records. Interpretation of treatment and outcomes was limited due to heterogeneity in real-world data, subjectivity in individual physician interpretation, lack of data collection on lines of therapy, and other factors. Because of the relatively short window of follow-up, the data were not optimal for robust analyses of treatments received and outcomes. Further, rates of CDK4/6 inhibitor use were relatively low, likely due to the study period (2014-2020) and the high proportion of our study population being from outside the United States. Although a number of CDK4/6 inhibitors were approved within this time frame, approval in many of the countries in this study was later than that in the United States.38, 39, 40, 41, 42, 43, 44, 45 Finally, comparisons of TFST, OS, and TTF in HER2-low versus HER2 IHC 0 in HR-negative subgroups were limited by small sample sizes, and the study was not powered for these comparisons.
Conclusions
HER2-low status has become clinically actionable with the availability of T-DXd as a treatment option, and this study provides greater understanding of patients with HER2-low unresectable/metastatic BC. We found that up to two-thirds of patients previously diagnosed with HER2-negative unresectable/metastatic BC may now be eligible for new HER2-low-directed treatments and confirmed that HER2-low is more common in patients with HR-positive disease. We found no difference in the characteristics of or clinical outcomes in patients with HER2-low versus HER2 IHC 0; therefore further research is needed to determine whether HER2-low is a prognostic indicator. Finally, our study showed that concordance between historical scores and rescores was >80% after standardized low-end HER2 expression training. Positive agreement for historical scoring versus rescoring was numerically lower for HER2 IHC 0 than for HER2-low, which suggests that targeted reassessment to determine eligibility for new treatment options could be beneficial for certain patients. The efficacy of T-DXd shown in the DESTINY-Breast04 study, combined with appropriate patient selection for this new treatment option, is anticipated to improve outcomes for these patient populations.
Acknowledgements
Medical writing support, under the direction of the authors, was provided by Nicole Lopez, PhD, and JoAnna Anderson, PhD, CMPP, of Articulate Science, LLC and Alexia Kalligeraki, PhD, of Helios Medical Communications Ltd, and was funded by AstraZeneca in accordance with Good Publication Practice (GPP) guidelines (http://www.ismpp.org/gpp-2022).
Funding
This study is sponsored by AstraZeneca. In March 2019, AstraZeneca entered into a global development and commercialization collaboration agreement with Daiichi Sankyo for trastuzumab deruxtecan (T-DXd; DS-8201). The study sponsor was responsible for study design and conduct; management, analysis, and interpretation of data; and the decision to submit the manuscript for publication. All authors made the final decision to submit the manuscript for publication and attest to the accuracy and completeness of the data.
Disclosure
All authors received nonfinancial support (assistance with manuscript preparation) from Articulate Science, LLC, and Helios Medical Communications Ltd funded by AstraZeneca. Additional disclosures are as follows: GV reports consulting or advisory fees from Agilent, AstraZeneca, Daiichi Sankyo, and Roche; participation in speakers bureaus and data safety monitoring advisory boards for Agilent, AstraZeneca, Daiichi Sankyo and MSD Oncology, and Roche; personal travel fees from AstraZeneca and Roche; and grants from Roche. MB reports consulting or advisory fees from AstraZeneca and Roche Canada; and contracted research funding from Pfizer Canada. NN reports grants from Chugai, Daiichi Sankyo, Eisai, Lilly, Mochida, Novartis, and Pfizer; and participation in speakers bureaus for AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Lilly, Novartis, and Pfizer. ET reports consulting or advisory fees from Daiichi Sankyo and Lilly; and fees for non-CME services from AstraZeneca, Daiichi Sankyo, and Lilly. SB reports consulting or advisory fees from AstraZeneca, Lilly, MSD, Novartis, Pfizer, Roche, and Sanofi; and contracted research funding from AstraZeneca, Lilly, GSK, MSD, Novartis, Pfizer, and Roche. FPL reports consulting or advisory fees from Agendia, AstraZeneca, Daiichi Sankyo, Eisai, Exact Sciences, GSK, MammaPrint, MSD, Myriad Genetics, Novartis, Pfizer, Roche, Seagen, and Veracyte; contracted research funding from AstraZeneca, MammaPrint, MSD, Myriad Genetics, Roche, and Veracyte; and personal travel fees from AstraZeneca and Seagen. NH reports participation in speakers bureaus for AstraZeneca, Chugai, Genomic Health, Kirin, Novartis, and Pfizer. JS reports research funding from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GSK, Eli Lilly, MSD, Novartis, Pfizer, Roche, and Sanofi; and partnerships with Roche. RTS reports consulting and non-CME service fees from AstraZeneca, Gilead, Lilly, Novartis, and Pfizer. AMB reports consulting or advisory fees from AbbVie, Agendia, Bayer, Biotheranostics, Coherus BioSciences, Daiichi Sankyo, Lilly, Eisai, Genentech/Roche, General Electric, Gilead Sciences, Immunomedics, Merck, Michael J. Hennessy Associates, Myriad Genetics, Novartis, OncLive, Pfizer, Puma Biotechnology, Seagen, and Tyme; and contracted research funding from AstraZeneca, Daiichi Sankyo, Lilly, Genentech/Roche, Gilead Sciences, Merck, Novartis, and Puma Biotechnology. CSO reports consulting or advisory fees from Roche and Seagen; and participation in speakers bureaus for Lilly. FS reports no conflict of interests. GH reports being a shareholder in Rhythm Biosciences Limited. DV reports a consulting or advisory role with OPEN Health; employment with AstraZeneca and may own stock or stock options in that company. GDJ reports a consulting or advisory role with AstraZeneca/MedImmune. AM is an employee of Daiichi Sankyo and may own stock or stock options in that company. AL and VGM are employees of AstraZeneca and may own stock or stock options in that company.
Data sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Disclaimers
Medical writing support was funded by AstraZeneca in accordance with Good Publication Practice (GPP) guidelines (http://www.ismpp.org/gpp-2022). The manuscript was reviewed for medical accuracy by AstraZeneca and Daiichi Sankyo; however, the authors retained full control of the content and made the final decisions for all aspects of this article.
Supplementary data
References
- 1.Wolff A.C., Hammond M.E., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer. V.4.2023. © National Comprehensive Cancer Network, Inc.; 2023. All rights reserved. Available at https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419. Accessed August 01, 2023. [To view the most recent and complete version of the guideline, go online to NCCN.org].
- 3.Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Loibl S., Poortmans P., Morrow M., Denkert C., Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi: 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 5.Arteaga C.L., Sliwkowski M.X., Osborne C.K., Perez E.A., Puglisi F., Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9(1):16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 6.Schettini F., Chic N., Brasó-Maristany F., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schalper K.A., Kumar S., Hui P., Rimm D.L., Gershkovich P. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med. 2014;138(2):213–219. doi: 10.5858/arpa.2012-0617-OA. [DOI] [PubMed] [Google Scholar]
- 8.Enhertu (fam-trastuzumab deruxtecan-nxki). Prescribing information. Daiichi Sankyo, Inc; 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s024lbl.pdf Available at.
- 9.Enhertu (trastuzumab deruxtecan). Summary of product characteristics. Daiichi Sankyo Europe GmbH; 2023. https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf Available at.
- 10.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moy B., Rumble R.B., Carey L.A. Chemotherapy and targeted therapy for human epidermal growth factor receptor 2-negative metastatic breast cancer that is either endocrine-pretreated or hormone receptor-negative: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40(26):3088–3090. doi: 10.1200/JCO.22.01533. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Premarket approval. PATHWAY anti-HER-2/Neu (4B5) rabbit monoclonal primary antibody. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P990081S047 Available at.
- 13.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 14.Raghavendra AS, Liu DD, Mouabbi JA, Tripathy D. Prevalence of HER2-low among stage I-III, metastatic breast cancer patients and their outcomes by HER2 status. Paper presented at the San Antonio Breast Cancer Symposium Annual Meeting. December 6-10, 2022; San Antonio, Texas. abstr HER2-04.
- 15.Jiang C., Perimbeti S., Deng L., Shapiro C.L., Gandhi S. Clinical outcomes of de novo metastatic HER2-low breast cancer: a National Cancer Database analysis. NPJ Breast Cancer. 2022;8(1):135. doi: 10.1038/s41523-022-00498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gampenrieder S.P., Rinnerthaler G., Tinchon C., et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23(1):112. doi: 10.1186/s13058-021-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miglietta F., Griguolo G., Bottosso M., et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021;7(1):137. doi: 10.1038/s41523-021-00343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott M., Vandenberghe M.E., Scorer P., Boothman A.M., Barker C. Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. J Clin Oncol. 2021;39(suppl 15):1021. [Google Scholar]
- 19.Paik S., Bryant J., Tan-Chiu E., et al. Real-world performance of HER2 testing – National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94(11):852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]
- 20.Perez E.A., Suman V.J., Davidson N.E., et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 21.Wolff A.C., Hammond M.E., Schwartz J.N., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 22.Pfitzner B.M., Lederer B., Lindner J., et al. Clinical relevance and concordance of HER2 status in local and central testing – an analysis of 1581 HER2-positive breast carcinomas over 12 years. Mod Pathol. 2018;31(4):607–615. doi: 10.1038/modpathol.2017.171. [DOI] [PubMed] [Google Scholar]
- 23.Cho-Phan C.D., Snider J., Zhang L., McGregor K., Schrock A.B., Castellanos E. Concordance of HER2+ status by IHC/ISH and ERBB2 status by NGS in a real-world clinicogenomic database and analysis of outcomes in patients (pts) with metastatic breast cancer (mBC) J Clin Oncol. 2021;39(suppl 15):1036. [Google Scholar]
- 24.Fernandez A.I., Liu M., Bellizzi A., et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022;8(4):1–4. doi: 10.1001/jamaoncol.2021.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambein K., Van Bockstal M., Vandemaele L., et al. Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: clinical and pathobiological relevance. Am J Clin Pathol. 2013;140(4):561–566. doi: 10.1309/AJCP4A7KTAYHZSOE. [DOI] [PubMed] [Google Scholar]
- 26.Rüschoff J, Penner A, Ellis IO, et al. Proficiency assessment of HER2-low breast cancer scoring with the Ventana PATHWAY 4B5 and Dako HercepTest HER2 assays and the impact of pathologist training. Paper presented at the San Antonio Breast Cancer Symposium Annual Meeting. December 6-10, 2022; San Antonio, Texas. abstr HER2-13.
- 27.Prat A, Modi S, Tsurutani J, et al. Determination of HER2-low status in tumors of patients with unresectable and/or metastatic breast cancer in DESTINY-Breast04. Paper presented at the San Antonio Breast Cancer Symposium Annual Meeting. December 6-10, 2022; San Antonio, Texas. abstr HER2-18.
- 28.Harbeck N, Modi S, Jacot W, et al. Trastuzumab deruxtecan vs treatment of physician’s choice in patients with HER2-low unresectable and/or metastatic breast cancer: subgroup analyses from DESTINY-Breast04. Paper presented at the San Antonio Breast Cancer Symposium Annual Meeting. December 6-10, 2022; San Antonio, Texas. abstr P1-11-01.
- 29.Moutafi M., Robbins C.J., Yaghoobi V., et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest. 2022;102(10):1101–1108. doi: 10.1038/s41374-022-00804-9. [DOI] [PubMed] [Google Scholar]
- 30.Gustavson M, Haneder S, Spitzmueller A, et al. Novel approach to HER2 quantification: digital pathology coupled with AI-based image and data analysis delivers objective and quantitative HER2 expression analysis for enrichment of responders to trastuzumab deruxtecan (T-DXd; DS-8201), specifically in HER2-low patients. Cancer Res. 2021;81(suppl 4):PD6-01. abstr PD6-01.
- 31.Diéras V, Deluche E, Lusque A, et al. Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: a phase II study with biomarkers analysis (DAISY). Paper presented at the San Antonio Breast Cancer Symposium Annual Meeting. December 7-10, 2021; San Antonio, Texas. abstr PD8-02.
- 32.Allison K.H., Hammond M.E., Dowsett M., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 33.Franchet C., Djerroudi L., Maran-Gonzalez A., et al. [2021 update of the GEFPICS’ recommendations for HER2 status assessment in invasive breast cancer in France] Mise a jour 2021 des recommandations du GEFPICS pour l'evaluation du statut HER2 dans les cancers infiltrants du sein en France. Ann Pathol. 2021;41(6):507–520. doi: 10.1016/j.annpat.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Carlino F., Diana A., Ventriglia A., et al. HER2-low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter, retrospective cohort study. Cancers (Basel) 2022;14(20):4981. doi: 10.3390/cancers14204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denkert C., Seither F., Schneeweiss A., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 36.Mutai R., Barkan T., Moore A., et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peiffer D.S., Zhao F., Chen N., et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the National Cancer Database. JAMA Oncol. 2023;9(4):500–510. doi: 10.1001/jamaoncol.2022.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eli Lilly and Company Lilly receives US FDA approval of VerzenioTM (abemaciclib) https://investor.lilly.com/news-releases/news-release-details/lilly-receives-us-fda-approval-verzeniotm-abemaciclib Available at.
- 39.European Medicines Agency Verzenios (abemaciclib) https://www.ema.europa.eu/en/documents/overview/verzenios-epar-medicine-overview_en.pdf Available at.
- 40.US Food and Drug Administration Palbociclib (IBRANCE Capsules). FDA. https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance-capsules Available at.
- 41.Pfizer Oncology IBRANCE® (palbociclib) receives approval in European Union for the treatment of women with HR+/HER2- metastatic breast cancer. https://www.pfizer.com/news/press-release/press-release-detail/ibrance_palbociclib_receives_approval_in_european_union_for_the_treatment_of_women_with_hr_her2_metastatic_breast_cancer Available at.
- 42.US Food and Drug Administration Ribociclib (Kisqali) https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali Available at.
- 43.Novartis. Novartis Kisqali® (ribociclib) receives EU approval as first-line treatment for HR+/HER2− locally advanced or metastatic breast cancer in combination with any aromatase inhibitor. Novartis. https://www.novartis.com/news/media-releases/novartis-kisqali-ribociclib-receives-eu-approval-first-line-treatment-hrher2-locally-advanced-or-metastatic-breast-cancer-combination-any-aromatase-inhibitor Available at.
- 44.Roncato R., Angelini J., Pani A., et al. CDK4/6 inhibitors in breast cancer treatment: potential interactions with drug, gene, and pathophysiological conditions. Int J Mol Sci. 2020;21(17):6350. doi: 10.3390/ijms21176350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price G.L., Sudharshan L., Ryan P., et al. Real world incidence and management of adverse events in patients with HR+, HER2- metastatic breast cancer receiving CDK4 and 6 inhibitors in a United States community setting. Curr Med Res Opin. 2022;38(8):1319–1331. doi: 10.1080/03007995.2022.2073122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.