Abstract
Cryptosporidiosis is a serious disease in malnourished children and in people with malignancies or AIDS. Current rodent models for evaluating drug therapy against cryptosporidiosis have many limitations, including the need for a high inoculum, the absence of symptoms resembling those seen in humans, and the need to maintain exogenous immunosuppression. We have developed a gamma interferon knockout (GKO) mouse model with which to evaluate therapies against C. parvum and have used paromomycin for evaluation of this model. The GKO model offers considerable improvements over other systems, since it requires no additional immunosuppression and adult mice can be infected with as few as 10 oocysts (compared with 107 for SCID mice). Infected mice develop profound gastrointestinal dysfunction due to extensive infection and severe mucosal damage involving the entire small intestine. Clinical symptoms, which include depression, anorexia, weight loss, and wasting, result in death within 2 to 4 weeks. The time of death depends on the oocyst challenge dose. Paromomycin modulated parasitological and clinical parameters in highly predictable and significant ways, including prevention of death. In addition, examination of the extensively infected gut provided an important insight into the dynamics between a specific drug treatment, its impact on the extent and the site of parasite distribution, and clinical outcome. These uniform symptoms of weight loss, wasting, and death are powerful new parameters which bring this model closer to the actual disease seen in humans and other susceptible mammalian species.
Infections caused by Cryptosporidium parvum lead to chronic diarrhea and wasting in people with AIDS, those with malignancies, and malnourished children (4, 10). Therapeutic agents currently available for treatment of such infections include paromomycin (20) and nitazoxanide (5, 6), but they are only partially effective. Many chemotherapeutic and immunotherapeutic agents have been evaluated in cell culture and/or various animal models (1, 12, 19, 21). The most commonly used rodent models include the dexamethasone-treated rat or mouse (2, 13, 22) and the congenitally immunodeficient SCID mouse (11, 14, 17). Although these animals can be infected with C. parvum, the sites of infection are generally limited to the pylorus, segments in the small intestine, the cecum, and the colon. These sites of infection do not closely resemble the pattern found in immunodeficient humans, in whom the entire small intestine can be colonized. Infected SCID mice normally remain healthy for several weeks and then develop chronic infections involving the hepatobiliary (HB) tract. The infectious dose required to induce consistent infections in the rodent models is 106 to 107 oocysts, which is manifold higher than the ∼100 oocysts needed to infect human volunteers (7). In addition, these animals do not develop symptoms that are directly related to their luminal infection, as do humans.
Mice bearing a targeted disruption of the gamma interferon (IFN-γ) gene (IFN-γ knockout [GKO] mice) are remarkably susceptible to infection with C. parvum (15). Moreover, the infection profile in GKO mice closely mimics that seen in humans and animals with severe clinical cryptosporidiosis. Because GKO mice are far more susceptible to cryptosporidiosis than any other rodent (15), we designed the present study to examine the suitability of the GKO mouse as a potential model for the evaluation of therapeutic drugs.
MATERIALS AND METHODS
Three sets of GKO mouse experiments were conducted. The first set identified parameters useful in establishing the model for drug evaluation. It describes the course and clinical outcome of small-inoculum infection with C. parvum, including the parasite distribution in the gut and the extent of associated mucosal lesions at two time points. Our prior studies did not quantify the extent of the disease (15). The second set of experiments was designed to explore the minimum parasite dose required to consistently induce infection in all animals. In the third set of experiments, paromomycin was used to validate the model for drug evaluation. Paromomycin, though noncurative, is a consistently partially effective agent in both humans and animal models. Its use also allowed a comparison of the GKO mouse model with other currently used animal models. The oocysts used in these experiments were from the GCH1 isolate, which we have maintained via serial passage in calves for more than 6 years. It has been described in detail previously (17) and is infectious to mice, calves, and humans.
Infection of GKO mice with C. parvum and its pathogenesis.
Twenty-one 8-week-old male GKO mice (C57BL/6 background), purchased from Jackson Laboratories, were housed in microisolators, and each was challenged with 5,000 C. parvum oocysts (3, 19). Seven male non-GKO C57BL/6 mice were also challenged as a control group. All mice were monitored for oocyst shedding three times per week by counting acid-fast-staining oocysts in 2 μl of feces. Body mass was measured weekly, and overall clinical appearance was assessed daily. Seven mice were euthanized 15 days after challenge for histologic analysis. All surviving mice were euthanized 32 days after challenge. Mucosal scores were determined at eight gastrointestinal sites: the stomach, the duodenum, three equally spaced mid-small intestinal sites, the terminal ileum, the cecum, and the colon. The scores of the eight gut sections were combined to calculate the mucosal score for each mouse, reflecting the extent of C. parvum infection in the luminal portion of the intestine. Each site was scored as follows: 0, no infection; 1, very-difficult-to-find parasite forms; 2, sparse but easy-to-find parasite forms; 3, abundantly present but focally distributed parasite forms; 4, extensive presence of parasite forms, covering most mucosal surfaces; or 5, extensive presence of parasite forms, covering the entire mucosal surfaces. The highest possible score was 40 (8 × 5). The HB tracts of all mice were also examined histologically.
Oocyst dose-response.
Thirty-five 6-week-old male GKO mice were randomized into five groups of seven mice each and maintained inside microisolators. In one experiment, members of groups 1 to 4 were challenged orally with 5,000, 1,000, 500, and 100 oocysts, respectively. Group 5 served as an uninfected control group. A second, confirmatory experiment was performed, with members of groups 1 to 4 receiving 5,000, 1,000, 100, and 10 oocysts, respectively. Infected mice were monitored as described above.
Dose-response of infected mice to treatment with paromomycin.
Twenty-six 4-week-old male GKO mice were randomized and challenged with 5,000 oocysts at 7 weeks of age. Four days later, these mice began to excrete oocysts. Beginning on day 5 of infection, members of groups 1 (six mice), 2 (six mice), and 3 (seven mice) were respectively treated with 2,000, 1,000 and 500 mg of paromomycin/kg of body weight/mouse/day, given in two divided doses for 10 days. A fourth infected group of seven mice received a placebo (phosphate-buffered saline). Mice were euthanized 15 days after challenge, and mucosal scores were determined. This experiment was repeated with 100 oocysts as the challenge dose.
Statistical analysis.
Data were analyzed with the Statistical Package for Social Sciences (Software Products SPSS Inc., Chicago, Ill.). Analysis of variance was conducted for all comparisons of multiple groups. If groups were significantly different by analysis of variance at the P < 0.05 level, then subgroup analysis was done. When parametric tests were inappropriate, standard nonparametric tests (detailed in Results) were used. Differences for which the two-tailed P value was ≤0.05 are reported as significant. We also report regression analyses using r2 values, which are more conservative and robust than r values. To normalize oocyst shedding data, the numbers of oocysts detected were log transformed. To avoid taking the log of zero when no oocysts were detected, we used the common statistical maneuver of adding 0.5 to all oocyst shedding scores before transformation.
RESULTS
Clinical symptoms and mucosal scores.
All mice challenged with 5,000 oocysts excreted oocysts within 4 days. The number of oocysts peaked on or about day 10 and declined until day 32 postchallenge (Fig. 1). Of the 21 infected mice, 4 died on day 12, 1 died on day 14, and 1 expired on day 16 after challenge. The body weights of infected mice declined from day 7 after challenge until the end of the experiment. On day 23, the C57 mice were significantly heavier than the GKO mice (means ± standard deviations, 28.90 ± 1.06 and 22.74 ± 0.68 g, respectively; P < 0.001), as they were on day 33 (31.75 ± 0.35 and 21.78 ± 0.52 g, respectively; P < 0.001). By day 10, the mice were hunched, emaciated, and reluctant to move, huddled in one corner of their cage, had scruffy and stained coats, and showed markedly decreased food consumption. They clinically deteriorated until the end of the experiment.
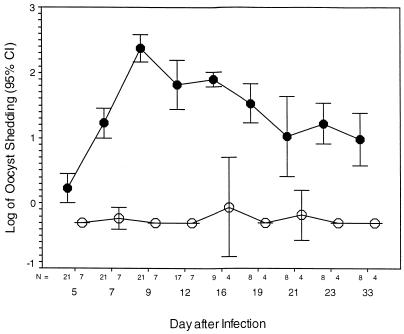
FIG. 1.
GKO mice (C57 background) (•) and control C57 mice (○) were each infected with 5,000 oocysts 28 days after observation was begun. At all times after infection, the log-transformed oocyst shedding score was significantly greater in GKO mice than in control C57 mice (P < 0.001 at all time points). The overall oocyst shedding score (log mean ± standard deviation) for the GKO group was 1.38 ± 0.08, while that for the C57 control group was −0.26 ± 0.02 (P < 0.001). CI, confidence interval.
Table 1 contrasts the mucosal score distributions for the eight sites on days 15 and 32 after challenge. The scores were similar on both days, with a consistent, high level of infection in the small intestine and terminal ileum. Fifteen days after challenge, parasites were mostly confined to the villous epithelium, with few architectural changes evident. In contrast, there was profound small intestinal mucosal disorganization 32 days after challenge, with extensive infection evident. Unlike the anti-IFN-γ-conditioned SCID mouse (18), the extents of infection in the pylorus and colon were mild. The cecal and colonic mucosal scores decreased significantly between days 15 and 32 after challenge.
TABLE 1.
Distributions and extents of mucosal infections at days 15 and 32 postchallengea
| Groupb | Mucosal scorec (mean ± SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stomach | Duodenum | SI-1d | SI-2d | SI-3d | Terminal ileum | Cecum | Colon | Total | |
| A | 1.1 ± 0.6 | 3.3 ± 1.2 | 4.2 ± 0.9 | 5.0 ± 0.0 | 4.4 ± 1.1 | 4.1 ± 1.2 | 3.0 ± 1.5 | 2.2 ± 0.7 | 29.2 ± 3.9 |
| B | 2.4 ± 0.7 | 1.0 ± 0.0 | 4.5 ± 0.7 | 5.0 ± 0.0 | 4.5 ± 0.7 | 4.1 ± 1.4 | 1.0 ± 0.0 | 1.4 ± 0.3 | 24.1 ± 2.5 |
After challenge with 5,000 oocytes per mouse.
Mice were euthanized on day 15 (group A) or day 32 (group B) after challenge.
0, no infection; 1, difficult-to-find parasite forms; 2, sparse but easy-to-find parasite forms; 3, abundant presence, but focally distributed; 4, extensive presence, covering most mucosal surfaces; 5, extensive presence, covering entire mucosal surfaces.
Equally spaced sites in small intestine.
In all of the mice in these and in subsequent experiments, the HB tract was free of infection. No mice developed clinical jaundice before death. Furthermore, serum liver enzyme values were assayed in a subset of mice before euthanization, and no elevations in values were found (data not shown).
Oocyst dose-response.
There were no differences in the shedding of oocytes by mice challenged with either 5,000 or 1,000 oocysts; the values had peaked on day 9 or 10 after challenge in both experiments. Oocyst shedding peaked 5 days later (on day 14 after challenge) and 10 days later (on day 19 after challenge) in mice inoculated with 100 oocysts and 10 oocysts, respectively. Regardless of the inoculum size, all mice eventually reached the same level of shedding (Fig. 2). The timing of death was related to the inoculum size, with deaths in the small-inoculum groups being delayed in a dose-dependent fashion compared to those in the higher-inoculum groups. By day 19, three mice from each group of seven receiving 5,000, 1,000, or 100 oocysts had either died or were euthanized to prevent further suffering. The original inoculum was highly predictive of the mean weight, in grams, of the surviving mice (r2 = 0.350, P = 0.001). Members of the group receiving 10 oocysts began to show symptoms of wasting on day 20. Both experiments showed similar patterns of shedding and weight loss.
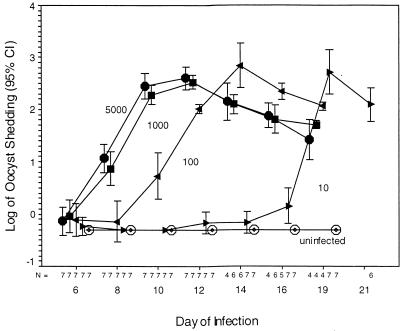
FIG. 2.
Groups of seven GKO mice were infected with 0 (○), 10 (▸), 100 (◂), 1,000 (■), or 5,000 (•) oocysts. The time to peak patency was related to the inoculum, and before mice began to die of the infection, oocyst excretion was temporally related to the initial inoculum. Each order-of-magnitude fall in the inoculum led to a 2- to 3-day delay to the time to peak patency. In the displayed as well as a confirmatory experiment, only 6 of 14 mice infected with 5,000 oocysts were alive on day 14, whereas 11 of 14 mice infected with 100 oocysts were alive on day 14 (χ2 = 3.743, P = 0.053). All mice given 10 oocysts became infected. On day 12, mouse no. 3 and 4 were positive; on day 14, mouse no. 2 and 4 (but not no. 3) were positive; on day 16, mouse no. 1, 2, 5, 6, and 7 (but not no. 3 or 4) were positive; and on day 19, all mice were positive. Thus, when a 10-oocyst inoculum is used, shedding may initially be at the limits of detection, yet it still rises to the same level as in mice infected with a larger inoculum. CI, confidence interval.
Responses of infected mice to different doses of paromomycin.
Mice challenged with 5,000 oocysts excreted high levels of oocysts by 5 days after challenge. Mice in the paromomycin-treated groups were protected from the clinical depression, weight loss, and mortality suffered by the untreated placebo group. All mice in the three paromomycin-treated groups, except for one mouse that died in a laboratory accident, survived the 15-day experiment. Mice treated with the lowest dose of paromomycin (500 mg/kg/day) still shed considerable numbers of oocysts (Fig. 3); curiously, however, they (as well as the other paromomycin-treated mice) showed no apparent clinical symptoms of infection. The mean weight ± the standard deviation of the mice treated with any dose of paromomycin fell from 18.52 ± 0.27 g on day 5 to 17.27 ± 0.32 g on day 15 (−1.25 g), whereas the weight of the untreated mice fell from 18.67 ± 0.44 g on day 5 to 14.80 ± 0.38 g on day 15 (−3.87 g), an over threefold difference. This difference in weight was very highly significant on each of the days after therapy was begun (P = 0.002, 0.001, and 0.002 on days 9, 12, and 15, respectively). In the experiment in which 5,000 oocysts were used as the challenge dose, two of seven placebo-treated mice died 9 days after infection (4 days into treatment) and a third mouse died on day 12. These three mice were the smallest within the group, suggesting that a higher body mass was protective against early death.
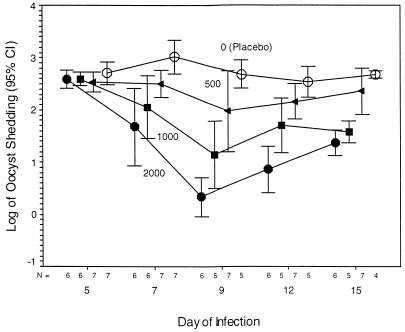
FIG. 3.
GKO mice were infected with 5,000 oocysts, and treatment with 0 (○), 500 (◂), 1,000 (■), or 2,000 (•) mg of paromomycin/kg/day began on day 5 of infection. There was a highly significant inverse relationship between fecal shedding of oocysts and the treatment dose administered to the mice (P < 0.0001). On day 9, 2,000 mg of paromomycin/kg/day decreased the fecal shedding of oocysts by ∼2.5 log units. Oocyst shedding was very highly significantly decreased by paromomycin in a dose-dependent fashion (r2 = 0.5142, 0.7215, 0.7752, and 0.6703 on days 7, 9, 12, and 15, respectively; P < 0.0001). In multiple regression analysis, the dose of paromomycin was very highly significant (P < 0.0001) whereas the day of the experiment was not significant (P = 0.2060). CI, confidence interval.
Paromomycin treatment induced a shift in the distribution and extent of infection that was associated with an improved clinical outcome. At necropsy, the entire small intestine of each mouse in the placebo group was distended and lacked muscular tone; there was little or no food in the stomach, the liver was pale, and the kidneys appeared somewhat congested. The appearance of the viscera of the paromomycin-treated mice was unremarkable.
As for the mice infected with 5,000 oocysts, the mucosal scores in the paromomycin-treated groups were very significantly decreased, in a dose-related fashion, compared to those of the untreated mice. Linear regression revealed a highly significant relationship (r = 0.859, F = 56.31, P < 0.001) in which ∼74% of the variance was explained by the dose of paromomycin alone (r2 = 0.7379). Table 2 reflects the impact of 10 days of paromomycin treatment on the extent and the site distribution of parasites in the gut compared with that of the placebo-treated group. Paromomycin treatment reduced, in a dose-dependent fashion, parasite colonization of the proximal half of the small intestine. This reduction was also reflected, in a dose-dependent fashion, in oocyst shedding shortly after the onset of treatment (Fig. 3, days 7 and 9). At the same time that paromomycin treatment reduced colonization proximally, it paradoxically caused or allowed the parasite to subsequently become established in the colon and cecum more predominantly than in the placebo group (Table 2). This was associated with a subsequent increase in oocyst shedding in all three paromomycin-treated mouse groups during the second half of the treatment period (Fig. 3, days 12 and 15).
TABLE 2.
Distribution and extent of mucosal infections by treatmenta
| Treatment | Mucosal scoreb (mean ± SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stomach | Duodenum | SI-1c | SI-2c | SI-3c | Terminal ileum | Cecum | Colon | Total | |
| Paromomycin | |||||||||
| 2,000 mg | 1.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.5 | 0.8 ± 0.4 | 0.8 ± 0.4 | 2.1 ± 0.9 | 3.0 ± 0.8 | 2.1 ± 0.7 | 10.3 ± 0.8 |
| 1,000 mg | 0.8 ± 0.4 | 0.4 ± 0.5 | 1.4 ± 1.5 | 3.2 ± 1.6 | 3.2 ± 1.7 | 1.8 ± 0.8 | 3.8 ± 0.4 | 2.2 ± 1.0 | 16.6 ± 5.4 |
| 500 mg | 1.5 ± 0.5 | 0.4 ± 0.5 | 0.6 ± 0.5 | 1.5 ± 0.7 | 4.8 ± 0.3 | 3.5 ± 0.7 | 4.4 ± 0.5 | 0.7 ± 0.7 | 18.4 ± 1.7 |
| None (placebo) | 1.5 ± 1.0 | 3.7 ± 1.5 | 4.7 ± 0.5 | 4.5 ± 1.0 | 4.2 ± 0.9 | 4.5 ± 0.5 | 2.7 ± 0.5 | 1.7 ± 0.5 | 28.0 ± 1.5 |
Measured at necropsy, 15 days after challenge with 5,000 oocytes per mouse, and at the end of a 10-day treatment period.
0, no infection; 1, difficult-to-find parasite forms; 2, sparse but easy-to-find parasite forms; 3, abundant presence, but focally distributed; 4, extensive presence, covering most mucosal surfaces; 5, extensive presence, covering entire mucosal surfaces.
Equally spaced sites in small intestine.
The kinetics of infection and the response to treatment in the experiment in which 100 oocysts were used as the challenge dose differed from those seen for infection with 5,000 oocysts (Fig. 4 and 5). Again, paromomycin-treated mice were protected from clinical symptoms, weight loss, and death. First, 8 days after infection, the mean oocyst shedding score was ∼2 orders of magnitude lower than that on day 4 for the mice infected with 5,000 oocysts. When challenged with 5,000 oocysts, two of the placebo-treated mice had died by day 9, whereas in mice infected with 100 oocysts, two of the placebo-treated group had died by day 13. Treatment with 2,000 mg of paromomycin/kg/day decreased oocyst shedding to nearly undetectable levels, and treatment with 500 or 1,000 mg/kg/day prevented the increase in oocyst shedding seen with the placebo treatment group. Only one mouse in the placebo group survived to day 18 after infection, illustrating the high mortality rate seen even after infection with only 100 oocysts. In contrast, all of the mice but one in all of the other groups survived (Fig. 4). No significant weight gain occurred after infection in the placebo-treated infected group (Fig. 5). In contrast, uninfected mice grew normally, and paromomycin-treated mice demonstrated some growth (in a consistently dose-dependent intermediate fashion) during the early period of the infection. Mucosal scores showed a very highly significant dose-dependent decrease with treatment (r2 = 0.9322, P < 0.001) for each of the six proximal intestinal sites. Mucosal scores for the cecum and colon were highest in the mice treated with 500 mg of paromomycin/kg/day, reminiscent of the paradoxical increase in scores seen in the mice infected with 5,000 oocysts. However, because only one placebo-treated mouse survived sufficiently long to have its mucosal scores assessed, this impression cannot have a statistical significance assigned to it.
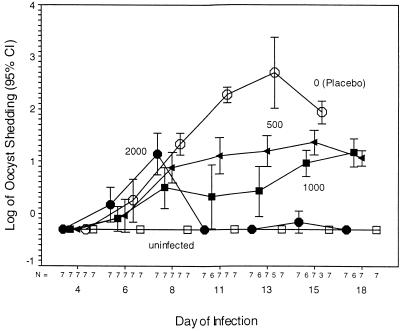
FIG. 4.
The effect of paromomycin treatment (0 [○], 500 [◂], 1,000 [■], or 2,000 [•] mg/kg/day) on oocyst shedding was assessed in mice that had been infected with 100 oocysts. Treatment was begun on day 8 of infection. By day 11 and thereafter, there was a dose-related decrease in oocyst shedding in all groups receiving paromycin treatment. This relationship was highly significant on all days (F = 235.35, P < 0.001, regression analysis). On day 18, there were no mice in the untreated group because all (7 of 7) had died, whereas in the treated groups only 1 of 21 had died (by Fisher’s exact test, P < 0.001 for differences in survival). CI, confidence interval.
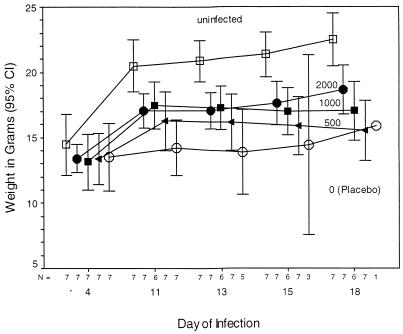
FIG. 5.
The weights of uninfected GKO mice (□) and of GKO mice infected with 100 oocysts on day 0 and treated with paromomycin (0 [○], 500 [◂], 1,000 [■], or 2,000 [•] mg/kg/day) on day 8 and thereafter are displayed. Four days after infection, there were no differences in weight among the treatment groups. In contrast, by 11 days after inoculation and thereafter, there was a highly significant difference in the weights of the uninfected control mice and the untreated (placebo) control group (means ± standard deviations, 21.31 ± 0.38 g and 14.25 ± 0.57 g, respectively; P < 0.001). The infected, untreated mice weighed only 67% as much as the uninfected mice. Paromomycin treatment very significantly blunted this decrease in weight in a dose-dependent fashion (F = 25.881, P < 0.001). CI, confidence interval.
DISCUSSION
GKO mice are profoundly susceptible to infection, weight loss, and death after infection with 107 GCH1 C. parvum oocysts (15). We have found that an infectious dose of as few as 10 oocysts establishes a lethal infection in these mice. These results indicate that of the animal models studied to date, the GKO mouse is by far the most susceptible to cryptosporidiosis. Infection reproducibly leads to high levels of luminal infection, with the symptoms and signs of infection being temporally related to the size of the infectious inoculum. We used the drug paromomycin to optimize this model and found that it led to consistent decreases in the infectious burden and prevented death. These characteristics strongly suggest that this model will be useful in the evaluation of anti-Cryptosporidium drugs.
In the search for effective therapy against persistent C. parvum infection, the use of a suitable immunodeficient model is highly desirable. The animal model should (i) allow a rapid and consistent establishment of a persistent infection in the adult, (ii) require a low infectious dose, and (iii) exhibit clinical symptoms such as a profound watery diarrhea, dehydration, malabsorption, weight loss, and emaciation, as well as death. The parasite should ideally colonize a major portion of the upper gastrointestinal tract and have the ability to spread to the HB tract. Of the rodent models that are being used to screen drugs for effectiveness against cryptosporidiosis, none meets all of the above criteria. These include the normal neonatal mouse (9, 16), the neonatal or adult SCID mouse (11, 14, 17), the dexamethasone-suppressed adult mouse (22), the immunosuppressed rat (2, 13), and the anti-IFN-γ-conditioned weaned SCID (18) models. Each model has its advantages and limitations.
The GKO mouse offers a number of significant advantages over the existing rodent models. The most significant advantage is the rapid development of clinical symptoms of wasting and weight loss. No other model has this characteristic. A second major advantage is the infection of the entire small intestine (unlike the patchy infection seen in the other models), which closely resembles the parasite distribution in severely infected or immunocompromised humans. We believe that it is this extensive infection of the small intestine that accounts for the rapid intestinal dysfunction seen in GKO mice. The ability of paromomycin to decrease the small intestinal parasite burden, as well as to prevent death, reinforces this conclusion. A third major advantage is the very low dose of oocysts (10) needed to infect GKO mice, compared with the 106 to 107 required in other models. This dose is even lower than the minimum calculated to be required to infect humans in one volunteer trial (7). These characteristics bring the GKO model closer to the situation in humans than even the calf and the piglet models of diarrhea, which require higher doses to induce clinical disease (8, 17). It is of note that the use of an inoculum of 10 oocysts (instead of 5,000 or 107) did not reduce the eventual severity of sickness; rather, it only increased the prepatent time. A fourth advantage of the GKO model is the number of clinical parameters that can be used for analyzing the impact of drug therapy on acute infection. In addition to oocyst shedding and mucosal scores, the two key parameters used in other rodent models, weight loss, physical appearance, behavior and well-being, gross gut appearance, and death are also available for evaluation in the GKO mouse model. Fifth, there is no need to use fragile neonatal mice, which are difficult to handle, given the exquisite sensitivity of adult GKO mice to infection. Sixth, the GKO model is rapid, requiring 3 weeks for completion of a drug assay, and requires no further conditioning, as do the immunosuppressed rodent and the IFN-γ-conditioned SCID mouse models.
One potential drawback to this model is the absence of HB disease. HB tract involvement in SCID or anti-IFN-γ-conditioned mice occurs only with chronic infection. The acute nature of cryptosporidiosis in GKO mice does not allow for chronic disease. A second drawback is the potential loss of mice in the placebo group, as was seen in the last experiment. This can be overcome by using older mice, which appear to survive longer, and increasing the group size. Third, despite the profound wasting manifested by weight loss, C. parvum-infected GKO mice do not develop diarrhea, a characteristic shared with all other rodent models. They do, however, display other symptoms consistent with gut dysfunction, as do immunocompromised humans.
Overall, these results strongly support the utility of the GKO mouse as a model superior to others currently used for drug discovery and evaluation. Treatment with paromomycin had a significant impact on the outcome of C. parvum infection, since it improved considerably the clinical symptoms, ameliorated parasite-induced weight loss, improved (and altered the distribution of) mucosal scores, and prevented death during the period of observation. These protective effects were dose related and demonstrated the ability of this model to show the efficacy of even drugs that are not curative. In the quest for curative drugs for this infection, this model will also likely provide a strong challenge for any putatively curative agent, since remnant subclinical infection during treatment will likely evolve into symptomatic infection and death once treatment ceases. We believe the GKO mouse is a powerful tool for the evaluation of, among others, anti-Cryptosporidium therapy, with a number of significant incremental advantages over prior models.
ACKNOWLEDGMENT
This work was supported in part by contract no. NO1-AI-75321 from NIH-NIAID.
REFERENCES
- 1.Blagburn B L, Soave R. Prophylaxis and chemotherapy: human and animal. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis—1997. Boca Raton, Fla: CRC Press; 1997. pp. 111–128. [Google Scholar]
- 2.Brasseur P, Lemeteil D, Ballet J J. Rat model for human cryptosporidiosis. J Clin Microbiol. 1988;26:1037–1039. doi: 10.1128/jcm.26.5.1037-1039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Current W L. Techniques and laboratory maintenance of Cryptosporidium. In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals—1990. Boston, Mass: CRC Press; 1990. pp. 31–49. [Google Scholar]
- 4.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis L J, Soave R, Dudley R E, Fessel J W, Faulkner S, Mamakos J P. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Nitazoxanide (NTZ) for AIDS-related cryptosporidial diarrhea (CD): an open-label safety, efficacy and pharmacokinetic study, abstr. LM50; p. 289. [Google Scholar]
- 6.Doumbo O, Rossignol J F, Pichard E, et al. Nitazoxanide in treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am J Trop Med Hyg. 1997;56:637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 7.DuPont H L, Chappel C L, Sterling C R, Okhuysen P O, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 8.Fayer R, Andrews C, Ungar B L P, Blagburn B L. Efficacy of hyperimmune bovine colostrum for prophylaxis of cryptosporidiosis in neonatal calves. J Parasitol. 1989;75:393–397. [PubMed] [Google Scholar]
- 9.Fayer R, Guidry A, Blagburn B L. Immunotherapeutic efficacy of bovine colostral immunoglobulins from a hyperimmunized cow against cryptosporidiosis in neonatal mice. Infect Immun. 1990;58:2962–2965. doi: 10.1128/iai.58.9.2962-2965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment and diagnosis. Adv Parasitol. 1998;40:30–87. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 11.Mead J R, You X, Pharr J E, Belenkaya Y, Arrowood M J, Fallon M T, Schinazi R F. Evaluation of maduramicin and alborixin in a SCID mouse model of chronic cryptosporidiosis. Antimicrob Agents Chemother. 1995;39:854–858. doi: 10.1128/aac.39.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 13.Rehg J E, Hancock M L, Woodmansee D B. Characterization of a dexamethasone-treated rat model of cryptosporidial infection. J Infect Dis. 1988;158:1406–1407. doi: 10.1093/infdis/158.6.1406. [DOI] [PubMed] [Google Scholar]
- 14.Rohlman V C, Kuhls T L, Mosier D A, Crawford D L, Hawkins D R, Abrams V L. Therapy with atovaquone for Cryptosporidium parvum infection in neonatal severe combined immunodeficiency mice. J Infect Dis. 1993;168:258–260. doi: 10.1093/infdis/168.1.258-a. [DOI] [PubMed] [Google Scholar]
- 15.Theodos C M, Sullivan K L, Griffiths J K, Tzipori S. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect Immun. 1997;65:4761–4769. doi: 10.1128/iai.65.11.4761-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzipori S, Campbell I, Angus T. The therapeutic effect of 16 antimicrobial agents on Cryptosporidium infection in mice. Aust J Exp Biol Med Sci. 1982;60:187–190. doi: 10.1038/icb.1982.20. [DOI] [PubMed] [Google Scholar]
- 17.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzipori S, Rand W, Theodos C. Evaluation of a two-phase scid mouse model preconditioned with anti-interferon-γ monoclonal antibody for drug testing against Cryptosporidium parvum. J Infect Dis. 1995;172:1160–1164. doi: 10.1093/infdis/172.4.1160. [DOI] [PubMed] [Google Scholar]
- 19.Tzipori S. Cryptosporidium parvum: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:188–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 20.White A C, Chappell C L, Hayat C S, Kimball K T, Flanigan T P, Goodgame R W. Paromomycin for cryptosporidiosis in AIDS: a prospective, double-blind trial. J Infect Dis. 1994;170:419–424. doi: 10.1093/infdis/170.2.419. [DOI] [PubMed] [Google Scholar]
- 21.Woods K M, Nesterenko M V, Upton S J. Efficacy of 101 antimicrobials and other agents in the development of Cryptosporidium parvum in vitro. Ann Trop Med Parasitol. 1996;60:603–615. doi: 10.1080/00034983.1996.11813090. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Healey M C. The immunosuppressive effects of dexamethasone administered in drinking water to C57BL/6N mice infected with Cryptosporidium parvum. J Parasitol. 1993;79:626–630. [PubMed] [Google Scholar]