Abstract
Recently, nanobiotechnology has attracted a lot of attention, as it is a rapidly emerging field that is still growing and developing efficient and advanced therapeutic protocols under the umbrella of nanomedicine. It can revolutionize solutions to biomedical problems by developing effective treatment protocols and therapeutics. However, focus and research are still required to make these therapeutics more effective and safer to use. In this study, iron oxide nanoparticles were synthesized from Madhuca indica extract using green synthesis protocols. The nanoparticles were further characterized based on their absorption spectrum, size, structural morphology, and other related parameters. Biological assays were also performed to evaluate biological applications for the synthesized nanoparticles. In silico analysis was performed to assess the druglike properties of synthesized nanoparticles. The results proved an optimized synthesis of the iron oxide nanoparticles with the size of 56 nm confirmed by SEM. The FTIR analysis predicted the presence of nitro and carbonyl groups in the synthesized nanoparticles. The 81% DPPH inhibition confirmed the antioxidant activity, and the 96.20% inhibition of egg albumin protein confirmed the anti-inflamatory activity. Additionally, the 73.26% inhibition of α-amylase, which was more than that of the control used, confirmed the antidiabetic activity. The ADMET analysis confirmed the synthesized nanoparticles as potential therapeutic candidates as well. However, further evaluation for safety concerns is still required to use these FeONPs as potential therapeutic agents. This study can be proved as a significant contribution to the scientific community and a gateway to the future scientists who are willing to work on nanomedicine and nanobiotechnology. ADMET analysis confirmed the synthesized nanoparticles as potential therapeutic candidates as well. However, further evaluation for safety concerns is still required to use these FeONPs and potential therapeutic agents.
1. Introduction
The diagnosis, treatment, and prevention of diseases can be significantly enhanced by the utilization of nanomedicine-based technologies. A significant impact on human health has been reported in the recent literature studies related to nanomedicine and advanced therapeutic technologies.1 To cure numerous conditions that call for surgery, such as tumors and artery obstructions, scientists have been able to create precise devices that accurately measure the size of blood cells. The treatment of various liver diseases has been enhanced by the use of nanoparticles and liposomes with homing devices for the targeting of receptors overexpressed on the hepatic tissue.2−6 The green synthesis of nanoparticles is dependent upon a redox reaction that occurs due to the reductive capacity of extracellular or cellular components of the cell. Iron oxide nanoparticles have been used extensively over the past 20 years for a variety of purposes, including magnetic resonance imaging and to induce hyperthermia for cancer therapy.7−10 The metal cations are converted into the metallic form with zero charge at the size of a nanoparticle.10,11
Madhuca indica is medicinally important and is used to treat a variety of conditions, including diabetes, ulcers, hepatoprotection, pyrexia, infertility, analgesia, antioxidants, edema, inflammation, piles, emesis, dermatological disorders, burns, earthworms, headaches, and wounds.12,13 Therefore, the plant contains required medicinal properties that can possess good health effects, including hepatoprotection, anti-inflammatory, and antidiabetic properties.14 Several studies are available that report the various medicinal properties of Madhuca indica, but there is still no report available on the hepatoprotection and general biological examination of nanoparticles synthesized using Madhuca indica. The synthesis of iron oxide nanoparticles using Madhuca indica can be a useful contrivance for society and mankind.15
The nanoparticles from the Madhuca indica plant were greenly synthesized by the green method in this study. The method is very simple and easy to use and does not require special instrumentations or techniques. The nanoparticles were further evaluated for their characteristics and size using UV–vis spectroscopy, Fourier transoform infrared (FTIR) spectroscopy, and scanning electron microscopy (SEM). After the characterization, the nanoparticles were assessed for various biological properties, including the antidiabetic property, anti-inflammatory activity, and antioxidant activity. The in silico analysis was performed to study the interaction mechanism of the synthesized nanoparticles with liver tissues. The assessment of hepatoprotective properties was done by in vivo analysis of the mice using the standard protocols.16
This study would be a breakthrough in the field of nanomedicines and nanocarrier-based drug delivery, as the greenly synthesized nanoparticles are highly efficacious in terms of biological applications as compared to already available drugs and treatment strategies. This study will open a new pathway for scientists who want to work in the field of nanobiotechnology and nanocarrier-based drug delivery. The greenly synthesized nanoparticles are potential candidates for efficacious and targeted drug delivery in the era of advanced therapeutics. However, some further work and clinical trials are required to use nanobased materials as nanomedicines and nanocarriers.
2. Materials and Methods
2.1. Sample Collection
The sample was collected from the Botanical Garden of the Department of Botany, University of Punjab, Lahore, Pakistan. The fresh leaves of the plant Madhuca indica J.F. Gmel (syn. Madhuca latifolia) were plucked and preserved in polythene bags and transferred to the Molecular Biotechnology and Bioinformatics Lab, University of Central Punjab, Lahore, Pakistan.
2.2. Preparation of the Plant Extract
The leaves of Madhuca indica J.F. Gmel were washed, shadow dried, crushed, suspended in distilled water, filtered with Whatmann no. 1 filter paper, and stored in the refrigerator at 4 °C to be used in the synthesis of iron oxide nanoparticles.
2.3. Preparation of the FeSO4 Stock Solution
The 1 M stock solution of FeSO4 was prepared in distilled water and stored in a reagent bottle. The molar mass of FeSO4 used was 278.01, therefore the preparation of 1000 mL of stock requires 278.01 g of FeSO4. For the preparation of 50 mL of FeSO4 stock solution, 13.90 g of FeSO4 was dissolved in 50 mL of distilled water, which was calculated from the given formula calculations.
For 50 mL of the working solution,
Therefore, 13.90 g of FeSO4 is required to prepare 50 mL of the working solution.
2.4. Preparation of the Working Solution of FeSO4
The working solution of 25 mM was prepared from the already prepared 1 M stock solution of FeSO4. For this purpose, 1.25 mL of the 1 M stock solution was added to the 48.75 mL of distilled water, and the mixture was stirred gently for the proper mixing of the solution. For mixing, a magnetic stirrer was used. The calculations of the required volume of stock solution were done using the given formula M1 × V1 = M2 × V2.
2.5. Green Synthesis of FeONPs from Plant Extract
The working solution and leaf extract were intermixed in a ratio of 1:9 v/v to make 200 mL in a reagent bottle. The combination was incubated for 24 h at 37 °C, and the spectrophotometric values were noted. After centrifuging the mixture at 4500 rpm for 30 min, the supernatant was removed and the process was repeated three times.17 The precipitate was then dried in the hot air oven for 1 h at 80 °C to obtain the powder form of the FeONPs that was utilized for further experimental procedures.
2.6. Characterization of FeONPs
The greenly synthesized FeONPs were characterized for their wavelength absorption, size, geometry, and structural consequences. For this purpose, various analyses were performed. The chemical and physical parameters of the synthesized nanoparticles were also analyzed.
2.7. UV–Visible Spectroscopy
The optimized formation of FeONPs was validated and verified by using UV–visible spectroscopy. The bands of surface plasmon resonance were monitored in the range of 200–700 nm, corresponding to the absorption of colloidal ferric oxide nanoparticles in the wavelength area of 250 nm-350 nm.18 The absorption spectrum at the ideal wavelength indicates the formation of iron oxide nanoparticles in the solution.
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
A Fourier transform infrared (FTIR) spectrophotometer was used to determine the functional groups responsible for iron oxide nanoparticle production and to capture the FTIR spectra. The FTIR spectrum was recorded between 4000 and 400 cm–1, and the solution was spun for 30 min at 10 000 rpm for analysis of the presence of functional groups.
2.9. Scanning Electron Microscopy (SEM)
The structure of the generated nanoparticles was ascertained using scanning electron microscopy (SEM). Backscattered and secondary electrons, which are created when the input electron beam travels over and interacts with the sample surface, are used in the surface imaging technique known as SEM to create an image of the sample.19 Dried samples were put on double-conductive tape fastened to a sample holder and coated with platinum–gold. At 80 kV voltage, structural consequences of FeONPs were obtained. ImageJ was used to calculate the diameter of the greenly synthesized FeONPs. OriginPro was used to plot data.
2.10. In Silico Analysis of the FeONPs
The in silico analysis was performed to analyze the toxicity and chemical parameters for greenly synthesized FeONPs. The interaction of the FeONPs with liver receptors was studied, and the absorption of FeONPs was also analyzed by computational methods.
2.11. Structure Formation of Synthesized FeONPs
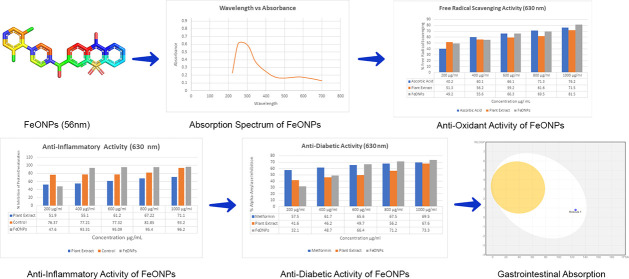
The structure of greenly synthesized FeONPs was drawn with the help of ChemDraw.20 The structure of ferrous sulfate (FeSO4) was drawn and converted into iron oxide (Fe2O3). The carbonyl functional group was attached to the Fe atom, acting as a bridge between two Fe atoms. The structure was then saved in the SDF format for further use. Figure 1 shows the chemical structure of the synthesized iron oxide nanoparticles.
Figure 1.
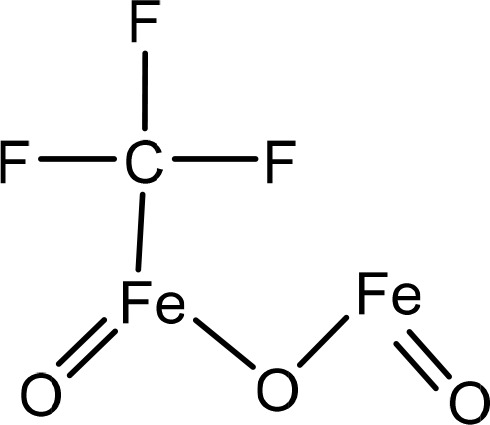
Chemical structure of greenly synthesized FeONPs drawn using ChemDraw.
2.12. Optimization of Chemical and Structural Diversity
Low-energy molecules are the most stable, so it is important to test hypotheses to determine which structure has the lowest energy value to optimize a molecule’s shape.21 The optimization of the chemical and structural diversity was performed using the FROG (FRee Online druG) tool (https://mobyle.rpbs.univ-paris-diderot.fr). It is an online server by the RPBS web portal used for structural bioinformatics and drug design.22 The structure was submitted in SDF format, and the output options were adjusted to the PDB. The Emax value was adjusted to 50, the mc step value was increased to 100, and the Energy window value was set to 100. The conformational geometry was refined using FROG, and the server optimized and enhanced the geometry to provide the output in PDB format.
2.13. Toxicity Prediction
Toxicology testing is necessary to gather knowledge about the dangerous properties and activities of drugs and chemicals so that their negative impacts on human health and the environment may be accurately assessed.23 The toxicity of the greenly synthesized nanoparticles was analyzed through the Toxicity Checker of the Mcule online server (https://mcule.com/apps/toxicity-checker). The structure was submitted to the server in the Mol2 format in order to access the chemical and structural toxicity of the synthesized nanoparticle.
2.14. Absorption Prediction and Drug Likeness of FeONPs
Drug absorption is the process of a drug moving from its site of administration to the bloodstream, and is a critical concept in pharmacokinetics. ADMET factors, namely, absorption, metabolism, excretion, and toxicity, are important considerations in drug development. A good drug candidate should exhibit appropriate ADMET characteristics at a therapeutic dosage while also being effective against the intended target.24 The ADMET analysis of the designed nanoparticle was performed using SwissADME (http://www.swissadme.ch) in order to access the toxicity, absorption, and other drug-like parameters in the designed nanoparticle.25 Therefore, the structure’s SMILE code was submitted to SwissADME, and the results were further analyzed.
3. Results
3.1. Sample Collection and Preparation of Plant Extract
Fresh leaves of Madhuca indica were collected, cleaned, and stored in airtight bags. The leaves were then used to prepare an extract for the green synthesis of nanoparticles. The yellowish extract was filtered and stored in a reagent bottle at 4 °C to ensure no contamination. The extraction was done at optimized temperatures and times for optimal nanoparticle synthesis results. The reagent bottle was sealed with parafilm for safety. Figure 2 shows the leaf powder mixed in water and the plant extract, which were used for the green synthesis of ferrous oxide nanoparticles. The plant extract was yellowish and slightly viscous in nature.
Figure 2.
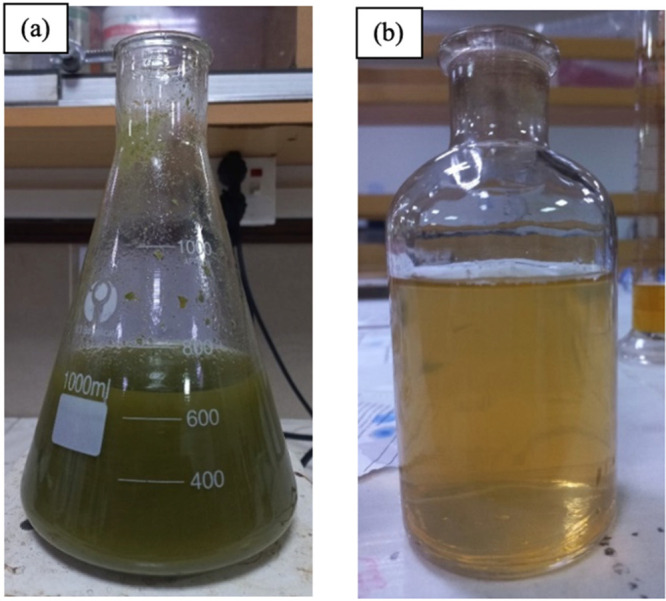
(a) Leaf powder solution in double-distilled water. (b) Plant leaf extract after filtration.
3.2. Preparation of FeSO4 Solutions
A stock solution of 1 M FeSO4 was prepared and stored in a covered reagent bottle due to the light sensitivity of FeSO4. Working solutions in various molarities were prepared from the stock solution for the optimal synthesis of FeONPs. The best molarity concentration was selected for the final preparation of FeONPs. A working solution of 25 mM was prepared from the stock solution and used for the synthesis of FeONPs. The solution was stored in a covered reagent bottle due to its light sensitivity.
3.3. Synthesis of FeONPs
After the 25 mM FeSO4 solution and the leaf extract were mixed, the color changed from yellowish to dark brown, indicating the synthesis of FeONPs. The solution was stored in an airtight reagent bottle covered with aluminum foil to protect it from light.26 The wavelength of the solution was scanned hourly using a UV–vis spectrophotometer for optimal intervals of incubation. After incubation for 24 h at 37 °C, the optimum wavelength was observed, and the solution was further used for characterization and analysis. The green synthesis of FeONPs was confirmed by the color change in the solution. The color change of the solution mixture after the addition of FeSO4 to the plant extract is given below in the Figure 3.
Figure 3.

Color change indication of the solution mixture after the addition of FeSO4 to the plant extract.
3.4. Characterization of FeONPs
3.4.1. UV–Visible Spectroscopy
The UV–visible spectrophotometer was used to analyze the green synthesis and stability of the FeONPs. The absorbance range of 200–800 nm was adjusted to detect the presence and stability of the FeONPs, which increased with the incubation time. The extracellular reduction of Fe+ ions indicated the formation of FeONPs. The ideal wavelength of 310 nm was observed in the UV–vis spectroscopy readings for FeONPs synthesized with a 25 mM FeSO4 solution. The absorption spectrum is presented in Figure 4.
Figure 4.
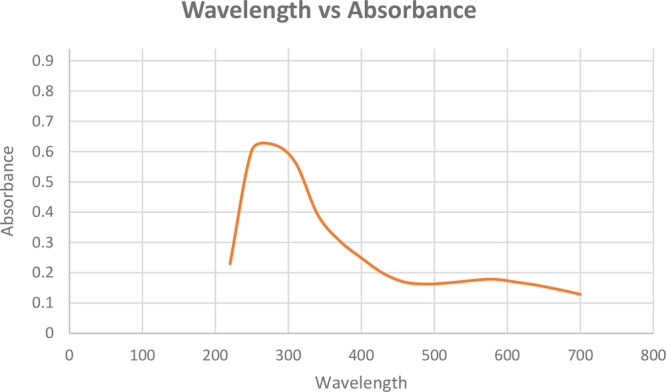
UV–visible spectrum for FeONPs synthesized using Madhuca indica plant extracts with 25 mM FeSO4.
3.4.2. Fourier Transform Infrared (FTIR) Spectrum
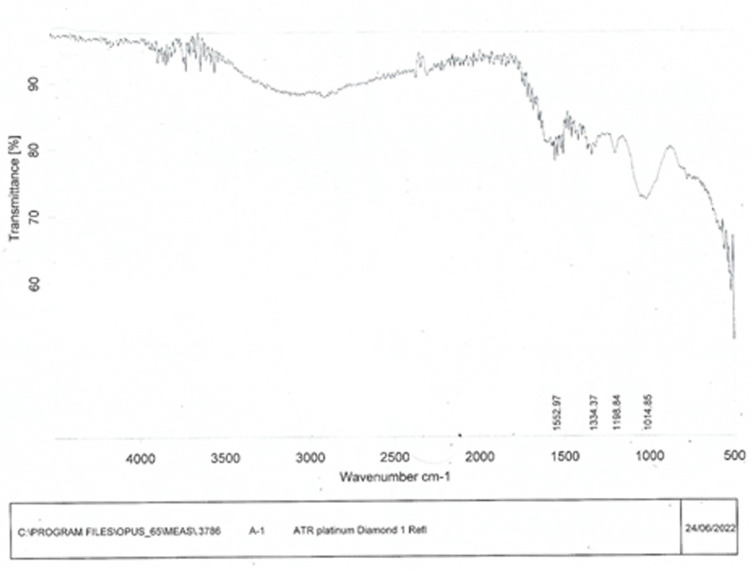
The FeONPs were capped and reduced by secondary metabolites. Fourier transform infrared (FTIR) spectroscopy was utilized for the detection of secondary metabolites in FeONPs. The FTIR spectra generated from FeONPs synthesized from Madhuca indica plant extract exhibited substantial absorption peaks at 1552.97, 1334.37, 1198.84, and 1014.85 cm–1, as shown in Figure 5. The peaks at 1552.97 and 1334.37 indicated the presence of the NO2 stretch (nitro group), and the peaks at 1198.84 indicated the presence of the strong C–OH stretch (carbonyl stretch) in the synthesized FeONPs. The peak at the 1014.85 indicated the presence of strong C–F (trifluoromethyl group) functional group on the FeONPs.
Figure 5.
FTIR spectrum of Ferric oxide nanoparticles synthesized using the leaf extract of Madhuca indica and FeSO4..
3.4.3. Scanning Electron Microscopy Analysis (SEM)
The surface shape and particle size of greenly produced FeONPs were determined using a scanning electron microscope (SEM), which uses a high-energy electron beam to produce images. FeONPs were found to be spherical and well-dispersed, with an average crystalline size of 50–60 nm. The mean diameter of the FeONPs was found to be 55 nm, as determined by ImageJ software. The images obtained by SEM and visualized by ImageJ software are shown in Figure 6.
Figure 6.
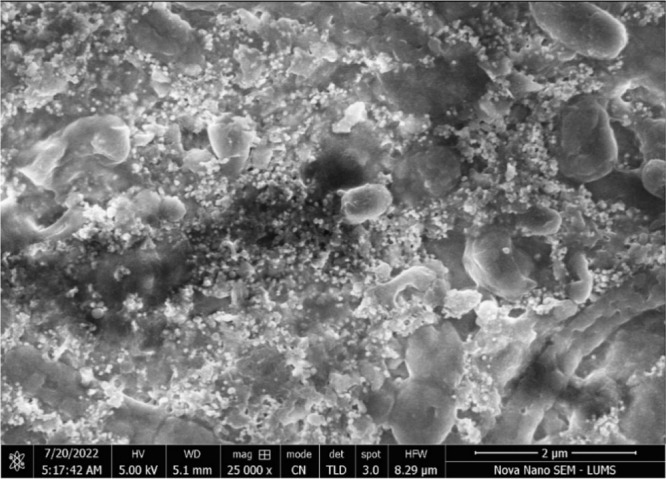
SEM image of greenly synthesized FeONPs at 2 μm scale.
A histogram of the nanoparticle sizes was created using OriginPro data analysis software. The diameter range of the greenly synthesized nanoparticles was found to be between 55 and 65 nm, with a mean size of 56 nm. The majority of the nanoparticles observed in the SEM images were within this range, making them ideal for drug delivery and other applications. The histogram of the diameter range for the greenly synthesized FeONPs is shown in Figure 7.
Figure 7.
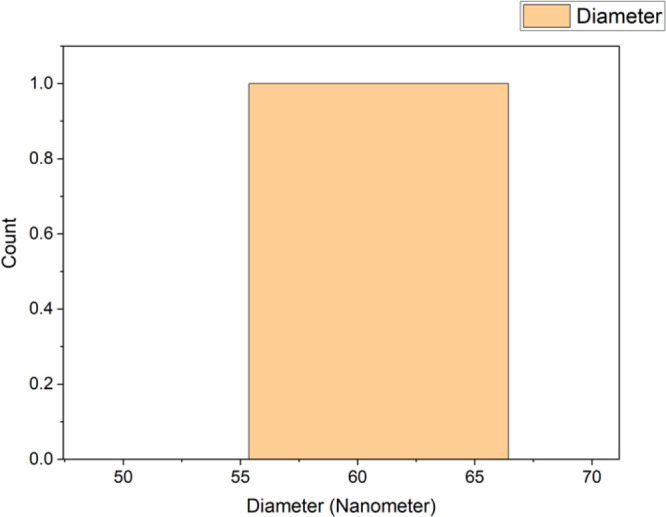
Histogram of nanoparticle size ranges computed using OriginPro.
3.4.4. Antioxidant Activity
The study analyzed the ability of different concentrations of plant extract, control (ascorbic acid), and FeONPs to scavenge free radicals using DPPH. The decrease in absorbance at 630 nm caused by the antioxidants was observed to promote the scavenging of radicals. The IC50 values for the plant extract, control, and FeONPs were calculated graphically and are presented in Table 1.
Table 1. Free Radical Scavenging Capability of the Plant Extract, the Control (Ascorbic Acid), and the FeONPs and Their IC50 Values.
| % free radical scavenging |
||||||
|---|---|---|---|---|---|---|
| FeONP concentration | ascorbic acid | plant extract | FeONPs R1 | FeONPs R2 | FeONPs R3 | mean R |
| 200 μg/mL | 40.2 | 51.3 | 49.2 | 49.1 | 49.2 | 49.16 ± 0.15 |
| 400 μg/mL | 60.1 | 56.2 | 55.6 | 55.6 | 55.6 | 55.6 ± 0.01 |
| 600 μg/mL | 66.1 | 59.2 | 66.3 | 66.2 | 66.2 | 66.23 ± 0.21 |
| 800 μg/mL | 71.3 | 61.6 | 69.5 | 69.5 | 69.6 | 69.53 ± 0.48 |
| 1000 μg/mL | 76.2 | 71.5 | 81.5 | 81.5 | 81.6 | 81.53 ± 0.49 |
The FeONPs showed the highest antioxidant activity at a concentration of 1000 μg/mL, with a value of 81.5% compared to the plant extract and control. The trend of antioxidant activity increased with increasing FeONP concentrations. Figure 8 presents the antioxidant activity trends for the FeONPs, the plant extract, and the control.
Figure 8.
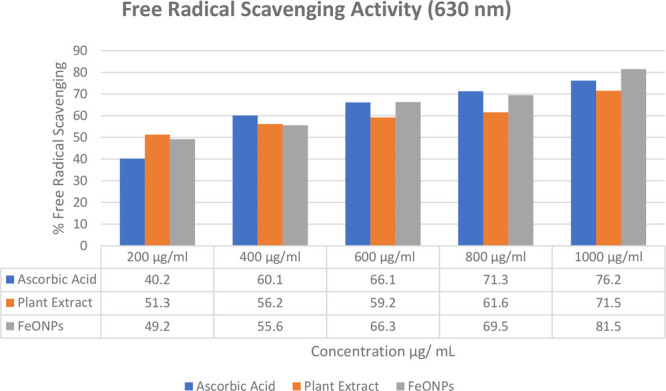
Antioxidant activities of the FeONPs, the plant extract, and the control
3.4.5. Anti-Inflammatory Activity
The maximum inhibition of protein denaturation for egg albumin protein was observed as 96.20% with the 500 μL/ml concentration of FeONPs and as 71.20% with the plant extract. The statistical analysis shows a significant relationship (p > 0.01) as compared to the control, i.e., aspirin (acetyl salicylic acid), which is clearly indicated in the Table 2. The results clearly indicate that greenly synthesized FeONPs are more effective in the inhibition of egg albumin protein compared to Madhuca indica plant extract and the control used in the experiment, as shown in Figure 9.
Table 2. Percentage Inhibition of Egg Albumin Protein by the FeONPs, the Plant Extract, and the Control.
| anti-inflammatory activity (630 nm) |
||||||
|---|---|---|---|---|---|---|
| control | plant extract | FeONPs |
||||
| FeONP concentration | R1 | R1 | R1 | R2 | R3 | mean ± SD |
| 200 μL/ml | 76.37 | 51.90 | 47.59 | 47.62 | 47.61 | 47.60 ± 0.02 |
| 400 μL/ml | 77.21 | 55.10 | 93.33 | 93.31 | 93.31 | 93.31 ± 0.66 |
| 600 μL/ml | 79.32 | 61.20 | 95.10 | 95.08 | 95.11 | 95.09 ± 0.42 |
| 800 μL/ml | 81.85 | 67.22 | 95.40 | 95.42 | 95.40 | 95.40 ± 0.24 |
| 1000 μL/ml | 93.20 | 71.20 | 96.20 | 96.21 | 96.20 | 96.20 ± 0.33 |
Figure 9.
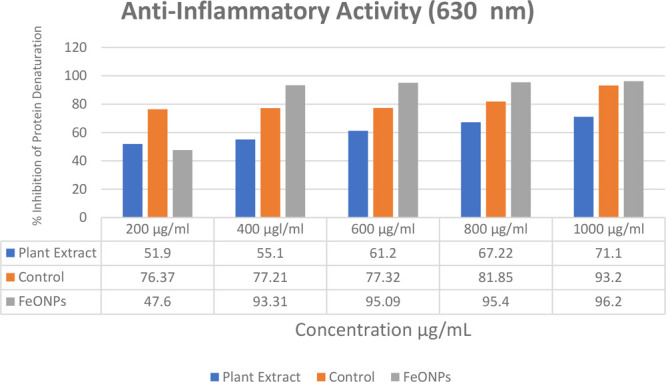
Trends in anti-inflammatory activities of the FeONPs, the plant extract, and the control.
3.4.6. Anti-Diabetic Activity
The study evaluated the antidiabetic activity by measuring the inhibition of α-amylase. Metformin HCl was used as a control, and the results showed 67% inhibition. Greenly synthesized FeONPs from Madhuca indica showed greater inhibition than the control and plant extract. Table 3 provides the percentage inhibition values and IC50 values for the FeONPs, the plant extract, and the control. Greenly synthesized FeONPs showed higher inhibition of α-amylase compared to the Madhuca indica plant extract and the control, i.e., metformin HCl. The maximum inhibition of 73.3% was observed at the concentration of 1000 μL/ml. The graphical representation of α-amylase inhibition is shown in Figure 10.
Table 3. Percent of Anti-Diabetic Activity and IC50 Values of the FeONPs, the Plant Extract, and the Control.
| anti-diabetic activity (630 nm) |
||||||
|---|---|---|---|---|---|---|
| % inhibition of α-amylase |
||||||
| FeONP concentration | control | plant extract | FeONPs R1 | FeONPs R2 | FeONPs R3 | mean |
| 200 μg/mL | 57.5 | 41.6 | 32.1 | 32.3 | 32.2 | 32.2 ± 0.02 |
| 400 μg/mL | 61.7 | 46.2 | 48.7 | 48.7 | 48.8 | 48.73 ± 0.02 |
| 600 μg/mL | 65.6 | 49.7 | 66.4 | 66.5 | 66.5 | 66.46 ± 0.03 |
| 800 μg/mL | 67.5 | 56.2 | 71.2 | 71.3 | 71.3 | 71.26 ± 0.01 |
| 1000 μg/mL | 69.5 | 67.6 | 73.3 | 73.4 | 73.4 | 73.36 ± 0.02 |
Figure 10.
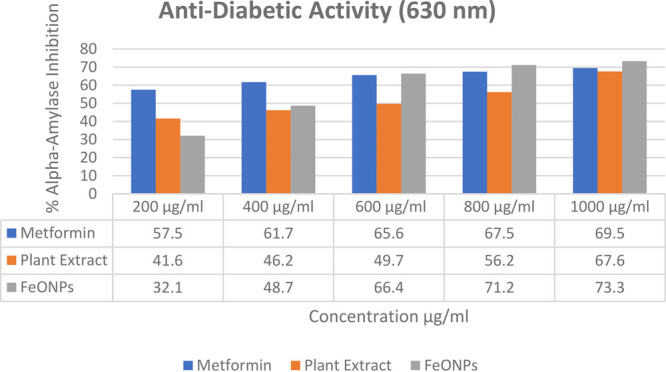
Trends in α-amylase inhibition among the control, the plant extract, and the FeONPs.
3.5. In Silico Analysis of the FeONPs
3.5.1. Structure Formation of Synthesized FeONPs
FeONPs (iron oxide nanoparticles) were synthesized, and their structure was determined using ChemDraw and FTIR. The carbonyl functional group acted as a bridge between two iron atoms on the iron oxide compound to form the final structure of FeONPs. The structure was saved in an SDF format, visualized on PyMol, and optimized for stability and refinement. Figure 11 shows the greenly synthesized FeONP structure visualized on PyMol.
Figure 11.
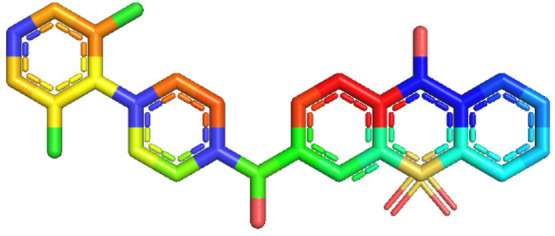
Structure of synthesized FeONPs visualized on PyMol visualization systems.
3.5.2. Optimization of Chemical and Structural Diversity
The molecular geometry was optimized, and the various atomic configurations were identified in order to determine the most stable form of the designed greenly synthesized FeONPs. The molecule with the lowest energy was selected because of the maximum stability of the lowest energy molecules. The finalized molecule with the lowest energy and maximum stability was downloaded from the RPBS web portal in the PDB format, and then it was utilized in further required analysis as well.
3.5.3. Toxicity Prediction
The Mcule online server predicted the greenly synthesized FeONPs to be nontoxic upon the evaluation by Toxicity Checker. Therefore, the results clearly declared that the FeONPs are nontoxic and nonharmful for use, as they do not possess any toxic effects or harmful properties. They can be further evaluated for safety in in vitro and in vivo experimentations. However, the computational results predict them to be nonharmful and nontoxic.
3.5.4. Absorption Prediction and Drug Likeness of FeONPs
SwissADME predicted the absorption and drug likeness along with the other drug like properties of greenly synthesized FeONPs. The absorption was computed by boiled-egg analysis, which represented a good HIA (human intestinal absorption) of the greenly synthesized FeONPs. Along with that, the bioavailability score was also observed as 0.55, indicating good absorption in the intestinal region of the human body. Figure 12 represents the boiled-egg analysis obtained by SwissADME. The compound has a molecular formula of C23H37Cl2N3O4S with a molecular weight of 522.53 g/mol.
Figure 12.
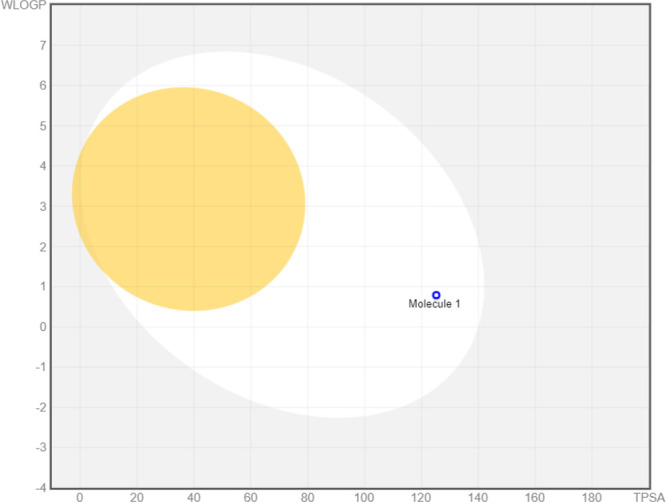
Boiled-egg analysis by SwissADME. The white region indicates good HIA.
It contains 33 heavy atoms and no aromatic heavy atoms and has a C(sp3) fraction of 0.91 with three rotatable bonds. There are seven H-bond acceptors and three H-bond donors present. The molar refractivity is 145.84, and the topological polar surface area (TPSA) is 125.20 Å2, as shown in Table 4.
Table 4. Physiochemical Properties of Greenly Synthesized FeONPs.
| physiochemical properties | |
|---|---|
| formula | C23H37Cl2N3O4S |
| molecular weight | 522.53 g/mol |
| no. heavy atoms | 33 |
| no. arom. heavy atoms | 0 |
| fraction C(sp3) | 0.91 |
| no. rotatable bonds | 3 |
| no. H-bond acceptors | 7 |
| no. H-bond donors | 3 |
| molar Refractivity | 145.84 |
The compound demonstrated high solubility in water, with solubility scores indicating good solubility. It was predicted to have good gastrointestinal absorption but minor skin permeability. The compound adhered to Lipinski’s Rule of 5, with only one violation due to its molecular weight. All other drug-likeness rules were followed with no violations. Based on these parameters, the greenly synthesized FeONPs show potential as therapeutic agents.
4. Discussion
Nanotechnology is increasingly being used in pharmaceutics and therapeutics to improve the durability, solubility, and bioavailability of drugs and for targeted drug delivery. It has the potential to improve therapeutic action, extend drug half-life, enhance hydrophobic drug solubility, and release pharmaceuticals in response to stimuli.27 However, studies have also examined the potential negative consequences or toxicological effects of nanotechnology, such as the hepatotoxicity and gut liver axis role of titanium dioxide nanoparticles on mice models. Quantitative proteome analysis was used to evaluate the effects of iron oxide nanoparticles on the liver, brain, and lungs in a rat model. Analysis of the 16S rDNA gene revealed that rats’ gut microbiota diversity increased in a dose-dependent way.28
Iron oxide nanoparticles have shown therapeutic potential in myocardial infarction, with the ability to increase the expression of the gap junction protein connexin 43 in cardio myoblasts for increased therapeutic potential. However, pathological investigations of these nanoparticles have also been reported, with recent studies showing their potential antibacterial activity against pathogens such as Staphylococcus aureus and Escherichia coli using phytofabricated iron oxide nanoparticles.29 The study analyzed the generated nanoparticles using various methods such as nitrogen adsorption–desorption, XRD, TEM, SEM, EDX, and FTIR spectroscopy. The MIC for S. aureus was found to be less than the detection limit, while the MIC for E. coli was 4 g/mL.
This study was conducted to synthesize ferrous oxide nanoparticles (FeONPs) using a green approach with the plant leaf extract of Madhuca indica. The plant is known for its therapeutic and medicinal properties, and the synthesized FeONPs were found to be in the appropriate size range and possessed good in vitro and in vivo biological properties. The FeONPs showed good antioxidation, anti-inflammatory, and antidiabetic activities, and the in vivo assessment on laboratory mice showed good results for the hepatoprotective assessment.
However, the study has several limitations, as the FeONPs could be studied under clinical trials and humanized conditions for finalize their safety and usage for various therapeutic properties.30 Additionally, the greenly synthesized FeONPs can be proved to be a considerable therapeutic option in liver injuries, including the liver cirrhosis and fibrosis, due to their optimal results in the liver-injured mice in in vivo investigations.31 Ferrous oxide nanoparticles (FeONPs) were synthesized using a green approach with a plant leaf extract of the Madhuca indica plant. The FeONPs were of appropriate size and showed good biological properties, including antioxidant, anti-inflammatory, and antidiabetic activity in vitro and hepatoprotective activity in vivo. Madhuca indica has been reported to have therapeutic and medicinal properties and is used to treat various conditions. The study demonstrated the potential of the green synthesis of FeONPs using Madhuca indica plant extract for biomedical applications.32
In 2018, Shah et al., reported some of the adverse immunological effects of iron oxide nanoparticles along with the iron-based drug complex formulations.33 However, the therapeutic benefits are more important to consider, as the toxic and adverse effects can be minimized; the positive matter is the therapeutic benefits that the iron oxide nanoparticles provide along with the targeted delivery of the drug, controlled release, and on-site delivery for better therapeutic applications.33,34 The immunotoxicity of iron oxide nanoparticles (IONPs) with various physicochemical properties and administration routes needs to be further studied. It is important to separate the toxicities unique to the iron core, iron ions, and covering materials. The greenly synthesized FeONPs can be utilized for the treatment of liver-related diseases and drug delivery due to their high stability, carrier capacity, and ability to include both hydrophilic and hydrophobic molecules. However, more research is required to enhance the safety parameters for FeONPs to be used as therapeutics. Colloidal drug carriers using nanoparticle delivery systems have been suggested, and nanoparticles (NPs) have been identified as a promising colloidal drug delivery system.
5. Conclusions
The FeONPs were synthesized from the plant leaf extract of Madhuca indica using a green approach that is simple and easy to use without any specified instrumentations. Additionally, the synthesized nanoparticles performed well in in vitro biological assessments and exhibited in vivo hepatoprotective properties in the laboratory mice. The greenly synthesized FeONPs can be utilized efficiently as therapeutics and drug delivery carriers as per the observed results in the various assessments. The FeONPs possessed good results as compared to their controls used in the antidiabetic, anti-inflammatory, and antioxidative studies. However, further clinical trials and in vivo studies are still required for the confirmation of safety and other standard requirements. This study will be one for the future scientists who are interested to explore the field of nanobiotechnology and nanomedicine, as it will provide a gateway to the scientists in the study of nanocarrier systems and drug delivery metabolisms.
Acknowledgments
The authors greatly acknowledge and express their gratitude to the Researchers Supporting Project number (RSP2023R335), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
Conceptualization, M.A., M.N., S.R., N.U., and T.A.; methodology, M.A., M.N., S.R., and N.U.; software, M.A.; validation, A.A.S.; formal analysis, T.A.; investigation, M.A., M.N., S.R., and N.U.; resources, M.A. and A.A.S.; data curation, T.A.; writing (original draft preparation), T.A. and M.N.; writing (review and editing), M.A., M.N., S.R., and N.U.; visualization, F.A. and N.U.; supervision, T.A., and S.N.; project administration, A.A.S. and M.A.; funding acquisition, T.A.
No external funding was received.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
References
- Mottaghitalab F.; Farokhi M.; Fatahi Y.; Atyabi F.; Dinarvand R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. Journal of controlled release. 2019, 295, 250–267. 10.1016/j.jconrel.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Saleem A.; Afzal M.; Naveed M.; Makhdoom S. I.; Mazhar M.; Aziz T.; Khan A. A.; Kamal Z.; Shahzad M.; Alharbi M.; Alshammari A. HPLC, FTIR and GC-MS Analyses of Thymus vulgaris Phytochemicals Executing In Vitro and In Vivo Biological Activities and Effects on COX-1, COX-2 and Gastric Cancer Genes Computationally. Molecules 2022, 27, 8512. 10.3390/molecules27238512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M.; Batool H.; Rehman S.u.; Javed A.; Makhdoom S. I.; Aziz T.; Mohamed A. A.; Sameeh M. Y.; Alruways M. W.; Dablool A. S.; Almalki A. A.; Alamri A. S.; Alhomrani M. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. 10.3390/pr10081521. [DOI] [Google Scholar]
- Aziz T.; Nadeem A. A.; Sarwar A.; Perveen I.; Hussain N.; Khan A. A.; Daudzai Z.; Cui H.; Lin L. Particle Nanoarchitectonics for Nanomedicine and Nanotherapeutic Drugs with Special Emphasis on Nasal Drugs and Aging. Biomedicines 2023, 11, 354. 10.3390/biomedicines11020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mamoori S. O. H.; Jabuk S. I.; Mahdi R. K.; Naji N. M.; Almaamori A. M. The Use of Nanotechnology in Medicine. Eur. J. Res. Devel. Sustain. 2022, 3 (4), 115–120. [Google Scholar]
- Xu C.; Akakuru O. U.; Zheng J.; Wu A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Frontiers in bioengineering and biotechnology. 2019, 7, 141. 10.3389/fbioe.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M.; Bukhari B.; Aziz T.; Zaib S.; Mansoor M. A.; Khan A. A.; Shahzad M.; Dablool A. S.; Alruways M. W.; Almalki A. A.; Alamri A. S.; Alhomrani M. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. 10.3390/molecules27134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat P.; Khan I.; Rehman A.; Jamil T.; Hayat A.; Rehman M. U.; Ullah N.; Sarwar A.; Alharbi A. A.; Dablool A. S.; Daudzai Z.; Alamri A. S.; Alhomrani M.; Aziz T. Myogenesis and Analysis of Antimicrobial Potential of Silver Nanoparticles (AgNPs) against Pathogenic Bacteria. Molecules 2023, 28, 637. 10.3390/molecules28020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoun S.; Arif R.; Jangid N. K.; Meena R. K. Green synthesis of nanoparticles using plant extracts: A review. Environmental Chemistry Letters. 2021, 19, 355–374. 10.1007/s10311-020-01074-x. [DOI] [Google Scholar]
- Wang Y.; Cai R.; Chen C. The nano–bio interactions of nanomedicines: Understanding the biochemical driving forces and redox reactions. Accounts of chemical research. 2019, 52, 1507–1518. 10.1021/acs.accounts.9b00126. [DOI] [PubMed] [Google Scholar]
- Ahmed R. H.; Mustafa D. E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. International Nano Letters. 2020, 10, 1–14. 10.1007/s40089-019-00291-9. [DOI] [Google Scholar]
- Khare P.; Kishore K.; Sharma D. K. Medicinal uses, Phytochemistry and Pharmacological profile of Madhuca longifolia. Asian Journal of Pharmacy and Pharmacology. 2018, 4, 570–581. 10.31024/ajpp.2018.4.5.5. [DOI] [Google Scholar]
- Reddy I. S. Madhuca indica: An untapped forest tree for its medicinal uses. Pharma Innov. 2022, 11, 1747–1751. [Google Scholar]
- Badukale N. A.; Panchale W. A.; Manwar J. V.; Gudalwar B. R.; Bakal R. L. Phytochemistry, pharmacology and botanical aspects of Madhuca indica: A review. Journal of Pharmacognosy and Phytochemistry. 2021, 10, 1280–1286. 10.22271/phyto.2021.v10.i2q.13987. [DOI] [Google Scholar]
- Morya S.; Kumari J.; Kumar D. An Overview: Nutritional and Therapeutic Comportment of Madhuca indica (Mahua). Agric. Food: e-Newsl. 2020, 2 (6), 349–351. [Google Scholar]
- Herlekar M.; Barve S.; Kumar R. Plant-mediated green synthesis of iron nanoparticles. J. Nanoparticles 2014, 2014, 140614. 10.1155/2014/140614. [DOI] [Google Scholar]
- Yu C.; Tang J.; Liu X.; Ren X.; Zhen M.; Wang L. Green biosynthesis of silver nanoparticles using Eriobotrya japonica (Thunb.) leaf extract for reductive catalysis. Materials. 2019, 12, 189. 10.3390/ma12010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranya S.; Vijayarani K.; Pavithra S. Green synthesis of iron nanoparticles using aqueous extract of Musa ornata flower sheath against pathogenic bacteria. Indian J. Pharm. Sci. 2017, 79, 688–694. 10.4172/pharmaceutical-sciences.1000280. [DOI] [Google Scholar]
- Mohammed A.; Abdullah A.. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics–HERVEX, Băile Govora, Romania, November 7–9, 2018; Asociatia FLUIDAS: Bucuresti, Romania, 2018; pp 7–9. https://fluidas.ro/hervex/proceedings2018/77-85.pdf.
- Brown T. ChemDraw by Perkin Elmer. The Science Teacher 2014, 81 (2), 67. [Google Scholar]
- Yang Y.; Jiménez-Negrón O. A.; Kitchin J. R. Machine-learning accelerated geometry optimization in molecular simulation. J. Chem. Phys. 2021, 154, 234704. 10.1063/5.0049665. [DOI] [PubMed] [Google Scholar]
- Leite T. B.; Gomes D.; Miteva M. A.; Chomilier J.; Villoutreix B. O.; Tufféry P. Frog: a FRee Online druG 3D conformation generator. Nucleic acids research. 2007, 35, W568–W572. 10.1093/nar/gkm289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens J. A.; Will Y. The significance of mitochondrial toxicity testing in drug development. Drug discovery today. 2007, 12, 777–785. 10.1016/j.drudis.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Jia C.-Y.; Li J.-Y.; Hao G.-F.; Yang G.-F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discovery Today. 2020, 25, 248–258. 10.1016/j.drudis.2019.10.014. [DOI] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific reports. 2017, 7, 42717. 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasundari J.; Praba P. S.; Jacob Y. B. A.; Vasantha V.; Shanmugaiah V. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Psidium guajava plant and their antibacterial activity. Chem. Sci. Rev. Lett. 2017, 6, 1244–1252. [Google Scholar]
- Kirtane A. R.; Verma M.; Karandikar P.; Furin J.; Langer R.; Traverso G. Nanotechnology approaches for global infectious diseases. Nature Nanotechnology. 2021, 16, 369–384. 10.1038/s41565-021-00866-8. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Zhou D.; Han S.; Zhou S.; Jia G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. 10.1186/s12989-019-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Li W.; Cheng S.; Liu J.; Wang S. Novel fabrication of bioengineered injectable chitosan hydrogel loaded with conductive nanoparticles to improve therapeutic potential of mesenchymal stem cells in functional recovery after ischemic myocardial infarction. Nanomedicine: Nanotechnology, Biology and Medicine. 2023, 47, 102616. 10.1016/j.nano.2022.102616. [DOI] [PubMed] [Google Scholar]
- Adityan S.; Tran M.; Bhavsar C.; Wu S. Y. Nano-therapeutics for modulating the tumour microenvironment: Design, development, and clinical translation. J. Controlled Release 2020, 327, 512–532. 10.1016/j.jconrel.2020.08.016. [DOI] [PubMed] [Google Scholar]
- Cornu R.; Béduneau A.; Martin H. Influence of nanoparticles on liver tissue and hepatic functions: A review. Toxicology. 2020, 430, 152344. 10.1016/j.tox.2019.152344. [DOI] [PubMed] [Google Scholar]
- Akintelu S. A.; Oyebamiji A. K.; Olugbeko S. C.; Folorunso A. S. Green synthesis of iron oxide nanoparticles for biomedical application and environmental remediation: a review. Eclética Química. 2021, 46, 17–37. 10.26850/1678-4618eqj.v46.4.2021.p17-37. [DOI] [Google Scholar]
- Shah A.; Dobrovolskaia M. A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine: Nanotechnology, Biology and Medicine. 2018, 14, 977–990. 10.1016/j.nano.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.; Yadav S.; Srivastava M.; Ganesh N.; Srivastava S. Promising anti-inflammatory bio-efficacy of saponin loaded silver nanoparticles prepared from the plant Madhuca longifolia. Asian. J. Nanosci. Mater. 2018, 5 (4), 313–326. 10.26655/AJNANOMAT.2022.4.5. [DOI] [Google Scholar]