Abstract
EA8212 BRIDGE is a phase 3 randomized trial comparing BCG vs GemDoce for BCG naïve high-risk non–muscle-invasive bladder cancer. This article provides an explanation for the rationale of the clinical trial and details the study design.
1. Background
For more than 40 yr, transurethral resection of bladder tumor (TURBT) followed by intravesical bacillus Calmette-Guérin (BCG) immunotherapy has been the standard treatment for patients with newly diagnosed high-risk non–muscle-invasive bladder cancer (NMIBC).
Although BCG may provide long-term recurrence free survival for some patients, 30–50% will experience disease recurrence within 2 yr of receiving BCG [1]. There is no accepted standard second-line medical therapy that is curative in the majority of patients, and thus many will undergo radical cystectomy. In addition, while BCG is well tolerated in the majority of patients, it is associated with both local and systemic toxicity, which can lead to BCG intolerance and early termination of treatment. Furthermore, worldwide BCG shortages have acutely impacted patient care and outcomes and are anticipated to lead to subsequent progression of disease [2].
Few trials in the past 30 yr have directly challenged BCG hegemony, largely because of its efficacy, with contemporary studies suggesting 12-mo high-grade recurrence-free survival (RFS) rates of 81–89% [3,4]. However, because of BCG shortages, toxicities causing intolerance, and imperfect efficacy, there is a clear need to find effective alternative therapies.
2. GemDoce
Single-agent intravesical gemcitabine chemotherapy has been used for bladder cancer for more than four decades. A randomized trial that evaluated gemcitabine vs one-third strength BCG (n = 120) for intermediate-risk NMIBC found no significant differences in recurrence or progression rates [5]. Furthermore, in a prospective randomized trial for patients with BCG-exposed NMTBC (n = 80), gemcitabine was associated with a 1-yr RFS rate of 47%, in comparison to 13% for a second induction course of BCG [6]. Similarly, single-agent docetaxel has been studied in several single-arm trials for BCG-exposed and unresponsive NMIBC, with a 1-yr RFS rate of 40% reported [7].
Since 2015, when the first retrospective study of combination intravesical gemcitabine and docetaxel (GemDoce) was published, this regimen has been increasingly used for BCG-exposed NMIBC, with multiple studies citing its efficacy as second-line therapy [8]. During BCG shortages, many urologists have turned to GemDoce as an alternative, allowing for the generation of preliminary data. In a retrospective single-institution study of 107 patients with BCG-naïve NMIBC, the 2-yr RFS rate was 82% [9]. A phase 2 prospective single-center study evaluating intravesical GemDoce for patients with BCG-naïve NMIBC revealed similar findings. With study accrual of all 25 patients having been completed, the 3-mo complete response (CR) rate was 100% (25/25) and the 12-mo CR rate for evaluable patients was 82% (14/17) [10]. Taken together, preliminary data regarding GemDoce for BCG-naïve NMIBC justify an expanded phase 3 randomized trial.
3. ECOG-ACRIN EA8212 (BRIDGE): study design
ECOG-ACRIN EA8212 is an ongoing phase 3 randomized trial evaluating intravesical BCG versus intravesical GemDoce for high-grade BCG-naïve NMIBC. Eligible patients include adults aged >18 yr with BCG-naive high-grade NMIBC (HG Ta, carcinoma in situ [CIS], or HG T1) without a history of urethral or upper tract urothelial cancer. Key exclusion criteria include the presence of sarcomatoid, plasmacytoid, micropapillary, or neuroendocrine variant histology, and prior intravesical chemotherapy other than perioperative use at the time of TURBT.
Patients are randomized to one of two treatment arms (Fig. 1) stratified by the presence of CIS, papillary disease, or concurrent CIS and papillary NMIBC (870 patients). Patients randomized to arm A will receive intravesical gemcitabine (1 g/50 ml normal saline [NS]) and docetaxel (40 mg/50 ml NS) weekly for 6 wk, with monthly maintenance for 2 yr. Patients randomized to arm B will receive intravesical BCG with a standard 6-wk induction course followed by 3-wk maintenance courses at 3, 6, 12, 18, 24, 30, and 36 mo. Similar to contemporary trials in this space, a mandatory biopsy for CIS patients will be performed at month 6.
Fig. 1 –
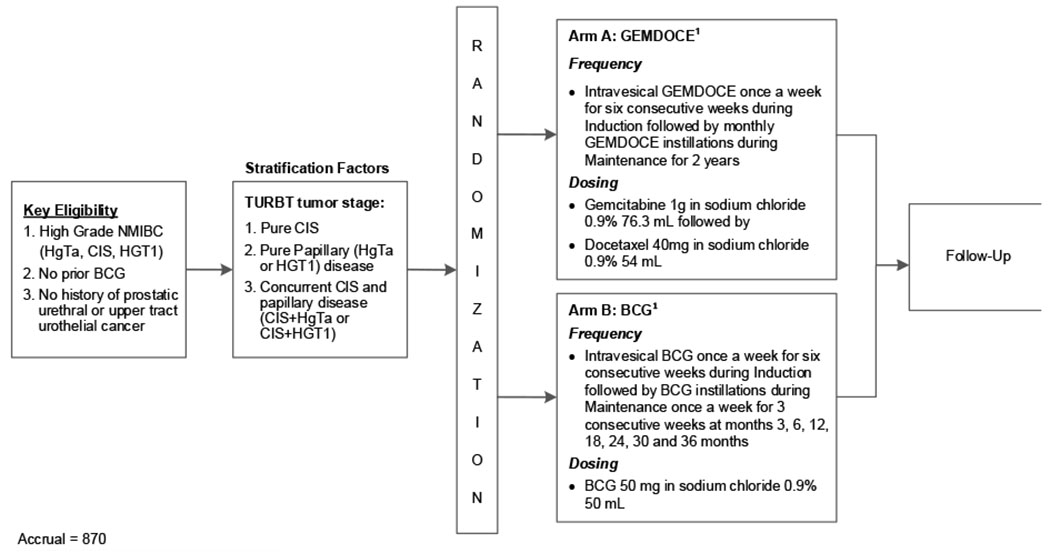
Study schema. BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ; HG high grade; NMIBC = non–muscle-invasive bladder cancer; TURBT = transurethral resection of bladder tumor.
The primary objective is to assess whether event-free survival for GemDoce is noninferior to that with standard BCG for patients with BCG-naïve high-grade NMIBC. Critical to the trial will be an understanding of the difference in patient experience between the two agents. Thus, a key secondary objective is to compare changes in cancer-specific and bladder cancer–specific quality of life. Other secondary objectives are to assess safety and toxicity and determine cystectomy-free and progression-free survival. The trial is being run through the National Clinical Trials Network and is available for all National Cancer Institute–funded Eastern Cooperative Oncology Group members to open to enrollment. The first patient was enrolled on February 7, 2023.
Acknowledgments:
This study is being conducted by the ECOG-ACRIN Cancer Research Group and is supported by the US National Institutes of Health/National Cancer Institute under grants U10CA180820, U10CA180794, UG1CA180830, UG1CA189854, UG1CA233180, UG1CA233196, UG1CA233290, and UG1CA233373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have nothing to disclose.
References
- 1.Kamat AM, Sylvester RJ, Böhle A, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol 2016;34:1935–44. 10.1200/JCO.2015.64.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Lim B, You D, et al. Association of bacillus Calmette–Guerin shortages with bladder cancer recurrence: a single-center retrospective study. Urol Oncol 2020;38:851.e11–7. 10.1016/j.urolonc.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Grimm MO, van der Heijden AG, Colombel M, et al. Treatment of high-grade non–muscle-invasive bladder carcinoma by standard number and dose of BCG instillations versus reduced number and standard dose of BCG instillations: results of the European Association of Urology Research Foundation randomised phase III clinical trial “NIMBUS”. Eur Urol 2020;78:690–8. 10.1016/j.eururo.2020.04.066 [DOI] [PubMed] [Google Scholar]
- 4.Matulay JT, Li R, Hensley PJ, et al. Contemporary outcomes of patients with nonmuscle-invasive bladder cancer treated with bacillus Calmette-Guérin: implications for clinical trial design. J Urol 2021;205:1612–21. 10.1097/JU.0000000000001633 [DOI] [PubMed] [Google Scholar]
- 5.Gontero P, Oderda M, Mehnert A, et al. The impact of intravesical gemcitabine and 1/3 dose bacillus Calmette-Guérin instillation therapy on the quality of life in patients with nonmuscle invasive bladder cancer: results of a prospective, randomized, phase II trial. J Urol 2013;190:857–62. 10.1016/j.juro.2013.03.097 [DOI] [PubMed] [Google Scholar]
- 6.Di Lorenzo G, Perdona S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 2010;116:1893–900. 10.1002/cncr.24914 [DOI] [PubMed] [Google Scholar]
- 7.Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guérin therapy. J Urol 2013;189:834–9. 10.1016/j.juro.2012.10.068 [DOI] [PubMed] [Google Scholar]
- 8.Steinberg RL, Thomas LJ, Brooks N, et al. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol 2020;203:902–9. 10.1097/JU.0000000000000688 [DOI] [PubMed] [Google Scholar]
- 9.McElree IM, Steinberg RL, Martin AC, et al. Sequential intravesical gemcitabine and docetaxel for bacillus Calmette-Guérin-naïve high-risk nonmuscle-invasive bladder cancer. J Urol 2022;208:589–99. 10.1097/JU.0000000000002740 [DOI] [PubMed] [Google Scholar]
- 10.Patel SH, Gabrielson A, Collins C, et al. Intravesical gemcitabine and docetaxel in the treatment of BCG-naïve non–muscle invasive urothelial carcinoma of the bladder: updates from a phase 2 trial. J Clin Oncol 2023;41(6 Suppl):507. 10.1200/JCO.2023.41.6_suppl.507 [DOI] [Google Scholar]


