Abstract
Rationale
Patients with obesity are at increased risk for developing acute respiratory distress syndrome (ARDS). Some centers consider obesity a relative contraindication to receiving extracorporeal membrane oxygenation (ECMO) support, despite growing implementation of ECMO for ARDS in the general population.
Objectives
To investigate the association between obesity and mortality in patients with ARDS receiving ECMO.
Methods
In this large, international, multicenter, retrospective cohort study, we evaluated the association of obesity, defined as body mass index ⩾ 30 kg/m2, with ICU mortality in patients receiving ECMO for ARDS by performing adjusted multivariable logistic regression and propensity score matching.
Measurements and Main Results
Of 790 patients with ARDS receiving ECMO in our study, 320 had obesity. Of those, 24.1% died in the ICU, compared with 35.3% of patients without obesity (P < 0.001). In adjusted models, obesity was associated with lower ICU mortality (odds ratio, 0.63 [95% confidence interval, 0.43–0.93]; P = 0.018). Examined as a continuous variable, higher body mass index was associated with decreased ICU mortality in multivariable regression (odds ratio, 0.97 [95% confidence interval, 0.95–1.00]; P = 0.023). In propensity score matching of 199 patients with obesity to 199 patients without, patients with obesity had a lower probability of ICU death than those without (22.6% vs. 35.2%; P = 0.007).
Conclusions
Among patients receiving ECMO for ARDS, those with obesity had lower ICU mortality than patients without obesity in multivariable and propensity score matching analyses. Our findings support the notion that obesity should not be considered a general contraindication to ECMO.
Keywords: obesity, extracorporeal membrane oxygenation, acute respiratory distress syndrome
At a Glance Commentary
Scientific Knowledge on the Subject
Few publications address the association between obesity and mortality in patients with acute respiratory distress syndrome (ARDS) supported with extracorporeal membrane oxygenation (ECMO). All prior studies have been single center, except for one registry-based study, each with inherent limitations. In the past three years, most publications related to ECMO use have focused predominantly on coronavirus disease (COVID-19)–related ARDS, leaving little evidence to support practice for non–COVID-19–related etiologies of ARDS.
What This Study Adds to the Field
This large, international, multicenter, retrospective cohort study examines data preceding the pandemic and is one of the first to present robust evidence that patients with obesity have lower ICU mortality than patients without in multivariable analysis corroborated by propensity score matching. Given the increasing prevalence of obesity and the fact that some centers consider obesity a contraindication to ECMO, our findings aid clinical decision making with respect to appropriate selection and management of patients with obesity who have ARDS and may benefit from ECMO. Our results support the hypothesis that obesity should not be considered a general contraindication to ECMO and advocate for the inclusion of patients with obesity in future clinical trials, where patients with higher degrees of obesity have been previously excluded from study populations.
The obesity epidemic remains a major driver of mortality worldwide, with the prevalence of obesity nearly tripling in the past half century (1, 2). As the proportion of patients with obesity admitted to ICUs increases (3, 4), our understanding of the impact of obesity on ICU outcomes continues to evolve. Whereas prior literature supports the concept of an “obesity paradox,” with better outcomes in patients with obesity compared with those without (5, 6), recent studies of critically ill patients, specifically with coronavirus disease (COVID-19), demonstrate no survival advantage with obesity in ICU patients compared with patients without obesity and suggest an age-dependent association (7–9). Notably, patients with obesity are at an increased risk for developing acute respiratory distress syndrome (ARDS) (10, 11) but are reported to have lower ARDS-specific mortality (12, 13).
The implementation of extracorporeal membrane oxygenation (ECMO) for patients with severe forms of ARDS has increased substantially over the past decade (14, 15), especially in light of growing evidence to support the use of ECMO in this setting (16–19). However, since the original version of the Extracorporeal Life Support Organization (ELSO) guidelines published in 2009, “size of patient” has been traditionally considered a relative contraindication to ECMO because of difficulties with cannulation, transport, and early mobilization or out of concern for poorer outcomes (4, 20–22). In addition, patients with higher body mass index (BMI) were excluded from high-quality trials examining the use of ECMO in ARDS (16), further highlighting the lack of familiarity with ECMO in this patient population and widening the data gap in supporting evidence-based care for such patients. The claim that obesity is a contraindication to ECMO has been challenged in recent years (23–26). Until recently, most of the evidence has been based on single-center, observational studies that included patients receiving extracorporeal life support for indications other than ARDS. One recent publication used the ELSO registry to evaluate the association of obesity on in-hospital mortality and reported lower mortality risk in higher classes of obesity (27). Nevertheless, confirmation of these results derived from more granular data with more precise effect estimates regarding the impact of obesity on outcomes in patients receiving ECMO for ARDS is still lacking, and patients with obesity, representing a substantial share of the population, may yet have ECMO withheld from them unnecessarily. We performed a large, international, multicenter, retrospective cohort study to investigate the association between obesity and mortality in patients with ARDS supported with ECMO. Some of the results of this study were previously reported in the form of an abstract (28).
Methods
Additional details on the methods are provided in the online supplement.
Data Sources
Data were collected from Columbia University Medical Center (New York), Alfred Hospital (Melbourne, Australia), St. Vincent’s Hospital (Sydney, Australia), Sorbonne University Assistance Publique–Hôpitaux de Paris (Paris, France), a previously published study (29), the Duke University School of Medicine (Durham, North Carolina), and the University of Milan (Milan, Italy) between July 1, 2012, and June 30, 2017, and combined with the LIFEGARDS (Ventilation Management of Patients with Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome) database that incorporates data from 23 ICUs across 10 countries (30), with respective institutional and ethics review board approvals obtained.
Study Participants
All adult patients supported with ECMO for ARDS for hypoxemic respiratory failure were included in the analysis. Peripartum and postpartum patients up to 3 months after delivery were excluded because of the inability to ascertain the contribution of pregnancy to BMI (31).
Outcomes
The primary outcome was ICU mortality in patients with obesity compared with those without, where obesity was defined as a BMI ⩾ 30 kg/m2 in accordance with World Health Organization definitions (31, 32). The association of BMI as a continuous variable, per 1 kg/m2 increase, with ICU mortality was examined using multivariable logistic regression. Secondary outcomes included ICU length of stay (LOS), hospital LOS, and number of days receiving ECMO and mechanical ventilation. Given the competing risk of death, mechanical ventilator–free, ECMO-free, and hospital-free days at 90 days in patients with obesity compared with those without were also calculated using previously described methods, considering zero free days for deceased patients (33).
Statistical Analysis
We followed the Strengthening the Reporting of Observational Studies in Epidemiology recommendations for reporting this study (see Figure E1 in the online supplement) (34). Continuous variables are expressed as mean (SD) or as median (interquartile range [IQR]) and categorical variables as counts and proportions. Normality of the data distribution was visually assessed using histograms. Proportions were compared using the χ2 test or Fisher exact test. Continuous variables were compared using Student’s t test or the Wilcoxon rank sum test.
Using a directed acyclic graph (see Figure E2) (35), a priori–defined pre-ECMO covariates presumed to be associated with the presence of obesity or patient outcomes were included in a multivariable logistic regression analysis to determine association of BMI with ICU mortality. These covariates were used to determine the propensity score of obesity for each patient. In the matched cohort analysis, taking into account the clustered structure of our dataset, patients with obesity were matched with patients without obesity according to the propensity score, using a 1:1 nearest neighbor matching procedure without replacement and a caliper width of 0.2, resulting in a relatively narrow difference between matched variables (see Figure E3). The McNemar test was subsequently performed.
Given the previously demonstrated nonlinear association between BMI and mortality (36), to evaluate for potential nonlinear associations between BMI and risk of ICU mortality, we used generalized additive models (GAMs) with the “gam” function with a smoothing function for BMI in R. We compared the Akaike information criterion of both linear models and GAMs to choose the model with the best fit. StataCorp 15.0 and R version 4.1.0 (The R Foundation for Statistical Computing) were used for these analyses.
Results
Among the participating centers, 790 patients receiving ECMO support for ARDS were included in the study, 350 of whom were recruited from the LIFEGARDS database. Demographics and precannulation clinical characteristics for the overall cohort and stratified by obesity are presented in Table 1. The average age was 44.2 years (SD, 15.5 yr), without a significant difference between the groups. In patients with obesity, the majority (58.8%) were women. APACHE (Acute Physiology and Chronic Health Evaluation) II scores were lower in patients with obesity compared with those without (P = 0.028). The etiology of respiratory failure was available in 534 patients, with bacterial, viral, and aspiration pneumonias being the most common, constituting 33.3%, 26.4%, and 14.6%, respectively, without a significant difference between the groups. Before ECMO cannulation, 63% of patients received neuromuscular blocking agents (NMBAs), 30% received inhaled nitric oxide (iNO), and 19% were placed in the prone position, with no significant differences in the use of these adjunctive therapies between groups except in the use of NMBAs, for which 280 patients with obesity (59.6%) versus 219 patients without (68.4%) received NMBAs (P = 0.014). Precannulation ratios of PaO2 to FiO2 (PaO2:FiO2) were 63.9 mm Hg (SD, 28.6 mm Hg) and 71.5 mm Hg (SD, 31.1 mm Hg) for patients with and without obesity, respectively (P = 0.001). Median plateau airway pressures before cannulation were 32 (IQR, 30–36) cm H2O for patients with obesity and 31 (IQR, 28–35) cm H2O for those without (P = 0.001; see Figure E4). Precannulation pH did not significantly differ between the groups. Further stratifications of our population by BMI and geographic origin are depicted in Tables E1 and E2, respectively.
Table 1.
Demographic, Clinical, and Mechanical Ventilation Characteristics before Extracorporeal Membrane Oxygenation Cannulation, for the Overall Cohort and Stratified by Obesity
| BMI (kg/cm2) |
|||||
|---|---|---|---|---|---|
| All Patients (n = 790) | Patients without Obesity (n = 470) | Patients with Obesity (n = 320) | P Value | SMD | |
| Age, yr | 44.2 ± 15.5 | 44.1 ± 16.8 | 44.4 ± 13.4 | 0.753 | 0.022 |
| Sex | 0.018 | 0.176 | |||
| Female | 286 (36.2) | 154 (32.8) | 132 (41.2) | — | — |
| Male | 504 (63.8) | 316 (67.2) | 188 (58.8) | — | — |
| BMI, kg/cm2 | 30.3 ± 9.2 | 24.6 ± 3.3 | 38.8 ± 8.3 | <0.001 | 2.255 |
| BMI ⩾ 30 kg/cm2 | 320 (40.5) | 0 (0.00) | 320 (100) | <0.001 | — |
| BMI ⩾ 35 kg/cm2 | 193 (24.4) | 0 (0.00) | 193 (60.3) | <0.001 | — |
| BMI ⩾ 40 kg/cm2 | 110 (13.9) | 0 (0.00) | 110 (34.4) | <0.001 | — |
| APACHE II score | 23.3 ± 9.7 (n = 660) | 23.9 ± 10.1 (n = 425) | 22.2 ± 8.9 (n = 235) | 0.028 | 0.175 |
| SOFA score | 10.0 ± 4.1 (n = 772) | 10.0 ± 4.4 (n = 461) | 9.9 ± 3.7 (n = 311) | 0.795 | 0.019 |
| Duration of IMV before ECMO, d | 2.00 (1.00–6.00) | 2.00 (1.00–6.00) | 3.00 (1.00–6.00) | 0.165 | 0.020 |
| Ventilation parameters | |||||
| FiO2, mm Hg | 100 (100–100) (n = 762) | 100 (100–100) (n = 447) | 100 (100–100) (n = 315) | 0.054 | 0.155 |
| PEEP, cm H2O | 14 (10–16) (n = 717) | 12 (10–15) (n = 423) | 15 (12–18) (n = 294) | <0.001 | 0.466 |
| Vt, ml/kg PBW | 6.33 ± 1.88 (n = 419) | 6.13 ± 1.63 (n = 287) | 6.79 ± 2.26 (n = 132) | 0.003 | 0.335 |
| Plateau pressure, cm H2O | 32 [29–35] (n = 529) | 31 [28–35] (n = 331) | 32 [30–36] (n = 198) | 0.001 | 0.328 |
| Driving pressure, cm H2O* | 18 [14–22] (n = 274) | 18 [14–23] (n = 148) | 18 [14–22] (n = 126) | 0.951 | 0.006 |
| Respiratory rate, breaths/min | 25.0 ± 7.6 (n = 686) | 24.5 ± 7.7 (n = 402) | 25.8 ± 7.3 (n = 284) | 0.023 | 0.176 |
| Precannulation arterial blood gas values | |||||
| pH | 7.24 ± 0.14 (n = 735) | 7.24 ± 0.14 (n = 433) | 7.24 ± 0.14 (n = 302) | 0.774 | 0.021 |
| PaCO2, mm Hg | 62.7 ± 26.7 (n = 628) | 61.0 ± 23.6 (n = 402) | 65.6 ± 31.2 (n = 226) | 0.055 | 0.166 |
| Worst PaO2:FiO2 ratio | 68.4 ± 30.3 (n = 755) | 71.5 ± 31.1 (n = 447) | 63.9 ± 28.6 (n = 308) | 0.001 | 0.257 |
| Rescue therapy before ECMO | |||||
| Neuromuscular blockade | 499 (63.2) | 280 (59.6) | 219 (68.4) | 0.014 | 0.185 |
| Inhaled nitric oxide | 234 (29.6) | 141 (30.0) | 93 (29.1) | 0.838 | 0.021 |
| Prone positioning | 152 (19.2) | 94 (20.0) | 58 (18.1) | 0.572 | 0.048 |
| HFOV | 36 (4.6) | 21 (4.5) | 15 (4.7) | 1.000 | 0.010 |
| RRT before ECMO | 226 (28.6) | 131 (27.9) | 95 (29.7) | 0.635 | 0.040 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; ECMO = extracorporeal membrane oxygenation; HFOV = high-frequency oscillatory ventilation; IMV = invasive mechanical ventilation; PBW = predicted body weight; PEEP = positive end-expiratory pressure; RRT = renal replacement therapy; SMD = standardized mean difference; SOFA = sequential organ failure assessment.
Data are expressed as mean ± SD, n (%), or median (interquartile range).
Plateau pressure minus PEEP.
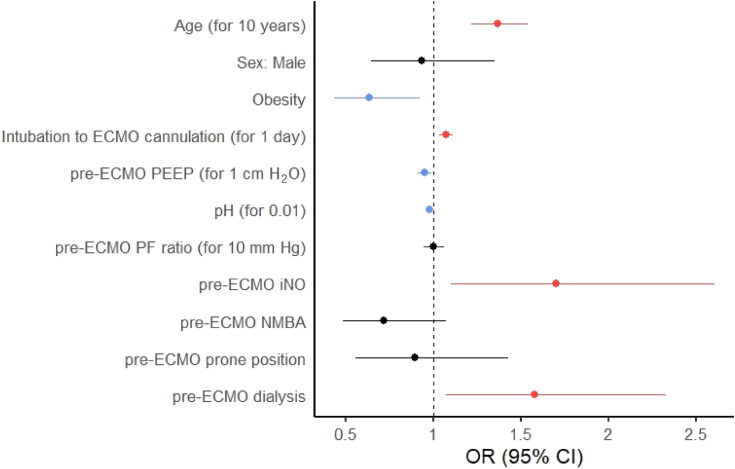
Of 320 patients with obesity, 77 (24.1%) died before discharge from the ICU, compared with 166 of 470 (35.3%) patients without obesity (P < 0.001). In multivariable analysis, obesity was associated with a lower risk of death in the ICU (odds ratio, 0.63 [95% confidence interval, 0.43–0.93]; P = 0.018), adjusted for age, sex, days of invasive mechanical ventilation (IMV) before cannulation, precannulation positive end-expiratory pressure (PEEP), pH, PaO2:FiO2, use of NMBA, iNO, prone positioning, and renal replacement therapy (RRT) (Figure 1; Table 2). Examined as a continuous variable, per 1 kg/m2, higher BMI was similarly associated with decreased ICU mortality in multivariable analysis (odds ratio, 0.97 [95% confidence interval, 0.95–1.00]; P = 0.023). Mortality stratified by BMI is depicted in Figure E5. Younger age, fewer days of IMV before ECMO, higher PEEP, higher pH, and not receiving iNO or RRT before ECMO were also associated with lower ICU mortality (Table 3). As APACHE II scores were not available for all patients, sensitivity analyses adjusting for APACHE II score in the multivariable logistic regression, examining the association of obesity and BMI as a continuous variable with ICU mortality, were performed on a smaller cohort (578 patients) and are shown in Tables E3 and E4, respectively. Sensitivity analyses adjusting for sequential organ failure assessment (SOFA) score are shown in Tables E5 and E6.
Figure 1.
Forest plot based on the results of multivariable analysis of the factors associated with ICU death in patients with obesity compared with those without obesity. Protective associations are shown in blue, harmful associations in red, and nonsignificant associations in black. CI = confidence interval; ECMO = extracorporeal membrane oxygenation; iNO = inhaled nitric oxide; NMBA = neuromuscular blocking agent; OR = odds ratio; PEEP = positive end-expiratory pressure; PF = PaO2:FiO2.
Table 2.
Variables Associated with ICU Death in Patients Supported by Extracorporeal Membrane Oxygenation in Multivariable Analysis Examining the Association of Obesity with Risk of Death in the ICU
| Odds Ratio for ICU Death (95% CI) | P Value | |
|---|---|---|
| Obesity | 0.63 (0.43–0.93) | 0.018 |
| Age, for 10-yr increase | 1.37 (1.22–1.54) | <0.001 |
| Sex, male | 0.93 (0.65–1.35) | 0.710 |
| Days of IMV before ECMO, for 1 d | 1.07 (1.03–1.11) | <0.001 |
| PEEP before ECMO, for 1 cm H2O increase | 0.95 (0.91–0.99) | 0.018 |
| pH before ECMO, for 0.01 increase | 0.98 (0.96–0.99) | <0.001 |
| PF ratio before ECMO, for 10 mm Hg increase | 1.00 (0.94–1.06) | 0.954 |
| Use of inhaled nitric oxide before ECMO | 1.70 (1.10–2.61) | 0.017 |
| Use of NMBA before ECMO | 0.72 (0.48–1.06) | 0.010 |
| Use of prone positioning before ECMO | 0.90 (0.55–1.43) | 0.653 |
| Need for dialysis before ECMO | 1.58 (1.07–2.33) | 0.020 |
Definition of abbreviations: CI = confidence interval; ECMO = extracorporeal membrane oxygenation; IMV = invasive mechanical ventilation; NMBA = neuromuscular blocking agents; PEEP = positive end-expiratory pressure; PF = PaO2:FiO2.
Table 3.
Variables Associated with ICU Death in Patients Supported by Extracorporeal Membrane Oxygenation in Multivariable Analysis Examining the Association of Body Mass Index as a Continuous Variable with Risk of Death in the ICU
| Odds Ratio for ICU Death (95% CI) | P Value | |
|---|---|---|
| BMI, per 1 kg/m2 increase | 0.97 (0.95–1.00) | 0.023 |
| Age, for 10-yr increase | 1.36 (1.21–1.53) | <0.001 |
| Sex, male | 0.93 (0.64–1.34) | 0.690 |
| Days of IMV before ECMO, for 1 d | 1.07 (1.04–1.12) | <0.001 |
| PEEP before ECMO, for 1 cm H2O increase | 0.96 (0.92–1.00) | 0.035 |
| pH before ECMO, for 0.01 increase | 0.98 (0.97–0.99) | <0.001 |
| PF ratio before ECMO, for 10 mm Hg increase | 1.00 (0.94–1.06) | 0.951 |
| Use of inhaled nitric oxide before ECMO | 1.75 (1.13–2.70) | 0.011 |
| Use of NMBA before ECMO | 0.73 (0.49–1.08) | 0.118 |
| Use of prone positioning before ECMO | 0.87 (0.54–1.40) | 0.578 |
| Need for dialysis before ECMO | 1.62 (1.10–2.39) | 0.015 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; ECMO = extracorporeal membrane oxygenation; IMV = invasive mechanical ventilation; NMBA = neuromuscular blocking agents; PEEP = positive end-expiratory pressure; PF = PaO2:FiO2.
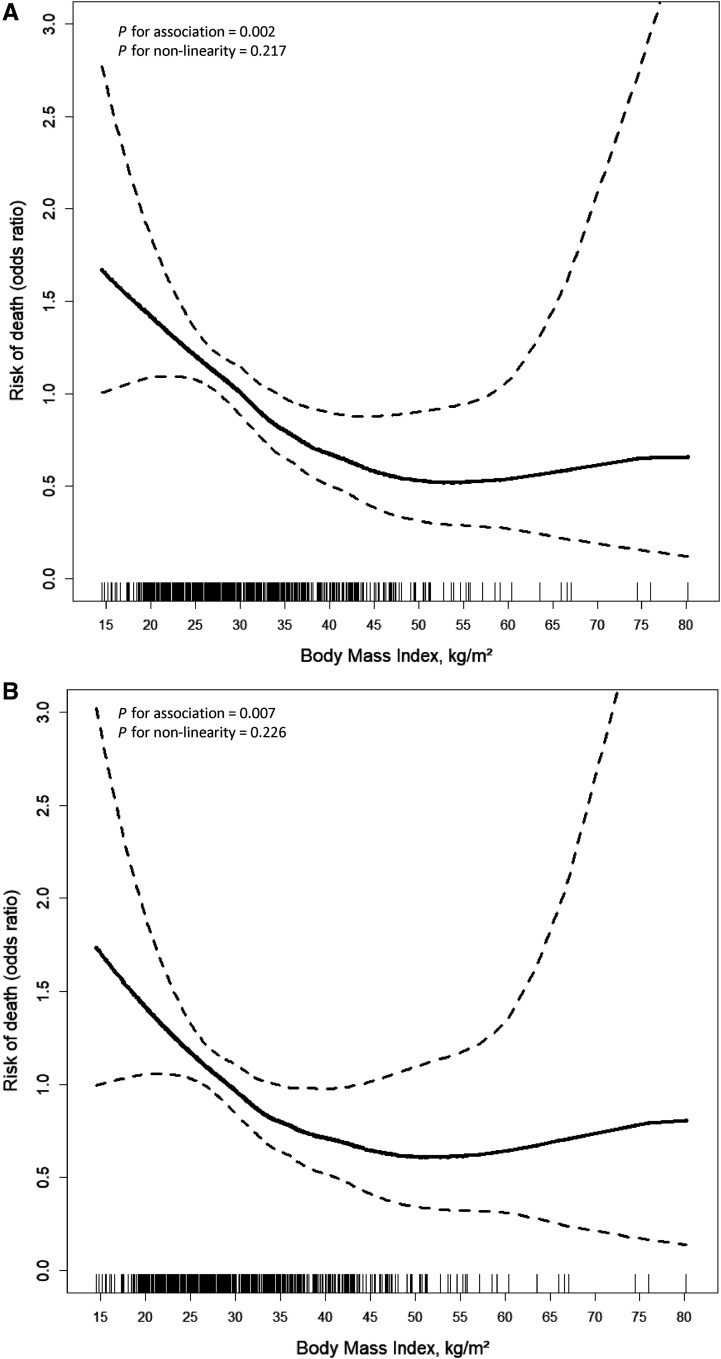
In GAMs, greater BMI was associated with a decreased risk of death, though this association may plateau at greater values of BMI (P for linearity = 0.002; Figure 2). In comparing linear models and GAMs, values were similar across all models, indicating that our findings are robust in multiple sensitivity analyses. We chose to display the GAM to demonstrate the lack of certainty in estimates for those with high BMIs and the possibility of a plateau or change in the direction of association between BMI and survival at very high values of BMI.
Figure 2.
(A and B) Odds ratios of mortality by BMI, unadjusted (A) and adjusted for age, sex, days of invasive mechanical ventilation before cannulation, precannulation positive end-expiratory pressure, pH, PaO2:FiO2, use of neuromuscular blocking agents, inhaled nitric oxide, prone positioning, and renal replacement therapy (B). The continuous association of BMI and odds of ICU death may differ somewhat from the primary model, as it is based on a generalized additive model that smooths the line segments to allow curved transitions over the ranges of BMI values. The solid line represents the estimated odds ratio. The dashed lines represent 95% confidence bands. BMI = body mass index.
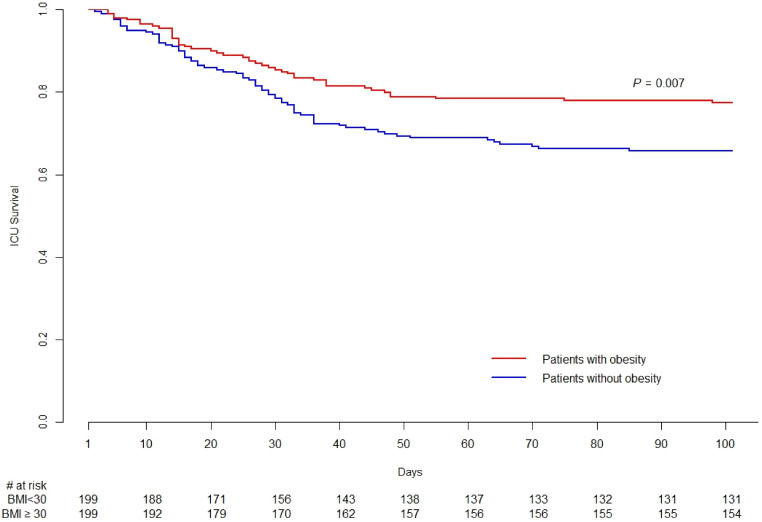
In propensity score matching analysis, 199 of 320 patients with obesity could be matched with 199 of the patients without obesity with good performance (see Table E7 and Figure E6) on age, sex, APACHE II score, respiratory rate, Vt, PEEP, plateau airway pressure, pH, PaCO2, PaO2:FiO2, use of high-frequency oscillatory ventilation, iNO, NMBA, prone positioning, and the receipt of RRT before cannulation (see Table E8). Demographic and clinical differences between patients with obesity, as well as the overall cohort, who could be matched versus those who went unmatched are depicted in Tables E9 and E10, respectively. Notably, matched patients in the overall cohort were sicker than patients who went unmatched, as evidenced by higher SOFA score, higher respiratory rate, higher plateau pressure, and lower PaO2:FiO2, despite higher use of NMBA before cannulation. In this analysis, once again, patients with obesity had a lower probability of death in the ICU (22.6% vs. 35.2%; P = 0.007; Figure 3).
Figure 3.
Kaplan-Meier graph for propensity score–matched populations. BMI = body mass index.
For the overall cohort, the median durations of ECMO and IMV were 10 (IQR, 7–18) days and 19 (IQR, 11–33) days, respectively (Table 4). The median LOSs in the ICU and the hospital were 24 (IQR, 13–39) days and 36 (IQR, 21–55) days, respectively. ICU and hospital LOSs, as well as duration of IMV and ECMO, did not differ significantly between patients with obesity and those without. There was no difference in ECMO-free days at 90 days between the groups. However, patients without obesity had fewer ventilator-free days and hospital-free days compared with patients with obesity, with medians of 50 (IQR, 0–75) days versus 60 (IQR, 0–75) days (P = 0.050) and 22 (IQR, 0–58 days) versus 40 (IQR, 0–58) days (P = 0.012), respectively. Furthermore, examining these variables in survivors, the median ECMO duration was 10 (IQR, 7–15) days compared with 14 (IQR, 6–25.5) days in nonsurvivors. Duration of IMV did not differ significantly between survivors versus nonsurvivors. In contrast, median ICU and hospital LOSs were significantly different, at 24 (IQR, 16–39) days and 41 (IQR, 26–60) days, respectively, in survivors, compared with 22 (IQR, 11–36.5) days and 28 (IQR, 13–44) days in nonsurvivors (Table 5; see Figure E7). Clinical outcomes among ICU survivors stratified by obesity are described in Table E11.
Table 4.
Clinical Outcomes for the Overall Cohort and Stratified by Obesity
| All Patients (n = 790) | Body Mass Index (kg/cm2) |
P Value | ||
|---|---|---|---|---|
| Patients without Obesity (n = 470) | Patients with Obesity (n = 320) | |||
| ECMO duration, d | 10.0 (6.00–18.0) | 10.0 (6.00–18.0) | 11.0 (7.00–16.0) | 0.450 |
| Mechanical ventilation duration, d | 19.0 (11.0–34.0) | 17.0 (10.0–34.0) | 20.0 (12.0–32.0) | 0.534 |
| Length of stay in ICU, d | 24.0 (14.0–39.0) | 24.0 (14.0–41.0) | 24.0 (14.5–37.0) | 0.850 |
| Length of stay in hospital, d | 36.0 (21.0–56.0) | 35.0 (20.0–58.0) | 37.0 (23.0–53.0) | 0.754 |
| ECMO-free days, at 90 d | 76.0 (0.0–82.0) | 74.0 (0.0–82.0) | 77.0 (18.0–82.0) | 0.098 |
| Ventilator-free days, at 90 d | 56.0 (0.0–75.0) | 49.0 (0.0–75.0) | 60.0 (0.0–75.0) | 0.031 |
| Hospital-free days, at 90 d | 32.0 (0.0–58.0) | 19.0 (0.0–58.0) | 40.0 (0.0–58.0) | 0.006 |
Definition of abbreviation: ECMO = extracorporeal membrane oxygenation.
Table 5.
Clinical Outcomes for ICU Survivors and Nonsurvivors
| Survivors (n = 547) | Nonsurvivors (n = 243) | P Value | |
|---|---|---|---|
| ECMO duration, d | 10.0 (7.00–15.0) | 14.0 (6.00–25.5) | <0.001 |
| Mechanical ventilation duration, d | 18.0 (11.0–32.0) | 21.0 (10.0–36.0) | 0.471 |
| Length of stay in ICU, d | 24.0 (16.0–39.0) | 22.0 (11.0–36.5) | 0.001 |
| Length of stay in hospital, d | 41.0 (26.0–60.0) | 28.0 (13.0–44.0) | <0.001 |
Definition of abbreviation: ECMO = extracorporeal membrane oxygenation.
Discussion
In this large, multicenter, international, observational, retrospective cohort study of patients receiving ECMO for ARDS, patients with obesity had lower ICU mortality compared with those without obesity. This finding persisted after adjustment for potential confounders and in sensitivity analyses using propensity score matching.
Our findings are consistent with prior single-center reports on the subject. Higher BMI was shown to be an independent predictor of 90-day survival in a 76-patient cohort of patients with COVID-19–associated ARDS receiving ECMO, of whom 29 had obesity (26). Comparably, 77 patients with obesity receiving ECMO for COVID-19–associated ARDS were found to have similar ICU and hospital mortality as patients without obesity (37). Last, in a study of 115 patients with BMI ⩾ 30 kg/m2 receiving ECMO for hypoxemic or hypercapnic respiratory failure, an overall mortality rate of 24.7% was reported across all obesity classes, with no association of BMI with mortality (24).
The largest study to date to evaluate this concept using the ELSO registry examined the association of BMI ⩾ 35 kg/m2 with in-hospital mortality in 18,529 patients, who were further subdivided into propensity score–matched groups of 4,707 patients with class II or greater obesity matched with 4,707 patients with BMI < 35 kg/m2 (27). Similar to our work, although using a different cutoff of BMI of ⩾35 kg/m2 to evaluate the impact of obesity, patients with higher BMI in the ELSO registry study had lower in-hospital mortality in propensity score matching, inverse propensity score–weighted, and multivariable analyses. Although our work confirms the central message of the ELSO registry study, there are notable distinctions between the two. As with many registry-based studies, though the sample size is appreciably larger, the quality and validity of the data in the ELSO registry are likely variable, with large percentages of missing data, such as one-quarter of patients missing pre-ECMO PEEP, for example. Our dataset, although smaller, is more granular and validated across multiple centers. Although we included patients receiving ECMO support for ARDS solely, the ELSO registry study based the query of ECMO indications on the International Classification of Diseases, Ninth and 10th Revisions, which inherently may carry inaccuracies, and included other etiologies of respiratory failures such as asthma, pulmonary hypertension, and bridge to lung transplantation. Our dataset is less informative than the ELSO registry data with respect to ECMO complications, discharge destination, and comorbid conditions, although our dataset includes APACHE II and SOFA scores to better characterize the latter. We have chosen to designate BMI ⩾ 30 kg/m2 as the definition of obesity, in accordance with World Health Organization definitions, as opposed to a BMI of ⩾35 kg/m2, which reflects class II obesity in the ELSO registry study, to be more precise in attributing the observed association to obesity. Furthermore, we focused more on the association of BMI per 1 kg/m2 increase to delineate the continuous association with mortality. Interestingly, although our conclusions are similar with respect to the association of obesity with mortality, BMI cutoff differences aside, our GAM plots differ in that the ELSO registry study reported mortality to be highest among patients with BMIs of 30–35 kg/m2, whereas we found a narrower confidence interval for that BMI range but not conclusively the highest mortality.
The notion that obesity is protective in surviving critical illness, otherwise known as the obesity paradox, has been previously described (6, 12, 38), with a number of suspected explanations for this phenomenon. The respiratory physiology of patients with obesity, of course, differs on average from that of other patients. In particular, increased chest wall elastance in patients with obesity has been proposed to offer a protective benefit of partially absorbing transpulmonary pressure, thereby decreasing ventilator-induced lung injury (39). In addition, higher plateau airway pressures generated by similar ventilator settings in patients with obesity may prompt lowering of ventilator volumes and pressures by clinicians, even though such pressures may reflect the combination of increased transpulmonary pressure and the increased chest wall elastance.
Although the aforementioned mechanisms may contribute to better survival in patients with obesity and ARDS, our findings specific to ECMO use in patients with obesity and the most severe forms of ARDS warrant further exploration. The difference in survival may be explained, in part, by the fact that patients with obesity are prone to increased pleural pressure, reduced FRC, and greater degrees of atelectasis relative to patients without obesity (40). In this setting, the severity of ARDS may have been overestimated in our patients with obesity relative to the patients without obesity, selecting for a population to be cannulated that, even with comparable PaO2:FiO2 ratios, may have had less severe pathologic forms of ARDS and a more readily reversible pathology, in particular, greater degrees of atelectasis, thus leading to collider bias. APACHE II scores were also notably lower in our obese group, suggesting the selection of a cohort with lower disease severity among the patients with obesity at the time of ECMO cannulation, thus raising the possibility of indication bias. In addition, despite a wide age distribution (see Figure E8), patients in our cohort were, on average, younger than a “typical” patient with ARDS, as previously reported in a large international cohort (with a mean age of 44 yr compared with a mean age of 61.5 yr), where the average BMI was notably 27.5 kg/m2, thus describing a typical patient with ARDS as overweight (41). This age difference, likely explained by strict patient selection before ECMO cannulation, could be contributing to the observed beneficial outcome and may reflect bias by indication as well. In addition, a median PEEP of 15 cm H2O in our group with obesity, even though significantly higher than in the group without obesity, suggests the potential underuse of PEEP in these patients, with the possibility that some proportion of patients with obesity may not have met indications for ECMO if managed differently. In that context, guiding optimal PEEP settings with bedside tools such as esophageal pressure (42) or electrical impedance tomography before ECMO consideration is desirable (43).
Our study has a number of strengths. First, we report on the largest cohort of patients with obesity receiving ECMO for ARDS, outside of the aforementioned ELSO registry study. Second, by virtue of being multicenter and international, our study is the first of its kind to examine mortality in patients with obesity receiving ECMO exclusively for ARDS across a wide range of settings, making our findings more generalizable. Last, we identified similar associations in propensity score matching analysis.
Our study has limitations. First, we anticipate that the study could be subject to selection bias, as ECMO was offered only to those who were deemed likely to benefit, whereby the selected patients with obesity could be relatively healthier compared with patients without obesity, although such a difference was not seen with the use of traditional scoring systems, with the exception of APACHE II.
Second, we lack data on patients who were deemed not candidates for ECMO support, for both patients with and without obesity. Third, although we report mechanical ventilation parameters, the use of NMBAs, and prone positioning, we do not have the means to assess differences in sedation and mobilization practices across the participating centers. Although practices within a given center should be similar for patients with and without obesity, nonetheless, the degree of obesity might affect the pharmacokinetics of sedatives or the ability to engage in physical rehabilitation, for instance. Notably, the rate of prone positioning before cannulation was lower in our combined cohort, compared with increased use seen in more recent years; increased use of prone positioning may select out a subset of patients with obesity who respond to this maneuver, thus obviating the need for ECMO (44). We used the direct acyclic graph to avoid collider bias but recognize that not all the variables that should ideally be included in the direct acyclic graph were available for analysis.
Fourth, our data do not include esophageal pressure measurements, which could be helpful in assessing the degree to which hypoxemia could be attributed to atelectasis in patients with obesity. Fifth, we lack information about our patients’ net fluid balance, which may be a contributor to hypoxemia in our population, and unfortunately, we lack surrogate measurements such as right and left ventricular function or diastolic dysfunction to approximate fluid status.
Last, we lack data on a comparator group of patients with obesity who received maximal mechanical ventilation in lieu of ECMO to ascertain the contribution of ECMO to the observed association, although such data would always be biased by the patients either not being selected for ECMO or being at a center that does not offer ECMO. In addition, as with all observational studies, our study has limitations intrinsic to the study design. Although we checked our data for inconsistencies and queried all investigators when outliers were detected, measurement error may remain unidentified in this large database. Similarly, unmeasured confounding may exist, as we lack data on, and thus have not been able to adjust for, all potential confounders.
Conclusions
Among patients receiving ECMO for ARDS, patients with obesity had lower ICU mortality than those without obesity, even after propensity score matching. Although unmeasured confounders likely remain, our findings support the notion that obesity should not be considered a general contraindication to ECMO.
Acknowledgments
Acknowledgment
The authors thank all the ICU doctors, nurses, and staff members for their tireless dedication to their work and for supporting this research.
LIFEGARDS collaborators: Pitié–Salpêtriàre Hospital, Medical Intensive Care Unit, Paris, France: Charles Edouard Luyt, Guillaume Lebreton, Pascal Leprince, Nicolas Brechot, Guillaume Franchineau, Ania Nieszkowska, and Guillaume Hekimian. IRCCS-ISMETT Istituto Mediterraneo per i Trapianti e terapie ad alta specializzazione - Department of Anesthesia and Intensive Care: Gennaro Martucci. Columbia University Medical Center/NewYork-Presbyterian Hospital: Matthew Bacchetta, Darryl Abrams, Madhavi Parekh, and Anil Trindade. Hiroshima University, Japan: Yoshiko Kida, Michihito Kyo, Tatsutoshi Shimatani, Nobuaki Shime, and Koichi Tanigawa. Royal Papworth Hospital NHS Foundation Trust, United Kingdom: Kamen Valchanov, Jo-anne Fowles, and Louise Meagher. Assistance Publique Hôpitaux de Marseille, Marseille, France: Laurent Papazian, and Sami Hraiech et Jean Marie Forel. Auckland City Hospital, New Zealand: Rachael Parke, Lianne McCarthy, Eileen Gilder, and Alastair McGeorge. University Hospital Saint-Luc, Belgium: Sophie Pierard, Luc-Marie Jacquet, and Olivier Van Caenegem. St. Vincent's Hospital, Sydney, Australia: Priya Nair and Claire Reynolds. Royal Prince Alfred Hospital, Sydney, Australia: Paul Forrest. Queen Mary Hospital, Hong Kong: Chan Wai Ming. Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Hong Kong: Harriet Kong. Academic Medical Center, University of Amsterdam: Matt Harmon. AP-HP, Bichat Hospital, Medical and Infectious Diseases Intensive Care Unit, Paris, France: Lilia Bouadma, Jean Francois Timsit, and Romain Sonneville. Cardiothoracic Surgery and Intensive Care Departments, Amiens University Hospital, France: Hervé Dupont, Joseph Nader, Marion Wasilewski, David Gilles, Faouzi Trojette, Mona Moubarak, and Thierry Caus. Bordeaux, France: Alexandre Ouattara. St. Vincent's Hospital, Melbourne: Espedito Faraone and Roger Smith.
Footnotes
Supported by the NIH and NHLBI grant K23 150280 (M.A.), NIH grant UL1TR001873 (M.R.B.), and NIH grants K23-HL133489 and R21-HL145506 (J.B.).
Author Contributions: D.R., T.P., C.R.R., G.G., M.S., and D.B. were involved in the study conception. D.R., T.P., C.R.R., G.G., M.A., M.R.B., J.B., M.S., and D.B. were involved in the trial design. D.R., T.P., and D.B. wrote the first draft of the manuscript. All authors contributed to the investigation and data collection and critically revised the manuscript for intellectual content. D.R., T.P., M.A., and M.R.B. provided statistical expertise. D.R., T.P., C.R.R., G.G., and M.S. were responsible for data integrity. D.R., T.P., C.R.R., G.G., M.S., and D.B. finalized the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202212-2293OC on July 19, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
LIFEGARDS collaborators:
Charles Edouard Luyt, Guillaume Lebreton, Pascal Leprince, Nicolas Brechot, Guillaume Franchineau, Ania Nieszkowska, Guillaume Hekimian, Matthew Bacchetta, Darryl Abrams, Madhavi Parekh, Anil Trindade, Yoshiko Kida, Michihito Kyo, Tatsutoshi Shimatani, Nobuaki Shime, Koichi Tanigawa, Kamen Valchanov, Jo-anne Fowles, Louise Meagher, Laurent Papazian, Sami Hraiech, Jean Marie Forel, Rachael Parke, Lianne McCarthy, Eileen Gilder, Alastair McGeorge, Sophie Pierard, Luc-Marie Jacquet, Olivier Van Caenegem, Priya Nair, Claire Reynolds, Paul Forrest, Chan Wai Ming, Harriet Kong, Matt Harmon, Lilia Bouadma, Jean Francois Timsit, Romain Sonneville, Hervé Dupont, Joseph Nader, Marion Wasilewski, David Gilles, Faouzi Trojette, Mona Moubarak, Thierry Caus, Espedito Faraone, and Roger Smith
References
- 1. Inci I, Klinzing S, Schneiter D, Schuepbach RA, Kestenholz P, Hillinger S, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation: an institutional experience and literature review. Transplantation . 2015;99:1667–1671. doi: 10.1097/TP.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 2. Hoetzenecker K, Donahoe L, Yeung JC, Azad S, Fan E, Ferguson ND, et al. Extracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J Thorac Cardiovasc Surg . 2018;155:1316–1328.e1. doi: 10.1016/j.jtcvs.2017.09.161. [DOI] [PubMed] [Google Scholar]
- 3. De Jong A, Molinari N, Pouzeratte Y, Verzilli D, Chanques G, Jung B, et al. Difficult intubation in obese patients: incidence, risk factors, and complications in the operating theatre and in intensive care units. Br J Anaesth . 2015;114:297–306. doi: 10.1093/bja/aeu373. [DOI] [PubMed] [Google Scholar]
- 4. Dennis DM, Trevenen M. Prevalence of obesity in an intensive care unit patient population. Intensive Crit Care Nurs . 2016;35:52–56. doi: 10.1016/j.iccn.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 5. Wardell S, Wall A, Bryce R, Gjevre JA, Laframboise K, Reid JK. The association between obesity and outcomes in critically ill patients. Can Respir J . 2015;22:23–30. doi: 10.1155/2015/938930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med . 2014;12:61. doi: 10.1186/1741-7015-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kooistra EJ, Brinkman S, van der Voort PHJ, de Keizer NF, Dongelmans DA, Kox M, et al. Body mass index and mortality in coronavirus disease 2019 and other diseases: a cohort study in 35,506 ICU patients. Crit Care Med . 2022;50:e1–e10. doi: 10.1097/CCM.0000000000005216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javidfar J, Zaaqoq AM, Labib A, Barnett AG, Hayanga JA, Eschun G, et al. COVID-19 Critical Care Consortium (COVID Critical) Morbid obesity’s impact on COVID-19 patients requiring venovenous extracorporeal membrane oxygenation: the COVID-19 critical care consortium database review. Perfusion . 2023 doi: 10.1177/02676591231156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. den Uil CA, Termorshuizen F, Rietdijk WJR, Sablerolles RSG, van der Kuy HPM, Haas LEM, et al. Dutch COVID-19 Research Consortium Age moderates the effect of obesity on mortality risk in critically ill patients with COVID-19: a nationwide observational cohort study. Crit Care Med . 2023;51:484–491. doi: 10.1097/CCM.0000000000005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax . 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anzueto A, Frutos-Vivar F, Esteban A, Bensalami N, Marks D, Raymondos K, et al. Ventila Group Influence of body mass index on outcome of the mechanically ventilated patients. Thorax . 2011;66:66–73. doi: 10.1136/thx.2010.145086. [DOI] [PubMed] [Google Scholar]
- 12. Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity paradox” in acute respiratory distress syndrome: a systematic review and meta-analysis. PLoS One . 2016;11:e0163677. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni YN, Luo J, Yu H, Wang YW, Hu YH, Liu D, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care . 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA . 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 15. de Chambrun P, Brodie D, Combes A. Appraising the real-life need for extracorporeal membrane oxygenation during the COVID-19 pandemic. Am J Respir Crit Care Med . 2021;204:2–4. doi: 10.1164/rccm.202104-0897ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med . 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 17. Abrams D, Ferguson ND, Brochard L, Fan E, Mercat A, Combes A, et al. ECMO for ARDS: from salvage to standard of care? Lancet Respir Med . 2019;7:108–110. doi: 10.1016/S2213-2600(18)30506-X. [DOI] [PubMed] [Google Scholar]
- 18. Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA . 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 19. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR Trial Collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet . 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 20. Ganslmeier P, Philipp A, Rupprecht L, Diez C, Arlt M, Mueller T, et al. Percutaneous cannulation for extracorporeal life support. Thorac Cardiovasc Surg . 2011;59:103–107. doi: 10.1055/s-0030-1250635. [DOI] [PubMed] [Google Scholar]
- 21. Keyser A, Philipp A, Zeman F, Lubnow M, Lunz D, Zimmermann M, et al. Percutaneous cannulation for extracorporeal life support in severely and morbidly obese patients. J Intensive Care Med . 2020;35:919–926. doi: 10.1177/0885066618801547. [DOI] [PubMed] [Google Scholar]
- 22. Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med . 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 23. Kon ZN, Dahi S, Evans CF, Byrnes KA, Bittle GJ, Wehman B, et al. Class III obesity is not a contraindication to venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg . 2015;100:1855–1860. doi: 10.1016/j.athoracsur.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 24. Galvagno SM, Jr, Pelekhaty S, Cornachione CR, Deatrick KB, Mazzeffi MA, Scalea TM, et al. Does weight matter? Outcomes in adult patients on venovenous extracorporeal membrane oxygenation when stratified by obesity class. Anesth Analg . 2020;131:754–761. doi: 10.1213/ANE.0000000000004454. [DOI] [PubMed] [Google Scholar]
- 25. Salna M, Fried J, Kaku Y, Brodie D, Sayer G, Uriel N, et al. Obesity is not a contraindication to veno-arterial extracorporeal life support. Eur J Cardiothorac Surg . 2021;60:831–838. doi: 10.1093/ejcts/ezab165. [DOI] [PubMed] [Google Scholar]
- 26. Daviet F, Guilloux P, Hraiech S, Tonon D, Velly L, Bourenne J, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care . 2021;11:157. doi: 10.1186/s13613-021-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peetermans M, Guler I, Meersseman P, Wilmer A, Wauters J, Meyns B, et al. Impact of BMI on outcomes in respiratory ECMO: an ELSO registry study. Intensive Care Med . 2023;49:37–49. doi: 10.1007/s00134-022-06926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudym D, Pham T, Dzierba A, Serra A, Agerstrand C, Abrams D, et al. Morbidity and mortality in the extremely obese patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med . 2018;197:A2568. [Google Scholar]
- 29. Petit M, Fetita C, Gaudemer A, Treluyer L, Lebreton G, Franchineau G, et al. Prone-positioning for severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation. Crit Care Med . 2022;50:264–274. doi: 10.1097/CCM.0000000000005145. [DOI] [PubMed] [Google Scholar]
- 30. Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: an international multicenter prospective cohort. Am J Respir Crit Care Med . 2019;200:1002–1012. doi: 10.1164/rccm.201806-1094OC. [DOI] [PubMed] [Google Scholar]
- 31. Lim CC, Mahmood T. Obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol . 2015;29:309–319. doi: 10.1016/j.bpobgyn.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 2021. https://cdn.who.int/media/docs/default-source/obesity/who-discussion-paper-on-obesity---final190821.pdf
- 33. Beitler JR, Ghafouri TB, Jinadasa SP, Mueller A, Hsu L, Anderson RJ, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med . 2017;195:1198–1206. doi: 10.1164/rccm.201609-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet . 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 35. Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc . 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 36. Sun YQ, Burgess S, Staley JR, Wood AM, Bell S, Kaptoge SK, et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear mendelian randomisation analyses. BMJ . 2019;364:l1042. doi: 10.1136/bmj.l1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balik M, Svobodova E, Porizka M, Maly M, Brestovansky P, Volny L, et al. The impact of obesity on the outcome of severe SARS-CoV-2 ARDS in a high volume ECMO centre: ECMO and corticosteroids support the obesity paradox. J Crit Care . 2022;72:154162. doi: 10.1016/j.jcrc.2022.154162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King P, Mortensen EM, Bollinger M, Restrepo MI, Copeland LA, Pugh MJ, et al. Impact of obesity on outcomes for patients hospitalised with pneumonia. Eur Respir J . 2013;41:929–934. doi: 10.1183/09031936.00185211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ball L, Serpa Neto A, Pelosi P. Obesity and survival in critically ill patients with acute respiratory distress syndrome: a paradox within the paradox. Crit Care . 2017;21:114. doi: 10.1186/s13054-017-1682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest . 1996;109:144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 41. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA . 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 42. Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med . 2012;38:395–403. doi: 10.1007/s00134-012-2490-7. [DOI] [PubMed] [Google Scholar]
- 43. Franchineau G, Bréchot N, Lebreton G, Hekimian G, Nieszkowska A, Trouillet JL, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med . 2017;196:447–457. doi: 10.1164/rccm.201605-1055OC. [DOI] [PubMed] [Google Scholar]
- 44. Audhya X, Bosch NA, Stevens JP, Walkey AJ, Law AC. Changes to hospital availability of prone positioning after the COVID-19 pandemic. Ann Am Thorac Soc . 2022;19:1610–1613. doi: 10.1513/AnnalsATS.202201-070RL. [DOI] [PMC free article] [PubMed] [Google Scholar]