Abstract
Thirty-one strains of Acinetobacter species, including type strains of the 18 genomic species and 13 clinical isolates, were compared by amplified ribosomal DNA restriction analysis (ARDRA), random amplified polymorphic DNA analysis (RAPD), and amplified fragment length polymorphism (AFLP) fingerprinting. ARDRA, performed with five different enzymes, showed low discriminatory power for differentiating Acinetobacter at the species and strain level. The standardized commercially available RAPD kit clearly enabled the discrimination of all Acinetobacter genomic species but showed great polymorphism between isolates of Acinetobacter baumannii. AFLP fingerprinting with radioactively as well as fluorescently labelled primers showed high discriminatory power for the identification of 18 Acinetobacter genomic species and typing of 13 clinical Acinetobacter isolates. Compared to radioactive AFLP, fluorescent AFLP was technically fast and simple to perform, and it permitted analysis with an automated DNA sequencer. Fluorescent AFLP seems particularly well suited for studying the epidemiology of nosocomial infections and outbreaks caused by Acinetobacter species.
Over the past 10 years, numerous outbreaks of nosocomial infections with Acinetobacter spp. have been reported, identifying Acinetobacter baumannii as the most predominant species involved. In hospitalized patients, Acinetobacter spp. frequently colonize the skin and upper respiratory tract and may cause various types of opportunistic infections (3). Risk factors for acquisition of these organisms include prolonged hospital stay, serious underlying disease, intravascular and intravesical catheterization, and treatment with broad-spectrum antibiotics (21, 24, 27, 33). Characteristics of Acinetobacter spp. may contribute to their epidemic behavior, such as the ability to acquire multiple antibiotic resistance (2) and the ability to survive on inanimate and dry surfaces for prolonged periods of time (13, 20, 37). In order to understand the epidemiology of Acinetobacter spp. in hospitalized patients and in the hospital environment, accurate identification of members of the genus at the species level is important. Delineation of species within the genus Acinetobacter is still the subject of extensive research. DNA-DNA hybridization studies have resulted in the identification of at least 18 DNA groups (genomic species) (5, 6, 12, 30). For strain typing, a number of genomic fingerprinting methods have been proposed. These include pulsed-field gel electrophoresis (14, 25), ribotyping (9, 12, 25), and PCR-based fingerprinting techniques such as random amplified polymorphic DNA analysis (RAPD) (15), repetitive extragenic palindromic sequence-based PCR (26), amplified ribosomal DNA restriction analysis (ARDRA) (35), and RNA spacer fingerprinting (11). A novel high-resolution genomic fingerprinting method, the amplified fragment length polymorphism (AFLP), has been shown to be applicable to a wide range of bacterial species including those of the genus Acinetobacter (10, 18, 19, 36).
In the present study, two generally used DNA typing techniques were compared to the AFLP technique, and their applicability was studied for the identification of Acinetobacter at the genomic species level and of A. baumannii at the strain level.
MATERIALS AND METHODS
Bacterial strains.
Eighteen different genomic species of Acinetobacter, as delineated by DNA-DNA hybridization, were studied (Table 1) (5, 6, 22, 30). Thirteen clinical Acinetobacter isolates were collected from samples of 13 different patients. All these isolates were collected within a period of 14 years in five different hospitals in The Netherlands. Five of them (A-1 to A-5) belonged to a well-characterized outbreak on a surgical ward (21). The other eight strains are epidemiologically unrelated; four of them (R-1, D-1, U-1, and E-1) are single isolates from larger outbreaks (7, 8). All outbreak isolates have been characterized previously by antibiograms, cell envelope protein electrophoretic typing, and ribotyping (10, 21). Presumptive identification of the 13 isolates was obtained by the analytical profile index procedure (API 20NE system; bioMérieux, Marcy l’Etoile, France). All strains were grown aerobically in Luria-Bertani medium (Difco Laboratories, Detroit, Mich.) and incubated in a rotary shaker at 37°C for 18 h. Bacterial strains were stored at −80°C in nutrient broth supplemented with 20% (wt/vol) glycerol.
TABLE 1.
Reference strains and clinical isolates used in the present study
| Strain | Genomic speciesa | Species name | Strain codec | Outbreakd | Specimen | Reference |
|---|---|---|---|---|---|---|
| 1 | 1 | A. calcoaceticus | ATCC 23055 | No | 6 | |
| 2 | 2 | A. baumannii | ATCC 19606 | No | 6 | |
| 3 | 3 | UNb | ATCC 19004 | No | 6 | |
| 4 | 4 | A. haemolyticus | ATCC 17906 | No | 6 | |
| 5 | 5 | A. junii | ATCC 17908 | No | 6 | |
| 6 | 6 | UN | ATCC 17979 | No | 6 | |
| 7 | 7 | A. johnsonii | ATCC 17909 | No | 6 | |
| 8 | 8 | A. lwoffii | NCTC 5866 | No | 6 | |
| 9 | 10 | UN | ATCC 17924 | No | 6 | |
| 10 | 11 | UN | ATCC 11171 | No | 6 | |
| 11 | 12 | A. radioresistens | IAM 13186 | No | 6 | |
| 12 | 13 | UN | ATCC 17903 | No | 29 | |
| 13 | 14 | UN | ATCC 17905 | No | 29 | |
| 14 | 15 | UN | MGH 98795 | No | 29 | |
| 15 | BJ14 | UN | CCUG 14816 | No | 9 | |
| 16 | BJ15 | UN | Adam Ac 606 | No | 9 | |
| 17 | BJ16 | UN | ATCC 17988 | No | 9 | |
| 18 | BJ17 | UN | SEIP Ac 87.314 | No | 9 | |
| 19 | 2 | A. baumannii | HK 20 | A-1 | Skin | 21 |
| 20 | 2 | A. baumannii | HK 70 | A-2 | Sputum | 21 |
| 21 | 2 | A. baumannii | HK 71 | A-3 | Wound | 21 |
| 22 | 2 | A. baumannii | HK 72 | A-4 | Wound | 21 |
| 23 | 2 | A. baumannii | HK 73 | A-5 | Urine | 21 |
| 24 | 3 | UN | HK 74 | No | Blood | |
| 25 | 2 | A. baumannii | HK 75 | No | Rectum | |
| 26 | 2 | A. baumannii | HK 76 | No | Sputum | |
| 27 | 2 | A. baumannii | HK 77 | No | Rectum | |
| 28 | 2 | A. baumannii | HK 21 | R-1 | Urine | 11 |
| 29 | 2 | A. baumannii | HK 22 | D-1 | Urine | 11 |
| 30 | 2 | A. baumannii | HK 23 | U-1 | Sputum | 11 |
| 31 | 2 | A. baumannii | HK 24 | E-1 | Bronchus | 11 |
Numbered according to the work of Bouvet and Grimont (5) for groups 1 to 12, according to the work of Tjernberg and Ursing (29) for groups 13 to 15, and according to the work of Bouvet and Jeanjean (6) for groups BJ14 to BJ17. Group 14 is identical to BJ13.
UN, unnamed genomic species.
CCUG, Culture Collection, University of Göteborg, Göteborg, Sweden; IAM, Institute of Applied Microbiology, The University of Tokyo, Tokyo, Japan; MGH, Collection of Malmö General Hospital, Malmö, Sweden; SEIP, Service des Entérobactéries de l’Institut Pasteur, Paris, France.
No, epidemiologically unrelated isolate; 1 to 5, outbreak number in Dutch cities: Amsterdam (A), Rotterdam (R), Dordrecht (D), Utrecht (U), or Enschede (E).
DNA isolation.
DNA was isolated as described previously (4). Extracted DNA was resolved in 100 μl of TE buffer (10 mM Tris, 1 mM EDTA [pH 8.0]) supplemented with 10 μg of RNase (Sigma, St. Louis, Mo.). Purified DNA was aliquoted and stored at −20°C. DNA concentrations were estimated by agarose gel electrophoresis against diluted samples of λ DNA (New England Biolabs, Inc., Beverly, Mass.).
ARDRA.
The ARDRA was performed as described previously (34). Briefly, amplification reactions were performed in a final volume of 50 μl containing 1.25 U of Taq polymerase, 100 μM (each) deoxynucleoside triphosphates (dNTPs), and 0.2 μM (each) primer in reaction buffer (1.5 mM MgCl2, 50 mM KCl in 10 mM Tris-HCl [pH 8.3]). Amplification was performed in a GeneAmp PCR System 9600 thermal cycler (Perkin-Elmer). After initial denaturation at 95°C for 5 min, the reaction mixture was run through 35 cycles of denaturation at 95°C for 45 s, annealing at 50°C for 45 s, and extension at 72°C for 1 min followed by a 7-min extension period at 72°C. The primers used were 5′TGGCTCAGATTGAACGCTGGCGGC (5′ end of 16S rRNA) and 5′TACCTTGTTACGACTTCACCCCA (3′ end of 16S rRNA) (23). Amplified products of approximately 1,500 bp each were visualized by agarose gel electrophoresis after staining with ethidium bromide (50 μg/ml). Amplified DNA (3 to 10 μl) was digested for 1 h at 37°C in 20-μl volumes of commercially supplied incubation buffer containing 5 U of restriction enzyme AluI (AGCT), CfoI (GCGC), MboI (GATC), MspI (CCGG), or RsaI (GTAC). Restriction was stopped by addition of 5 μl of 5× sample buffer (25% [wt/vol] glycerol, 0.5% [wt/vol] sodium dodecyl sulfate, 50 mM EDTA, 0.05% bromophenol blue). Restriction fragment patterns were separated by agarose gel electrophoresis at 150 V in 2× Tris-borate-EDTA (TBE) buffer and visualized after being stained with ethidium bromide (50 ng/ml). Gels were photographed under UV illumination.
RAPD.
The RAPD assay was performed as described previously (38) with the commercially available Ready-To-Go RAPD analysis kit (Pharmacia Biotech, Uppsala, Sweden). This product contains a RAPD Analysis Primer Set and Ready-To-Go RAPD Analysis Beads with thermostable polymerases (AmpliTAQ and Stoffel fragment), lyophilized buffer (10 mM Tris, 30 mM KCl, 3 mM MgCl2 [pH 8.3] in a 25-μl reaction volume), dNTPs (0.4 mM [each] in a 25-μl reaction volume), and bovine serum albumin (2.5 μg). Briefly, DNA was amplified by addition of 25 pmol of primer, H2O to a final volume of 25 μl, and one RAPD analysis bead to 10 ng of template DNA. The mixtures were subjected to 45 cycles of amplification (95°C for 60 s, 36°C for 60 s, and 72°C for 120 s for each cycle) with an initial incubation step at 95°C for 5 min, in a GeneAmp PCR System 9600 thermocycler (Perkin-Elmer). The six primers (RAPD Analysis Primer Set) used were AP1 (5′GGTGCGGGAA3′), AP2 (5′GTTTCGCTCC3′), AP3 (5′GTAGACCCGT3′), AP4 (5′AAGAGCCCGT3′), AP5 (5′AACGCGCAAC3′), and AP6 (5′CCCGTCAGCA3′) (1). Amplified fragments were separated by agarose gel electrophoresis at 150 V in 0.5× TBE buffer and were visualized after staining with ethidium bromide (10 μg/ml). Gels were photographed under UV illumination. The negative control contained all components except template DNA. Escherichia coli C1a DNA (Pharmacia Biotech) was used as a positive control.
AFLP.
All procedures relating to the preparation of AFLP templates were performed essentially as described by Janssen et al. (18) and Vos et al. (36). Briefly, purified chromosomal DNA (50 ng) was digested with 1 U of EcoRI (Pharmacia LKB Biotechnology, Uppsala, Sweden) and 1 U of MseI (New England Biolabs, Inc.). The EcoRI adapter was prepared by mixing equimolar amounts of the oligonucleotide sequences 5′CTCGTAGACTGCGTACC3′ and 5′AATTGGTACGCAGTC3′, which were heated until 65°C and slowly cooled to room temperature. Preparation of the MseI adapter was performed in the same manner by using the sequences 5′GACGATGAGTCCTGAG3′ and 5′TACTCAGGACTCATC3′. Ligation of adapters to the restriction fragments was performed overnight at 20°C in a final volume of 30 μl. The ligation mixture consisted of 50 ng of chromosomal DNA, 50 pmol (each) EcoRI and MseI adapter, 1.2 U of T4 DNA ligase (Pharmacia LKB Biotechnology), 1 mM ATP, and ligase buffer (10 mM Tris-acetate [pH 7.5], 10 mM magnesium acetate, 50 mM potassium acetate, 5 mM dithiothreitol, 50 ng of bovine serum albumin per μl). After ligation, the DNA was diluted with distilled water to a final volume of 500 μl and stored at −20°C until use.
AFLP reactions with radioactively labelled primers.
AFLP reactions with radioactively labelled primers were performed as described previously (21).
AFLP reactions with fluorescently labelled primers.
A Texas Red fluorescently labelled EcoA primer (Isogen Bioscience BV, Maarssen, The Netherlands) was used for DNA amplification in 10 μl of a reaction mixture containing 0.5 ng of template DNA, 20 ng of labelled Eco primer, 1 μl of 2 mM dNTPs, 60 ng of unlabelled MseC primer, 1 U of Taq polymerase (Perkin-Elmer) in 10 mM Tris-HCl (pH 8.3), 50 mM KCl, and 1.5 mM MgCl2. Amplification was performed in a GeneAmp PCR System 9600 thermal cycler (Perkin-Elmer) for 35 cycles of denaturation (30 s at 94°C), annealing (30 s at 65 to 56°C), and DNA molecule extension (60 s at 72°C). In the first 12 cycles, the annealing temperature was lowered by 0.7°C per cycle. After completion of the cycle program, 3 μl of loading buffer (Amersham Life Science, Cleveland, Ohio) was added to the reaction mixtures. Prior to gel loading, the amplicons were denatured by heating for 1 min at 95°C and rapid cooling on ice. Fluorescent amplified fragments were separated on a denaturing polyacrylamide gel (RapidGel-XL-6%; Amersham Life Science) in 1× TBE buffer (100 mM Tris, 100 mM boric acid, 2 mM EDTA [pH 8.0], 6 M urea) according to the manufacturer’s instructions in a Vistra 725 automated DNA sequencer (Amersham Life Science). A 2-μl sample of each reaction mixture was loaded on the gel. Gels were run at 1,500 V for 6 h.
Densitometric scanning and data processing.
ARDRA and RAPD photographs as well as AFLP autoradiograms were scanned with a densitoscanner (Scanjet 4C; Hewlett-Packard, Bracknell, United Kingdom), and images were stored as tagged image file format files with Deskscan II version 2.3 (Hewlett-Packard). Fluorescently labelled AFLP fingerprints were analyzed on the Vistra 725 DNA sequencer and stored as tagged image file format files with the Vistra 2 Tiff software (Amersham). Both types of images were processed with GelCompar 3.1 software (Applied Maths, Kortrijk, Belgium). Following conversion, normalization, and background subtraction with mathematical algorithms, levels of similarity between fingerprints were calculated with the Pearson product moment correlation coefficient (r). Cluster analysis was performed with the unweighted pair group method using average linkages (UPGMA).
RESULTS
ARDRA genomic typing.
The 16S rRNA gene of each of the 31 Acinetobacter strains was amplified and restricted with the enzymes AluI, CfoI, MboI, MspI, and RsaI. This resulted in five separate restriction patterns for each strain. The five patterns were digitized and subsequently combined in one overall pattern in one single analysis. Each enzyme generated up to 10 fragments per strain. The cumulative DNA patterns yielded a maximum of approximately 50 bands per strain which were subjected to cluster analysis. ARDRA could not distinguish the 18 different Acinetobacter genomic species because all the strains were clustered within a broad range of linkage levels between 37 and 88%. Two strains were linked below the 40% level, whereas several other strains showed almost identical restriction patterns resulting in clustering at high correlation levels (Fig. 1).
FIG. 1.
Digitized ARDRA patterns and dendrogram of 18 Acinetobacter genomic species (1 to 18) and 13 clinical Acinetobacter isolates (19* to 31*) obtained after restriction of the amplified 16S rRNA gene with five different enzymes (AluI, CfoI, MboI, MspI, and RsaI). For each strain, all restriction patterns have been combined into one single lane. The dendrogram was constructed with Gelcompar cluster analysis by UPGMA. Percentages of similarity and molecular weights are shown above the dendrogram. The strain code as presented in Table 1 is shown on the right.
ARDRA strain typing.
Analysis of the 13 clinical isolates showed that all the strains except one clustered with the A. baumannii type strain (ATCC 19606) at a linkage level of 78%. Strain 24 (HK 74) was linked to DNA group 3 (ATCC 19004) at a level of 87%. The five Amsterdam outbreak isolates (A-1 to A-5) and three epidemiologically unrelated strains, 25, 26, and 28, showed a similarity of 82%.
Analysis of combined clustering results of genomic strains and clinical isolates showed a clear overlap in linkage levels, indicating low discriminatory power for the method. Omission of any one of the restriction enzyme patterns from the overall ARDRA pattern further decreased the discriminatory power (data not shown).
RAPD genomic typing.
The 31 Acinetobacter strains were subjected to six PCRs with different primers individually present in the RAPD kit. With each primer, different PCR fingerprints of up to seven amplified fragments were generated. For each strain, all the bands obtained in the six RAPD assays were digitized and combined for cluster analysis (Fig. 2). Overall, RAPD was able to discriminate the 18 different Acinetobacter genomic isolates with similarity levels of 48% or lower.
FIG. 2.
Digitized RAPD patterns and dendrogram of 18 Acinetobacter genomic species (1 to 18) and 13 clinical Acinetobacter isolates (19* to 31*) obtained after separate PCRs with six different primers. For each strain, all six RAPD patterns have been combined into one single lane (1 to 6). The dendrogram was constructed with Gelcompar cluster analysis by UPGMA. Percentages of similarity and molecular weights are shown above the dendrogram. The strain code as presented in Table 1 is shown on the right.
RAPD strain typing.
Analysis of RAPD profiles of the 13 clinical isolates showed that all of them were allocated to the type strain of A. baumannii (ATCC 19606) at a correlation level of only 26%. This level increased in analysis of a separate gel with RAPD patterns of clinical isolates and the type strain of A. baumannii (data not shown). Five strains from the outbreak in Amsterdam and two unrelated isolates, 26 and 28, were linked at an average similarity of 75%. This group of seven strains was clearly separated from the other clinical Acinetobacter isolates.
The RAPD strain typing of the 13 clinical isolates has been performed by using all six primers which were enclosed in the RAPD kit. We investigated the minimal number of primers needed to obtain the same results as with the full set of six primers. These results showed that at least five different primers are needed for optimal RAPD fingerprinting.
AFLP genomic typing.
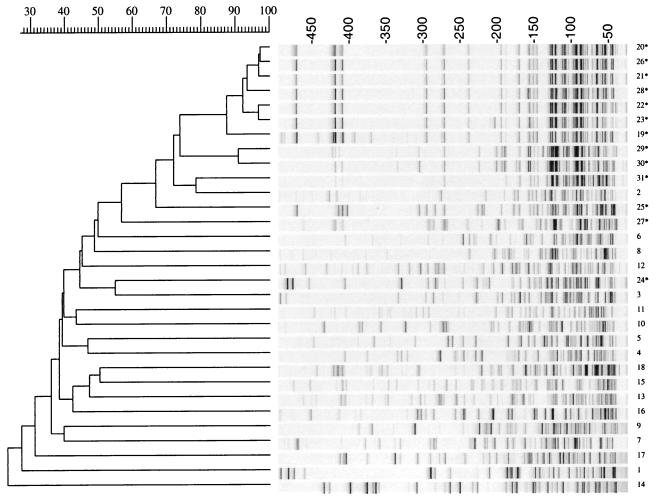
AFLP fingerprinting with radioactively labelled primers was compared to AFLP with fluorescently labelled primers. Cluster analysis of both methods gave comparable results (Fig. 3 and 4). The 18 genomic species were clearly distinguishable at correlation levels of 41 and 50% for the radioactive and fluorescent AFLP patterns, respectively.
FIG. 3.
Digitized radioactively labelled AFLP patterns and dendrogram of 18 Acinetobacter genomic species (1 to 18) and 13 clinical Acinetobacter isolates (19* to 31*) obtained after PCR on EcoA and MseC templates. The dendrogram was constructed with Gelcompar cluster analysis by UPGMA. Percentages of similarity and molecular weights are shown above the dendrogram. The strain code as presented in Table 1 is shown on the right.
FIG. 4.
Fluorescently labelled AFLP patterns and dendrogram of 18 Acinetobacter genomic species (1 to 18) and 13 clinical Acinetobacter isolates (19* to 31*) obtained after PCR on EcoA and MseC templates. The dendrogram was constructed with Gelcompar cluster analysis by UPGMA. Percentages of similarity and molecular weights are shown above the dendrogram. The strain code as presented in Table 1 is shown on the right.
AFLP strain typing.
Among the 13 clinical isolates, both AFLP methods clustered 12 strains with A. baumannii at correlation levels of 55 and 57%; strain 24 (HK 74) was clustered with Acinetobacter DNA group 3 (ATCC 19004). All five outbreak-related A. baumannii strains from Amsterdam and two epidemiologically unrelated strains (26 and 28) were clustered within one group at a cutoff value of 85% for the radioactive AFLP versus 87% for the fluorescent AFLP.
DISCUSSION
Discrimination of Acinetobacter at the species and strain level has been performed by a variety of phenotypic and genomic techniques. During the last decade, a number of PCR fingerprinting methods such as RAPD (15, 27), repetitive extragenic palindromic sequence-based PCR (26), ARDRA (37), RNA spacer fingerprinting (12), and 16S ribosomal DNA sequence analysis (17) have been found to be useful for typing clinical strains of Acinetobacter species. Recently, AFLP fingerprinting was shown to be a sensitive method for the identification of Acinetobacter genomic species (19). In the present study, we evaluated the discriminatory power of ARDRA, RAPD, and AFLP fingerprinting for the identification of Acinetobacter isolates by using a set of well-characterized strains. In addition, 13 clinical isolates, including five strains from one outbreak (A-1 to A-5), were used to compare the usefulness of the three genomic techniques for strain typing of Acinetobacter species.
ARDRA fingerprinting could not distinguish the 18 genomic species at low cutoff levels (<50%), in contrast to RAPD and AFLP. Only two strains (16 and 17) could be discriminated with linkage levels below 40%. Eight of the genomic species which were clustered in three different groups (4, 6, and 7; 5, 13, and 18; 9 and 10) could not be identified because linkage levels were above 80%. In the study of Vaneechoutte et al. (35), ARDRA was not able to identify six of these eight genomic species, namely, strains 4 and 7, 5 and 18, and 9 and 10, which is slightly better than our findings. This difference is most likely due to the computerized analysis of the ARDRA patterns instead of visual comparison of the patterns as performed by Vaneechoutte et al. Identification of the 13 clinical isolates was similar for ARDRA and AFLP, which identified 12 strains as A. baumannii and 1 as DNA group 3. Biotyping of all these strains, however, identified all 13 strains as A. baumannii. This difference was not surprising, since identification by use of biochemical tests does not differentiate the closely related DNA groups 1, 2, 3, and 13 (the Acinetobacter calcoaceticus-A. baumannii complex) (12).
The RAPD technique is being used increasingly in many laboratories for epidemiologic typing of a wide range of microorganisms including Acinetobacter (15, 27). Although the RAPD technique offers the advantages of simplicity and rapidity, a lack of reproducibility has been reported due to its high susceptibility to variation by primer and DNA concentration, DNA template quality, gel electrophoresis, and the type of DNA polymerase (31, 33). To overcome some of these problems, we used standardized concentrations of purified genomic DNA and a standardized RAPD kit. Besides the good discrimination of the 18 Acinetobacter genomic species with linkage levels below 50%, RAPD identification clustered the 13 clinical isolates with A. baumannii at a low cutoff level (<50%). However, a higher linkage level was obtained when separate gel analysis of RAPD patterns of only the clinical isolates in combination with the type strain of A. baumannii was performed. All strains were typeable by RAPD, and analysis of all possible RAPD pattern combinations showed that at least five primers were necessary to amplify enough fragments to obtain the same clustering of isolates. This indicates that one cannot speed up or perform cheaper RAPD fingerprinting by using a smaller number of primers. It is possible that the number of primers for RAPD typing can be reduced by using other primers (e.g., ERIC 1 and ERIC 2); however, it is obvious that several primers will be necessary to obtain high discriminatory power for RAPD analysis.
AFLP, a novel PCR-mediated DNA fingerprinting method, was first described in 1993 (39). It can be used for characterizations and comparisons of any DNA, irrespective of its origin or complexity (18, 36, 39). AFLP genotyping has been used for a number of different bacterial species including Acinetobacter (16, 18, 19). The AFLP has several advantages compared to various other typing methods, including discriminatory power, flexibility, reproducibility, and production of clear banding patterns suitable for computerized analysis. In this study, the level of AFLP reproducibility was determined by performing duplicate radioactive AFLP fingerprinting of all Acinetobacter genomic strains, which yielded homologous patterns (data not shown). Both AFLP and RAPD fingerprinting could clearly distinguish all the 18 Acinetobacter genomic species with linkage levels below 50%, in contrast to ARDRA. In a previous study, radioactive AFLP also was found to be a good method for the identification of Acinetobacter at the genomic level (19). Use of radioactive compounds, however, is laborious and expensive and needs special laboratory equipment and protection. Therefore, we investigated AFLP fingerprinting with the use of fluorescently labelled primers in an automated sequence apparatus. Despite differences in PCR volume, PCR program, thermocycler, and detection techniques, cluster analysis of radioactively and fluorescently labelled AFLP patterns showed comparable clustering of the different Acinetobacter strains with minimal differences in correlation levels. These results show that fluorescently labelled AFLP fingerprinting, in combination with direct analysis of patterns by use of an automated DNA sequencer, is an excellent alternative to radioactive AFLP.
Comparison of typing results with epidemiologic data for the clinical isolates gave concordant results for all three DNA fingerprinting methods. Outbreak strains were clearly discriminated although clustered with two or three unrelated strains, which suggests a clonal origin. This indicates that all three methods are useful to delineate outbreaks of nosocomial A. baumannii.
In this study, evaluation of different genomic fingerprinting methods was performed by computerized comparison of digitized fingerprinting patterns instead of visual comparison. Data analysis by computer offers the possibility of comparison of large numbers of patterns, formation of databases, and cluster analysis. ARDRA and RAPD photographs as well as AFLP autoradiograms had to be scanned, whereas fluorescently labelled AFLP fingerprints could be directly analyzed. Recently, Tenover et al. have published excellent guidelines concerning visual comparison and interpretation of chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis for bacterial strain typing (28). Supplementary guidelines for interpretation of cluster analysis data to identify and differentiate bacterial strains may be necessary in the near future, as computer-based image acquisition and analysis become more and more widely available in clinical laboratories.
In summary, the results of this study demonstrate that ARDRA has low discriminatory power for differentiating Acinetobacter at the species and strain level. The commercially available RAPD kit enabled the discrimination of Acinetobacter genomic species but showed great polymorphism between isolates of A. baumannii. This technique, however, seems to be useful for a rapid identification of outbreak-related strains in a routine clinical microbiology laboratory. The radioactive and fluorescent AFLP fingerprinting methods showed high discriminatory power for the identification of 18 Acinetobacter genomic species and typing of 13 clinical Acinetobacter isolates. Compared to radioactive AFLP, fluorescent AFLP is technically faster and simpler, and analysis is more accurate since scanning of the fingerprints for computerized analysis is not necessary. Therefore, fluorescent AFLP seems particularly well suited to the study of the epidemiology of nosocomial infections and outbreaks caused by Acinetobacter species.
ACKNOWLEDGMENTS
We thank Myrthe Otsen, Department of Infection and Immunity, University of Utrecht, Utrecht, The Netherlands, for technical advice, and Lenie Dijkshoorn, Department of Medical Microbiology, University Hospital Leiden, Leiden, The Netherlands, for the donation of some of the strains used in this study.
REFERENCES
- 1.Akopyanz N, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amyes S G B, Young H K. Mechanisms of antibiotic resistance in Acinetobacter spp.—genetics of resistance. In: Bergogne-Berezin E, Joly-Guillou M L, editors. Acinetobacter: microbiology, epidemiology, infections, management. Boca Raton, Fla: CRC Press, Inc.; 1996. pp. 185–223. [Google Scholar]
- 3.Bergogne-Bérézin E, Towner K J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-Van Dillen P M E, Van Der Noorda J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvet P J M, Grimont P A D. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov., and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 1986;36:228–240. [Google Scholar]
- 6.Bouvet P J M, Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989;140:291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 7.Dijkshoorn L, Wubbels J L, Beunders A J, Degener J E, Boks A L, Michel M F. Use of protein profiles to identify Acinetobacter calcoaceticus in a respiratory care unit. J Clin Pathol. 1989;42:853–857. doi: 10.1136/jcp.42.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkshoorn L, van Ooyen A, Hop W C J, Theuns M, Michel M F. Comparison of clinical acinetobacter strains using a carbon source growth assay. Epidemiol Infect. 1990;104:443–453. doi: 10.1017/s0950268800047452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkshoorn L, Aucken H M, Gerner-Smidt P, Kaufmann M E, Ursing J, Pitt T L. Correlation of typing methods for Acinetobacter isolates from hospital outbreaks. J Clin Microbiol. 1993;31:702–705. doi: 10.1128/jcm.31.3.702-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkshoorn L, Aucken H M, Gerner-Smidt P, Janssen P, Kaufmann M E, Garaizar J, Ursing J, Pitt T L. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–1525. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenstein B, Bernards A T, Dijkshoorn L, Gerner-Smidt P, Towner K J, Bouvet P J M, Daschner F D, Grundmann H. Acinetobacter species identification by using tRNA spacer fingerprinting. J Clin Microbiol. 1996;34:2414–2420. doi: 10.1128/jcm.34.10.2414-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991;29:277–282. doi: 10.1128/jcm.29.2.277-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Getchell-White S J, Donowitz L G, Gröschel D H M. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989;10:402–407. doi: 10.1086/646061. [DOI] [PubMed] [Google Scholar]
- 14.Gouby A, Carles-Nurit M, Bouziges N, Bourg G, Mesnard R, Bouvet P J M. Use of pulsed-field gel electrophoresis for investigation of hospital outbreaks of Acinetobacter baumannii. J Clin Microbiol. 1992;30:1588–1591. doi: 10.1128/jcm.30.6.1588-1591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gräser Y, Klare I, Halle E, Gantenberg R, Buchholz P, Jacobi H D, Presber W, Schönian G. Epidemiological study of an Acinetobacter baumannii outbreak by using polymerase chain reaction fingerprinting. J Clin Microbiol. 1993;31:2417–2420. doi: 10.1128/jcm.31.9.2417-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim A, Gerner-Smidt P, Liesack W. Phylogenetic relationship of the twenty-one DNA groups of the genus Acinetobacter as revealed by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:837–841. doi: 10.1099/00207713-47-3-837. [DOI] [PubMed] [Google Scholar]
- 18.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 19.Janssen P, Maquelin K, Coopman R, Tjernberg I, Bouvet P, Kerstens K, Dijkshoorn L. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int J Syst Bacteriol. 1997;47:1179–1187. doi: 10.1099/00207713-47-4-1179. [DOI] [PubMed] [Google Scholar]
- 20.Jawad A, Heritage J, Snelling A M, Gascoyne-Binzi D M, Hawkey P M. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol. 1996;34:2881–2887. doi: 10.1128/jcm.34.12.2881-2887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeleman J G M, Parlevliet G A, Dijkshoorn L, Savelkoul P H M, Vandenbroucke-Grauls C M J E. Nosocomial outbreak of multiresistant Acinetobacter baumannii on a surgical ward: epidemiology and risk factors for acquisition. J Hosp Infect. 1997;37:113–123. doi: 10.1016/s0195-6701(97)90181-x. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura Y, Ino T, Iizuka H. Acinetobacter radioresistens sp. nov. isolated from cotton and soil. Int J Syst Bacteriol. 1988;38:209–211. [Google Scholar]
- 23.Rossau R, Duhamel M, Jannes G, Decourt J L, van Heuverswyn H. The development of specific rRNA-derived oligonucleotide probes for Haemophilus ducreyi, the causative agent of chancroid. J Gen Microbiol. 1991;137:277–285. doi: 10.1099/00221287-137-2-277. [DOI] [PubMed] [Google Scholar]
- 24.Scerpella E G, Wanger A R, Armittage L, Ericsson C D. Nosocomial outbreak caused by a multiresistant clone of Acinetobacter baumannii: results of the case-control and molecular epidemiologic investigations. Infect Control Hosp Epidemiol. 1995;16:92–97. doi: 10.1086/647063. [DOI] [PubMed] [Google Scholar]
- 25.Seifert H, Gerner-Smidt P. Comparison of ribotyping and pulsed-field gel electrophoresis for molecular typing of Acinetobacter isolates. J Clin Microbiol. 1995;33:1402–1407. doi: 10.1128/jcm.33.5.1402-1407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelling A, Gerner-Smidt P, Hawkey P M, Heritage J, Parnell P, Porter C, Bodenham A R, Inglis T. Validation of use of whole-cell repetitive extragenic palindromic sequence-based PCR (REP-PCR) for typing strains belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii complex and application of the method to the investigation of a hospital outbreak. J Clin Microbiol. 1996;34:1193–1202. doi: 10.1128/jcm.34.5.1193-1202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Struelens M J, Carlier E, Maes N, Serruys E, Quint W G V, van Belkum A. Nosocomial colonization and infection with multiresistant Acinetobacter baumannii: outbreak delineation using DNA macro restriction analysis and PCR-fingerprinting. J Hosp Infect. 1993;25:15–32. doi: 10.1016/0195-6701(93)90005-k. [DOI] [PubMed] [Google Scholar]
- 28.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tjernberg I, Ursing J. Clinical strains of Acinetobacter classified by DNA-DNA hybridization. Acta Pathol Microbiol Immunol Scand. 1989;97:595–605. doi: 10.1111/j.1699-0463.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 30.Tyler K D, Wang G, Tyler S D, Johnson W M. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J Clin Microbiol. 1997;35:339–346. doi: 10.1128/jcm.35.2.339-346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valsangiacomo C, Baggi F, Gaia V, Balmelli T, Peduzzi R, Piffaretti J. Use of amplified fragment length polymorphism in molecular typing of Legionella pneumophila and application to epidemiological studies. J Clin Microbiol. 1995;33:1716–1719. doi: 10.1128/jcm.33.7.1716-1719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenbroucke-Grauls C M J E, Kerver A J H, Rommes J H, Jansen R, den Dekker C, Verhoef J. Endemic Acinetobacter anitratus in a surgical intensive care unit: mechanical ventilators as a reservoir. Eur J Clin Microbiol Infect Dis. 1988;7:485–489. doi: 10.1007/BF01962597. [DOI] [PubMed] [Google Scholar]
- 34.Vaneechoutte M, De Beenhouwer H, Claeys G, Verschraegen G, De Rouck A, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaneechoutte M, Dijkshoorn L, Tjernberg I, Elaichouni A, de Vos P, Claeys G, Verschraegen G. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1995;33:11–15. doi: 10.1128/jcm.33.1.11-15.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Muiper M, Zabeau M. AFLP: a new concept for DNA fingerprinting. Nucleic Acids Res. 1995;21:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wendt C, Dietze B, Dietz E, Rüden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zabeau M, Vos P. Selective restriction fragment amplification: a general method for DNA fingerprinting. European Patent Office; 1993. , publication 0 534 858 A1, bulletin 93/13. [Google Scholar]