Abstract
Purpose:
Increased fatty infiltration in paraspinal muscles has been recognized as a feature of muscle quality loss in people with Low Back Pain (LBP) and is highly associated with the severity of LBP and dysfunction. Reducing fatty infiltration has been recognized as a rehabilitation aim. An earlier systematic review published in 2014 revealed conflicting evidence for the reversibility of paraspinal muscle quality by means of exercise and no updates have been published since. A new systematic literature search is warranted.
Method:
Pubmed, CINAHL and Embase were searched from inception to July 2022. Randomized, non-randomized controlled trials (RCT and non-RCT) and single-arm trials were included if they reported the effect of exercise on paraspinal fatty infiltration in people with LBP. Effect sizes and statistical power were calculated for (1) exercise versus control, and (2) pre-post exercise changes. Available data from the RCTs were pooled via meta-analysis when appropriate. Otherwise, data were synthesized qualitatively.
Results:
Two RCTs, one non-RCT and three single-arm trials met the selection criteria. Data were not pooled due to substantial clinical heterogeneity. Effect sizes from the RCTs revealed no significant difference for exercise versus control. One single-arm trial with high risk of bias demonstrated a significant pre-post difference with moderate effect size, but only at one (T12-L1) of the investigated levels.
Conclusion:
Moderate quality evidence is available that paraspinal fatty infiltration is not reversible with exercise in people with LBP. More larger RCT’s are needed to draw firmer conclusions.
Keywords: Adipose tissue, Morphology, Physical Therapy, Rehabilitation
INTRODUCTION
Low back pain (LBP) is a prevalent condition worldwide [1]. Recurrent and chronic symptoms are common, leading to a high socioeconomic burden and healthcare utilization [2–4]. Complex multidirectional interrelationships between biological, psychological and social systems are plausible contributing factors to chronic LBP [5]. From a biological perspective, the quality of the paraspinal muscles has been suggested to being important in people with, or at risk of developing, chronic LBP [6].
Various parameters of paraspinal muscle health (e.g., size and composition) have been acknowledged as potentially important biological markers in people with LBP [6]. The burden of proof for muscle size and shape as a surrogate for degenerative muscle composition is inconclusive and contradictory [7,8]. The increase of fatty infiltration appears to be a good representation of degenerative muscle composition and is a feature of decreased muscle contractility and quality [7]. The accumulation of non-contractile fatty infiltration appears to occupy the space of atrophic muscle fibres and is particularly present between the epimysial, perimysial and lumbosacral borders of the lumbar multifidus and erector spinae [9]. The replacement of muscle tissue by non-contractile fat tissue is expected to lead to decreased muscle functional capacity [10], with lowered fatigue resistance to metabolic demands as a possible consequence [11]. Paraspinal fatty infiltration tends to be expressed to a greater magnitude in people with LBP compared to healthy age-matched controls [12], and is highly associated with the presence and severity of LBP [12,13], levels of disability [14, 15] and muscle dysfunction [16].
Exercise therapy is a well-recognized therapeutic intervention to improve pain, disability and muscle function in patients with LBP, however the exact mechanisms underpinning the effect of exercise remains elusive [17]. Reducing paraspinal fatty infiltration, accompanied by the increase of muscle contractility and functional capacity, has been considered a plausible mechanism of exercise [18].
An earlier systematic review on the effectiveness of exercise on lower trunk muscle morphology revealed conflicting evidence [19]. This review included papers up to April 2012 and no updates or other reviews have been published since. The outcomes of interest of the earlier review were muscle size and fatty infiltration. Moreover, studies with solely healthy participants were included as well to reach a conclusion about the reversibility of fatty infiltration. As such, the reversibility of paraspinal fatty infiltration with exercise in people with LBP remains unclear. Our study aimed to determine whether fatty infiltration in paraspinal muscles is reversible with exercise in people with LBP by summarizing all available primary research on this topic.
METHODS
The study protocol was pre-registered in PROSPERO (ID=CRD42020166890). The review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) guidelines [20].
Data sources and searches
The literature was searched comprehensively for relevant articles from inception to July 2022 via three electronic databases: Pubmed (Legacy interface), Embase and CINAHL. The PICO-approach was used to formulate two research questions: (1): “ Is exercise(I) – more effective than control – in reversing fatty infiltration in the paraspinal muscles(O) – in people with low back pain(P)” – and (2) “Is fatty infiltration in paraspinal muscles reversible(O) - by exercise(I) – in people with low back pain(P).
The search string for each database was developed in collaboration with a medical information specialist (JM) (see Appendix 1). A sensitive search strategy was preferred to minimise the omission of relevant trials. Exercise was excluded as a search term because it reduced the sensitivity of our search. In addition to the database searches, the references of the included articles and review articles were screened for additional publications.
Study selection
Two reviewers (EW and JP) independently selected the studies based on title and abstract. If selection criteria were met, full texts were evaluated. In case of disagreement, a third reviewer (AP) was consulted. Randomized controlled trials (RCT) and non-randomized controlled trials (non-RCT) if they met the following criteria: (1) the study only included people with LBP in the intervention groups, (2) patients were 18 years and older, (3) quantification of fatty infiltration of the paraspinal muscles was based on Magnetic Resonance Imaging (MRI) or Computed Tomography (CT), and (4) exercise was included as an intervention to reverse paraspinal fatty infiltration. In addition, single-arm trials with equivalent criteria were also included to investigate the magnitude and direction of pre-post exercise changes for the purpose of research question two.
The paraspinal muscles were defined as the lumbar multifidus and/or erector spinae muscles (longissimus, iliocostalis and spinalis). Studies were excluded if they used ultrasonography as imaging acquisition method due to low soft-tissue contrast to distinguish fatty infiltration from muscle tissue. For MRI studies, either quantitative or qualitative MRI measurement approaches were considered to measure fatty infiltration [13, 21]. In quantitative imaging analysis, fatty infiltration is separated from muscle tissue based on a pixel grayscale threshold [21]. In qualitative imaging analysis, fatty infiltration is visually graded using standard criteria: ‘normal’ for estimates of 0–10%, ‘slight’ for 10–50% and ‘severe’ for >50% of fat [13]. In studies using CT scans, the increase of fatty infiltration is quantified as a decline of muscle density, calculated by Hounsfield Units (HU). Muscle density correlates inversely with the amount of fatty infiltration [22].
Quality assessment and data extraction
Quality assessments for the RCTs were conducted according to the Cochrane Collaboration’s checklist for assessment of risk of bias [23], and the non-RCT and single-arm trials were assessed by the ROBINS-I tool [24]. The selected articles were independently rated for quality by two investigators (EW and JP). In case of disagreement, a third investigator (AP) was consulted to help resolve the disagreement. The risk of bias score was considered when interpreting the results, but was not considered for study selection. Risk of bias within and across the RCTs was defined as high, unclear or low [23]. Risk of bias within and across the non-RCTs was considered low, moderate, serious and critical [24]. The quality of the description of exercise was assessed using the Template for Intervention Description and Replication (TIDier) [25].
Data extraction was independently conducted by two reviewers (EW and JP) using a data extraction form that included: 1) study design; 2) description of patient group(s); 3) characteristics of the participants; 4) sample size; 5) imaging technique (i.e., MRI or CT); 6) type of exercise and control (frequency, intensity and duration for both exercise and control intervention) and 6) outcome measures.
Data synthesis
The reversibility of fatty infiltration by means of exercise was calculated using standardized mean differences (SMD) with 95% confidence intervals (95%CI). Effect sizes were considered small (Cohen’s d=0.2), moderate (Cohen’s d=0.5) and large (Cohen’s d=0.8) [26]. Statistical power was calculated to test the probability that effect sizes detect statistically significant results and was considered high if above 0.80 [26]. SMD and 95%CI were summarized per spinal level in separate forest plots for both exercise versus control, and pre-post exercise effects. Available exercise versus control data from the RCTs were pooled by meta-analysis if the assumptions for statistical and clinical heterogeneity were met [27]. Statistical heterogeneity was calculated using the I2 test [27]. Values higher than 50% were considered indicative for substantial statistical heterogeneity [27]. Clinical heterogeneity was assessed for study population (patient characteristics, type of LBP), exercise details (type, dose, frequency, intensity), control intervention details (type, dose, frequency, intensity) and trial setting [28]. The authors summarized the levels of evidence qualitatively if the data were considered statistically or clinical heterogenous (TABLE 1) [29].
TABLE 1.
Best Evidence Synthesis Criteria for Levels of Evidence
| Level of Evidence | Rating | Criteria a |
|---|---|---|
| High-quality evidence | +++ OR - - - |
Multiple studies with methodological quality rates as at least “good” OR one study with methodological quality rated as excellent and consistent findings (+ OR -) AND total sample size > 100 |
| Moderate-quality evidence | ++ OR - - |
Multiple studies with methodological quality rated as “fair” OR one study with methodological quality rated as “good” AND consistent findings (+ OR -) AND total sample size > 50 |
| Low-quality evidence | + OR - |
One study with methodological quality rated as “fair” AND consistent findings (+ OR -) |
| Conflicting evidence | + / - | Multiple studies AND conflicting findings |
| No evidence | ? | Only studies with methodological quality rated as “poor” or only studies with rates as “?” |
Adequacy of measurement property: +: positive, -: negative, ?: indeterminate.
RESULTS
Study selection
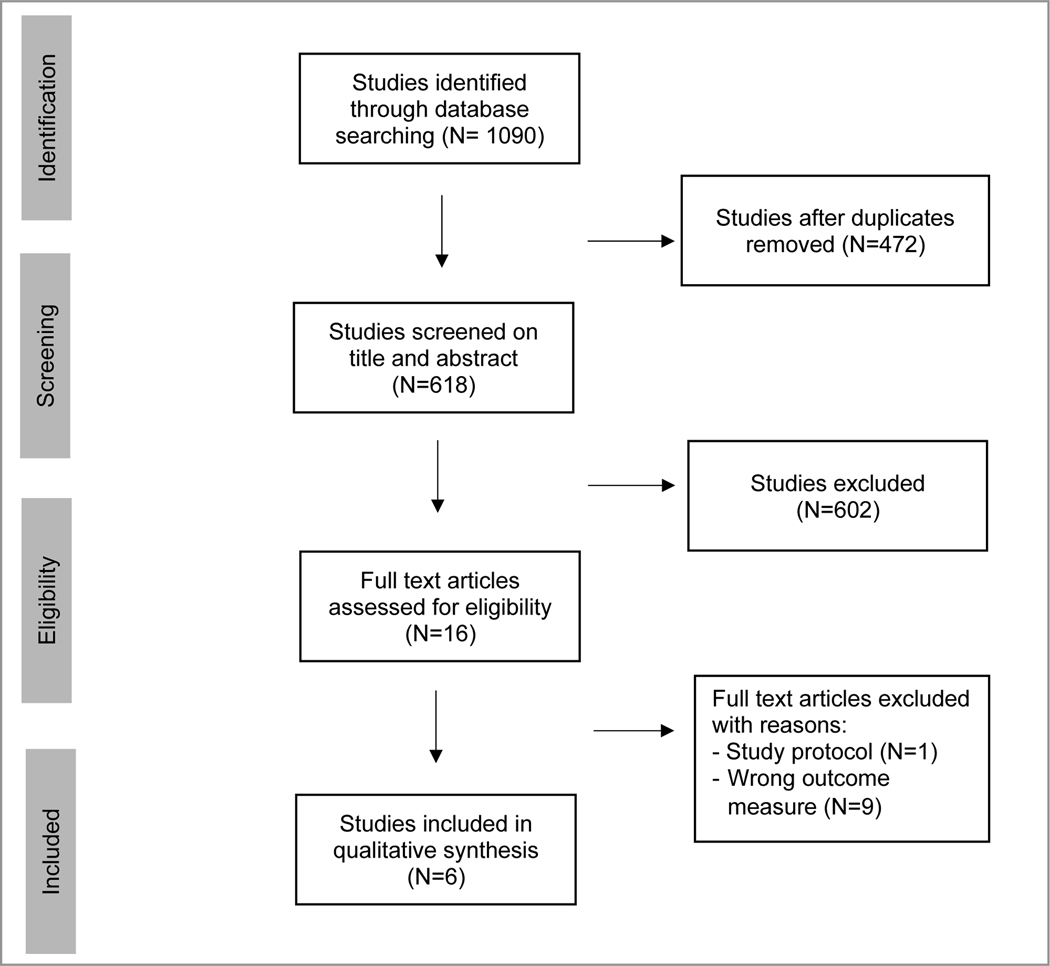
The literature search identified 1090 manuscripts. After removal of duplicates, 618 studies remained and were screened on title and abstract. Sixteen full text articles were assessed for eligibility. Six studies met the selection criteria and were included in this review (FIGURE 1).
FIGURE 1.
Prisma flow diagram
Study characteristics
Supplementary table 1 shows the characteristics of the six included studies. Two RCTs [30, 31], one non-RCT [32] and three single-arm trials [33–35] were included. A total of 161 patients were included in this review (41% (n=66) female; 59% (n=95) male). Of these patients, 118 underwent an exercise program. The sample size (with pre-post intervention imaging) per study varied from 14 [34] to 61 [30] participants. One RCT [30] included 124 participants in total, but performed pre-post imaging on 61 participants and only captured the T12-L1 vertebral level in 41 participants. As such, the total sample size for this study [30] was set to 61.
Image acquisition and fatty infiltration quantification
Different image acquisition techniques were used in the included studies. Four studies used MRI [32–35] and two studies used CT-imaging [30, 31]. Two studies quantified the erector spinae [30, 31], one study quantified the multifidus [33], and three studies combined the multifidus and erector spinae in the analysis [32, 34, 35]. Two RCTs [30, 31] using CT-imaging calculated the muscle density within a homogeneous part of the erector spinae. One MRI study [35] used T2-weighted sequences, two MRI studies [32, 34] used T1-weighted sequences and one MRI study [33] used a balanced fast-field echo sequence. Three of the four MRI-studies [32, 33, 35] used a multi-slice approach spanning the L3 to S1 vertebral levels, and one study [34] used a single-slice approach at the level L4 vertebral level.
Three of the four MRI studies [33–35] used a quantitative semi-automated threshold method to define the ‘true’ area of fatty infiltration based on pixel or voxel grayscale intensity [33–35]. One study determined the degree of fatty infiltration based on a qualitative grading scale based on visual inspection, with definitions ranging from normal to severe fatty infiltration.
Interventions
The exercise interventions varied in treatment period (8–16 weeks) and number of sessions (10–48 sessions). Three studies [32–34] performed machine-based lumbar extension training with different training intensities and session durations. One single-arm trial [35] used free-weight resistance training. One RCT [31] used a comprehensive group training, and one RCT [30] combined an exercise protocol combined with cognitive interventions, such as pain education and exposure to activities.
The control group of one RCT [30] underwent lumbar fusion and post-operative exercise was restricted to the first three months after surgery. The control group of another RCT [31] was managed by their general practitioner with no treatment or referral restrictions. The control group in one non-RCT [32] consisted of eight age-matched healthy male participants who received the same exercise program as the experimental group.
All studies reported the type of exercise, modes, location and training weeks adequately. Two of the six studies reported modification and adherence to exercise [31, 35]. None of the studies described all the items on the TIDieR checklist (see Supplementary TABLE 3).
Risk of bias
The two reviewers agreed 100% on the overall score using the Cochrane Risk of Bias Tool for Clinical Trials and ROBINS-I tool. Results of the risk of bias assessments are presented in Supplementary Table 2A and 2B. One RCT [31] scored low risk of bias and one RCT [30] high risk of bias. One RCT [30] scored unclear risk of bias on selective reporting and incomplete outcome data. The non-RCT [32] and single-arm trials [33–35] scored moderate risk of bias in the key domain bias due to confounding. One single-arm trial [35] scored critical risk of bias in bias due to missing data and selection of the reported results. One non-RCT [32] scored critical risk of bias for selection of participants into the study.
Data extraction
Exercise versus control
One RCT [31] with low risk of bias showed no significant difference in changes between the exercise and control group at L3-L4 and L4-L5. The other RCT [30] with high risk of bias showed significant differences in changes between the exercise versus control group at L3-L4 (p<0.05), but not T12-L1 (p>0.1). The reported main difference at L3-L4 for significant differences between exercise versus control group in this study was found to be due to a significant decrease in muscle density in the control group (lumbar fusion) .
Single-arm pre-post exercise changes
One RCT [31] with low risk of bias revealed no significant pre-post exercise changes in muscle density at L3-L4 and L4-L5. The other RCT [30] with high risk of bias demonstrated a significant increase in muscle density between baseline and after exercise at the T12-L1 vertebral level, but not at L3-L4. One non-RCT [32] with critical risk of bias identified a reduction of fatty infiltration in 50% of the participants with severe fatty infiltration levels, but not in participants with moderate levels of fatty infiltration at the study onset. However, this study reported no statistical evaluation for treatment effect estimates. One single-arm trial [35] with critical risk of bias demonstrated significant reductions of paraspinal fatty infiltration at the vertebral level of L3-L4 and L4-L5, but not L5-S1. Two other single-arm trials [33, 34] with moderate risk of bias found no significant reductions of fatty infiltration with exercise.
Data synthesis
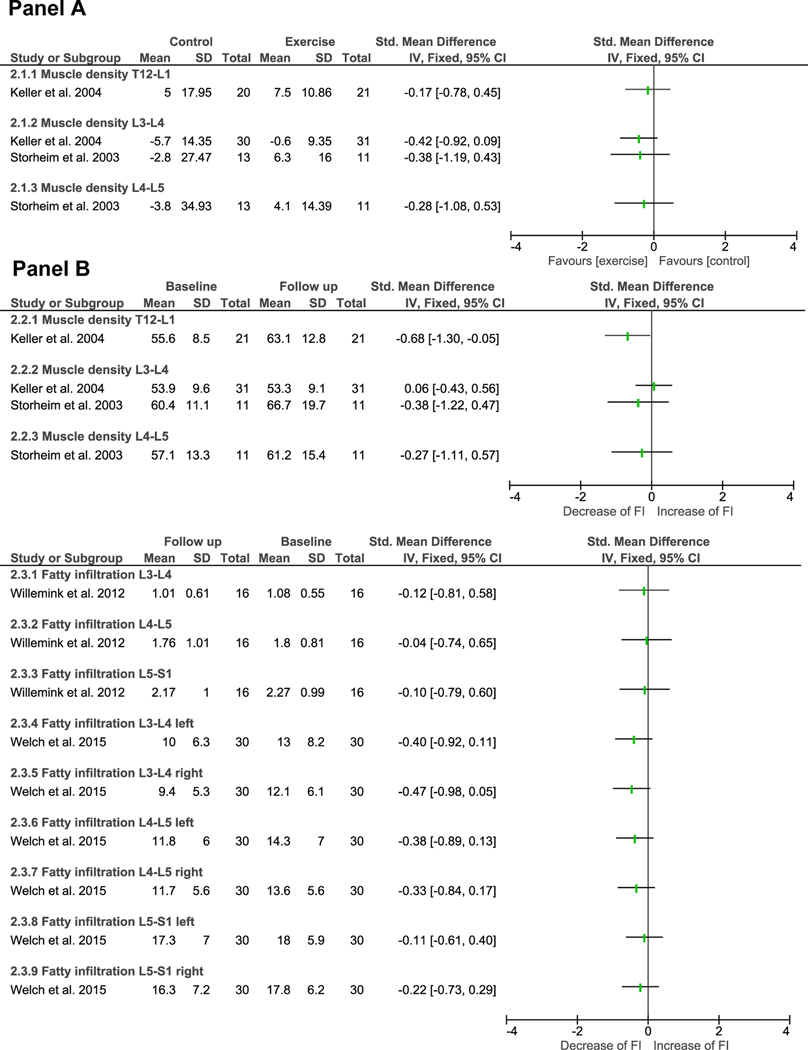
Forest plots summarizing the effect sizes are presented in FIGURE 2. Available data from the two RCTs [30, 31] were not pooled by meta-analysis because of substantial clinical heterogeneity (i.e., participants, intervention and control characteristics). The single-arm trials were not used to generate general conclusions about the reversibility of fatty infiltration, but only to investigate the direction and magnitude of pre-post exercise changes.
FIGURE 2.
Forest plots summarizing the effect sizes of exercise for changes in fatty infiltration in paraspinal muscles. Panel A summarises exercise versus control, and Panel B summarises pre-post exercise changes. An increase in muscle density corresponds with decrease of fatty infiltration as muscle density correlates inversely with the amount of fatty infiltration [22].
CI = Confidence Interval, FI = Fatty Infiltration; SD = Standard Deviation; Std = Standard, IV= Independent Variable, Total = number of participants.
Exercise versus control
Although one RCT [30] reported significant differences for changes following exercise versus control at L3-L4, we found no significant effect sizes with low statistical power for exercise versus control groups for both RCTs at T12-L1 (effect size= −0.17; statistical power = 0.08) [30], L3-L4 (effect size = −0.41; statistical power =0.37, effect size = −0.38; statistical power =0.16) [30, 31] and L4–5 (effect size =−0.28; statistical power = 0.20) (see FIGURE 2A) [31].
Single-arm pre-post exercise changes
Due to lack of reporting with regard to mean differences between pre-post intervention, it was not possible to calculate effect sizes for one non-RCT [32] and one single-arm trial [34]. One RCT [31] with low risk of bias demonstrated no significant pre-post effect sizes with low statistical power at L3-L4 (effect size = −0.27; statistical power = 0.10) and L4-L5 (effect size= −0.38; statistical power = 0.17). Another RCT [30] with high risk of bias demonstrated a significant pre-post intervention effect with moderate effects size and low statistical power (effect size = −0.68; statistical power = 0.61) at the T12-L1 vertebral level. However, no significant effects sizes with low statistical power were found at L3-L4 (effect size = 0.06; statistical power = 0.04) in this study.
No significant effect sizes for pre-post exercise effects were found for the single-arm trials. Although one single-arm trial [35] with critical risk of bias reported a significant pre-post intervention effect in their study, the calculated effects sizes in this review demonstrated no significant differences (see FIGURE 2B) with low statistical power (L3-L4 left = 0.21; L3-L4 right = 0.25; L4-L5 left = 0.18; L4-L5 right = 0.15).
DISCUSSION
This systematic review provides moderate quality evidence that paraspinal fatty infiltration is not reversible by means of exercise in people with LBP. However, more age-matched normative data is needed before clinically meaningful conclusions can be drawn. Our conclusion is based on the following observations: a) the calculated effect sizes from the RCTs [30, 31] for exercise versus control interventions were not significant with low statistical power, b) only one [30] out of six studies demonstrated a significant pre-post exercise change at the T12-L1 vertebral level, but had a moderate effects size and low statistical power. Moreover, this study [30] was of low methodological quality and the other five studies [31–35] showed no significant pre-post exercise changes.
This systematic review reached different conclusions compared to an earlier systematic review published in 2014 [19]. They provided conflicting evidence on the reversibility of muscle morphology following exercise, since approximately half of the included studies reported an improvement. Such differences are likely the results of differences in the selection criteria. Shahtahmassebi et al. (2014) included all studies reporting changes in either muscle size and fatty infiltration across participants with LBP and healthy participants. In contrast, our study only reported changes in fatty infiltration and only included patients with LBP in the intervention groups. This may clarify the different conclusions, because fatty infiltration tends to be expressed to a greater magnitude in people with LBP compared to healthy age-matched controls [12].
Lack of favourable outcomes for exercise in the current review can be due to several factors. These factors concern the generalisability or applicability of treatment effect estimates:
a). Image acquisition techniques and fatty infiltration quantification methods.
This systematic review included clinical studies that quantified fatty infiltration levels by either MRI or CT with substantial differences in image acquisition parameter which limited the possibility of pooling treatment effect estimates. High data variance due to substantial measurement variability for specific level approach (multi-slice, unislice) and sides (unilateral, bilateral) at which the fatty infiltration was present. Reporting fatty infiltration measurements for each spinal level is recommended, because a single spinal level appears to be not representative for the entire lumbar spine musculature [36]. We also observed differences in segmentations methods across studies to define the paraspinal muscles (in- or excluding epimuscular fat). The inclusion of epimuscular fatty infiltration between the aponeurosis and thoracolumbar fascia results in larger values for fat fraction [9], this is likely affecting the effect size of the reversibility of fatty infiltration by means of exercise therapy.
b). Exercise descriptions.
The exercise interventions included short treatment periods ranging from 8 to 16 weeks, and follow-up imaging was performed between 8 weeks and 1 year. The optimal duration and intensity of an exercise program to reduce intramuscular fatty infiltration effectively remains unknown [37]. It has been reported that chronic adaptations in the homeostatic myocellular environment are only evident after 8 weeks of exercise [38], and could even take longer due to multiple factors, such as training status (fat oxidation rate is higher in trained versus sedentary people) [39]. As such, it remains questionable whether an exercise and follow-up period of 8–16 weeks is sufficient to expect a decrease in fatty infiltration levels in people with LBP. Future research should focus on exercise programs with longer treatment durations. Only two [31, 35] out of six included studies reported the adherence to exercise. In addition, only one study [31] reported the delivered exercise as planned. Providing sufficient details about the execution of exercise is fundamental to replicate interventions and to interpret clinical importance [40, 41].
c). Generalization of outcomes across responder and non-responder patient subgroups.
It is likely that each type of LBP is characterized by its own physiological basis driven by different time-dependent structural remodelling mechanisms [6]. In fact, people with continuous chronic LBP (LBP for at least 3 months, 7 pain days per week) undergo accelerated paraspinal fatty infiltration compared to people with recurrent LBP (recurrent pain flare of at least 24 hours, followed by a pain-free episode of at least 1 month) and non-continuous chronic LBP (LBP for at least 3 months, 3–4 pain days a week) [42]. These characteristics to distinguish subgroups of LBP were not identified in the included studies. Only one [34] of the studies controlled for population-based confounding factors such as age, sex, race/ethnicity and body composition. Fatty infiltration increases to a greater magnitude in men versus women [43], increases by age [44], is highly associated with body composition [45] and the accumulation rate may differ between ethnicities [46]. As such, it is likely that the reversibility of fatty infiltration differs between patient subpopulations. Future prospective studies with well-defined patient subpopulations should address this issue.
d). Sample size of study population.
One RCT [30] that demonstrated a statistically significant pre-post intervention effect size at T12-L1 included the largest sample size (pre-post imaging in 61 participants and the T12-L1 vertebral level was captured in 41 patients). The other studies [31–35] which demonstrated no statistically significant exercise changes included smaller sample sizes in their intervention groups (n < 30). Small sample size could have contributed to statistical underpowered effect (type II error) [47]. As such, larger sample sizes are needed to increase statistical power [26].
Limitations
This systematic review should be interpreted in the light of some limitations already mentioned above. Furthermore, our conclusions were based on a small number of available studies with substantial methodological (study design, methodological quality) and clinical heterogeneity. As such, we were not able to pool available data by meta-analysis. We included studies with different fatty infiltration quantification methods and imaging acquisition methods, that limited the possibility to pool and compare data form different studies. Levels of evidence were defined as moderate quality evidence, because effect sizes were consistent among two RCTs for exercise versus control effects and among four studies with high risk of bias and one RCT with low risk of bias for pre-post exercise differences.
CONCLUSIONS
This systematic review provides moderate quality evidence that paraspinal fatty infiltration is not reversible by means of exercise in people with LBP. However, more clinical studies with consistent, and consensus-driven, methodologies, larger sample sizes and longer treatment duration are needed to draw firm conclusions about the reversibility of fatty infiltration by means of exercise in people with LBP.
Supplementary Material
FUNDING SUPPORT
Kenneth A. Weber II received funding from National Institute of Neurological Disorders and Stroke (grants K23NS104211 and L30NS108301). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors played no role in the study design, data collection, decision to publish, or preparation of the report.
FINANCIAL DISCLOSURE
The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
APPENDIX A
Search strings per database
PubMed:
((“fatty infiltration”[tiab] OR “fat infiltration”[tiab] OR “intramuscular adipose tissue”[tiab] OR “intermuscular adipose tissue”[tiab] OR “intra-muscular adipose tissue”[tiab] OR “intramuscular fat”[tiab] OR “intermuscular fat”[tiab] OR “fat content”[tiab] OR “cross sectional area”[tiab]) AND (“Back Muscles”[mh] OR “back muscles”[tiab] OR lumbar[tiab] OR “paraspinal muscles”[tiab] OR “paravertebral muscles”[tiab] OR multifidus[tiab] OR iliocostalis[tiab] OR longissimus[tiab] OR “erector spinae”[tiab]) AND (“Back Pain”[mh] OR “back pain”[tiab] OR “back pains”[tiab] OR “back ache”[tiab] OR “back aches”[tiab] OR “back complaint”[tiab] OR “back complaints”[tiab] OR “back dysfunction”[tiab] OR “back symptom”[tiab] OR “back symptoms”[tiab] OR backpain[tiab] OR backpains[tiab] OR backache[tiab] OR backaches[tiab] OR “lumbar pain”[tiab] OR “lumbar pains”[tiab] OR “lumbar ache”[tiab] OR “lumbar aches”[tiab] OR “lumbar complaint”[tiab] OR “lumbar complaints”[tiab] OR “lumbar dysfunction”[tiab] OR “lumbar symptom”[tiab] OR “lumbar symptoms”[tiab] OR “spinal pain”[tiab] OR “spinal pains”[tiab] OR “spinal ache”[tiab] OR “spinal aches”[tiab] OR “spinal complaint”[tiab] OR “spinal complaints”[tiab] OR “spinal dysfunction”[tiab] OR “spinal symptom”[tiab] OR “spinal symptoms”[tiab])) NOT (animals[mh] NOT humans[mh])
Embase:
((((‘fatty infiltration’/exp OR ‘fat infiltration’/exp OR ‘intramuscular adipose tissue’/exp OR ‘intermuscular adipose tissue’/exp OR ‘intramuscular fat’/exp OR ‘fat content’/exp OR ‘cross sectional area’/exp) OR (‘fatty infiltration’ OR ‘fat infiltration’ OR ‘intramuscular adipose tissue’ OR ‘intermuscular adipose tissue’ OR ‘intra-muscular adipose tissue’ OR ‘intramuscular fat’ OR ‘intermuscular fat’ OR ‘fat content’ OR ‘cross sectional area’):ti,ab) AND ((‘back muscle’/exp OR ‘lumbar muscle’/exp OR ‘paraspinal muscle’/exp OR ‘multifidus muscle’/exp OR ‘iliocostalis muscle’/exp OR ‘longissimus muscle’/exp OR ‘erector spinae muscle’/exp) OR (‘back muscles’ OR lumbar OR ‘paraspinal muscles’ OR ‘paravertebral muscles’ OR multifidus OR iliocostalis OR longissimus OR ‘erector spinae’):ti,ab) AND ((‘backache’/exp OR ‘spinal pain’/exp) OR (‘back pain’ OR ‘back pains’ OR ‘back ache’ OR ‘back aches’ OR ‘back complaint’ OR ‘back complaints’ OR ‘back dysfunction’ OR ‘back symptom’ OR ‘back symptoms’ OR backpain OR backpains OR backache OR backaches OR ‘lumbar pain’ OR ‘lumbar pains’ OR ‘lumbar ache’ OR ‘lumbar aches’ OR ‘lumbar complaint’ OR ‘lumbar complaints’ OR ‘lumbar dysfunction’ OR ‘lumbar symptom’ OR ‘lumbar symptoms’ OR ‘spinal pain’ OR ‘spinal pains’ OR ‘spinal ache’ OR ‘spinal aches’ OR ‘spinal complaint’ OR ‘spinal complaints’ OR ‘spinal dysfunction’ OR ‘spinal symptom’ OR ‘spinal symptoms’):ti,ab)) NOT ([animals]/lim NOT [humans]/lim)) AND [embase]/lim
CINAHL:
((“fatty infiltration” OR “fat infiltration” OR “intramuscular adipose tissue” OR “intermuscular adipose tissue” OR “intra-muscular adipose tissue” OR “intramuscular fat” OR “intermuscular fat” OR “fat content” OR “cross sectional area”) AND (MH “Multifidus Muscles” OR MH “Erector Spinae Muscles” OR “back muscles” OR lumbar OR “paraspinal muscles” OR “paravertebral muscles” OR multifidus OR iliocostalis OR longissimus OR “erector spinae”) AND (MH “Back Pain+” OR “back pain” OR “back pains” OR “back ache” OR “back aches” OR “back complaint” OR “back complaints” OR “back dysfunction” OR “back symptom” OR “back symptoms” OR backpain OR backpains OR backache OR backaches OR “lumbar pain” OR “lumbar pains” OR “lumbar ache” OR “lumbar aches” OR “lumbar complaint” OR “lumbar complaints” OR “lumbar dysfunction” OR “lumbar symptom” OR “lumbar symptoms” OR “spinal pain” OR “spinal pains” OR “spinal ache” OR “spinal aches” OR “spinal complaint” OR “spinal complaints” OR “spinal dysfunction” OR “spinal symptom” OR “spinal symptoms”)) NOT (MH “Animals” NOT MH “Human)
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Becker A, Held H, Redaelli M, et al. (2010) Low Back Pain in Primary Care. Spine (Phila Pa 1976) 35:1714–1720. 10.1097/BRS.0b013e3181cd656f [DOI] [PubMed] [Google Scholar]
- 2.Hestbaek L, Leboeuf-Yde C, Manniche C (2003) Low back pain: what is the long-term course? A review of studies of general patient populations. Eur Spine J 12:149–165. 10.1007/s00586-002-0508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore M, Sadosky A, Stacey BR, et al. (2012) The Burden of Chronic Low Back Pain. Spine (Phila Pa 1976) 37:E668–E677. 10.1097/BRS.0b013e318241e5de [DOI] [PubMed] [Google Scholar]
- 4.van der Gaag WH, Enthoven WTM, Luijsterburg PAJ, et al. (2019) Natural History of Back Pain in Older Adults over Five Years. J Am Board Fam Med 32:781–789. 10.3122/jabfm.2019.06.190041 [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan P, Caneiro JP, O’Keeffe M, O’Sullivan K (2016) Unraveling the Complexity of Low Back Pain. J Orthop Sports Phys Ther 46:932–937. 10.2519/jospt.2016.0609 [DOI] [PubMed] [Google Scholar]
- 6.Goubert D, van Oosterwijck J, Meeus M, et al. (2016) Structural changes of lumbar muscles in non-specific low back pain. Pain Physician 19:E985–E1000 [PubMed] [Google Scholar]
- 7.Hodges PW, James G, Blomster L, et al. (2015) Multifidus Muscle Changes After Back Injury Are Characterized by Structural Remodeling of Muscle, Adipose and Connective Tissue, but Not Muscle Atrophy. Spine (Phila Pa 1976) 40:1057–1071. 10.1097/BRS.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 8.Niemeläinen R, Briand M-M, Battié MC (2011) Substantial Asymmetry in Paraspinal Muscle Cross-Sectional Area in Healthy Adults Questions Its Value as a Marker of Low Back Pain and Pathology. Spine (Phila Pa 1976) 36:2152–2157. 10.1097/BRS.0b013e318204b05a [DOI] [PubMed] [Google Scholar]
- 9.Berry DB, Padwal J, Johnson S, et al. (2018) Methodological considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskelet Disord 19:135. 10.1186/s12891-018-2059-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamrick MW, McGee-Lawrence ME, Frechette DM (2016) Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne) 7:. 10.3389/fendo.2016.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konopka AR, Wolff CA, Suer MK, Harber MP (2018) Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol Integr Comp Physiol 315:R461–R468. 10.1152/ajpregu.00030.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mengiardi B, Schmid MR, Boos N, et al. (2006) Fat Content of Lumbar Paraspinal Muscles in Patients with Chronic Low Back Pain and in Asymptomatic Volunteers: Quantification with MR Spectroscopy. Radiology 240:786–792. 10.1148/radiol.2403050820 [DOI] [PubMed] [Google Scholar]
- 13.Kjaer P, Bendix T, Sorensen JS, et al. (2007) Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med 5:2. 10.1186/1741-7015-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teichtahl AJ, Urquhart DM, Wang Y, et al. (2015) Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J 15:1593–1601. 10.1016/j.spinee.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 15.Chang J-W, Chen Y-A, Wang S-W, et al. (2015) Relationship between paraspinal muscles fat infiltration and daily activity function in patients with lumbar spinal stenosis. Physiotherapy 101:e214. 10.1016/j.physio.2015.03.382 [DOI] [Google Scholar]
- 16.Hildebrandt M, Fankhauser G, Meichtry A, Luomajoki H (2016) Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. Man Ther 25:eS562. 10.1016/j.math.2016.05.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon R, Bloxham S (2016) A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare 4:22. 10.3390/healthcare4020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen BK, Febbraio MA (2008) Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol Rev 88:1379–1406. 10.1152/physrev.90100.2007 [DOI] [PubMed] [Google Scholar]
- 19.Shahtahmassebi B, Hebert JJ, Stomski NJ, et al. (2014) The Effect of Exercise Training on Lower Trunk Muscle Morphology. Sport Med 44:1439–1458. 10.1007/s40279-014-0213-7 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535–b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortin M, Omidyeganeh M, Battié MC, et al. (2017) Evaluation of an automated thresholding algorithm for the quantification of paraspinal muscle composition from MRI images. Biomed Eng Online 16:61. 10.1186/s12938-017-0350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelke K, Museyko O, Wang L, Laredo J-D (2018) Quantitative analysis of skeletal muscle by computed tomography imaging—State of the art. J Orthop Transl 15:91–103. 10.1016/j.jot.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Gotzsche PC, et al. (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928–d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, et al. (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann TC, Glasziou PP, Boutron I, et al. (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348:g1687–g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 26.Cohen J (1992) A power primer. Psychol Bull 112:155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagnier JJ, Moher D, Boon H, et al. (2012) Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol 12:111. 10.1186/1471-2288-12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furlan AD, Pennick V, Bombardier C, van Tulder M (2009) 2009 Updated Method Guidelines for Systematic Reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 34:1929–1941. 10.1097/BRS.0b013e3181b1c99f [DOI] [PubMed] [Google Scholar]
- 30.Keller A, Brox JI, Gunderson R, et al. (2004) Trunk Muscle Strength, Cross-sectional Area, and Density in Patients With Chronic Low Back Pain Randomized to Lumbar Fusion or Cognitive Intervention and Exercises. Spine (Phila Pa 1976) 29:3–8. 10.1097/01.BRS.0000103946.26548.EB [DOI] [PubMed] [Google Scholar]
- 31.Storheim K, Holm I, Gunderson R, et al. (2003) The Effect of Comprehensive Group Training on Cross-sectional Area, Density, and Strength of Paraspinal Muscles in Patients Sick-Listed for Subacute Low Back Pain. J Spinal Disord Tech 16:271–279. 10.1097/00024720-200306000-00008 [DOI] [PubMed] [Google Scholar]
- 32.Mooney V, Gulick J, Perlman M, et al. (1997) Relationships Between Myoelectric Activity, Strength, and MRI of Lumbar Extensor Muscles in Back Pain Patients and Normal Subjects. J Spinal Disord 10:348???356. 10.1097/00002517-199708000-00011 [DOI] [PubMed] [Google Scholar]
- 33.Willemink MJ, van Es HW, Helmhout PH, et al. (2012) The Effects of Dynamic Isolated Lumbar Extensor Training on Lumbar Multifidus Functional Cross-Sectional Area and Functional Status of Patients With Chronic Nonspecific Low Back Pain. Spine (Phila Pa 1976) 37:E1651–E1658. 10.1097/BRS.0b013e318274fb2f [DOI] [PubMed] [Google Scholar]
- 34.Berry DB, Padwal J, Johnson S, et al. (2019) The effect of high-intensity resistance exercise on lumbar musculature in patients with low back pain: a preliminary study. BMC Musculoskelet Disord 20:290. 10.1186/s12891-019-2658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welch N, Moran K, Antony J, et al. (2015) The effects of a free-weight-based resistance training intervention on pain, squat biomechanics and MRI-defined lumbar fat infiltration and functional cross-sectional area in those with chronic low back. BMJ Open Sport Exerc Med. 10.1136/bmjsem-2015-000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodges PW, Bailey JF, Fortin M, Battié MC (2021) Paraspinal muscle imaging measurements for common spinal disorders: review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur Spine J 30:3428–3441. 10.1007/s00586-021-06990-2 [DOI] [PubMed] [Google Scholar]
- 37.Addison O, Marcus RL, LaStayo PC, Ryan AS (2014) Intermuscular Fat: A Review of the Consequences and Causes. Int J Endocrinol 2014:1–11. 10.1155/2014/309570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bird SR, Hawley JA (2017) Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med 2:e000143. 10.1136/bmjsem-2016-000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purdom T, Kravitz L, Dokladny K, Mermier C (2018) Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr 15:1–10. 10.1186/s12970-018-0207-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conn VS (2012) Unpacking the Black Box. West J Nurs Res 34:427–433. 10.1177/0193945911434627 [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM (2010) Commentary: External validity of results of randomized trials: disentangling a complex concept. Int J Epidemiol 39:94–96. 10.1093/ije/dyp305 [DOI] [PubMed] [Google Scholar]
- 42.Goubert D, De Pauw R, Meeus M, et al. (2017) Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J 17:1285–1296. 10.1016/j.spinee.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 43.Crawford RJ, Volken T, Ni Mhuiris Á, et al. (2019) Geography of Lumbar Paravertebral Muscle Fatty Infiltration. Spine (Phila Pa 1976) 44:1294–1302. 10.1097/BRS.0000000000003060 [DOI] [PubMed] [Google Scholar]
- 44.Crawford RJ, Filli L, Elliott JM, et al. (2016) Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. Am J Neuroradiol 37:742–748. 10.3174/ajnr.A4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teichtahl AJ, Urquhart DM, Wang Y, et al. (2015) Physical inactivity is associated with narrower lumbar intervertebral discs, high fat content of paraspinal muscles and low back pain and disability. Arthritis Res Ther 17:114. 10.1186/s13075-015-0629-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford RJ, Elliott JM, Volken T (2017) Change in fatty infiltration of lumbar multifidus, erector spinae, and psoas muscles in asymptomatic adults of Asian or Caucasian ethnicities. Eur Spine J 26:3059–3067. 10.1007/s00586-017-5212-6 [DOI] [PubMed] [Google Scholar]
- 47.Banerjee A, Chitnis U, Jadhav S, et al. (2009) Hypothesis testing, type I and type II errors. Ind Psychiatry J 18:127. 10.4103/0972-6748.62274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song J, Araghi K, Dupont MM, et al. (2022) Association between muscle health and patient-reported outcomes after lumbar microdiscectomy: early results. Spine J. 10.1016/j.spinee.2022.05.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.