Abstract
Swine infectious agents, especially viruses, are potential public health risks associated with the use of pig organs for xenotransplantation in humans. Therefore, there is a need for better characterization of swine viruses and for the development of diagnostic tests for their detection. We report here isolation of a novel strain of porcine circovirus (PCV) from pigs with postweaning multisystemic wasting syndrome (PMWS). Affected pigs exhibited severe interstitial pneumonia and lymphoid depletion. The complete nucleotide sequence (1,768 nucleotides) of the genome of the PCV isolate was determined and compared with the sequence of the PCV strain isolated from PK-15 cells. Sequence comparison revealed significant differences between the two PCV strains, with an overall DNA homology of 76%. Two major open reading frames (ORFs) were identified. ORF1 was more conserved between the two strains, with 83% nucleotide homology and 86% amino acid homology. ORF2 was more variable, with nucleotide homology of 67% and amino acid homology of 65%. PCR and in situ hybridization demonstrated abundant viral DNA in various organs of pigs with PMWS. In situ hybridization demonstrated that this strain of PCV targets multiple organs and infects macrophages, lymphocytes, endothelial cells, and epithelial cells.
The infectious agents of swine are receiving increased attention due to the potential use of pig organs for xenotransplantation in humans. Some of these agents are poorly studied, and/or their significance to swine health is unknown. Porcine circovirus (PCV) is one such agent and is considered to be widespread in swine (7, 33, 35). PCV is a member of the family Circoviridae, which consists of DNA viruses with a circular, single-stranded genome. Other members include chicken anemia virus and psittacine beak and feather disease virus (28, 38) in animals and several plant viruses, including subterranean clover stunt virus, coconut foliar decay virus, and banana bunchy top virus (4, 15, 29). PCV was first isolated in 1974 as a persistent contaminant of the continuous porcine kidney cell line PK-15 (ATCC CCL31) (34, 37), and the PCV strain isolated from PK-15 cells (PCV PK-15) has been well characterized (3, 20, 23). No common antigenic determinants or DNA sequence homologies among animal circoviruses have been detected (39).
Serological surveys using indirect immunofluorescence or immunoperoxidase assays indicate that antibodies to PCV PK-15 are very common in North American and European swine herds. Antibodies to PCV were found in 85% of the sera of slaughter pigs in Germany (35), in 53% of the samples in a survey of swine in the United States (18), in 86% of randomly collected serum samples in Britain (8), and in 92% of randomly collected serum samples in Ireland (3). A survey conducted with an enzyme-linked immunosorbent assay in Germany showed that in almost all swine herds tested (with one exception), PCV infection was common (33). Antibodies reacting with PCV PK-15 have also been detected in humans, mice, and cattle (32).
Despite the common occurrence of PCV infection, the clinical significance of PCV in swine and other species is not known. Limited experimental transmission studies suggest that the PCV PK-15 strain may not cause any overt disease in swine (2). However, several reports suggesting that PCV is associated with postweaning multisystemic wasting syndrome (PMWS) in growing pigs (6, 13, 14, 27), and possibly with congenital tremors in newborn pigs (17), have been published recently. PMWS is a newly emerged porcine disease syndrome, characterized clinically by progressive dyspnea, emaciation, and lymph node enlargement and pathologically by a wide range of inflammatory lesions that most often include lymphohistiocytic to granulomatous lymphadenitis, interstitial pneumonia, hepatitis, interstitial nephritis, and pancreatitis (6, 13, 14).
The purpose of this study was to determine whether PCV isolated from pigs with PMWS is related to PCV PK-15, as well as to develop PCR and in situ hybridization assays for the detection of PCV in clinical samples. Such methods should be useful in elucidating the role of PCV in swine disease syndromes and for providing PCV-free xenografts. This report describes the isolation and genetic characterization of a new PCV strain (PCV ISU-31) from tissues of pigs with PMWS which is significantly different from the PCV strain previously isolated from the PK-15 cell line.
MATERIALS AND METHODS
Clinical history and tissue samples.
A group of 1,200 pigs weighing 75 to 85 lb in an eastern Iowa grower-finisher herd had a 3 to 4% incidence of dyspnea, depression, and ill thrift. These pigs had been farrowed in Canada and weaned at 14 to 21 days, transported to a nursery unit in northern Iowa, and moved to the grower-finisher herd when they weighed 50 to 60 lb. Affected pigs were minimally responsive to treatment with various antibiotics. Microscopic examination of tissues from one dead pig submitted to the Iowa State University Veterinary Diagnostic Laboratory revealed severe lymphohistiocytic interstitial pneumonia and severe depletion of tonsilar and splenic lymphoid tissue. Moderate numbers of macrophages in the tonsil contained intracytoplasmic clusters of globular amphophilic inclusion bodies. Immunohistochemistry revealed porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the lung, and PRRSV was isolated from pooled lung, tonsil, and lymph node. PRRSV immunohistochemistry and virus isolation were performed as described previously (10, 12). Findings for this pig were consistent with both PMWS and porcine reproductive and respiratory syndrome (PRRS), and additional clinically affected pigs (five live and one dead) were obtained from the same herd for further studies. Five of the six pigs had microscopic lesions consistent with PMWS.
Samples of lymph node, lung, liver, kidney, tonsil, and large and small intestine were collected from all animals and stored at −70°C. Total DNA was isolated from 25 mg of each tissue sample and used for PCR experiments. For histopathologic studies, samples were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. Five-micrometer-thick sections for histologic examination and in situ hybridization were cut from the same blocks. Samples of lung, liver, and lymph node stored at −70°C were used for virus isolation.
DNA isolation, PCR amplification, cloning, and sequencing.
DNA was isolated from lung, liver, spleen, intestine, lymph node, and tonsil samples of animals with PMWS. The DNA was isolated from 25 mg of each tissue sample with a QIAamp Tissue Kit (Qiagen, Santa Clarita, Calif.). PCR amplification was performed with 100 ng of DNA in a reaction mixture with five different sets of primers designed on the basis of the sequence of the PCV PK-15 strain. As a negative control for PCR, DNA isolated from PK-15 cells was used. PCR was performed in 30 cycles with the following parameters: denaturation at 94°C for 20 s, annealing at 61°C for 20 s, and elongation at 72°C for 45 s. PCR products were analyzed by agarose gel electrophoresis. Only in PCR with primers PCVF (5′-CAGCGGCAGCACCTCGGCAGCGTCAGT-3′) and PCV507 (5′-TCCAATCACGCTGCTGCATCTTCCCGC-3′) was a specific 530-bp PCR product amplified from tissue DNA of all samples tested. The PCR product amplified from the lymph node of one animal was isolated, sequenced, and cloned into a pGEM-T vector (Promega, Madison, Wis.). The resulting plasmid, pPSP.PCV1, was used for in situ hybridization. Two specific primers, PCV75.1 (5′-CGGAAGGATTATTCAGCGTGAACACCC-3′) and PCV389 (5′-GCTGTGAGTACCTTGTTGGAGAGCGGG-3′), were synthesized and used to amplify the rest of the genome of this PCV isolate, referred to as PCV ISU-31, from the same DNA sample. The 1.5-kb DNA fragment was amplified and sequenced. Sequencing data were analyzed with the GeneWorks (IntelliGenetics, Inc., Mountain View, Calif.) and MacVector (International Biotechnologies, Inc., New Haven, Conn.) DNA analysis programs. During virus isolation, total DNA was isolated from the monolayer of experimentally infected and noninfected PK-15 cells with the QIAamp Tissue Kit (Qiagen) and used in PCR with the primers described above.
In situ hybridization.
Lymph node, spleen, tonsil, liver, lung, heart, kidney, pancreas, nasal turbinate, and large and small intestine samples were fixed in 10% buffered formalin and processed for in situ hybridization as described previously for coronaviruses (31), with slight modifications. The plasmid pPSP.PCV1, linearized with restriction enzyme NcoI (Stratagene, La Jolla, Calif.), was used as a template to make an antisense fluorescein-labeled RNA probe. In order to make a sense RNA probe, plasmid pPSP.PCV1 was linearized with restriction enzyme PstI (Stratagene), and RNA was synthesized with T7 RNA polymerase (Stratagene). In situ hybridization was performed as described previously (31) with slight modification. Sections of 5-μm thickness were deparaffinized, rehydrated, and treated with proteinase K (50 μg/ml). Before hybridization, tissues were incubated with 200 μl of hybridization buffer by using a CoverWell chamber (Grace Bio-Labs, Bend, Oreg.) at 95°C for 10 min. Sections were hybridized, washed, and treated with RNase A. Then, sections were incubated with anti-fluorescein alkaline phosphatase, washed, incubated with 4-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Boehringer Mannheim, Indianapolis, Ind.), and counterstained with nuclear fast red. Tissues from two negative control pigs, two pigs experimentally infected with PRRSV, and nondenatured RNase-treated PMWS tissues hybridized with the sense RNA probe served as negative controls.
Virus isolation.
Tissues from lung, liver, and lymph node of the pig with PMWS from which PCV ISU-31 was PCR amplified were pooled and homogenized in minimal essential medium containing penicillin (100 U/μl) and streptomycin (100 μg/ml) to make an approximately 10% suspension. The sample was clarified, filtered through a 0.22-μm-pore-size filter, and used as an inoculum for further studies. Virus isolation was carried out in PK-15 cell culture shown by PCR to be free of PCV PK-15 contamination. Two 75-cm2 flasks of semiconfluent PK-15 cells were inoculated with 3 ml of inocula and incubated at 37°C in CO2 atmosphere for virus adsorption. Then, 15 ml of minimal essential medium supplemented with 10% fetal bovine serum and antibiotics was added, and flasks were incubated for 4 h more. Following incubation, cells were treated with 300 mM d-glucosamine (36) for 30 min and then incubated for a further 48 h. One flask was used for total DNA isolation, and another flask was passaged into fresh 75-cm2 tissue culture flasks for further propagation. Cells were passaged every 2 days and treated with d-glucosamine 24 h after each passage. After passages 1 to 4, total DNA was isolated from infected and noninfected cells and tested by PCR for the presence of PCV DNA, as described above. Infected cells were also tested by indirect immunofluorescence assay (IIFA) with polyclonal serum collected from pigs with clinical signs of PMWS. For IIFA, infected cells were distributed into 12-well cell culture plates and fixed with cold methanol 24 h after glucosamine treatment. After fixation, cells were washed twice with phosphate-buffered saline (PBS) and incubated with primary polyclonal serum (1:10 dilution in PBS) at 37°C for 1 h. The cell cultures were washed twice with PBS and immunostained with a 1/50 dilution of fluorescein isothiocyanate-conjugated goat anti-swine immunoglobulin G antibodies (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) in PBS at 37°C for 30 min. After a wash with PBS, cells were examined with a fluorescence microscope for the presence of specific fluorescent staining.
Nucleotide sequence accession number.
The EMBL nucleotide sequence database accession number of the sequence reported in this paper is AJ223185.
RESULTS
Gross and histopathologic findings.
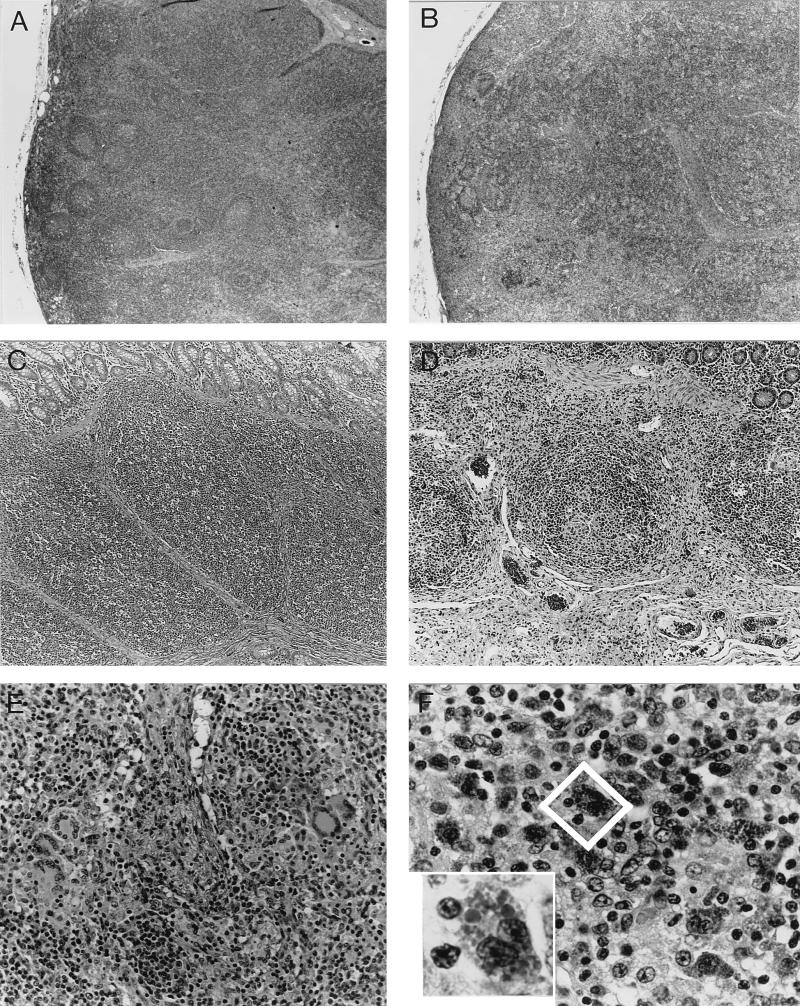
Six of the seven pigs examined in this study had lesions consistent with PMWS. Lungs were noncollapsed, rubbery, and mottled red to pale tan. Inguinal, tracheobronchial, and mesenteric lymph nodes were moderately to markedly enlarged and pale. Microscopic examination revealed severe lymphohistiocytic interstitial pneumonia with mild to marked type 2 pneumocyte hyperplasia. Alveoli contained neutrophils, eosinophils, macrophages, and fibrinonecrotic debris. In some areas there was marked multifocal fibrosis of airway laminae propriae and bronchiolitis obliterans. Peyer’s patches, tonsils, and spleens exhibited marked depletion of lymphocytes and variable infiltrates of macrophages and fewer multinucleate giant cells. Predominantly in the Peyer’s patches, occasional macrophages within lymphocyte-depleted foci contained multiple globular basophilic to amphophilic intracytoplasmic inclusion bodies. Infiltrates of lymphocytes and macrophages were inconsistently present within liver, pancreas, kidney, and gastric tunica muscularis samples. Immunohistochemistry revealed PRRSV antigen in the lungs of two of the pigs. Typical histological lesions observed in lymphoid tissues of pigs with PMWS are shown in Fig. 1.
FIG. 1.
Photomicrographs of normal porcine tissues (A and C) and tissues from pigs with PMWS (B, D, E, and F). (A) Normal porcine lymph node with multiple distinct lymphoid follicles. (B) Lymph node from a pig with PMWS. The node is moderately depleted of lymphocytes and lacks follicles. (C) Peyer’s patch from a normal pig. The submucosa is entirely filled with lymphocytes. (D) Peyer’s patch from a pig with PMWS exhibits severe lymphoid depletion. (E) Lymph node from a pig with PMWS. Sinuses contain multinucleate giant cells. (F) Peyer’s patch from a pig with PMWS showing multiple macrophages in a moderately depleted Peyer’s patch filled with amphophilic intracytoplasmic inclusion bodies. Inset, higher magnification of a binucleate macrophage (outlined in center of panel) that is distended with globular intracytoplasmic inclusion bodies.
PCR amplification and genetic characterization of a new PCV isolate.
Because the intracytoplasmic clusters of basophilic inclusions were consistent with PCV infection, DNA was isolated from lung, liver, spleen, intestine, lymph node, and tonsil samples of animals with lesions of PMWS. Circovirus DNA was successfully amplified from all tested samples. From one sample, the entire genome of PCV was amplified in two overlapping fragments, cloned, and sequenced.
Analysis of sequencing data showed that PCV ISU-31 has a 1,768-bp DNA genome. The genome of PCV ISU-31 was 9 nucleotides longer than the genome of PCV PK-15. Significant differences were found between these two strains, with an overall DNA homology of 76%. Two major open reading frames (ORFs), ORF1 and ORF2, located in opposite orientations, were detected in the genome of PCV ISU-31. Two noncoding regions, 83 and 44 bp long, were located between ORF1 and ORF2. ORF1 in the PCV ISU-31 genome is 942 bp long and is predicted to encode a protein of 314 amino acids. In comparison, ORF1 of PCV PK-15 is 936 bp long, with a predicted protein of 312 amino acids. The homology in ORF1 between these two strains was 83% at the nucleotide level and 86% at the amino acid level. Figure 2A shows the alignment of the predicted ORF1 proteins of PCV ISU-31 and PCV PK-15, with three variable regions. Variable region 1 is at the 5′ end of the protein, where there is a 3-amino-acid deletion (Arg, Ser, Gly) in PCV PK-15. Variable region 2 is in the middle of the protein (amino acids 180 to 198), and variable region 3 (amino acids 281 to 314) is at the 3′ end and has 1 amino acid deleted in the predicted protein of PCV ISU-31 ORF1. ORF1 is predicted to encode a 36-kDa protein and possibly represents the major structural protein of PCV (23). It also possesses homology with putative replication-associated proteins of plant circoviruses, similar to that detected previously for PCV PK-15 (20, 23). All three domains predicted by Koonin and Ilyina (19) for Rep proteins were detected, as well as the nucleotide binding site GPPGCGKS (Fig. 2A). Three potential N-glycosylation sites were detected in the ISU-31 ORF1, compared to one site in ORF1 of PCV PK-15. The major ORF2 of the PCV ISU-31 genome is 699 bp long and is predicted to encode a 233-amino-acid protein of 28 kDa, which is similar in size to ORF2 of PCV PK-15. ORF2 was more variable than ORF1, with DNA and amino acid homologies of 67 and 65%, respectively (Fig. 2B). Comparison of the genomes of PCV ISU-31 and chicken anemia virus did not reveal any homology between these two viruses at the DNA or protein level.
FIG. 2.
Comparative alignments of ORF1 and ORF2 predicted proteins (A and B) and origins of replication (C) of PCV ISU-31 and PCV PK-15. (A) Alignment of the ORF1-encoded proteins of PCV ISU-31 and PCV PK-15. Domains typical of Rep proteins (1 to 3) and the nucleotide binding site (4) are underlined. (B) Alignment of the ORF2-encoded proteins of PCV ISU-31 and PCV PK-15. (C) DNA alignment of replication origins of PCV ISU-31 and PCV PK-15. The inverted repeat of the putative stem-loop structure is underlined by arrows, the conserved nonanucleotide sequence is shown in boldface type, and the three 6-bp repeats are underlined.
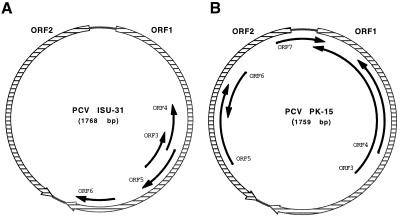
Figure 3 shows the genetic maps of PCV ISU-31 and PCV PK-15. In addition to the two large ORFs, a set of smaller ORFs with coding capacities of more than 45 amino acids were detected in the genomes of both strains. The patterns of the smaller ORFs are significantly different. Only small ORF3 and ORF4 in the PCV ISU-31 genome have counterparts in the PCV PK-15 genome, but the proteins predicted for these ORFs are truncated at the C termini. The sizes of the predicted proteins of PCV ISU-31 and PCV PK-15 are 57 and 206 amino acids, respectively, for ORF3 and 55 and 115 amino acids, respectively, for ORF4. At this time, little information is known about the significance, if any, of the smaller ORFs.
FIG. 3.
Genome organizations of PCV ISU-31 (A) and PCV PK-15 (B). The two major ORFs are indicated by hatched boxes; black arrows show the positions and orientations of ORFs with the potential to encode proteins of more than 45 amino acids.
We also sequenced PCV from tissues from a pig with PMWS in another herd. The nucleotide sequence of this PCV strain, designated PCV ISU-70, was amplified from total DNA isolated from lymph nodes with the primers described above. The sequence of PCV ISU-70 was similar to the sequence of the PCV ISU-31 strain, with a genome size of 1,768 nucleotides and an overall DNA homology of 97% and homologies of 98 and 94% in ORF1 and ORF2, respectively. DNA homology of PCV ISU-70 with PCV PK-15 was 76%. Genome comparisons showed that the PCV strains from the pigs with PMWS are closely related to each other and are significantly different from PCV PK-15.
In order to test the PK-15 cells used in our experiments for the presence of circovirus contamination, we tested DNA preparations isolated from the continuous pig kidney (PK-15) cell line in PCR with primers specific for PCV PK-15. All attempts to amplify PCV DNA from noninfected PK-15 cells failed. This suggested that the PK-15 cell line we used in our experiments was free of PCV; hence, this cell line was used for virus isolation.
In situ hybridization.
For in situ hybridization, we used tissues from pigs with PMWS which were positive for PCV by PCR. In situ hybridization was performed with sense and antisense RNA probes specific for ORF1 of PCV ISU-31.
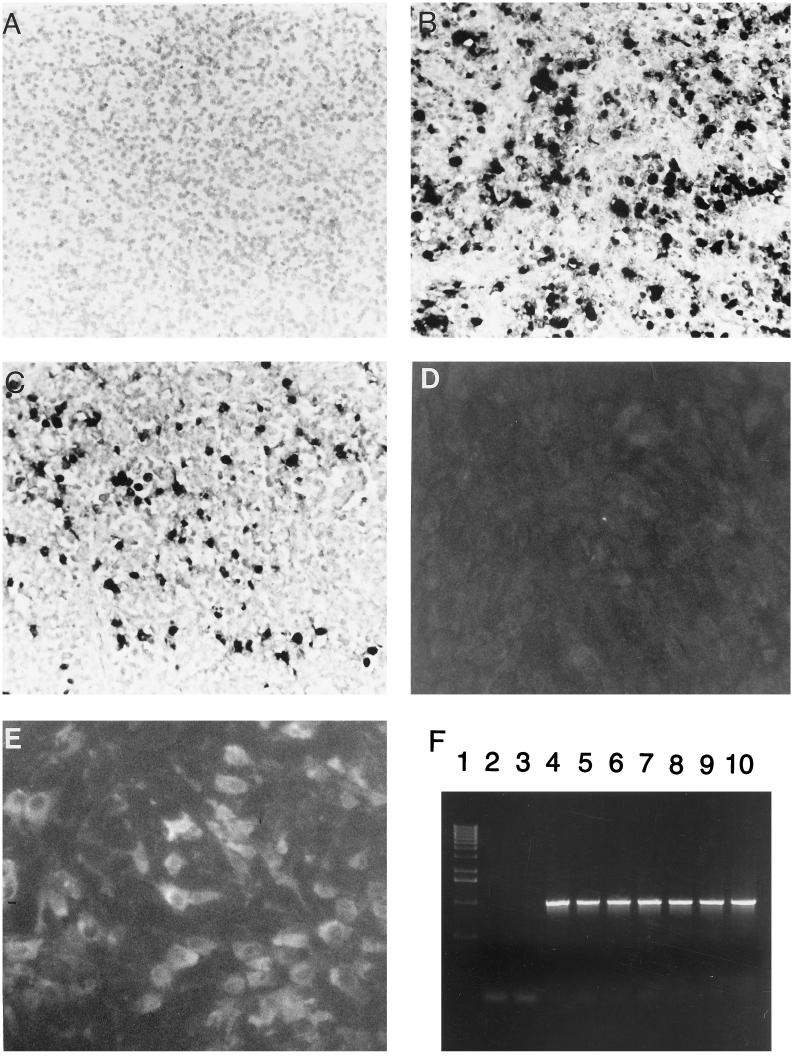
In situ hybridization with the antisense RNA probe demonstrated PCV nucleic acid as a dark purple reaction product in the nucleus, cytoplasm, or both of numerous cells in tissues of pigs with PMWS (Fig. 4B). With the sense RNA probe, signal was present only in the nucleus of the infected cells (Fig. 4C). All negative control slides lacked reaction product (Fig. 4A).
FIG. 4.
Photomicrograph of lymph node from a normal control pig (A) and a pig with PMWS (B and C) hybridized with an antisense RNA probe (A and B) and a sense RNA probe (C). Panels D and E show results of immunofluorescent staining of uninfected PK-15 cells (D) and cells infected with PCV ISU-31 (E) with a polyclonal serum, which was confirmed to be PCV positive by PCR, from a pig with PMWS. A serum dilution of 1:10 was used. (F) Detection of PCV in tissues of pigs with PMWS by PCR with primers PCV75 and PCV1073. Lane 1, molecular weight marker; lane 2, negative (no DNA) control; lane 3, PCR of DNA isolated from noninfected PK-15 cells; lanes 4 to 10, PCR of DNA samples isolated from lymph nodes of pigs with PMWS.
PCV-infected cells were detected in spleen, lymph node, tonsil, liver, heart, lung, nasal turbinate, kidney, pancreas, and large and small intestine samples. The infected cells were predominantly macrophages and small mononuclear cells consistent with lymphocytes in spleen, lymph node, and tonsil. In large and small intestines, the infected cells were predominantly enterocytes, macrophages, and small mononuclear cells consistent with lymphocytes in the lamina propria, Peyer’s patches, and lymphoid follicles. In lungs, the infected cells were predominantly alveolar macrophages, lymphocytes, and fusiform cells around the airway (most likely fibroblasts or smooth muscle cells). In the nasal turbinate, the infected cells were fusiform cells in the submucosa. Kupffer cells and a few hepatocytes were the predominant virus-infected cells in the liver. In heart samples, hybridization signal was present in endothelial cells of capillaries. In pancreas samples, the infected cells were predominantly pancreatic acinar epithelial cells, pancreatic duct epithelial cells, fusiform cells around the pancreatic ducts, and endothelial cells. In the kidneys, the infected cells were predominantly fusiform interstitial cells in the cortex and a few renal tubular epithelial cells.
Isolation of virus.
We also attempted isolation of PCV in PK-15 cells. Pooled tissue homogenates of lungs, lymph nodes, and liver of one animal were used for inoculation of PCV-free PK-15 cells. No signs of cytopathic effect were observed. Virus replication was monitored by PCR. All flasks of PK-15 cells inoculated with tissue homogenates were positive by PCR. IIFA was also performed on infected PK-15 cells. Results of IIFA correlated with the PCR data and are shown in Fig. 4D and E. The propagated virus (passage 4) was sequenced and found to be identical to the virus amplified from the original inoculum. We also used primers PCV75 (5′-GGGTGTTCACGCTGAATAATCCTTCCG-3′) and PCV1073 (5′-CCAGGACTACAATATCCGTGTAACC-3′) to amplify a 1,013-bp fragment of PCV genome from tissues of four other pigs diagnosed with PMWS from the case described above. All tissues were PCR positive (Fig. 4F), and sequencing data revealed 100% homology with the genome of PCV ISU-31.
DISCUSSION
PMWS appears to be an emerging disease problem in North American and European swine herds (6, 14, 27, 30). Morbidity is reported to range from 5 to 50% in affected herds, and the case fatality rate approaches 100%. Although PCV infection has been associated with PMWS, the role of PCV infection in the pathogenesis of PMWS is uncertain, particularly since the PCV strain isolated from PK-15 cells is reported to be nonpathogenic (2).
In this study, PCV was detected by PCR and in situ hybridization in tissues of all six pigs exhibiting lesions consistent with PMWS. In two of these pigs, PRRSV antigen was demonstrated by immunohistochemistry. PRRSV is the causative agent of PRRS and is widespread in North America and Europe (1). PRRSV is a single-stranded positive sense RNA virus which belongs to the Arteriviridae family and causes respiratory disease and reproductive failure in pigs (24–26). PRRSV can be easily detected and differentiated from PCV by PCR, in situ hybridization, immunohistochemistry, and IIFA (5, 9, 10, 16, 21). Although both PRRS and PMWS are characterized by lymphohistiocytic interstitial pneumonia, lesions present in lymphoid tissues in the pigs in this study were characteristic of PMWS. Whereas PRRSV induces marked follicular hyperplasia of lymphoid tissues (11, 12), depletion of lymphoid tissues and replacement by macrophages and multinucleate giant cells is the hallmark of PMWS (6, 14); this lesion was consistently found in the pigs with PMWS in this study and was the major feature upon which the diagnosis of PMWS was based.
PCV was isolated from tissues of one animal and genetically characterized. Genome comparisons showed significant differences between this strain, designated PCV ISU-31, and the previously characterized PCV strain isolated as a contaminant of the porcine continuous cell line PK-15. Overall nucleotide sequence homology was 76%. ORF1 was the most conserved, with 83% nucleic acid identity and 86% amino acid identity. ORF2 was more variable, with a nucleic acid identity of 67% and amino acid identity of 65%. The patterns of small ORFs also were significantly different. Whether these genetic changes are reflected in phenotypic differences such as biological properties is not known at this time.
Because pPSP.PCV1, used for in situ hybridization, contains an insert of the PCV genome, including 36 nucleotides of noncoding region and 494 nucleotides from the 5′ end of ORF1, the antisense RNA probe was expected to detect positive-strand viral DNA and double-stranded replicative-form DNA in the nucleus of the PCV-infected cells and mRNA of ORF1 in the cytoplasm of the PCV-infected cells. The sense strand RNA probe was expected to detect only the double-stranded replicative-form DNA. The nuclear staining resulting from hybridization with the sense strand RNA probe in this study suggests that the replicative intermediate form of PCV is present primarily in the nucleus of infected cells. Comparisons of consecutive slides hybridized with sense and antisense probes showed that the number of positive cells detected with the antisense strand probe was three to four times higher than that detected with the sense strand probe, making the antisense probe a much better choice for the detection of PCV in clinical samples. Many positive cells with only cytoplasmic staining were detected by the antisense probe, suggesting a significant accumulation of ORF1 mRNA and/or genomic DNA during viral replication.
The infected cells included macrophages, small mononuclear cells consistent with lymphocytes, endothelial cells, enterocytes, and pancreatic acinar epithelial cells. This is in contrast to PCV PK-15, which caused infection only in cells of mononuclear phagocyte lineage (2, 3, 22). PCV PK-15 has been shown to be nonpathogenic in pigs (2, 35). The abundant presence of PCV ISU-31 in the tissues of pigs with PMWS suggests a potentially important role for this PCV strain in PMWS; however, additional studies are needed to understand the significance of PCV in PMWS. The PCR and in situ hybridization tests developed in this study provide molecular tools for epidemiological studies of PCV infection and should be beneficial to further studies of the role of PCV in PMWS.
The common occurrence of PCV infection in swine, the demonstration of PCV-specific antibodies in humans (32), the genetic diversity among PCV strains, and the ability of PCV to cause persistent infection at least in vitro suggest the need for risk assessment of PCV as a potential zoonotic agent, considering the immense interest in the use of pigs in xenotransplantation. The PCR and in situ hybridization tests described here should provide the ability to screen pigs for PCV infection in order to provide safer donors for xenotransplantation.
REFERENCES
- 1.Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997;55:309–316. doi: 10.1016/s0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- 2.Allan G M, McNeilly F, Cassidy J P, Reilly G A C, Adair B, Ellis W A, McNulty M S. Pathogenesis of porcine circovirus, experimental infections of colostrum deprived piglets and examination of pig fetal material. Vet Microbiol. 1995;44:49–64. doi: 10.1016/0378-1135(94)00136-k. [DOI] [PubMed] [Google Scholar]
- 3.Allan G M, Phenix K V, Todd D, McNulty M S. Some biological and physico-chemical properties of Porcine Circovirus. J Vet Med B. 1994;41:17–26. doi: 10.1111/j.1439-0450.1994.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 4.Boevink P, Chu P W G, Keese P. Sequence of Subterranean Clover Stunt Virus DNA: affinities with the Geminiviruses. Virology. 1995;207:354–361. doi: 10.1006/viro.1995.1094. [DOI] [PubMed] [Google Scholar]
- 5.Botner A. Diagnosis of PRRS. Vet Microbiol. 1997;55:295–301. doi: 10.1016/s0378-1135(96)01333-8. [DOI] [PubMed] [Google Scholar]
- 6.Clark E G. Proceedings of the American Association of Swine Practitioners. Quebec City, Quebec, Canada: American Association of Swine Practitioners; 1997. Post-weaning multisystemic wasting syndrome; pp. 499–501. [Google Scholar]
- 7.Dulac G C, Ahmad A. Porcine circovirus antigens in PK-15 cell line (ATCC CCL-33) and evidence of antibodies to circovirus in Canadian pigs. Can J Vet Res. 1989;53:431–433. [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards S, Sands J J. Evidence of circovirus infection in British pigs. Vet Rec. 1994;134:680–681. doi: 10.1136/vr.134.26.680. [DOI] [PubMed] [Google Scholar]
- 9.Halbur P G, Andrews J J, Huffman E L, Paul P S, Meng X-J, Niyo Y. Development of streptavidin-biotin immunoperoxidase procedure for the detection of the porcine reproductive and respiratory syndrome virus antigen in porcine lung. J Vet Diagn Investig. 1994;6:254–257. doi: 10.1177/104063879400600219. [DOI] [PubMed] [Google Scholar]
- 10.Halbur P G, Miller L D, Paul P S, Meng X J, Huffman E L, Andrews J J. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of three-week-old colostrum-deprived pigs. Vet Pathol. 1995;32:200–204. doi: 10.1177/030098589503200218. [DOI] [PubMed] [Google Scholar]
- 11.Halbur P G, Paul P S, Frey M L, Landgraf J, Eernisse K, Meng X J, Andrews J J, Lum M A, Rathje J A. Comparison of the antigen distribution of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1996;33:159–170. doi: 10.1177/030098589603300205. [DOI] [PubMed] [Google Scholar]
- 12.Halbur P G, Paul P S, Frey M L, Landgraf J, Eernisse K, Meng X J, Lum M A, Andrews J J, Rathje J A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 13.Harding J C. Proceedings of the American Association of Swine Practitioners. Quebec City, Quebec, Canada: American Association of Swine Practitioners; 1997. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation; p. 503. [Google Scholar]
- 14.Harding J C S, Clark E G. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS) Swine Health Prod. 1997;5:201–203. [Google Scholar]
- 15.Harding R M, Burns T M, Hafner G, Dietzgen R G, Dale J L. Nucleotide sequence of one component of the banana bunchy top virus genome contains a putative replicase gene. J Gen Virol. 1993;74:323–328. doi: 10.1099/0022-1317-74-3-323. [DOI] [PubMed] [Google Scholar]
- 16.Haynes J S, Halbur P G, Sirinarumitr T, Paul P S, Meng X J, Huffman E L. Temporal and morphologic characterization of the distribution of porcine reproductive and respiratory syndrome virus (PRRSV) by in situ hybridization in pigs infected with isolates of PRRSV that differ in virulence. Vet Pathol. 1997;34:39–43. doi: 10.1177/030098589703400106. [DOI] [PubMed] [Google Scholar]
- 17.Hines R K, Lukert P D. Proceedings of the American Association of Swine Practitioners. Chicago, Ill: American Association of Swine Practitioners; 1994. Porcine circovirus as a cause of congenital tremors in newborn pigs; pp. 344–345. [Google Scholar]
- 18.Hines R K, Lukert P D. Porcine circovirus: a serological survey of swine in the United States. Swine Health Prod. 1995;3:71–73. [Google Scholar]
- 19.Koonin E V, Ilyina T V. Computer assisted dissection of rolling circle DNA replication. BioSystems. 1993;30:241–268. doi: 10.1016/0303-2647(93)90074-m. [DOI] [PubMed] [Google Scholar]
- 20.Mankertz A, Persson F, Mankertz J, Blaess G, Buhk H-J. Mapping and characterization of the origin of DNA replication of porcine circovirus. J Virol. 1997;71:2562–2566. doi: 10.1128/jvi.71.3.2562-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mardassi H, Wilson L, Mounir S, Dea S. Detection of porcine reproductive and respiratory syndrome virus and efficient differentiation between Canadian and European strains by reverse transcription and PCR amplification. J Clin Microbiol. 1994;32:2197–2203. doi: 10.1128/jcm.32.9.2197-2203.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeilly F, Allan G M, Foster J C, Adiar B M, McNulty M S. Effect of porcine circovirus infection on porcine alveolar macrophage function. Vet Immunol Immunopathol. 1996;49:295–306. doi: 10.1016/0165-2427(95)05476-6. [DOI] [PubMed] [Google Scholar]
- 23.Meehan B M, Creelan J L, McNulty M S, Todd D. Sequence of porcine circovirus DNA: affinities with plant circoviruses. J Gen Virol. 1997;78:221–227. doi: 10.1099/0022-1317-78-1-221. [DOI] [PubMed] [Google Scholar]
- 24.Meng X J, Paul P S, Halbur P G, Lum M A. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch Virol. 1995;140:745–755. doi: 10.1007/BF01309962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng X J, Paul P S, Halbur P G, Morozov I. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1995;76:3181–3188. doi: 10.1099/0022-1317-76-12-3181. [DOI] [PubMed] [Google Scholar]
- 26.Meulenberg J J, Petersen den Besten A, de Kluyver E, van Nieuwstadt A, Wensvoort G, Moormann R J. Molecular characterization of Lelystad virus. Vet Microbiol. 1997;55:197–202. doi: 10.1016/S0378-1135(96)01335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayar G P S, Andre H, Lihua L. Detection and characterization of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. Can Vet J. 1997;38:384–386. [PMC free article] [PubMed] [Google Scholar]
- 28.Ritchie B W, Niagro F D, Lukert P D, Steffens W L, Latimer K S. Characterization of a new virus from cockatoos with psittacine beak and feather disease. Virology. 1989;171:83–88. doi: 10.1016/0042-6822(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 29.Rohde W, Randles J W, Langridge P, Harold D. Nucleotide sequence of a circular single stranded DNA associated with coconut foliar decay virus. Virology. 1990;176:648–651. doi: 10.1016/0042-6822(90)90038-s. [DOI] [PubMed] [Google Scholar]
- 30.Segales J, Sitjar M, Domongo M, Dee S, Del Pozo M, Noval R, Saeristan C, De las Heras A, Ferro A, Latimer K S. First report of post weaning multisystemic wasting syndrome in pigs in Spain. Vet Rec. 1997;141:600–601. [PubMed] [Google Scholar]
- 31.Sirinarumitr T, Paul P S, Kluge J P, Halbur P G. In situ hybridization technique for the detection of swine enteric and respiratory coronaviruses, transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCV), in formalin-fixed paraffin-embedded tissues. J Virol Methods. 1996;56:149–160. doi: 10.1016/0166-0934(95)01901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischer I, Bode J, Timm H, Peters D, Rasch R, Pociuli S, Gerike E. Presence of antibodies reacting with porcine circovirus in sera of humans, mice, and cattle. Arch Virol. 1995;140:1427–1439. doi: 10.1007/BF01322669. [DOI] [PubMed] [Google Scholar]
- 33.Tischer I, Bode L, Peters D, Pociuli S, Germann B. Distribution of antibodies to porcine circovirus in swine populations of different breeding farms. Arch Virol. 1995;140:737–743. doi: 10.1007/BF01309961. [DOI] [PubMed] [Google Scholar]
- 34.Tischer I, Geldblom H, Vettermann W, Koch M A. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64–66. doi: 10.1038/295064a0. [DOI] [PubMed] [Google Scholar]
- 35.Tischer I, Mields W, Wolff D, Vagt M, Greim W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271–276. doi: 10.1007/BF01314286. [DOI] [PubMed] [Google Scholar]
- 36.Tischer I, Peters D, Rasch R, Pociuli S. Replication of porcine circovirus: induction by glycosamine and cell cycle dependence. Arch Virol. 1987;96:39–57. doi: 10.1007/BF01310989. [DOI] [PubMed] [Google Scholar]
- 37.Tischer I, Rasch R, Tochtermann G. Characterization of papovavirus- and picornavirus-like particles in permanent pig kidney cell lines. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1974;226:153–167. [PubMed] [Google Scholar]
- 38.Todd D, Creelan J L, Mackie D P, Rixon F, McNulty M S. Purification and biochemical characterization of chicken anaemia agent. J Gen Virol. 1990;71:819–823. doi: 10.1099/0022-1317-71-4-819. [DOI] [PubMed] [Google Scholar]
- 39.Todd D, Niagro F D, Ritchie B W, Curran W, Allan G M, Lukert P D, Latimer K S, Steffens W L, McNulty M S. Comparison of three animal viruses with circular single-stranded DNA genomes. Arch Virol. 1991;117:129–135. doi: 10.1007/BF01310498. [DOI] [PubMed] [Google Scholar]