Abstract
Introduction:
Heart involvement is a common problem in systemic sclerosis. Recently, a definition of systemic sclerosis primary heart involvement had been proposed. Our aim was to establish consensus guidance on the screening, diagnosis and follow-up of systemic sclerosis primary heart involvement patients.
Methods:
A systematic literature review was performed to investigate the tests used to evaluate heart involvement in systemic sclerosis. The extracted data were categorized into relevant domains (conventional radiology, electrocardiography, echocardiography, cardiac magnetic resonance imaging, laboratory, and others) and presented to experts and one patient research partner, who discussed the data and added their opinion. This led to the formulation of overarching principles and guidance statements, then reviewed and voted on for agreement. Consensus was attained when the mean agreement was ⩾7/10 and of ⩾70% of voters.
Results:
Among 2650 publications, 168 met eligibility criteria; the data extracted were discussed over three meetings. Seven overarching principles and 10 guidance points were created, revised and voted on. The consensus highlighted the importance of patient counseling, differential diagnosis and multidisciplinary team management, as well as defining screening and diagnostic approaches. The initial core evaluation should integrate history, physical examination, rest electrocardiography, trans-thoracic echocardiography and standard serum cardiac biomarkers. Further investigations should be individually tailored and decided through a multidisciplinary management. The overall mean agreement was 9.1/10, with mean 93% of experts voting above 7/10.
Conclusion:
This consensus-based guidance on screening, diagnosis and follow-up of systemic sclerosis primary heart involvement provides a foundation for standard of care and future feasibility studies that are ongoing to support its application in clinical practice.
Keywords: Systemic sclerosis, cardiac involvement, consensus guidance, screening, diagnosis, follow-up
Introduction
Systemic sclerosis (SSc) is a chronic connective tissue disorder characterized by vasculopathy, inflammation/autoimmunity and fibrosis1–3 that may affect different organs and at different times during the disease evolution, resulting in heterogeneous clinical scenarios. Cardiac involvement in SSc is frequently referred to as “the silent killer.” In the EUSTAR cohort study from Elhai et al., 4 SSc primary heart involvement (SSc-pHI) was deemed to be the cause of 12% of SSc-related deaths. Similar data were also seen in a combined Australian-Canadian cohort by Hao et al., 5 who identified 9% of the mortality events in their prevalent cohort as being related to myocardial involvement. In both studies, cardiac involvement was not defined according to pre-defined criteria and the adjudication was by physician opinion.
Different sets of expert consensus algorithms are available for the detection, follow-up, and treatment of SSc-pHI patients. Among them, the UK Systemic Sclerosis Study Group first provided guidance for physicians, stressing the importance of examining both symptomatic and asymptomatic patients, as well as the need to take the general population’s cardiovascular risk factors into account. 6 More recently, a Greek cardiology-rheumatology collaboration group proposed a management algorithm that was based on a two-step approach to evaluate SSc patients and placed different tests in different tiers of priority. 7
Indeed, there are a plethora of first-, second- and third-level tests that can be performed on patients with SSc for the identification and follow-up of cardiac complications. However, each of them identifies only one or a few specific manifestations of SSc-pHI: for example, resting electrocardiography (ECG) and monitoring mostly detect fixed conduction defects and arrhythmia, resting standard trans-thoracic echocardiography (sTTE) identifies motion abnormality and contractility impairment, while cardiac magnetic resonance (CMR) is a more sensitive multiparametric test that can also detect tissue characteristics indicative of inflammatory and fibrotic changes. 8 Given the diverse manifestations included in the “cardiac scleroderma spectrum,” the different tests should allow for comprehensive but feasible evaluation, taking into consideration time, cost, and availability.
This article will support physicians in identifying SSc-pHI in daily practice by (1) reviewing the literature for cardiac diagnostic tests used in SSc and (2) providing consensus guidance for the screening, diagnosis and monitoring of SSc-pHI.
Methods
Systematic literature review
Patient-Exposure-Outcome (PEO) questions were formulated, investigating the use of assessments to evaluate cardiac structure and function in SSc (Supplement Annex 1). Using the search string applied for our recent literature review (SLR), 9 a systematic literature review was performed on three databases (EMBASE, PubMed, Web of Science), from inception to 31 December 2019. Articles in English, including ⩾10 adult SSc patients or cohorts in which SSc patient data could be separately extracted, with cardiac involvement or cardiac evaluation as primary target, were included. Non-human studies, pediatric age (<18 years), secondary cardiac involvement, articles in a language other than English, full-text not available and literature reviews (after careful checking of the bibliography for any articles not included in the evaluation) represented the main exclusion criteria. Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) recommendations were followed, where applicable.
Study selection and data abstraction
A single author (C.B.) performed the de-duplication using the reference software EndNote. The articles were then screened according to title and abstract evaluation by two reviewers (C.B., G.D.L.), with a third author giving inputs when disagreements occurred (M.H.B.). Finally, full texts were evaluated by authors in pairs (G.H. and K.B., Y.A.S. and A.B., G.D.L. and C.B., A.L. and A.D., R.B.D. and G.M.M., A.G. and I.M., Y.I. and A.X.), with a third evaluator (M.H.B.) resolving disagreements. To test for consistency, 5% of the papers were evaluated both for title, abstract, and full texts by all extractors.
Outcomes
Data were extracted in agreement with the formulated PEO questions. The design of the study, the criteria used to select the patients, number of patients and female prevalence were also extracted from the manuscripts. In addition, data regarding the test used in the cardiac evaluation and the specific parameters were extracted. Data were presented in terms of absolute frequencies (percentage), mean ± standard deviation, median (interquartile range) according to the terms used in the manuscript of origin.
Expert committee meetings
Aside from the convenors (P.S. and M.M.-C.) and methodologists (C.B. and M.H.B.), the expert committee included 18 senior members from Europe (n = 15), North America (n = 2), and Asia (n = 1), comprising 9 cardiologists (E.B., L.G., S.M., A.P., A.L.P.C., S.P., C.T., A.D., A.R.) and 9 SSc experts including rheumatologist, dermatologists and immunologists (R.M., P.S., T.K., O.D,. Y.A., C.D., D.K., D.E.F., M.K.). The committee participated in a series of virtual meetings between November 2020 and July 2021. Input from a patient research partner (PRP-IG) was also provided during all meetings and voting processes.
Methodology of formulation of each statement
The results of the SLR were presented to the expert committee during three meetings, covering different topics (Laboratory and ECG for the first; sTTE for the second, cardiovascular magnetic resonance (CMR) and “other tests” for the third). The data were separated according to the nature of the patients included, namely whether there was a high suspicion of or previously diagnosed heart involvement (with investigations therefore applied for diagnostic or monitoring purposes, respectively) or no known heart involvement (with tests therefore screening in nature). If reported, the comparison with the control group was also presented.
The data were presented and discussed by the expert committee, whose members were asked to specify which of the discussed tests they would recommend, in which category of patients and when (in terms of both timing in the disease course and frequency).
The results of each meeting were then summarized into statements (C.B., M.H.B., M.M.-C., P.S.), which underwent further revision by the expert committee: first in terms of content, then for clarity. Finally, the revised statements were voted upon for agreement, with a scale ranging from 1 (strongly disagree) to 10 (strongly agree). Each statement required a mean agreement ⩾7/10 and by ⩾70% of voters to be accepted as a consensus statement.
Results
Data from the systematic literature review
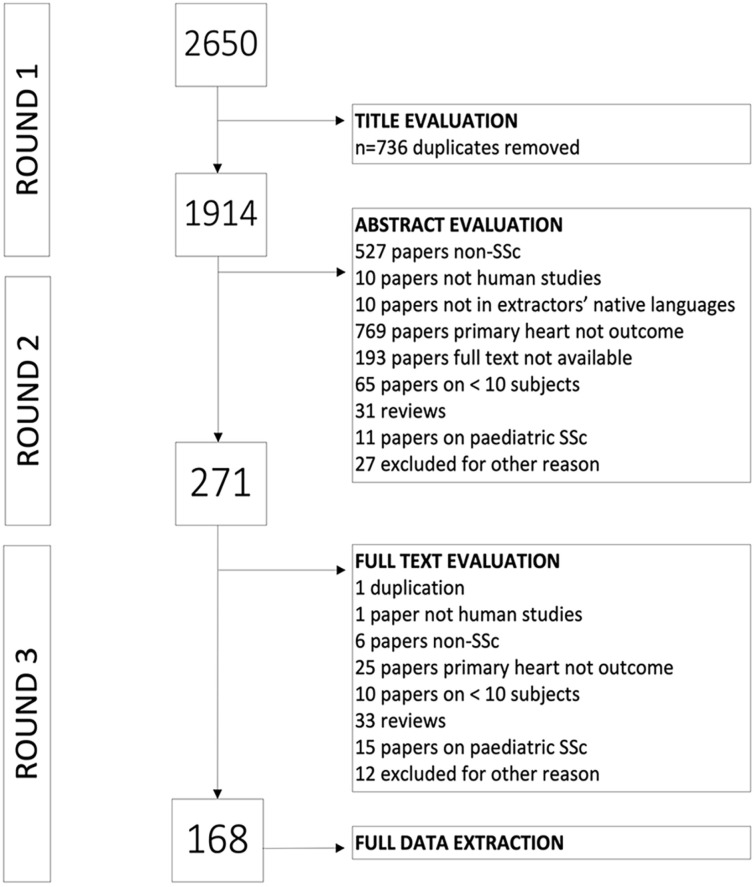
Among 2650 publications retrieved from the three databases, 168 manuscripts underwent data extraction (see PRISMA graph, Figure 1). The reproducibility exercise confirmed a level of agreement of 94% on manuscript selection and data extraction.
Figure 1.
PRISMA scheme of the evaluation and selection procedure of scientific articles for the systematic review of the literature.
The 168 articles reported cross-sectional (n = 70), prospective (n = 50), and retrospective (n = 23) studies. Among 28,723 patients included in the manuscripts, (n = 23,396, 83.3% were female) from 164 articles, 15.1% to 100% were classified as SSc by the American College of Rheumatology/EUropean League Against Rheumatism 2013 criteria (n = 45) or American Rheumatology Association 1980 criteria (n = 75), although multiple sets of criteria (n = 24) or unspecified criteria (n = 24) were also recorded. Patients were mostly enrolled in the studies as consecutive cases (n = 100), as subgroups of patients without cardiac involvement or pulmonary arterial hypertension (PAH) or suspicion of the presence of either one or the other (n = 54). The remaining studies comprised patients presenting with cardiac involvement or known cardiac symptoms (n = 11). Among 97 manuscripts with controls, 2964 age- and sex-matched healthy individuals constituted the control groups.
The results of the SLR are reported in the supplementary files (Supplementary Tables 1–8), divided according to the predefined domains used, thus listing the parameters reported for the single test category. Overall, heterogeneity of data was observed, in terms of both tests used and parameters reported, which were frequently derived from small samples of patients involved.
Meeting sessions: creating the consensus guidance
The discussion held during the three virtual meetings allowed the generation of a list of 17 statements, which were divided into “overarching principles” and “consensus guidance statements.” After content and linguistic revision, the whole committee voted over the 7 overarching principles and the 10 consensus guidance statements to reach agreement (Tables 1–3). None of the originally created statements were discarded, either for agreement lower than the established threshold (<7/10 agreement) or for a low number of voters above the predefined cutoff (<70% of the committee). The overall mean agreement of the guidance points was 9.1/10, with mean 93% of experts voting above 7/10.
Table 1.
Overarching principles of the consensus guidance for the screening, diagnosis and follow-up of systemic sclerosis primary heart involvement.
| Overarching principles | n voting | Mean agreement (OK if ⩾7) | % voters <70% (OK if ⩽30) | |
|---|---|---|---|---|
| OP1 | These recommendations refer to the definition of systemic sclerosis–related primary heart involvement (SSc-pHI) 9 | 12 | 8.42 | 17 |
| OP2 | SSc-pHI should be considered particularly in the early stages of the disease, but it may also be present and develop throughout the disease course of a patient with SSc | 13 | 9.38 | 8 |
| OP3 | The patient should be counseled about the symptoms and consequences of SSc-pHI to raise their awareness and to ensure the importance of reporting symptoms to the physician | 14 | 9.71 | 7 |
| OP4 | Where suspicion for SSc-pHI exists, acute and chronic coronary syndromes should be considered and managed in line with current guidelines | 14 | 9.21 | 0 |
| OP5 | The differential diagnosis and management of SSc-pHI should be undertaken by a multi-disciplinary team that comprises cardiologist(s) (with necessary subspecialist expertise as indicated) and rheumatologists with SSc expertise | 15 | 9.00 | 7 |
| OP6 | Screening refers to the assessment of asymptomatic patients with no known SSc-pHI, who can be further stratified into those who are considered “at higher risk” and those who should be considered “at lower risk” of developing heart involvement | 14 | 8.43 | 14 |
| OP7 | Diagnosis refers to the assessment of patients presenting with symptoms and/or signs and/or investigations compatible with possible SSc-pHI | 15 | 8.47 | 13 |
Table 2.
Guidance statements of the consensus guidance for the screening, diagnosis, and follow-up of systemic sclerosis primary heart involvement.
| Consensus guidance statements | n voting | Mean agreement (OK if ⩾7) | % voters <7 (OK if ⩽30%) | |
|---|---|---|---|---|
| ST1 | The diagnostic workup of SSc-pHI should comprise an integration of history (cardiac red flag symptoms), physical examination, and laboratory/imaging/ electrocardiography results and should be tailored to the individual | 14 | 9.86 | 0 |
| ST2 | Physicians should counsel patients and caregivers in layperson language, providing detailed information on SSc-pHI, its symptoms and signs, diagnostic and monitoring procedures. The information should highlight the importance of reporting symptoms to the multidisciplinary team | 14 | 9.86 | 0 |
| ST3 | Screening for SSc-pHI should be performed in every patient at time of SSc diagnosis. Follow-up evaluations should be considered | 15 | 8.80 | 7 |
| ST4 | Asymptomatic SSc patients with no history of heart involvement should have a core annual assessment, which may coincide with annual pulmonary arterial hypertension surveillance. Core assessment would comprise electrocardiogram, standard trans-thoracic echocardiography and serum cardiac biomarkers, such as hs-troponin, NT-proBNP, or BNP |
15 | 9.33 | 0 |
| ST5 | Screening with Cardiac Magnetic Resonance may be considered in asymptomatic patients with no history of heart involvement and on a case-by-case basis | 14 | 8.21 | 21 |
| ST6 | Symptoms suggestive of SSc-pHI should trigger-specific assessment. This includes initial core evaluation with electrocardiogram, standard trans-thoracic echocardiography and serum cardiac biomarkers, such as hs-troponin, NT-proBNP, or BNP | 14 | 9.93 | 0 |
| ST7 | Cardiac magnetic resonance should be included as part of the diagnostic work up where suspicion for SSc-pHI remains following positive findings from the initial core evaluation | 13 | 9.23 | 0 |
| ST8 | In patients with confirmed SSc-pHI or clinically suspected myocarditis, with or without myocardial abnormalities on cardiac magnetic resonance, endomyocardial biopsy may be indicated in line with European Society of Cardiology guidelines and position statements, after exclusion of coronary artery disease | 12 | 8.67 | 17 |
| ST9 | Where SSc-pHI is confirmed, Holter monitoring is recommended as the first-line assessment to evaluate for the arrhythmia burden and standard trans-thoracic echocardiography for the evaluation of the cardiac chambers and function. Other tests may be considered in consultation with appropriate cardiology expertise | 12 | 8.50 | 0 |
| ST10 | Management of confirmed SSc-pHI (including frequency of monitoring and nature of testing) should be tailored to the individual patient’s clinical scenario, discussed, and agreed upon by the multidisciplinary team | 13 | 9.31 | 0 |
SSc: systemic sclerosis; SSc-pHI: systemic sclerosis–related primary heart involvement; BNP: B-type natriuretic peptide; NT-proBNP: N-terminal pro-brain natriuretic peptide; hs-troponin: high-sensitivity troponin.
Table 3.
Flowchart of the assessments of SSc patients, according to their cardiac disease status.
| Status | Medical history | Clinical examination | Laboratory biomarkers | ECG | sTTE | CMR | Further tests | |
|---|---|---|---|---|---|---|---|---|
| No prior diagnosis of SSc-pHI | Asymptomatic—low risk | Annual | Annual | Annual | Annual resting ECG | Annual | May be considered on a case-by-case basis | To be guided by the MDT according to the results of the core assessment, on a case-by-case evaluation |
| Asymptomatic—high risk | Annual | Annual | Annual | Annual—ECG Holter may be considered | Annual | May be considered on a case-by-case basis | ||
| Symptomatic | Annual, unless timing adjusted by the MDT | Annual, unless timing adjusted by the MDT | Annual, unless timing adjusted by the MDT | Annual ECG/ECG Holter, unless timing adjusted by the MDT | Annual | Should be considered if suspicion remains | ||
| Diagnosed with SSc-pHI | Annual, unless timing adjusted by the MDT Specific follow-up assessments set tailored by the MDT |
To be guided by the MDT according to the diagnosis and treatment, on a case-by-case evaluation | ||||||
CMR: cardiac magnetic resonance; ECG: electrocardiography; MDT: multidisciplinary team; sTTE: standard trans-thoracic echocardiography; SSc: systemic sclerosis; SSc-pHI: systemic sclerosis–related primary heart involvement.
Overarching principles
These recommendations refer to the definition of SSc-pHI. 9
SSc-pHI should be considered particularly in the early stages of the disease, but it may also be present and develop throughout the disease course of a patient with SSc.
The patient should be counseled about the symptoms and consequences of SSc-pHI to raise their awareness and to ensure the importance of reporting symptoms to the physician.
The World Scleroderma Foundation/Heart Failure Association definition created during the previous steps of this initiative proposed that “SSc-pHI comprises cardiac abnormalities that are predominantly attributable to SSc rather than other causes and/or complications (including non-SSc-specific cardiac conditions (e.g. ischemic heart disease, arterial hypertension, drug toxicity, other cardiomyopathies, primary valvular disease) or SSc non-cardiac conditions (e.g. PAH, renal involvement, interstitial lung disease)); SSc-pHI may be subclinical and must be confirmed through diagnostic investigation; The pathogenesis of SSc-pHI comprises one or more of inflammation, fibrosis, and vasculopathy”. 9 The expert committee further supported the previously proposed definition of SSc-pHI, confirming SSc-pHI patients as the main target population of the current step of the project. In addition, the committee underlined the possibility for SSc-pHI to manifest at any stage of the disease, but with closer attention in the early disease phase in diffuse cutaneous SSc. Finally, there was overall agreement that the patient should be actively and specifically questioned about cardiac red flag symptoms and be educated and motivated to report such symptoms during medical consultations.
4. Where suspicion for SSc-pHI exists, acute and chronic coronary syndromes should be considered and managed in line with current guidelines.
5. The differential diagnosis and management of SSc-pHI should be undertaken by a multidisciplinary team (MDT) that comprises cardiologist(s) (with necessary subspecialist expertise as indicated) and rheumatologists with SSc expertise.
Multidisciplinary management between cardiology and non-cardiology SSc experts was strongly recommended, when possible and feasible. Physicians caring for patients with SSc may bring those with high-risk “scleroderma” profile features to the attention of cardiologists to support more rapid cardiac assessment as indicated. Similarly, cardiologists may recommend a timelier assessment based on specific signs or symptoms, taking into consideration the differential diagnosis and other cardiac complications not primarily related to SSc. The evaluation and ongoing management of patients by a cardiologist experienced with SSc were suggested where feasible.
6. Screening refers to the assessment of asymptomatic patients with no known SSc-pHI, who can be further stratified into those who are considered “at higher risk” and those who should be considered “at lower risk” of developing heart involvement.
7. Diagnosis refers to the assessment of patients presenting with symptoms and/or signs and/or investigations compatible with possible SSc-pHI.
The expert committee agreed that “screening” refers to the assessment of patients with no known history of heart involvement and/or those considered to be at higher risk of SSc-pHI. “Diagnosis” refers to the assessment of patients presenting with symptoms/signs compatible with SSc-pHI. The identification of patients at higher risk of SSc-pHI was highlighted as an area of particular importance, with more effective definition and refinement of clinical suspicion considered an important unmet need. From the literature to date, a high-risk SSc-pHI clinical profile includes various demographic, serological, and clinical features, which are summarized in Table 4. The committee decided not to specifically mention risk factors for SSc-pHI in the statement itself, given the heterogeneity of the cardiac outcomes or cardiac definitions used to identify them and the need to replicate the results using the currently proposed definition of SSc-pHI, which may not only confirm the same but identify new predictors for this complication.
Table 4.
Risk factors for cardiac outcomes or cardiac disease identified in previous studies of SSc patients.
| Risk factor | Cardiac outcome or definition of SSc-cardiac disease | Reference |
|---|---|---|
| Male gender | Left ventricular systolic dysfunction on TTE | Allanore et al. 10 |
| Black ethnicity | Pericarditis | Laing et al. 11 |
| Easter European | Palpitations, conduction block, diastolic and systolic LV dysfunction | Walker et al. 12 |
| Older age of onset | Left ventricular systolic dysfunction on TTE | Allanore et al. 10 |
| Conduction blocks, diastolic dysfunction | Carreira et al. 13 | |
| More severe cardiac disease according to the Medsger severity scale | Medsger et al. 14 | |
| Diffuse cutaneous SSc | At least one of the following: pericarditis, congestive heart failure, severe arrhythmias, and/or atrioventricular conduction abnormalities | Ferri et al. 15 |
| Diastolic dysfunction and conduction block | Hunzelmann et al. 16 | |
| Palpitations | Walker et al. 12 | |
| Supra-ventricular or ventricular tachycardia | Kostis et al. 17 | |
| Higher mRSS | Presence of LGE and higher ECV on CMR | Dumitru et al. 18 |
| Higher T1 values on CMR | Lee et al., 19 Terrier et al. 20 | |
| Anti-Topoisomerase I antibodies | Conduction disturbances and abnormal ECGs | Hunzelmann et al. 16 |
| Cardiomyopathy, symptomatic pericarditis, or an arrhythmia requiring treatment | Steen and Medsger 21 | |
| Anti-Ku antibodies | Cardiac involvement defined as left-sided congestive heart failure (LV ejection fraction < 45%) or pericarditis by TTE or CMR imaging, arrhythmia requiring treatment, or conduction defect | Rodriguez-Reyna et al. 22 |
| Anti-histone antibodies | Abnormal ECG | Hesselstrandet al. 23 |
| Anti-RNA polymerase antibodies | Any of the following: symptomatic pericarditis, clinical evidence of LV congestive heart failure not attributable to any other condition, or conduction defect or arrhythmias requiring treatment | Kuwana et al. 24 |
| Anti-U3RNP antibodies | Cardiomyopathy, symptomatic pericarditis, or an arrhythmia requiring treatment | Steen and Medsger 21 |
| Digital ulcers | Left ventricular systolic dysfunction on TTE | Allanore et al. 10 |
| Higher ECV and higher MPR on CMR | Dumitru et al. 18 | |
| Increased T2 ratio on CMR | Mavrogeniet al. 25 | |
| Tendon friction rubs | Cardiac involvement not defined | Steen and Medsger 26 |
| Myositis | Left ventricular systolic dysfunction on TTE | Allanore et al. 10 |
| Lung involvement | Left ventricular systolic dysfunction on TTE | Allanore et al. 10 |
| Higher T1 values on CMR | Terrier et al. 20 | |
| Higher HAQ-DI score | Severe heart (or kidney) involvement | Steen and Medsger 27 |
| More severe heart disease based on the ECG, ejection fraction, and NYHA functional class findings | Morita et al. 28 | |
| Late NVC pattern | Moderate to severe cardiac disease defined with the Medsger severity scale | Medsger et al. 14 |
CMR: cardiac magnetic resonance; ECG: electrocardiogram; ECV: extracellular volume; EF: ejection fraction; HAQ-DI: Health assessment questionnaire–Disability Index; LV: left ventricle; MPR: myocardial perfusion reserve; mRSS: modified Rodnan’s skin score; NVC: nailfold videocapillaroscopy; NYHA: New York Heart Association; SSc: systemic sclerosis; TTE: trans-thoracic echocardiography.
Consensus statements
The diagnostic workup of SSc-pHI should comprise an integration of history (cardiac red flag symptoms), physical examination, and laboratory/imaging/ECG results and should be tailored to the individual.
The importance of including cardiac evaluation as part of regular SSc patient assessment to detect SSc-pHI early was supported by the availability of non-invasive tests and the prognostic importance. Presence of symptoms (cardiac red flags such as dyspnea, chest pain, palpitations, syncope, dizziness) and cardiovascular physical examination raising the suspicion for cardiac involvement were deemed a pivotal part of the medical consultation.
2. Physicians should counsel patients and caregivers in layperson language, providing detailed information on SSc-pHI, its symptoms and signs, diagnostic, and monitoring procedures. The information should highlight the importance of reporting symptoms to the MDT.
Further emphasizing overarching principle 3, the committee agreed on a specific statement on the importance of promoting the understanding of the patients about cardiac SSc-related complications, educating and motivating the patients to report such symptoms; as well as on the assessments needed for its screening, diagnosis, and follow-up evaluation.
3. Screening for SSc-pHI should be performed in every patient at the time of SSc diagnosis. Follow-up evaluations should be considered.
4. Asymptomatic SSc patients with no history of heart involvement should have a core annual assessment, which may coincide with annual pulmonary arterial hypertension surveillance. Core assessment would comprise electrocardiogram, sTTE, and serum cardiac biomarkers such as high-sensitivity troponin (hs-troponin), NT-proBNP, or BNP.
5. Screening with CMR may be considered in asymptomatic patients with no history of heart involvement and on a case-by-case basis.
Given the possibility of SSc-pHI in the early inflammatory stages of SSc, the expert committee suggested assessment for pHI should take place from the time of SSc diagnosis. The expert committee advised at least one annual assessment with hs-troponin and NT-proBNP for unselected stable/asymptomatic patients to identify patients with possible subclinical abnormalities if appropriate. C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and creatine kinase (CK) were also suggested every year, as a nonspecific workup, which could indicate cardiac disease (acknowledging articular and/or inflammatory muscle involvement may confound these tests). The expert committee indicated BNP as more reliable in patients with renal failure when compared to NT-proBNP (which is recommended in patients with systolic heart failure). It was also recommended that physicians should be aware of statin use and elevated CK levels, often asymptomatic. Regarding ECG, the expert committee suggested annual resting ECG to pick up fixed abnormalities, while annual ECG-Holter may be considered in selected patients with a higher-risk profile, if feasible. As a general consideration, the expert committee stressed the importance of taking concomitant medications (i.e. β-blockers, antidepressants) and metabolic disorders (such as potassium disorders) into account, when evaluating conduction parameters, such as QTc interval. Moreover, there was a suggestion to focus more thoroughly on abnormalities that need a prompt change of treatment, such as atrial fibrillation, malignant arrhythmias (i.e. nonsustained or sustained ventricular tachycardia) and major conduction disorders (leading to pacemaker or cardiac defibrillator implantation). All other abnormalities might be considered minor (as requiring treatments, such as β-blockers). As with previous consensus guidance papers, there was no agreement regarding the standardized performance of CMR for screening purposes, due to the lack of robust evidence. Although asymptomatic patients may have CMR abnormalities, the prognostic significance has not been fully established, therefore CMR cannot be currently recommended as part of a standard screening despite its unquestionable potential for detailed assessment of structural and functional manifestations of SSc-pHI. Although availability, feasibility, and cost have also limited this as a general screening measure, access to CMR in several centers has paved the way for acquiring and capitalizing on an unprecedented level of data so far. Regarding sTTE, an annual assessment was suggested in line with PAH screening of asymptomatic patients. For those patients with a high-risk profile and development of other organ involvement, as well as borderline results in a previous assessment, a case-by-case evaluation of the timing of the assessment was recommended, in accordance with a cardiology assessment. In general, the expert committee stressed the importance of sTTE to include both two-chamber and four-chamber (biplane) and advocated high-skill training of sonographers to ensure consistency among tests performed at tertiary centers or peripheral centers. In case of doubt, the expertise of a tertiary center should be considered.
6. Symptoms suggestive of SSc-pHI should trigger a specific assessment. This includes initial core evaluation with electrocardiogram, sTTE and serum cardiac biomarkers, such as hs-troponin, NT-proBNP, or BNP.
7. CMR should be included as part of the diagnostic workup where suspicion for SSc-pHI remains following positive findings from the initial core evaluation.
8. In patients with confirmed SSc-pHI or clinically suspected myocarditis, with or without myocardial abnormalities on CMR, endomyocardial biopsy may be indicated in line with ESC guidelines and position statements, after exclusion of coronary artery disease.
In patients with symptoms or unstable clinical presentation, the same above-mentioned laboratory tests were suggested as a minimum annual evaluation, with timing and additional laboratory tests guided by history and other diagnostic assessments. Resting ECG should be repeated during or immediately before the Cardiology consultation, as well as a Holter ECG, with frequency and modality tailored to the clinical context and specific need as per the cardiologist’s evaluation. The expert committee agreed that Holter ECG should report both qualitative (presence) and quantitative (number) alterations. A similar personalized evaluation of sTTE abnormalities was suggested to trigger cardiology consultation and guide further re-evaluation. Similarly, the expert committee agreed on patient selection and on the need for MDT discussion to consider additional diagnostic tests (including CMR) and differential diagnosis (including ischemic, infective, metabolic causes). Additional tests, such as nuclear medicine tests (scintigraphy, positron emission tomography (PET) scan), coronary angiography, and coronary CT, were considered appropriate after cardiology evaluation. Endomyocardial biopsy should be performed according to the European Society of Cardiology (ESC) guidelines, regardless of CMR findings, after exclusion of coronary artery disease, based upon cardiology evaluation.29–33
9. Where SSc-pHI is confirmed, Holter monitoring is recommended as the first-line assessment to evaluate for the arrhythmia burden and sTTE for the evaluation of the cardiac chambers and function. Other tests may be considered in consultation with appropriate cardiology expertise.
10. Management of confirmed SSc-pHI (including frequency of monitoring and nature of testing) should be tailored to the individual patient’s clinical scenario, discussed, and agreed upon by the MDT.
Finally, the expert committee recommended the importance of multidisciplinary care when following up patients with a diagnosis of SSc-pHI. This included both the nature of the testing (mostly relying on milestone assessments such as sTTE and Holter-ECG in relation to the specific cardiac manifestation) to be further adapted to the individual case and the frequency of the monitoring to be performed.
Discussion
This initiative led to the development of a consensus guidance on the screening, diagnosis and follow-up assessments for SSc-pHI.
Diagnostic tests in SSc-pHI: data from the literature
Most of the current literature included “first-line” assessments for SSc-pHI, including patients with or without cardiac involvement or cardiac symptoms. Growing evidence is accumulating from CMR studies, allowing concomitant anatomical, functional and tissue characterization. However, less evidence was available for conventional radiology, myocardial scintigraphy and coronary artery studies.
Higher levels of cardiac-specific laboratory biomarkers, such as NT-proBNP and Troponin I, have been observed in SSc patients compared to healthy controls 34–37 Studies using CMR and sTTE confirmed that all cardiac chambers and structures may be involved in SSc-pHI, with abnormalities of myocardial wall motion, contractility, and relaxation reported. In addition, sTTE has demonstrated significantly abnormal values of right ventricular function and tissue Doppler data,38–43 while CMR data were consistent with histopathological evaluation of endomyocardial biopsy and autopsy samples, indicating inflammatory and fibrotic tissue changes.19,34,44–48 ECG studies have detected a meaningful number of arrhythmias, although the definition ranged from benign isolated ectopics to major malignant ventricular arrhythmias, and no studies have compared an SSc group with matched healthy controls or between cardiac involved and noninvolved SSc patients.
The details of the cohorts and nature of the patients identified in the SLR were not clear, and there was significant heterogeneity of information, in terms of both tests/parameters applied to patients and details given. Some cardiac imaging studies identified underlying pathology but not necessarily with clinically overt disease and were mostly derived from simple association studies. The relatively low quality of evidence means the consensus agreement is based more on eminence than evidence, also influenced by the fact that the local organization of the different Health Systems across Europe, North America, and Asia may be extremely variable.
Principles of SSc-pHI management
The need for the active participation of the patient in the care process emerged as a pillar in the management of SSc-pHI: Patient involvement in clinical practice and clinical research is well established and contributes to our understanding on which interventions may have a positive impact on quality of life, morbidity, and mortality. 49 This was also important to further raise the awareness of the clinician, with particular emphasis on the need to counsel patients in a lay language, to inform them about possible cardiac symptoms and diagnostic procedures, as well as the importance to report to the MDT.
The pivotal role of multidisciplinary management of SSc-pHI was another central feature, whose additional value has been previously shown for SSc-PAH. 50 Scleroderma and cardiology expertise are both pivotal in considering and excluding differential or concomitant diagnoses, as well as in suggesting second-/third-level assessments on a case-by-case basis.
Screening, diagnosis and follow-up evaluation of SSc-pHI
Other screening programs are currently practiced in SSc, such as the screening for PAH, 51 which is recommended once a year by the European Society of Cardiology/European Respiratory Society. 52 As for other screening procedures, the evaluation of cardiac status may be performed more frequently depending on the clinical presentation. In comparison to the core assessment including clinical examination, sTTE, rest ECG, and laboratory tests, there is no convincing basis to support Holter and stress electrophysiology as part of routine screening, with their use driven by symptoms or outcome of other tests. Nevertheless, Holter ECG may be a promising and powerful screening tool, and it is well accepted by the patient given its non-invasive nature. Therefore, it remains part of the research agenda to validate use in a systematic prospective registry and inform on cutoffs with diagnostic and/or prognostic value, to support its application in routine screening assessment.
Timing for sTTE as screening test was recommended as once a year, also in line with the PAH-screening standard, with possible shortening of the timing on a case-by-case basis according to cardiologist and rheumatologist judgment. Although tertiary cardiology centers with SSc expertise would be the ideal setting for the performance of sTTE, this is unrealistic and not necessarily in the patient’s interest. Given the differences among health systems, the scientific community should be advocating highly skilled training of echocardiographers to ensure consistency in the reporting whether a tertiary or a peripheral center is performing the examination.
Despite growing evidence for the potential role of CMR in detecting several manifestations of SSc-pHI, currently available data do not yet allow a recommendation for its routine use in all patients. It is recognized that even completely asymptomatic patients may show CMR abnormalities and that CMR is a surrogate for the gold-standard, endomyocardial biopsy. CMR provides important data on tissue characterization with preliminary data suggesting prognostic implication, but these findings require confirmation in larger prospective studies. Against this background, the current consensus guidance included CMR to (1) be considered on a case-by-case basis, including in asymptomatic patients without history of heart involvement and (2) be included as part of the diagnostic workup where suspicion remains following positive findings in the initial core evaluation.
Comparison with current approaches
In comparison to the diagnostic work-up proposed by Bournia et al., we did not indicate clearly which assessments should be included in the second tier, rather this should be decided on a case-by-case basis in line with a tailored approach managed by an MDT. Our consensus included laboratory biomarkers in the annual cardiac workup, ECG, and sTTE for the screening of asymptomatic patients, given the increasing evidence of their role for the evaluation of myocardial stress and microvascular disease.53,54 This is particularly the case of hs-troponin, which is a promising biomarker for the detection of myocardial involvement.53–55 In addition, NT-proBNP and BNP are already part of the screening algorithm for SSc-PAH and are already available to the physician as a useful guidance to further understand the cardiac context. 56
The consensus best practice from Bissell et al. 6 stressed the importance of the MDT in the management of SSc-pHI, including recognition of wider cardiac disease, attributing an active role to the caring rheumatologist in the evaluation of coronary artery disease (CAD), lipid profile, and glycate hemoglobin. Our consensus guidance considered acute and chronic CAD as a necessary differential diagnosis to be always considered and tested according to specific guidelines and recommendations. In addition, we continue to recommend yearly assessment of symptomatic patients, with further adjustment of timing and nature of assessments on a case-by-case basis. This was stressed in this article for the patients with definite cardiac involvement, in which the whole decision algorithm was patient-tailored by the multispecialty team.
In comparison with both Bournia et al. and Bissell et al., our article had the benefit of increasing evidence on the use of CMR6,7,48 and therefore proposes that it may be considered by the MDT for screening purposes and should be included in the diagnostic work-up where suspicion remains after the core evaluation. This remains part of the research agenda, although recent publications have identified possible risk factors for the detection of CMR changes, such as higher mRSS, presence of digital ulcers, and increased cardiac biomarkers. 18 These results further support the MDT evaluation, with the rheumatologist being pivotal to assess skin involvement and peripheral vasculopathy, the role of biomarkers in the annual cardiac evaluation and tests, and the added value of CMR from a diagnostic and prognostic perspective, as well as to monitor response to treatment. 57
Strengths and limitations
Our study has some clear strengths. The expert panel comprised international specialists dealing with the breadth of SSc-pHI, including cardiologists with subspecialty expertise, rheumatologists, and immunologists. Moreover, we included patient research partners throughout this initiative.
The main limitations are that SLR highlighted poorly detailed and inconsistent data with a lack of well-controlled prospective data in this field. Guidance on state-of-the-art tests was therefore largely based on expertise (rather than a sizable evidence base), similar to the previous consensus document. 6 Unfortunately, no validation is currently available to support the statements provided, either retrospective or prospective. We recommend ongoing research to refine the suggested guidance, in particular the investigations that would not be captured by routine assessment of other indications, such as the screening for PAH.
Conclusion
This consensus initiative for SSc-pHI provides initial guidance for screening and diagnostic work up. A next step will be validation in a real-life cohort with the future ambition of identifying the high-risk SSc profile and evaluating interventions to prevent and treat this life-threatening complication and its manifestations.
Supplemental Material
Supplemental material, sj-pdf-1-jso-10.1177_23971983231163413 for Consensus on the assessment of systemic sclerosis–associated primary heart involvement: World Scleroderma Foundation/Heart Failure Association guidance on screening, diagnosis, and follow-up assessment by Cosimo Bruni, Maya H Buch, Aleksandra Djokovic, Giacomo De Luca, Raluca B Dumitru, Alessandro Giollo, Ilaria Galetti, Alexia Steelandt, Konstantinos Bratis, Yossra Atef Suliman, Ivan Milinkovic, Anna Baritussio, Ghadeer Hasan, Anastasia Xintarakou, Yohei Isomura, George Markousis-Mavrogenis, Sophie Mavrogeni, Luna Gargani, Alida LP Caforio, Carsten Tschöpe, Arsen Ristic, Sven Plein, Elijah Behr, Yannick Allanore, Masataka Kuwana, Christopher P Denton, Daniel E Furst, Dinesh Khanna, Thomas Krieg, Renzo Marcolongo, Alessia Pepe, Oliver Distler, Petros Sfikakis, Petar Seferovic and Marco Matucci-Cerinic in Journal of Scleroderma and Related Disorders
Acknowledgments
M.H.B. is an NIHR Senior Investigator. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Author contributions: Criterion 1:
(a) Substantial contributions to study conception and design: C.B., M.H.B., D.E.F., M.M.-C., P.S..
(b) Substantial contributions to acquisition of data: C.B., A.D., G.D.L., R.B.D., A.G., I.G., A.S., K.B., Y.A.S., I.M., A.B., G.H., A.X,. Y.I.
(c) Substantial contributions to analysis and interpretation of data: C.B., A.D., S.M., L.G., A.L.P.C., C.T., A.R., S.P., E.B., Y.A., M.K., C.D., D.E.F., D.K., T.K., R.M., A.P., O.D., P.S., M.M.-C.
Criterion 2: Drafting the article or revising it critically for important intellectual content: all authors.
Criterion 3: Final approval of the version of the article to be published: all authors.
COI statement: The Editor/Editorial Board Member of JSRD is an author of this paper; therefore, the peer-review process was managed by alternative members of the Board and the submitting Editor/Board member had no involvement in the decision-making process.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Aleksandra Djokovic: no conflicts of interest to declare. Alessandro Giollo received consulting fees and/or honoraria from Novartis, Lilly, and Galapagos. Alessia Pepe: no conflicts of interest to declare. Alexia Steelandt: no conflicts of interest to declare. Alida L.P. Caforio: no conflicts of interest to declare. Anastasia Xintarakou: no conflicts of interest to declare. Anna Baritussio: no conflicts of interest to declare. Arsen Ristic: no conflicts of interest to declare. Carsten Tschöpe: no conflicts of interest to declare. Christopher P. Denton has received consultancy fees and/or research grant funding from Actelion, GlaxoSmithKline, Bayer, Sanofi-Aventis, Inventiva, Boehringer Ingelheim, Roche, CSL Behring, UCB Pharma, Leadiant Biosciences, Corbus, and Acceleron. Cosimo Bruni received consulting fees and/or honoraria from Actelion, Eli-Lilly, Boehringer Ingelheim; research grants from Gruppo Italiano Lotta alla Sclerodermia (GILS), European Scleroderma Trials and Research Group (EUSTAR), and Scleroderma Research Foundation (SRF), Scleroderma Clinical Trials Consortium (SCTC); and educational grants from AbbVie. Cosimo Bruni was supported by an Italian Ministry of University and Research PhD Scholarship, and this study is part of his PhD thesis. Daniel E. Furst reports grant/research support from Corbus, Galapagos GSK, Pfizer, Talaris, CSL Behring, Mitsubishi; Consultant fees from Actelion, Amgen, Corbus, Galapagos, Novartis, Pfizer, Roche/Genentech, Talaris, CSL Behring, and Boehringer Ingelheim. Dinesh Khanna reports grant support from NIH, Immune Tolerance Network, Bayer, BMS, Horizon, and Pfizer; consultant for Acceleron, Actelion, AbbVie, Amgen, Bayer, Boehringer Ingelheim, Chemomab, CSL Behring, Genentech/Roche, Horizon, Merck, Mitsubishi Tanabe Pharma, Prometheus Leadership/Equity position—Chief Medical Officer, and Eicos Sciences, Inc. Elijah R Behr: no conflicts of interest to declare. George Markousis-Mavrogenis: no conflicts of interest to declare. Ghadeer Hasan: no conflicts of interest to declare. Giacomo De Luca received honoraria from SOBI, Novartis, Pfizer, MSD, and Celgene. Ivan Milinkovic received honoraria/support from Boehringer Ingelheim and Hemofarm-Stada. Kostantinos Bratis: no conflicts of interest to declare. Luna Gargani received consultancy fees from GE Healthcare, Philips Healthcare, and Caption Health outside the submitted work. Marco Matucci-Cerinic received consultancies from Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, and Roche. Masataka Kuwana received consultancy fees and/or research grant funding from AbbVie, Astellas, Bayer, Boehringer Ingelheim, Chugai, Corbus, Eisai, Horizon, Janssen, Mochida, Nippon Shinyaku, Ono Pharmaceuticals, Pfizer, and Mitsubishi Tanabe. Maya H. Buch: no conflicts of interest to declare. Oliver Distler has/had consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, AbbVie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, AstraZeneca, Baecon, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, 4P Science, Galapagos, Glenmark, Horizon, Inventiva, Janssen, Kymera, Lupin, Medscape, Miltenyi Biotec, Mitsubishi Tanabe, MSD, Novartis, Prometheus, Redxpharma, Roivant, Sanofi, and Topadur; patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143); research grants from Kymera, Mitsubishi Tanabe. Petar Seferovic: no conflicts of interest to declare. Petros Sfikakis reports consultancy fee from Actelion, Pfizer, Genesis, MSD, UCB, Boehringer Ingelheim, Enorasis, Farmaserv, Lilly, Gliead, AbbVie, and Novartis; grants/research support from AbbVie, Roche, Pfizer, Faran, Amgen, Jannsen, Boehringer Ingelheim, and Gilead. Raluca B. Dumitru: no conflicts of interest to declare. Renzo Marcolongo: no conflicts of interest to declare. Sophie Mavrogeni: no conflicts of interest to declare. Sven Plein: no conflicts of interest to declare. Thomas Krieg reports consultancy fee and grant funding from Actelion. Yannick Allanore reports personal fees from Actelion, Bayer, BMS, Boehringer, and Curzion and grants and personal fees from Inventiva, Roche, and Sanofi. Yohei Isomura: no conflicts of interest to declare. Yossra Atef Suliman: no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received endorsement from the World Scleroderma Foundation and the Heart Failure Association of the European Society of Cardiology. Cosimo Bruni was supported by an Italian Ministry of University and Research PhD Scholarship.
Public patient involvement: A patient research partner (IG) was involved in the project.
ORCID iDs: Cosimo Bruni  https://orcid.org/0000-0003-2813-2083
https://orcid.org/0000-0003-2813-2083
Giacomo De Luca  https://orcid.org/0000-0002-5306-7714
https://orcid.org/0000-0002-5306-7714
Yossra Atef Suliman  https://orcid.org/0000-0003-2919-1966
https://orcid.org/0000-0003-2919-1966
George Markousis-Mavrogenis  https://orcid.org/0000-0001-8192-6670
https://orcid.org/0000-0001-8192-6670
Masataka Kuwana  https://orcid.org/0000-0001-8352-6136
https://orcid.org/0000-0001-8352-6136
Christopher P Denton  https://orcid.org/0000-0003-3975-8938
https://orcid.org/0000-0003-3975-8938
Dinesh Khanna  https://orcid.org/0000-0001-6822-3401
https://orcid.org/0000-0001-6822-3401
Data sharing: All data relevant to the study are included in the article or uploaded as supplementary information.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relate Disorders 2017; 2(3): 137–152. [Google Scholar]
- 2. De Luca G, Cavalli G, Campochiaro C, et al. Interleukin-1 and systemic sclerosis: getting to the heart of cardiac involvement. Front Immunol 2021; 12: 653950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caforio ALP, De Luca G, Baritussio A, et al. Serum organ-specific anti-heart and anti-intercalated disk autoantibodies as new autoimmune markers of cardiac involvement in systemic sclerosis: frequency, clinical and prognostic correlates. Diagnostics (Basel) 2021; 11(11): 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elhai M, Meune C, Boubaya M, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017; 76(11): 1897–1905. [DOI] [PubMed] [Google Scholar]
- 5. Hao Y, Hudson M, Baron M, et al. Early mortality in a multinational systemic sclerosis inception cohort. Arthritis Rheumatol 2017; 69(5): 1067–1077. [DOI] [PubMed] [Google Scholar]
- 6. Bissell LA, Anderson M, Burgess M, et al. Consensus best practice pathway of the UK Systemic Sclerosis Study group: management of cardiac disease in systemic sclerosis. Rheumatology (Oxford) 2017; 56(6): 912–921. [DOI] [PubMed] [Google Scholar]
- 7. Bournia VK, Tountas C, Protogerou AD, et al. Update on assessment and management of primary cardiac involvement in systemic sclerosis. J Scleroderma Relat Disord 2018; 3(1): 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruni C, Ross L. Cardiac involvement in systemic sclerosis: getting to the heart of the matter. Best Pract Res Clin Rheumatol 2021; 35(3): 101668. [DOI] [PubMed] [Google Scholar]
- 9. Bruni C, Buch MH, Furst DE, et al. Primary systemic sclerosis heart involvement: a systematic literature review and preliminary data-driven, consensus-based WSF/HFA definition. J Scleroderma Relat Disord 2022; 7(1): 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allanore Y, Meune C, Vonk MC, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010; 69(1): 218–221. [DOI] [PubMed] [Google Scholar]
- 11. Laing TJ, Gillespie BW, Toth MB, et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum 1997; 40(4): 734–742. [DOI] [PubMed] [Google Scholar]
- 12. Walker UA, Tyndall A, Czirják L, et al. Geographical variation of disease manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research (EUSTAR) group database. Ann Rheum Dis 2009; 68(6): 856–862. [DOI] [PubMed] [Google Scholar]
- 13. Carreira PE, Carmona L, Joven BE, et al. Differences associated with age at onset in early systemic sclerosis patients: a report from the EULAR Scleroderma Trials and Research Group (EUSTAR) database. Scand J Rheumatol 2019; 48(1): 42–51. [DOI] [PubMed] [Google Scholar]
- 14. Medsger TA, Jr, Bombardieri S, Czirjak L, et al. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003; 21(3 Suppl. 29): S42–S46. [PubMed] [Google Scholar]
- 15. Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(2): 139–153. [DOI] [PubMed] [Google Scholar]
- 16. Hunzelmann N, Genth E, Krieg T, et al. The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology (Oxford) 2008; 47(8): 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kostis JB, Seibold JR, Turkevich D, et al. Prognostic importance of cardiac arrhythmias in systemic sclerosis. Am J Med 1988; 84(6): 1007–1015. [DOI] [PubMed] [Google Scholar]
- 18. Dumitru RB, Bissell LA, Erhayiem B, et al. Predictors of subclinical systemic sclerosis primary heart involvement characterised by microvasculopathy and myocardial fibrosis. Rheumatology (Oxford) 2021; 60(6): 2934–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee DC, Hinchcliff ME, Sarnari R, et al. Diffuse cardiac fibrosis quantification in early systemic sclerosis by magnetic resonance imaging and correlation with skin fibrosis. J Scleroderma Relat Disord 2018; 3(2): 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terrier B, Dechartres A, Gouya H, et al. Cardiac intravoxel incoherent motion diffusion-weighted magnetic resonance imaging with T1 mapping to assess myocardial perfusion and fibrosis in systemic sclerosis: association with cardiac events from a prospective cohort study. Arthritis Rheumatol 2020; 72(9): 1571–1580. [DOI] [PubMed] [Google Scholar]
- 21. Steen VD, Medsger TA, Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43(11): 2437–2444. [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez-Reyna TS, Hinojosa-Azaola A, Martinez-Reyes C, et al. Distinctive autoantibody profile in Mexican Mestizo systemic sclerosis patients. Autoimmunity 2011; 44(7): 576–584. [DOI] [PubMed] [Google Scholar]
- 23. Hesselstrand R, Scheja A, Shen GQ, et al. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology (Oxford) 2003; 42(4): 534–540. [DOI] [PubMed] [Google Scholar]
- 24. Kuwana M, Kaburaki J, Okano Y, et al. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum 1994; 37(1): 75–83. [DOI] [PubMed] [Google Scholar]
- 25. Mavrogeni SI, Bratis K, Karabela G, et al. Cardiovascular Magnetic Resonance Imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflamm Allergy Drug Targets 2015; 14(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 26. Steen VD, Medsger TA, Jr. The palpable tendon friction rub: an important physical examination finding in patients with systemic sclerosis. Arthritis Rheum 1997; 40(6): 1146–1151. [DOI] [PubMed] [Google Scholar]
- 27. Steen VD, Medsger TA, Jr. The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum 1997; 40(11): 1984–1991. [DOI] [PubMed] [Google Scholar]
- 28. Morita Y, Muro Y, Sugiura K, et al. Results of the Health Assessment Questionnaire for Japanese patients with systemic sclerosis—measuring functional impairment in systemic sclerosis versus other connective tissue diseases. Clin Exp Rheumatol 2007; 25(3): 367–372. [PubMed] [Google Scholar]
- 29. Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34(33): 2636–2648. [DOI] [PubMed] [Google Scholar]
- 30. Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38(35): 2649–2662. [DOI] [PubMed] [Google Scholar]
- 31. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42(36): 3599–3726. [DOI] [PubMed] [Google Scholar]
- 32. Seferović PM, Tsutsui H, McNamara DM, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur J Heart Fail 2021; 23(6): 854–871. [DOI] [PubMed] [Google Scholar]
- 33. Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2021; 18(3): 169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hromádka M, Seidlerová J, Suchý D, et al. Myocardial fibrosis detected by magnetic resonance in systemic sclerosis patients—Relationship with biochemical and echocardiography parameters. Int J Cardiol 2017; 249: 448–453. [DOI] [PubMed] [Google Scholar]
- 35. Nordin A, Svenungsson E, Björnådal L, et al. Troponin I and echocardiography in patients with systemic sclerosis and matched population controls. Scand J Rheumatol 2017; 46(3): 226–235. [DOI] [PubMed] [Google Scholar]
- 36. Karaahmet T, Tigen K, Gurel E, et al. Impact of systemic sclerosis on electromechanical characteristics of the heart. Heart Vessels 2010; 25(3): 223–228. [DOI] [PubMed] [Google Scholar]
- 37. Karadag DT, Sahin T, Tekeoglu S, et al. Evaluation of left and right ventricle by two-dimensional speckle tracking echocardiography in systemic sclerosis patients without overt cardiac disease. Clin Rheumatol 2020; 39(1): 37–48. [DOI] [PubMed] [Google Scholar]
- 38. Nogradi A, Porpaczy A, Porcsa L, et al. Relation of right atrial mechanics to functional capacity in patients with systemic sclerosis. Am J Cardiol 2018; 122(7): 1249–1254. [DOI] [PubMed] [Google Scholar]
- 39. Dag S, Budulgan M, Dilek B, et al. Relation of asymmetric dimethylarginine and cardiac involvement in systemic sclerosis. Acta Reumatol Port 2014; 39(3): 228–235. [PubMed] [Google Scholar]
- 40. Ayman AA, Alashry SE. Tissue Doppler assessment of right ventricular function in female patients with limited form of systemic sclerosis. Egypt Heart J 2013; 65: 191–197. [Google Scholar]
- 41. Ciurzynski M, Bienias P, Irzyk K, et al. Heart diastolic dysfunction in patients with systemic sclerosis. Arch Med Sci 2014; 10(3): 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poanta L, Dadu R, Tiboc C, et al. Systolic and diastolic function in patients with systemic sclerosis. Eur J Intern Med 2009; 20(4): 378–382. [DOI] [PubMed] [Google Scholar]
- 43. Nešković JS, Ristić A, Petronijević M, et al. B-type natriuretic peptide as a marker of different forms of systemic sclerosis. J Med Biochem 2018; 37(4): 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Markousis-Mavrogenis G, Bournia VK, Panopoulos S, et al. Cardiovascular magnetic resonance identifies high-risk systemic sclerosis patients with normal echocardiograms and provides incremental prognostic value. Diagnostics (Basel) 2019; 9(4): 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ntusi NA, Piechnik SK, Francis JM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014; 16: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barison A, Gargani L, De Marchi D, et al. Early myocardial and skeletal muscle interstitial remodelling in systemic sclerosis: insights from extracellular volume quantification using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 2015; 16(1): 74–80. [DOI] [PubMed] [Google Scholar]
- 47. Thuny F, Lovric D, Schnell F, et al. Quantification of myocardial extracellular volume fraction with cardiac MR imaging for early detection of left ventricle involvement in systemic sclerosis. Radiology 2014; 271(2): 373–380. [DOI] [PubMed] [Google Scholar]
- 48. Gargani L, Todiere G, Guiducci S, et al. Early detection of cardiac involvement in systemic sclerosis: the added value of magnetic resonance imaging. JACC Cardiovasc Imaging 2019; 12(5): 927–928. [DOI] [PubMed] [Google Scholar]
- 49. van Leeuwen NM, Boonstra M, Huizinga TWJ, et al. Illness perceptions, risk perceptions and worries in patients with early systemic sclerosis: a focus group study. Musculoskeletal Care 2020; 18(2): 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coirier V, Lescoat A, Fournet M, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: comparison of DETECT algorithm to decisions of a multidisciplinary team, in a competence centre. Rev Med Interne 2017; 38(8): 502–507. [DOI] [PubMed] [Google Scholar]
- 51. Bruni C, De Luca G, Lazzaroni MG, et al. Screening for pulmonary arterial hypertension in systemic sclerosis: a systematic literature review. Eur J Intern Med 2020; 78: 17–25. [DOI] [PubMed] [Google Scholar]
- 52. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 53. Barsotti S, Stagnaro C, d’Ascanio A, et al. High sensitivity troponin might be a marker of subclinical scleroderma heart involvement: a preliminary study. J Scleroderma Relat Disorders 2017; 2(3): 183–187. [Google Scholar]
- 54. Jha M, Wang M, Steele R, et al. NT-proBNP, hs-cTnT, and CRP predict the risk of cardiopulmonary outcomes in systemic sclerosis: findings from the Canadian Scleroderma Research Group. J Scleroderma Relat Disord 2022; 7(1): 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hughes M, Lilleker JB, Herrick AL, et al. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis 2015; 74(5): 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coghlan JG, Denton CP, Grünig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73(7): 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Panopoulos S, Mavrogeni S, Vlachopoulos C, et al. Cardiac magnetic resonance imaging before and after therapeutic interventions for systemic sclerosis-associated myocarditis. Rheumatology (Oxford) 2022. Epub ahead of print 9 September. DOI: 10.1093/rheumatology/keac504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jso-10.1177_23971983231163413 for Consensus on the assessment of systemic sclerosis–associated primary heart involvement: World Scleroderma Foundation/Heart Failure Association guidance on screening, diagnosis, and follow-up assessment by Cosimo Bruni, Maya H Buch, Aleksandra Djokovic, Giacomo De Luca, Raluca B Dumitru, Alessandro Giollo, Ilaria Galetti, Alexia Steelandt, Konstantinos Bratis, Yossra Atef Suliman, Ivan Milinkovic, Anna Baritussio, Ghadeer Hasan, Anastasia Xintarakou, Yohei Isomura, George Markousis-Mavrogenis, Sophie Mavrogeni, Luna Gargani, Alida LP Caforio, Carsten Tschöpe, Arsen Ristic, Sven Plein, Elijah Behr, Yannick Allanore, Masataka Kuwana, Christopher P Denton, Daniel E Furst, Dinesh Khanna, Thomas Krieg, Renzo Marcolongo, Alessia Pepe, Oliver Distler, Petros Sfikakis, Petar Seferovic and Marco Matucci-Cerinic in Journal of Scleroderma and Related Disorders