Abstract
Two multiplex PCR enzyme immunoassays (PCR-EIAs) were developed for Staphylococcus aureus exotoxin gene screening as an alternative to the conventional biological assays, which depend on detectable amounts of toxins produced. One set of oligonucleotide primers and probes was designed to search for enterotoxin A to E genes (entA, entB, entC, entD, and entE), and the other one was designed to detect the staphylococcal exfoliative toxin genes (eta and etb) and the toxic shock syndrome toxin 1 gene (tst). Oligonucleotide primers were used as published previously, modified or newly developed to meet the requirements of both good size-distinguishable amplification bands of multiplex PCR and the temperature limit of the uracil DNA glycosylase system for carryover protection. Amplification products were visualized by agarose gel electrophoresis, and specificity was controlled with the aid of a DNA EIA system using oligonucleotide probes derived from the sequences of the S. aureus toxin genes. PCR procedures were performed by using template nucleic acids extracted from a panel of S. aureus reference strains and from a collection of 50 clinical strains. The PCR results were compared with those of immunological toxin production assays. This multiplex PCR-EIA system offers an alternative method for the rapid, sensitive, specific, and simultaneous detection of the clinically important exotoxin potency of isolated S. aureus strains for diagnostic purposes as well as research studies.
Many Staphylococcus aureus strains produce one or more of a group of specific exotoxins that include staphylococcal enterotoxins (SEs), staphylococcal exfoliative toxins (ETs), and toxic shock syndrome toxin 1 (TSST-1). The SEs, which cause staphylococcal food poisoning, are classified by serological criteria into five major groups (A to E, referred to as SEA to SEE, respectively), and SEC can be further subdivided into SEC1, SEC2, and SEC3, based on differences in minor epitopes (1, 3, 4, 6, 11, 12, 23). The ETs are responsible for the staphylococcal scalded-skin syndrome, and currently two different toxin serotypes (A and B, referred to as ETA and ETB, respectively) are known (16, 27). TSST-1 is the major exotoxin etiologically involved in staphylococcal toxic shock syndrome, especially in menstrual cases (5, 25).
Conventional methods for the detection of such toxin-producing S. aureus strains are based on immunological procedures measuring the toxin in the culture supernatants of suspected S. aureus strains or in contaminated food extracts or in patient specimens (26). These methods are always dependent on detectable amounts of toxins. In recent years, protein sequences have been established for all these toxins, as well as nucleotide sequences for the corresponding genes (tst, entA, entB, entC1 to entC3, entD, entE, eta, and etb) (2, 8, 10, 13, 14, 18, 20, 22). DNA-DNA hybridization techniques have been developed that use gene fragments or oligonucleotide probes that react with either one or more of the respective toxin genes (24). Recently, specific oligonucleotide primers for PCR have been described for analysis of S. aureus strains for the presence of toxin genes, mainly for scientific reasons (9, 17). However, there is still need for rapid, specific, and, especially, simultaneous detection of the genes for production of all of these exotoxins of isolated S. aureus strains for diagnostic and epidemiological purposes.
Here we report two multiplex PCR enzyme immunoassays (PCR-EIAs)—one designed to detect the staphylococcal enterotoxin genes, the other designed to detect the tst gene and the et genes. The respective PCR protocols were evaluated by using S. aureus template DNA extracted from a panel of 23 toxin-producing reference strains and a collection of 50 clinically isolated strains. The specificity was controlled by the aid of a DNA EIA system using oligonucleotide probes derived from the nucleotide sequences of the S. aureus toxin genes. PCR results were compared with those of immunological toxin production assays.
(This study was presented in part at the 35th Annual Meeting of the Infectious Diseases Society of America, San Francisco, Calif., 1997.)
MATERIALS AND METHODS
Bacterial strains.
The 23 toxin-producing reference strains (see Tables 3 and 4) were American Type Culture Collection strains, were part of the strain collection of our institute, or were kindly supplied by W. Witte (Robert-Koch-Institut, Wernigerode, Germany) and N. El Solh (Institut Pasteur, Paris, France) and had been previously defined by virtue of their respective toxin production. In addition to standard PCR controls for contamination events, S. aureus Cowan 1 and Staphylococcus epidermidis ATCC 20044 served as negative controls, with both run with each set of PCR and DNA-EIA reactions. All clinical strains were isolated in our diagnostic laboratory. A total of 50 isolates from 43 patients were identified biochemically by the automated ID 32 Staph system (API Systems S. A., Montalieu Vercieu, France) and confirmed as S. aureus by Pastorex Staph-Plus (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). For nucleic acid (NA) isolation, S. aureus strains were subcultured on blood agar. A single colony was then inoculated into brain heart infusion broth (BHI, Merck, Darmstadt, Germany) and incubated overnight with shaking at 37°C.
TABLE 3.
Results of testing reference strains for staphylococcal enterotoxin genotype derived from agarose gel analysis of multiplex PCR and colorimetric microtiter plate DNA EIA
| Reference strain | Toxin phenotype | Result by:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarose gel analysisa
|
Colorimetric assay (OD450/630 ratio)
|
||||||||||
| sea | seb | sec | sed | see | sea | seb | sec | sed | see | ||
| KN543 | SEA | + | − | − | − | − | 0.853 | 0.037 | 0.062 | 0.021 | 0.018 |
| KN556 | SEA | + | − | − | − | − | 0.799 | 0.036 | 0.062 | 0.019 | 0.019 |
| KN560a | SEA | + | − | − | − | − | 1.035 | 0.063 | 0.030 | 0.030 | 0.018 |
| H390 | SEA, SED | + | − | − | + | − | 0.867 | 0.041 | 0.063 | 0.817 | 0.019 |
| ATCC 14458 | SEB | − | + | − | − | − | 0.021 | 1.364 | 0.117 | 0.050 | 0.022 |
| 62/92b | SEB | − | + | − | − | − | 0.018 | 1.169 | 0.066 | 0.054 | 0.036 |
| H288a | SEB | − | + | − | − | − | 0.028 | 1.540 | 0.037 | 0.140 | 0.018 |
| st 20783 | SEB | − | + | − | − | − | 0.016 | 0.981 | 0.055 | 0.041 | 0.021 |
| ATCC 19095 | SEC | − | − | + | − | − | 0.015 | 0.043 | 1.335 | 0.021 | 0.025 |
| 1229/93b | SEC | − | − | + | − | − | 0.015 | 0.038 | 1.406 | 0.022 | 0.020 |
| H494 | SEC | − | − | + | − | − | 0.021 | 0.024 | 2.200 | 0.026 | 0.018 |
| ATCC 23235 | SED | − | − | − | + | − | 0.013 | 0.042 | 0.123 | 1.214 | 0.021 |
| 1634/93b | SED | − | − | − | + | − | 0.019 | 0.024 | 0.128 | 1.475 | 0.018 |
| ATCC 27664 | SEE | − | − | − | − | + | 0.021 | 0.034 | 0.099 | 0.020 | 1.819 |
| Cowan 1c | − | − | − | − | − | 0.014 | 0.083 | 0.039 | 0.019 | 0.017 | |
| ATCC 20044d | − | − | − | − | − | 0.020 | 0.031 | 0.110 | 0.024 | 0.018 | |
−, negative; +, positive (judged by eye).
Kindly provided by W. Witte (Robert Koch-Institut Wernigerode, Germany).
S. aureus negative control strain.
S. epidermidis negative control strain.
TABLE 4.
Results of testing reference strains for staphylococcal ETs and TSST-1 genotype derived from agarose gel analysis of multiplex PCR-EIA and colorimetric microtiter plate DNA EIA
| Reference strain | Toxin pheno- type(s) | Result by:
|
|||||
|---|---|---|---|---|---|---|---|
| Agarose gel analysisa
|
Colorimetric assay (OD450/630 ratio)
|
||||||
| eta | etb | tst | eta | etb | tst | ||
| 1930/95b | ETA | + | − | − | 1.308 | 0.017 | 0.013 |
| BM 10458c | ETA | + | − | − | 1.284 | 0.028 | 0.028 |
| BM 10431c | ETA, ETB | + | + | − | 0.961 | 1.932 | 0.017 |
| BM 10143c | ETB | − | + | − | 0.045 | 1.922 | 0.031 |
| 161/93b | TSST-1 | − | − | + | 0.019 | 0.019 | 1.450 |
| 150 | TSST-1 | − | − | + | 0.024 | 0.021 | 2.166 |
| 235 | TSST-1 | − | − | + | 0.017 | 0.018 | 1.705 |
| KN813 | TSST-1 | − | − | + | 0.020 | 0.022 | 1.870 |
| KN820 | TSST-1 | − | − | + | 0.020 | 0.019 | 1.792 |
| Cowan 1d | − | − | − | 0.034 | 0.020 | 0.036 | |
| ATCC 20044e | − | − | − | 0.024 | 0.019 | 0.024 | |
−, negative; +, positive (judged by eye).
Kindly provided by W. Witte (Robert Koch-Institut Wernigerode, Germany).
Kindly provided by N. El Solh (Centre National de Référence des Staphylocoques, Institut Pasteur, Paris, France).
Staphylococcus aureus, negative control strain.
Staphylococcus epidermidis, negative control strain.
DNA isolation procedures.
Total NAs were isolated from 0.5 ml of a brain heart infusion broth culture. Cells were pelleted by centrifugation at 5,000 × g for 20 min (Biofuge 13; Heraeus, Hanau, Germany), resuspended in 185 μl of TE buffer (20 mM Tris chloride, 2 mM EDTA [pH 8.0]) with 15 μl of recombinant lysostaphin (15 mg/ml) from AMBI (Tarrytown, N.Y.), and incubated at 37°C for 0.5 h. NAs were subsequently extracted with a QIAamp tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer’s recommendations. NA samples were eluted with distilled water and adjusted to a final concentration of 1 μg/ml according to A260 values.
PCR.
Oligonucleotide primers are listed in Table 1. Primarily, we tested primer sequences published by Johnson et al. (17). Modifications or newly designed PCR primers were tested to meet the requirements of a multiplex PCR to obtain good size-distinguishable amplification bands as well as the requirements of the uracil DNA glycosylase (UNG) system with an inferior limit of annealing temperature at 55°C. These new or modified primers and the biotinylated oligonucleotide probes were designed by computerized sequence analysis with PC/Gene, release 18.0 (IntelliGenetics, Mountain View, Calif.) and Primer Premier 4.04 (Premier Biosoft, Palo Alto, Calif.). The oligonucleotides were obtained from MWG Biotech (Ebersberg, Germany).
TABLE 1.
Base sequences, gene locations, and predicted sizes of PCR products for the S. aureus exotoxin-specific oligonucleotide primers
| Genea | Primer | Oligonucleotide sequence (5′→3′) | Nucleotide location within gene | Size of PCR product (bp) |
|---|---|---|---|---|
| sea | SEA-3b | CCT TTG GAA ACG GTT AAA ACG | 487–507 | 127 |
| SEA-4b | TCT GAA CCT TCC CAT CAA AAA C | 592–613 | ||
| seb | SEB-1c | TCG CAT CAA ACT GAC AAA CG | 634–653 | 477 |
| SEB-4b | GCA GGT ACT CTA TAA GTG CCT GC | 1088–1110 | ||
| secd | SEC-3b | CTC AAG AAC TAG ACA TAA AAG CTA GG | 665–690 | 271 |
| SEC-4b | TCA AAA TCG GAT TAA CAT TAT CC | 913–935 | ||
| sed | SED-3b | CTA GTT TGG TAA TAT CTC CTT TAA ACG | 354–380 | 319 |
| SED-4b | TTA ATG CTA TAT CTT ATA GGG TAA ACA TC | 644–672 | ||
| see | SEE-3b | CAG TAC CTA TAG ATA AAG TTA AAA CAA GC | 482–510 | 178 |
| SEE-2c | TAA CTT ACC GTG GAC CCT TC | 640–659 | ||
| tst | TST-3 | AAG CCC TTT GTT GCT TGC G | 51–69 | 445 |
| TST-6 | ATC GAA CTT TGG CCC ATA CTT T | 474–495 | ||
| eta | ETA-3b | CTA GTG CAT TTG TTA TTC AAG ACG | 374–397 | 119 |
| ETA-4b | TGC ATT GAC ACC ATA GTA CTT ATT C | 468–492 | ||
| etb | ETB-3b | ACG GCT ATA TAC ATT CAA TTC AAT G | 51–75 | 262 |
| ETB-4 | AAA GTT ATT CAT TTA ATG CAC TGT CTC | 286–312 |
Sequences and locations were derived from the published nucleotide sequences for SEA (7), SEB (18), SEC1 to SEC3 (10, 13), SED (2), SEE (14), TSST-1 (8), and ETA and ETB (20).
Modified oligonucleotide primers based on primer sequences published by Johnson et al. (17).
Oligonucleotide primer sequences as described by Johnson et al. (17).
The primer sequences used here cover all three subtypes of SEC.
First, preliminary trials with different annealing temperatures (50, 52.5, 55, and 57.5°C), cycle numbers (25, 30, and 35), amounts (1.0, 1.25, 1.5, and 2.0 U), and brands of DNA polymerases (Taq DNA polymerase; Boehringer, Mannheim, Germany; Expand high-fidelity PCR system, Boehringer; PrimeZyme, Biometra, Göttingen, Germany; AmpliTaq DNA polymerase, Applied Biosystems, Foster City, Calif.; and AmpliTaq Gold DNA polymerase, Applied Biosystems) and with various concentrations of magnesium chloride (1.0, 1.5, 2.0, 2.5, 3.0, 3.5, and 4.0 mM) were performed to define the optimal PCR conditions (data not shown).
The amplification was performed in an automated thermocycler with a hot bonnet (Hybaid, Teddington, United Kingdom). The optimized thermal cycling conditions for both multiplex PCRs were 30 cycles of denaturation at 95°C for 1 min (2 min for the first cycle), annealing at 55°C for 1 min, and polymerization at 72°C for 2 min (5 min for the last cycle). Optimized amplifications were carried out in 0.5-ml tubes in a reaction volume of 50 μl containing 200 μM dATP, dCTP, and dGTP. Instead of dTTP, dUTP was used in a concentration of 600 μM. The master mix (PCR Core Kit Plus; Boehringer) contained 10 mM Tris-HCl (pH 8.9), 50 mM KCl, 3 mM magnesium chloride, 1.0 U of UNG, and 0.5 pmol of primer. This reaction mixture was incubated for 10 min at room temperature (UNG reaction condition). For UNG inactivation, prevention of primer-dimer formation and denaturation of template DNA, after addition of template DNA (5 to 10 ng), the mixture was incubated at 95°C for 5 min and stored on ice before addition of 1.25 U of Taq DNA polymerase (Boehringer, Mannheim, Germany).
Amplified products (10 μl) were resolved by 3.0% agarose gel electrophoresis at 150 V for 1 to 2 h. The gel was then stained with ethidium bromide, and the bands were visualized under UV illumination at 254 nm.
Hybridization procedures.
Designed 5′-biotinylated oligonucleotide probes for the eight toxins tested (Table 2) were separately used for hybridization of amplified DNA from the multiplex PCRs in a generic DNA EIA (GEN-ETI-K DEIA; Sorin, Saluggia, Italy) according to the manufacturer’s recommendations. Briefly, strips coated with streptavidin were incubated with an optimized concentration of the biotinylated probes (20 pg/μl for each probe) at 5°C overnight. After addition of 100 μl of hybridization buffer, the strips were incubated with 20 μl of the 1:10-diluted denatured PCR products at the optimal hybridization temperature (55°C for all used probes) for 1 h. Following hybridization, the samples were treated with 100 μl of a 1:50-diluted anti-double-stranded DNA mouse antibody solution for 1 h. The detection of the DNA-antibody bond was performed by addition of an enzyme tracer system (antimouse antibody labelled with horseradish peroxidase), and the results were measured after incubation with the chromogen-substrate solution by using a vertical reading photometer. The optical density at 450/630 nm (OD450/630 ratio) of the resulting color was measured, and the A630 was subtracted from the A450. A cutoff of 0.150 absorbance units above the mean value of determinations of toxin-negative reference strains was defined as a positive reaction.
TABLE 2.
Base sequences and gene locations for the S. aureus exotoxin-specific 5′-biotinylated oligonucleotide probes
| Genea | Probeb | Oligonucleotide sequence (5′→3′) | Nucleotide location within gene |
|---|---|---|---|
| sea | SEA-7B | GGA GTT GGA TCT TCA AGC AAG ACG | 531–554 |
| seb | SEB-3B | GAG AAT AGC TTT TGG TAT GAC ATG | 901–924 |
| secc | SEC-5B | AAC GGC AAT ACT TTT TGG TAT GAT | 772–795 |
| sed | SED-5B | TAA AGC CAA TGA AAA CAT TGA TTC A | 382–406 |
| see | SEE-5B | CTT TGG CGG TAA GGT GCA AAG AGG C | 594–618 |
| tst | TST-5B | AAG CCA ACA TAC TAG CGA AGG AAC | 348–371 |
| eta | ETA-5B | CCA TGC AAA AGC AGA AGT TTC AGC | 414–437 |
| etb | ETB-5B | TAC CAC CTA ATA CCC TAA TAA TCC AA | 190–215 |
Sensitivity testing.
For determination of the sensitivities of the PCR assays and hybridization procedures, the minimal amount of staphylococcal DNA that would be successfully detected was determined. Purified DNA of reference strains was quantitated by using a spectrophotometer, and 10-fold serial dilutions starting at 1 μg/ml were subsequently subjected to multiplex PCR and DNA EIA. Upon completion of PCR, an aliquot from each tube was analyzed by electrophoresis and placed into the DNA EIA as described above. To compare the influence of the multiplex procedure versus that of a single-PCR application, DNA of the toxin-positive reference strains was tested as a single target versus equal amounts of a mixture of all gene targets.
Specificity testing.
Samples of DNA from the target toxin-negative S. aureus strain Cowan 1 and from a set of coagulase-negative staphylococci, from pyrogenic exotoxin and streptolysin O-producing strains of Streptococcus pyogenes, and from other gram-positive microorganisms (microcooci, stomatococci, and bacilli) of the strain collection of our institute were tested. Ten nanograms of template DNA was used per reaction.
Determination of staphylococcal toxins.
Culture filtrates of the S. aureus strains were tested for the presence of SEA to SEE by an EIA (Ridascreen set A, B, C, D, E; R-Biopharm, Darmstadt, Germany), and, except for SEE, by a semiquantitative reversed passive latex agglutination test (SET-RPLA; Unipath, Basingstoke, Hampshire, United Kingdom). The presence of TSST-1 was determined by the same method (TST-RPLA; Unipath). An immunoblot with sheep anti-ETA antibody (Toxin Technology, Sarasota, Fla.), alkaline phosphatase-conjugated donkey anti-sheep immunoglobulin G (Sigma, St. Louis, Mo.), and purified ETA (Toxin Technology) as a positive control was used for the detection of ETA production.
RESULTS
Optimization of PCR conditions.
Titration of Mg2+ concentration of the reaction buffer revealed an optimum of 3 mM for both multiplex PCRs. The primer sets allowed PCR amplification of the target gene fragments with 25, 30, or 35 cycles. Thirty cycles was found to be optimal for both multiplex systems. The optimal thermal profile was 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min for both multiplex PCRs. The Expand High Fidelity PCR system (Boehringer, Mannheim, Germany) and the use of other DNA polymerases had no advantages over our multiplex PCR systems.
DNA EIA.
Analysis of the nucleotide sequences published for the target genes indicated that the 24- to 26-base oligonucleotide probes (Table 2) encode sequences unique to the toxins from which they were deduced. Hybridization experiments with these gene probes were performed with PCR amplification products derived from single and multiplex PCRs as described above. The optimal concentration for coating the microtiter plates with the biotinylated probes was 20 pg/μl for each probe. The results indicated a complete correlation between the amplification products and their corresponding hybridization. No unspecific hybridization was observed among the different PCR products of all staphylococcal exotoxins tested.
Sensitivity of multiplex PCR and DNA EIA.
Both multiplex primer sets successfully amplified the appropriate regions of the target toxin genes from a DNA dilution containing as little as 50 pg (ranging from 1 to 50 pg) for single PCRs and in multiplex PCRs as little as 100 pg (ranging from 1 to 100 pg) of DNA of a DNA mixture isolated from reference strains containing the test toxin genes. In a given multiplex PCR mixture consisting of DNA from every type of target gene, the resulting band intensities of the larger amplicons were reduced compared to the intensities of the smaller amplicons and to the artificially pooled single PCR, but this does not affect the test in practice. The sensitivity of subsequent DNA EIAs was always 10 to 100 times higher than that of both the single and multiplex PCRs (data not shown).
Specificity of the staphylococcal oligonucleotide primers and probes.
Initially, the specific primer pairs published by Johnson et al. (17) were tested for their applicability to multiplex PCR for detection of the staphylococcal toxin genes. To meet the requirements of good size-distinguishable amplification bands of multiplex PCR and of the temperature limit of the UNG system for carryover protection, it was necessary to modify and partly select new primers directed to specific nucleotide sequences within the target genes. The eight modified or newly developed pairs of toxin-specific oligonucleotide primers and the eight newly derived toxin-specific biotinylated oligonucleotide probes were selected by computer analysis with previously described nucleotide sequences for the corresponding genes of SEA (7), SEB (18), SEC1 to SEC3 (10, 13), SED (2), SEE (14), TSST-1 (8), and ETA and ETB (20). A total of 23 reference strains of S. aureus were used as templates for the primer sets, each with 5 to 10 ng of bacterial DNA in the PCR mixture. The DNA of all S. aureus exotoxin-producing reference strains was specifically amplified by the primers and confirmed by oligonucleotide probes of the DNA EIA; thus, the corresponding genes were correctly recognized (Tables 3 and 4). The sizes of the amplicons were identical to those predicted from the primer design (Table 1). Figures 1 and 2 show representative gels for the PCR product patterns of both multiplex PCRs. In addition, none of the primer pairs and the corresponding probes reacted with any strains of non-SE-producing S. aureus strains, other coagulase-positive staphylococci (S. intermedius or S. schleiferi subsp. coagulans), a spectrum of coagulase-negative staphylococcal species (S. epidermidis, S. haemolyticus, S. hominis, S. warneri, S. capitis, and S. simulans), and other related gram-positive genera, including stomatococci, micrococci, and Bacillus spp. (data not shown). Because of the close relationship between SEB and SEC and other members of the pyrogenic exotoxin family, we tested pyrogenic exotoxin and streptolysin O-producing strains of S. pyogenes in our multiplex PCR system, and no specific amplification and hybridization were observed.
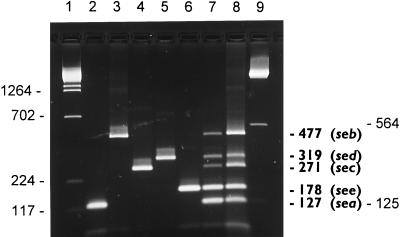
FIG. 1.
Agarose gel electrophoresis patterns showing PCR-amplified products in multiplex PCR for the staphylococcal enterotoxin genes. Lanes 1, DNA molecular weight marker λ HindIII; 2, sea; 3, seb; 4, sec; 5, sed; 6, see; 7, multiplex PCR with all enterotoxin genes simultaneously (sea to see); 8, artificial arrangement of the amplification fragments of sea to see; 9, DNA molecular weight marker λ BstE II. Sizes are marked in base pairs on the left and right.
FIG. 2.
Agarose gel electrophoresis patterns showing PCR-amplified products in multiplex PCR for the staphylococcal ET genes (eta and etb) and TSST-1 gene (tst). Lanes 1: DNA molecular weight marker λ HindIII; 2, eta; 3, etb; 4, tst; 5, multiplex PCR with both ET genes and the TSST-1 gene simultaneously; 8, artificial arrangement of the amplification fragments of eta, etb, and tst; 9, DNA molecular weight marker λ BstE II. Sizes are marked in base pairs on the left and right.
Testing of S. aureus isolates by multiplex PCR-EIA and comparison with toxin phenotype.
Amplicons with predicted sizes and a positive hybridization reaction were detected in all reference strains analyzed (Tables 3 and 4). Also, there was 100% correlation between the results of the multiplex PCR-EIA and the toxin phenotype testing of the reference strains.
Within 50 clinical S. aureus isolates obtained from 43 patients, a total of 19 positive amplification results were found. All could be confirmed by the respective hybridization procedure. In 11 clinical strains from different patients, we detected various genes for enterotoxins, in 7 strains we detected the TSST-1 gene, and in 1 strain we detected the ETA gene (Table 5).
TABLE 5.
Detection of exotoxin-producing S. aureus reference strains (n = 23) and clinical isolates (n = 50) by immunoassays and multiplex PCR-EIA
| Toxin phenotype | No. of strains | No. of positive samples detected by:
|
No. of differencesa | |
|---|---|---|---|---|
| Immuno- assays | Multiplex PCR-EIA | |||
| SEA | 10 | 9 | 10 | 1 |
| Reference strains | 4 | 4 | 4 | 0 |
| Clinical isolates | 6 | 5 | 6 | 1 |
| SEB | 6 | 6 | 6 | 0 |
| Reference strains | 4 | 4 | 4 | |
| Clinical isolates | 2 | 2 | 2 | |
| SEC | 5 | 5 | 5 | 0 |
| Reference strains | 3 | 3 | 3 | |
| Clinical isolates | 2 | 2 | 2 | |
| SED | 4 | 4 | 4 | 0 |
| Reference strains | 3 | 3 | 3 | |
| Clinical isolates | 1 | 1 | 1 | |
| SEE | 1 | 1 | 1 | 0 |
| Reference strains | 1 | 1 | 1 | |
| Clinical isolates | 0 | |||
| ETA | 4 | 3 | 4 | 0 |
| Reference strains | 3 | 3 | 3 | |
| Clinical isolates | 1 | 1 | 1 | |
| ETB | 2 | 2 | 2 | 0 |
| Reference strains | 2 | 2 | 2 | |
| Clinical isolates | 0 | |||
| TSST-1 | 12 | 12 | 12 | 0 |
| Reference strains | 5 | 5 | 5 | |
| Clinical isolates | 7 | 7 | 7 | |
| Double producerb | 7 | 7 | 7 | 0 |
| Reference strains | 2 | 2 | 2 | |
| Clinical isolates | 5 | 5 | 5 | |
| Nonproducerc | 39 | 0 | 0 | 0 |
| Reference strains | 3 | 0 | 0 | |
| Clinical isolates | 36 | 0 | 0 | |
Differences between the test results from immunological and PCR methods.
Double exotoxin producers: one reference strain with SEA and SED, one reference strain with ETA and ETB, four clinical strains with SEA and TSST-1, and one clinical strain with SEC and TSST-1, respectively.
Nonproducer of staphylococcal exotoxins tested.
Both multiplex PCR systems could detect exotoxin double producers in reference strains (one SEA plus SED and one ETA plus ETB) in one step. In the clinical strains, we observed five strains carrying two exotoxin genes: one strain carried the sec and tst genes, and four strains carried the sea and tst genes (Table 5). Thus, an excellent correlation was found between the results of genotype and phenotype testing. In only one strain positive for sea by multiplex PCR-EIA could SEA toxin production not be detected by both immunological tests used.
DISCUSSION
The ability of S. aureus strains to produce SEs, ETs, and/or TSST-1 is an important property with various clinical implications. Determination is still mainly based on immunological methods for toxin detection which are time- and labor-consuming. Furthermore, these methods depend on the concentration of toxin expressed and thus can be negatively influenced by various factors. Also, for enterotoxins, differences in the toxin production levels by S. aureus strains grown in natural substrate and laboratory media have been described previously (15).
We have developed a multiplex PCR-EIA test system which allows the simultaneous detection of the enterotoxin (sea to see), ET (eta and etb), and TSST-1 (tst) genes. We could prove this system to be sensitive and specific for the respective genes by using staphylococcal cultures. Because our objective was to develop a simple and fast method for use in routine diagnosis, we preferred an NA preparation procedure that was simpler than the classical phenol-chloroform extraction method. Samples for PCR can be prepared in 1 h without an obvious risk of sample cross-contamination. The use of alternative DNA polymerase systems, so-called expanded and/or high-fidelity systems, had no advantages over our multiplex PCRs, although they are described as being able to increase the yield of amplification by reducing the mismatch pausing associated with Taq DNA polymerase.
Strict precautions must be taken to avoid false-positive amplification because of contamination of the reaction mixture both with target DNA from staphylococcal cells and by carryover of amplified target DNA from previous PCR cycles. Therefore, to prevent false-positive results, we used the UNG system (21). This enzyme can be used with dUTP to eliminate PCR contaminations from previous DNA synthesis reactions by degrading uracil-containing DNA. Since it has been reported that UNG from Escherichia coli remains partially active or regains activity leading to degradation of the dU-containing PCR product, it is necessary to keep the reaction mixture at a higher temperature (70°C) or to freeze the product immediately after the last amplification step (19). Because UNG shows activity only below 55°C, it is recommended to choose annealing temperatures at or above this temperature in order to avoid degradation of newly synthesized dU-containing amplicons by possible residual UNG activity after the inactivation step of this enzyme. To meet this recommendation, we had to modify or design new primers composed of nucleotides allowing such temperatures. A higher annealing temperature is also recommended to increase the specificity of the PCR. The use of a DNA EIA system as a hybridization step after the PCR procedure offers a simple and reliable specificity control of the amplicons.
The multiplex PCR-EIA test system described here was primarily designed for the detection of exotoxin genes in S. aureus in culture. Research is in progress to apply this system to the direct detection of toxigenic S. aureus strains in human specimens and food material.
ACKNOWLEDGMENTS
We thank Susanne Deiwick and Martina Schulte for excellent technical assistance. We are grateful to M. Herrmann for critical suggestions on the manuscript.
REFERENCES
- 1.Avena R M, Bergdoll M S. Purification and some physicochemical properties of enterotoxin C, Staphylococcus aureus strain 361. Biochemistry. 1967;6:1474–1480. doi: 10.1021/bi00857a033. [DOI] [PubMed] [Google Scholar]
- 2.Bayles K W, Iandolo J J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989;171:4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergdoll M S, Borja C R, Avena R M. Identification of a new enterotoxin as enterotoxin C. J Bacteriol. 1965;90:1481–1485. doi: 10.1128/jb.90.5.1481-1485.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergdoll M S, Borja C R, Robbins R N, Weiss K F. Identification of enterotoxin E. Infect Immun. 1971;4:593–595. doi: 10.1128/iai.4.5.593-595.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergdoll M S, Crass B A, Reiser R F, Robbins R N, Davis J P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;i:1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 6.Bergdoll M S, Surgalla M J, Dack G M. Staphylococcal enterotoxin: identification of a specific precipitating antibody with enterotoxin neutralizing property. J Immunol. 1959;83:334–338. [PubMed] [Google Scholar]
- 7.Betley M J, Mekalanos J J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988;170:34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomster-Hautamaa D A, Kreiswirth B N, Kornblum J S, Novick R P, Schlievert P M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986;261:15783–15786. [PubMed] [Google Scholar]
- 9.Bohach, G. A., B. N. Kreiswirth, R. P. Novick, and P. M. Schlievert. 1989. Analysis of toxic shock syndrome isolates producing staphylococcal enterotoxins B and C1 with use of southern hybridization and immunologic assays. Rev. Infect. Dis. 11(Suppl. 1):S75–81. [DOI] [PubMed]
- 10.Bohach G A, Schlievert P M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987;209:15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- 11.Casman E P. Further serological studies of staphylococcal enterotoxin. J Bacteriol. 1960;79:849–856. doi: 10.1128/jb.79.6.849-856.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casman E P, Bennett R W, Dorsey A E, Issa J A. Identification of a fourth staphylococcal enterotoxin, enterotoxin D. Biochemistry. 1967;94:1875–1882. doi: 10.1128/jb.94.6.1875-1882.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couch J L, Betley M J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989;171:4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couch J L, Soltis M T, Betley M J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988;170:2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Lucía E, Goyache J, Orden J A, Blanco J L, Ruiz Santa-Quiteria J A, Domínguez L, Suárez G. Production of enterotoxin A by supposedly nonenterotoxigenic Staphylococcus aureus strains. Appl Environ Microbiol. 1989;55:1447–1451. doi: 10.1128/aem.55.6.1447-1451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson A D, Spero L, Cades J S, de Cicco B T. Purification and characterization of different types of exfoliative toxin from Staphylococcus aureus. Infect Immun. 1979;24:679–684. doi: 10.1128/iai.24.3.679-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson W M, Tyler S D, Ewan E P, Ashton F E, Pollard D R, Rozee K R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C L, Khan S A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986;166:29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 20.Lee C Y, Schmidt J J, Johnson-Winegar A D, Spero L, Iandolo J J. Sequence determination and comparison of the exfoliative toxin A and toxin B genes from Staphylococcus aureus. J Bacteriol. 1987;169:3904–3909. doi: 10.1128/jb.169.9.3904-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longo M C, Berninger M S, Hartley J L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990;93:125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- 22.O’Toole P W, Foster T J. Nucleotide sequence of the epidermolytic toxin A gene of Staphylococcus aureus. J Bacteriol. 1987;169:3910–3915. doi: 10.1128/jb.169.9.3910-3915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiser R F, Robbins R N, Noleto A L, Khoe G P, Bergdoll M S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984;45:625–630. doi: 10.1128/iai.45.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rifai S, Barbancon V, Prevost G, Piemont Y. Synthetic exfoliative toxin A and B DNA probes for detection of toxigenic Staphylococcus aureus strains. J Clin Microbiol. 1989;27:504–506. doi: 10.1128/jcm.27.3.504-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlievert P M, Shands K N, Dan B B, Schmid G P, Nishimura R D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 26.Thompson N E, Razdan M, Kuntsmann G, Aschenbach J M, Evenson M L, Bergdoll M S. Detection of staphylococcal enterotoxins by enzyme-linked immunosorbent assays and radioimmunoassays: comparison of monoclonal and polyclonal antibody systems. Appl Environ Microbiol. 1986;51:885–890. doi: 10.1128/aem.51.5.885-890.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiley B B, Glasgow L A, Rogolsky M. Staphylococcal scalded-skin syndrome: development of a primary binding assay for human antibody to the exfoliative toxin. Infect Immun. 1976;13:513–520. doi: 10.1128/iai.13.2.513-520.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]