Abstract
OBJECTIVE
To study the interaction among HLA genotype, early probiotic exposure, and timing of complementary foods in relation to risk of islet autoimmunity (IA).
RESEARCH DESIGN AND METHODS
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows 8,676 children with increased genetic risk of type 1 diabetes. We used a Cox proportional hazards regression model adjusting for potential confounders to study early feeding and the risk of IA in a sample of 7,770 children.
RESULTS
Any solid food introduced early (<6 months) was associated with increased risk of IA if the child had the HLA DR3/4 genotype and no probiotic exposure during the 1st year of life. Rice introduced at 4–5.9 months compared with later in the U.S. was associated with an increased risk of IA.
CONCLUSIONS
Timing of solid food introduction, including rice, may be associated with IA in children with the HLA DR3/4 genotype not exposed to probiotics. The microbiome composition under these exposure combinations requires further study.
Graphical Abstract
Introduction
Class II HLA haplogenotypes account for about one-half of the genetic risk for islet autoimmunity (IA) and the later progression to type 1 diabetes (1). In addition to genes, environmental factors, including early diet, have been shown to be associated with the risk of IA (2). Probiotic use any time during the first 27 days of life was inversely associated with IA among children with the high-risk HLA DR3/4 genotype for type 1 diabetes in The Environmental Determinants of Diabetes in the Young (TEDDY) study (3). The objective of the current study was to investigate the interaction among timing of introduction of complementary foods, HLA genotype, and timing of first probiotic exposure in relation to IA in the TEDDY cohort.
Research Design and Methods
TEDDY is a prospective cohort study involving three clinical centers in the U.S. (Colorado, Georgia/Florida, Washington State), and three in Europe (Finland, Germany, and Sweden). The detailed study design and methods have been described previously (4–6). The study population is presented in Supplementary Fig. 1, and population characteristics in Supplementary Table 2. The final sample size was 7,770. The food exposures and categorization of timing are described in Table 1.
Table 1.
Food exposures
| Categorization of timing of food introduction by age (months) | |||
|---|---|---|---|
| Food | Early or short duration | Late (reference) | |
| Exclusive breastfeeding | <4 | ≥4 | |
| Any breastfeeding | <4 | ≥4 | |
| Any infant formula | <4 | 4 to <6 | ≥6 |
| Any solid food† | <4 | 4 to <6 | ≥6 |
| All cereals | <4 | 4 to <6 | ≥6 |
| Gluten-containing cereals | <4 | 4 to <6 | ≥6 |
| Nongluten-containing cereals | <4 | 4 to <6 | ≥6 |
| Fruits and berries | <4 | 4 to <6 | ≥6 |
| Root vegetables | <4 | 4 to <6 | ≥6 |
| Other vegetables than roots | <4 | 4 to <6 | ≥6 |
| Regular cow’s milk | <4 | 4 to <6 | ≥6 |
| Any meat‡ | <4 | 4 to <6 | ≥6 |
| Egg | ≤9 | >9 | |
| Rice* | <4 | 4 to <6 | ≥6 |
| Oat* | <4 | 4 to <6 | ≥6 |
All cereals (including gluten and nongluten), fruits and berries, all vegetables (including roots), milk products, eggs, any meat (including red meat, poultry, fish and seafood, processed meats).
Including all red meat, poultry, fish, and seafood.
Preliminary analyses suggested that nongluten cereals played a role in the associations between any solid food and the outcomes, and therefore, we additionally studied two of the most commonly consumed nongluten baby cereals, rice and oat, and their timing of introduction in relation to outcomes separately by country.
Infant gut microbiota goes through significant changes over the 1st year of life (7). Therefore, we also studied the timing of the initial probiotic exposure either from dietary supplements or from infant formula during the first 52 weeks. We also considered only early exposures before 26 weeks of age. We did not analyze findings during the first 4 weeks of life, as reported earlier (3), because these subgroup numbers were insufficient. Probiotics mainly included Lactobacillus reuteri and Lactobacillus rhamnosus. The length of probiotic use was not examined in this observational study.
IA
Persistent confirmed IA was defined by the presence of one or several autoantibodies against GAD (GADA), IA-2 antigen (IA-2A), or insulin (IAA) at each of the two TEDDY laboratories on two or more consecutive visits. The detailed study design and methods have been previously published (4,5). The timing of seroconversion was defined as the age of the first persistent confirmed autoantibody sample and the right-censored time as the age when the last blood sample available was determined as negative for IA.
Statistical Analysis
A Cox proportional hazards regression model was used to investigate the association between timing of food exposures and the risk of IA in the TEDDY cohort. Interactions between timing of food exposure and HLA genotype (DR3/4 compared with any other genotype than DR3/4) and between timing of food exposure and first probiotics were studied while controlling for country, whether any first-degree relative had type 1 diabetes, and sex of the child. Response variables included the risk of developing IA overall, IAA only as the first-appearing autoantibody (IAA-first), GADA only as the first-appearing autoantibody (GADA-first), or multiple autoantibodies appearing simultaneously. We also conducted three-way interaction models to examine whether the association between timing of selected foods and the risk of IA was modified by HLA DR3/4 and by the first exposure to probiotics. All statistical analyses were done using SAS 9.4 software (SAS/STAT 15.2).
Results
Main Effects
Early introduction of gluten-containing cereals was associated with a decreased risk of any IA, GADA-first, and multiple autoantibodies (Supplementary Tables 3–6). Wheat (consumed alone or with another cereal) accounted for 90% of the first exposures to gluten-containing cereals before 6 months of age.
Subgroups
There was an interaction between timing of introduction of fruit and berries and HLA genotype (DR3/4 vs. other) when multiple autoantibodies were studied as an outcome. Similarly, an interaction between timing of any solid food and first probiotics within the first 52 weeks in relation to multiple autoantibodies was observed. Furthermore, the interactions between timing of egg introduction and first probiotics in relation to IAA-first and GADA-first were found (Table 2).
Table 2.
Timing of introduction of selected complementary foods and risk of developing IA by HLA genotype or by use of probiotics during the first 52 weeks of life
| HLA genotype | Use of probiotics during the first 52 weeks*** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing of first food exposure (months) and outcome | n | Affected, n | Other than HLA DR3/4 HR (95% CI), P* | n | Affected, n | HLA DR3/4 HR (95% CI), P* | n | Affected, n | No probiotic exposure before 52 weeks of age HR (95% CI), P** | n | Affected, n | Probiotic exposure before or at 52 weeks of age HR (95% CI), P** |
| Any solid foods | ||||||||||||
| Any IA | ||||||||||||
| <4 | 1,840 | 145 | 0.93 (0.66, 1.30), 0.656 | 1,192 | 159 | 1.31 (0.90, 1.91), 0.153 | 2,367 | 234 | 1.20 (0.88, 1.62), 0.245 | 665 | 70 | 0.91 (0.57, 1.44), 0.678 |
| 4 to <6 | 2,393 | 243 | 1.09 (0.78, 1.50), 0.620 | 1,528 | 219 | 1.31 (0.91, 1.89), 0.151 | 3,070 | 364 | 1.31 (0.98, 1.76), 0.069 | 851 | 98 | 0.91 (0.59, 1.42), 0.688 |
| ≥6 | 495 | 48 | 1 | 322 | 36 | 1 | 621 | 55 | 1 | 196 | 29 | 1 |
| Interaction P | 0.209 | 0.154 | ||||||||||
| IAA-first | ||||||||||||
| <4 | 1,840 | 56 | 0.96 (0.54, 1.69), 0.880 | 1,192 | 60 | 1.78 (0.90, 3.53), 0.098 | 2,367 | 88 | 1.45 (0.85, 2.46), 0.174 | 665 | 28 | 0.90 (0.42, 1.93), 0.777 |
| 4 to <6 | 2,393 | 92 | 1.13 (0.65, 1.94), 0.670 | 1,528 | 76 | 1.70 (0.86, 3.34), 0.126 | 3,070 | 131 | 1.55 (0.92, 2.60), 0.101 | 851 | 38 | 0.90 (0.43, 1.87), 0.769 |
| ≥6 | 495 | 17 | 1 | 322 | 10 | 1 | 621 | 17 | 1 | 196 | 10 | 1 |
| Interaction P | 0.290 | 0.396 | ||||||||||
| GADA-first | ||||||||||||
| <4 | 1,840 | 55 | 0.84 (0.48, 1.46), 0.543 | 1,192 | 68 | 1.09 (0.64, 1.86), 0.754 | 2,367 | 94 | 0.93 (0.60, 1.44), 0.749 | 665 | 29 | 1.17 (0.53, 2.59), 0.692 |
| 4 to <6 | 2,393 | 118 | 1.28 (0.76, 2.15), 0.350 | 1,528 | 101 | 1.12 (0.66, 1.89), 0.667 | 3,070 | 174 | 1.22 (0.80, 1.86), 0.358 | 851 | 43 | 1.22 (0.57, 2.60), 0.609 |
| ≥6 | 495 | 18 | 1 | 322 | 18 | 1 | 621 | 27 | 1 | 196 | 9 | 1 |
| Interaction P | 0.307 | 0.732 | ||||||||||
| Multiple autoantibodies | ||||||||||||
| <4 | 1,840 | 69 | 0.86 (0.54, 1.38), 0.531 | 1,192 | 99 | 1.28 (0.81, 2.03), 0.289 | 2,367 | 122 | 1.29 (0.85, 1.95), 0.234 | 665 | 46 | 0.77 (0.45, 1.34), 0.358 |
| 4 to <6 | 2,393 | 129 | 1.14 (0.73, 1.76), 0.572 | 1,528 | 142 | 1.37 (0.87, 2.14), 0.172 | 3,070 | 212 | 1.61 (1.08, 2.41), 0.020 | 851 | 59 | 0.73 (0.43, 1.23), 0.234 |
| ≥6 | 495 | 27 | 1 | 322 | 24 | 1 | 621 | 29 | 1 | 196 | 22 | 1 |
| Interaction P | 0.236 | 0.028 | ||||||||||
| Cereals (24 missing) | ||||||||||||
| Any IA | ||||||||||||
| <4 | 1,101 | 81 | 0.86 (0.63, 1.19), 0.371 | 762 | 89 | 1.03 (0.74, 1.45), 0.844 | 1,501 | 135 | 1.06 (0.80, 1.40), 0.689 | 362 | 35 | 0.76 (0.48, 1.19), 0.224 |
| 4 to <6 | 2,807 | 273 | 1.01 (0.78, 1.32), 0.927 | 1,744 | 262 | 1.22 (0.91, 1.63), 0.184 | 3,585 | 428 | 1.25 (0.98, 1.59), 0.072 | 966 | 107 | 0.84 (0.59, 1.20), 0.335 |
| ≥6 | 805 | 82 | 1 | 527 | 62 | 1 | 952 | 89 | 1 | 380 | 55 | 1 |
| Interaction P | 0.447 | 0.051 | ||||||||||
| IAA-first | ||||||||||||
| <4 | 1,101 | 32 | 0.88 (0.52, 1.47), 0.617 | 762 | 33 | 1.26 (0.71, 2.25), 0.435 | 1,501 | 49 | 1.05 (0.67, 1.66), 0.820 | 362 | 16 | 1.02 (0.50, 2.07), 0.960 |
| 4 to <6 | 2,807 | 101 | 0.96 (0.63, 1.48), 0.858 | 1,744 | 94 | 1.50 (0.90, 2.52), 0.121 | 3,585 | 154 | 1.24 (0.84, 1.85), 0.277 | 966 | 41 | 0.96 (0.53, 1.73), 0.891 |
| ≥6 | 805 | 32 | 1 | 527 | 19 | 1 | 952 | 33 | 1 | 380 | 18 | 1 |
| Interaction P | 0.402 | 0.522 | ||||||||||
| GADA-first | ||||||||||||
| <4 | 1,101 | 31 | 0.79 (0.47, 1.32), 0.364 | 762 | 34 | 0.89 (0.53, 1.50), 0.252 | 1,501 | 52 | 0.93 (0.61, 1.44), 0.756 | 362 | 13 | 0.69 (0.33, 1.43), 0.315 |
| 4 to <6 | 2,807 | 130 | 1.16 (0.76, 1.77), 0.502 | 1,744 | 125 | 1.29 (0.83, 2.00), 0.697 | 3,585 | 207 | 1.38 (0.96, 1.99), 0.085 | 966 | 48 | 0.94 (0.53, 1.66), 0.832 |
| ≥6 | 805 | 30 | 1 | 527 | 27 | 1 | 952 | 37 | 1 | 380 | 20 | 1 |
| Interaction P | 0.957 | 0.368 | ||||||||||
| Multiple autoantibodies | ||||||||||||
| <4 | 1,101 | 40 | 0.82 (0.52, 1.27), 0.371 | 762 | 53 | 0.91 (0.60, 1.38), 0.660 | 1,501 | 70 | 0.95 (0.66, 1.37), 0.782 | 362 | 23 | 0.76 (0.44, 1.33), 0.337 |
| 4 to <6 | 2,807 | 137 | 0.97 (0.68, 1.39), 0.877 | 1,744 | 169 | 1.21 (0.85, 1.72), 0.298 | 3,585 | 238 | 1.24 (0.90, 1.69), 0.184 | 966 | 68 | 0.84 (0.54, 1.29), 0.420 |
| ≥6 | 805 | 48 | 1 | 527 | 43 | 1 | 952 | 55 | 1 | 380 | 36 | 1 |
| Interaction P | 0.430 | 0.240 | ||||||||||
| Gluten-containing cereals (134 missing) | ||||||||||||
| Any IA | ||||||||||||
| <4 | 294 | 14 | 0.49 (0.28, 0.84), 0.010 | 213 | 22 | 0.81 (0.52, 1.27), 0.359 | 410 | 31 | 0.68 (0.46, 0.99), 0.042 | 97 | 5 | 0.52 (0.21, 1.28), 0.155 |
| 4 to <6 | 1,624 | 162 | 0.97 (0.77, 1.21), 0.765 | 1,057 | 160 | 1.01 (0.80, 1.27), 0.918 | 2,116 | 254 | 0.95 (0.79, 1.14), 0.580 | 565 | 68 | 1.13 (0.82, 1.56), 0.454 |
| ≥6 | 2,723 | 259 | 1 | 1,725 | 230 | 1 | 3,421 | 365 | 1 | 1,027 | 124 | 1 |
| Interaction P | 0.397 | 0.636 | ||||||||||
| IAA-first | ||||||||||||
| <4 | 294 | 7 | 0.65 (0.295, 1.43), 0.281 | 213 | 7 | 0.73 (0.33, 1.62), 0.442 | 410 | 14 | 0.84 (0.47, 1.48), 0.539 | 97 | 0 | – |
| 4 to <6 | 1,624 | 58 | 0.88 (0.61, 1.28), 0.509 | 1,057 | 52 | 0.92 (0.63, 1.36), 0.670 | 2,116 | 83 | 0.84 (0.61, 1.14), 0.255 | 565 | 27 | 1.15 (0.70, 1.91), 0.578 |
| ≥6 | 2,723 | 100 | 1 | 1,725 | 86 | 1 | 3,421 | 138 | 1 | 1,027 | 48 | 1 |
| Interaction P | 0.992 | 0.798 | ||||||||||
| GADA-first | ||||||||||||
| <4 | 294 | 4 | 0.33 (0.12, 0.90), 0.030 | 213 | 9 | 0.73 (0.36, 1.47), 0.377 | 410 | 10 | 0.51 (0.26, 0.98), 0.042 | 97 | 3 | 0.67 (0.20, 2.21), 0.505 |
| 4 to <6 | 1,624 | 82 | 1.18 (0.84, 1.66), 0.330 | 1,057 | 75 | 1.04 (0.74, 1.46), 0.823 | 2,116 | 128 | 1.12 (0.86, 1.48), 0.404 | 565 | 29 | 1.09 (0.65, 1.82), 0.748 |
| ≥6 | 2,723 | 105 | 1 | 1,725 | 102 | 1 | 3,421 | 158 | 1 | 1,027 | 49 | 1 |
| Interaction P | 0.319 | 0.804 | ||||||||||
| Multiple autoantibodies | ||||||||||||
| <4 | 294 | 3 | 0.19 (0.06, 0.59), 0.004 | 213 | 12 | 0.76 (0.41, 1.38), 0.365 | 410 | 12 | 0.46 (0.25, 0.83), 0.010 | 97 | 3 | 0.52 (0.16, 1.67), 0.271 |
| 4 to <6 | 1,624 | 74 | 0.78 (0.57, 1.07), 0.127 | 1,057 | 106 | 1.16 (0.87, 1.53), 0.317 | 2,116 | 136 | 0.91 (0.71, 1.16), 0.429 | 565 | 44 | 1.16 (0.78, 1.72), 0.465 |
| ≥6 | 2,723 | 147 | 1 | 1,725 | 147 | 1 | 3,421 | 214 | 1 | 1,027 | 80 | 1 |
| Interaction P | 0.063 | 0.916 | ||||||||||
| Nongluten-containing cereals (29 missing) | ||||||||||||
| Any IA | ||||||||||||
| <4 | 1,029 | 79 | 0.89 (0.65, 1.23), 0.486 | 712 | 83 | 0.99 (0.71, 1.38), 0.948 | 1,415 | 128 | 1.01 (0.77, 1.32), 0.946 | 326 | 34 | 0.83 (0.53, 1.31), 0.418 |
| 4 to <6 | 2,830 | 270 | 0.98 (0.75, 1.27), 0.870 | 1,759 | 261 | 1.15 (0.87, 1.53), 0.323 | 3,601 | 424 | 1.17 (0.93, 1.47), 0.192 | 988 | 107 | 0.84 (0.59, 1.19), 0.326 |
| ≥6 | 850 | 87 | 1 | 561 | 69 | 1 | 1,018 | 100 | 1 | 393 | 56 | 1 |
| Interaction P | 0.523 | 0.092 | ||||||||||
| IAA-first | ||||||||||||
| <4 | 1,029 | 28 | 0.90 (0.54, 1.50), 0.675 | 712 | 35 | 1.08 (0.61, 1.89), 0.796 | 1,415 | 31 | 0.91 (0.59, 1.42), 0.696 | 326 | 32 | 1.19 (0.58, 2.43), 0.927 |
| 4 to <6 | 2,830 | 99 | 0.93 (0.61, 1.41), 0.723 | 1,759 | 107 | 1.29 (0.79, 2.08), 0.307 | 3,601 | 100 | 1.09 (0.75, 1.58), 0.648 | 988 | 106 | 0.97 (0.54, 1.76), 0.773 |
| ≥6 | 850 | 28 | 1 | 561 | 26 | 1 | 1,018 | 34 | 1 | 393 | 20 | 1 |
| Interaction P | 0.586 | 0.475 | ||||||||||
| GADA-first | ||||||||||||
| <4 | 1,029 | 30 | 0.80 (0.48, 1.35), 0.406 | 712 | 32 | 0.93 (0.55, 1.56), 0.776 | 1,415 | 50 | 0.94 (0.62, 1.45), 0.788 | 326 | 12 | 0.72 (0.34, 1.52), 0.386 |
| 4 to <6 | 2,830 | 129 | 1.12 (0.74, 1.69), 0.595 | 1,759 | 126 | 1.34 (0.87, 2.07), 0.186 | 3,601 | 206 | 1.35 (0.95, 1.93), 0.095 | 988 | 49 | 0.98 (0.56, 1.73), 0.943 |
| ≥6 | 850 | 32 | 1 | 561 | 28 | 1 | 1,018 | 40 | 1 | 393 | 20 | 1 |
| Interaction P | 0.888 | 0.451 | ||||||||||
| Multiple autoantibodies | ||||||||||||
| <4 | 1,029 | 40 | 0.91 (0.58, 1.41), 0.660 | 712 | 49 | 0.88 (0.58, 1.33), 0.543 | 1,415 | 67 | 0.96 (0.67, 1.39), 0.844 | 326 | 22 | 0.81 (0.46, 1.42), 0.456 |
| 4 to <6 | 2,830 | 136 | 0.99 (0.69, 1.41), 0.936 | 1,759 | 169 | 1.17 (0.83, 1.65), 0.376 | 3,601 | 237 | 1.23 (0.91, 1.66), 0.188 | 988 | 68 | 0.82 (0.53, 1.27), 0.380 |
| ≥6 | 850 | 49 | 1 | 561 | 47 | 1 | 1,018 | 59 | 1 | 393 | 37 | 1 |
| Interaction P | 0.480 | 0.213 | ||||||||||
| Fruits and berries (37 missing) | ||||||||||||
| Any IA | ||||||||||||
| <4 | 1,053 | 69 | 0.69 (0.51, 0.94), 0.017 | 690 | 84 | 1.03 (0.77, 1.39), 0.835 | 1,341 | 116 | 0.85 (0.67, 1.09), 0.199 | 402 | 37 | 0.83 (0.53, 1.30), 0.413 |
| 4 to <6 | 2,481 | 248 | 0.96 (0.76, 1.22), 0.751 | 1,584 | 230 | 1.09 (0.85, 1.40), 0.514 | 3,124 | 367 | 1.03 (0.85, 1.25), 0.759 | 941 | 111 | 1.00 (0.69, 1.44), 0.987 |
| ≥6 | 1,169 | 118 | 1 | 756 | 98 | 1 | 1,566 | 167 | 1 | 359 | 49 | 1 |
| Interaction P | 0.120 | 0.617 | ||||||||||
| IAA-first | ||||||||||||
| <4 | 1,053 | 26 | 0.76 (0.46, 1.28), 0.303 | 690 | 32 | 1.12 (0.68, 1.83), 0.665 | 1,341 | 32 | 0.96 (0.64, 1.44), 0.843 | 402 | 14 | 0.81 (0.38, 1.71), 0.575 |
| 4 to <6 | 2,481 | 101 | 1.19 (0.80, 1.77), 0.397 | 1,584 | 89 | 1.12 (0.73, 1.72), 0.603 | 3,124 | 80 | 1.16 (0.83, 1.62), 0.375 | 941 | 45 | 1.11 (0.60, 2.05), 0.746 |
| ≥6 | 1,169 | 38 | 1 | 756 | 33 | 1 | 1,566 | 33 | 1 | 359 | 16 | 1 |
| Interaction P | 0.290 | 0.880 | ||||||||||
| GADA-first | ||||||||||||
| <4 | 1,053 | 29 | 0.61 (0.38, 0.96), 0.034 | 690 | 33 | 0.95 (0.60, 1.51), 0.834 | 1,341 | 46 | 0.70 (0.49, 1.02), 0.061 | 402 | 16 | 1.01 (0.50, 2.07), 0.970 |
| 4 to <6 | 2,481 | 106 | 0.83 (0.59, 1.18), 0.303 | 1,584 | 10 | 1.20 (0.82, 1.75), 0.348 | 3,124 | 169 | 0.98 (0.74, 1.30), 0.865 | 941 | 47 | 1.12 (0.62, 2.03), 0.710 |
| ≥6 | 1,169 | 56 | 1 | 756 | 43 | 1 | 1,566 | 81 | 1 | 359 | 18 | 1 |
| Interaction P | 0.283 | 0.772 | ||||||||||
| Multiple autoantibodies | ||||||||||||
| <4 | 1,053 | 30 | 0.54 (0.34, 0.84), 0.006 | 690 | 57 | 1.08 (0.75, 1.56), 0.682 | 1,341 | 64 | 0.84 (0.61, 1.17), 0.305 | 402 | 23 | 0.69 (0.40, 1.21), 0.198 |
| 4 to <6 | 2,481 | 127 | 0.90 (0.65, 1.25), 0.533 | 1,584 | 144 | 1.09 (0.80, 1.49), 0.602 | 3,124 | 202 | 1.04 (0.80, 1.35), 0.756 | 941 | 69 | 0.86 (0.55, 1.34), 0.504 |
| ≥6 | 1,169 | 67 | 1 | 756 | 64 | 1 | 1,566 | 96 | 1 | 359 | 35 | 1 |
| Interaction P | 0.035 | 0.507 | ||||||||||
| Egg (470 missing) | ||||||||||||
| Any IA | ||||||||||||
| ≤9 | 3,098 | 286 | 0.99 (0.80, 1.23), 0.947 | 2,020 | 282 | 1.00 (0.80, 1.24), 0.974 | 4,082 | 445 | 0.94 (0.79, 1.12), 0.515 | 1,036 | 123 | 1.16 (0.85, 1.58), 0.350 |
| >9 | 1,353 | 450 | 1 | 886 | 126 | 1 | 1,663 | 192 | 1 | 576 | 69 | 1 |
| Interaction P | 0.801 | 0.466 | ||||||||||
| IAA-first | ||||||||||||
| ≤9 | 3,098 | 107 | 0.93 (0.66, 1.31), 0.682 | 2,020 | 97 | 0.96 (0.67, 1.37), 0.811 | 4,082 | 166 | 1.09 (0.81, 1.47), 0.554 | 1,036 | 38 | 0.63 (0.40, 1.01), 0.053 |
| >9 | 1,353 | 54 | 1 | 886 | 47 | 1 | 1,663 | 64 | 1 | 576 | 37 | 1 |
| Interaction P | 0.911 | 0.038 | ||||||||||
| GADA-first | ||||||||||||
| ≤9 | 3,098 | 130 | 1.02 (0.74, 1.42), 0.898 | 2,020 | 132 | 1.08 (0.78, 1.50), 0.651 | 4,082 | 200 | 0.86 (0.67, 1.11), 0.245 | 1,036 | 62 | 2.26 (1.29, 3.97), 0.004 |
| >9 | 1,353 | 55 | 1 | 886 | 53 | 1 | 1,663 | 91 | 1 | 576 | 17 | 1 |
| Interaction P | 0.904 | 0.004 | ||||||||||
| Multiple autoantibodies | ||||||||||||
| ≤9 | 3,098 | 135 | 0.83 (0.63, 1.11), 0.210 | 2,020 | 176 | 0.95 (0.72, 1.23), 0.677 | 4,082 | 236 | 0.85 (0.68, 1.06), 0.153 | 1,036 | 75 | 1.01 (0.6, 1.48), 0.942 |
| >9 | 1,353 | 81 | 1 | 886 | 86 | 1 | 1,663 | 119 | 1 | 576 | 48 | 1 |
| Interaction P | 0.321 | 0.475 | ||||||||||
Boldface indicates significance at P < 0.05.
Adjusted for country, first-degree family member with type 1 diabetes status, sex of the child, and probiotic exposure during the 1st year of life (52 weeks).
Adjusted for country, first-degree family member with type 1 diabetes status, sex of the child, and high-risk genotype (HLA DR3/4).
When the timing of first probiotic exposure was studied in categories <26 weeks, and ≥26 weeks, or none, slightly stronger associations were found, but they did not affect the interpretation of the results.
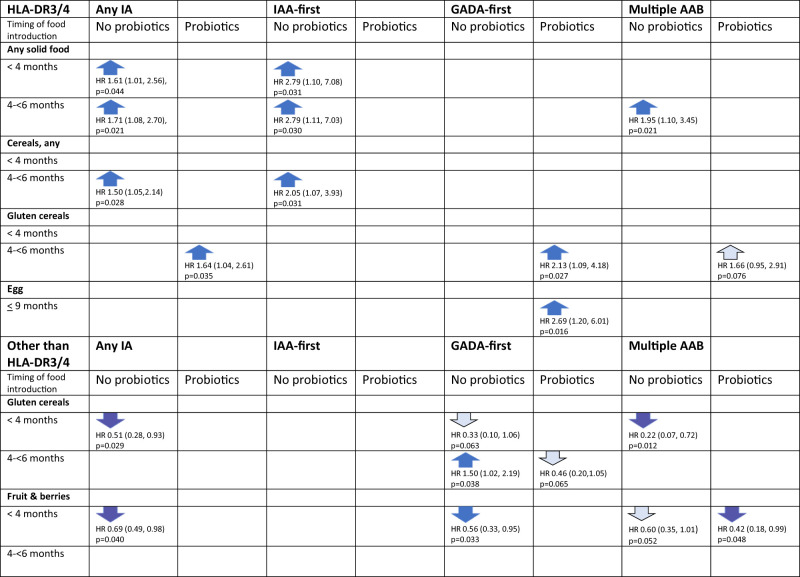
Both HLA genotype and probiotic exposure together modified the association between timing of any solid food introduction and risk of the outcomes (Fig. 1 and Supplementary Table 7). Among children who carried HLA DR3/4 and who were not exposed to probiotics during their first 52 weeks of life, early introduction of any solid food was associated with an increased risk of any IA, IAA-first, and multiple autoantibodies. However, if probiotics were introduced before 52 weeks, none of these associations were present in the subgroup of children with HLA DR3/4 (Fig. 1). The change in direction in the association by probiotics at <52 weeks was found only among children carrying a DR3 allele. Duration of breastfeeding was not associated with the risk of IA.
Figure 1.
Timing of the introduction of foods and the risk of developing any IA, IAA-first, GADA-first, and multiple autoantibodies by HLA genotype and by probiotic exposure by 52 weeks of age, showing only the statistically significant associations. The HR from the Cox proportional hazard model (with 95% CI) uses the reference of ≥6 months, except >9 months for egg. Dark-colored arrows flag P < 0.05, and light-colored arrows flag 0.05 < P < 0.09. Statistically significant three-way interactions between HLA genotype, timing of probiotic exposure, and timing of gluten cereals introduction: P = 0.034 for any IA and P = 0.019 for GADA-first, and between HLA genotype, timing of probiotic exposure, and timing of egg introduction: P = 0.023 for multiple autoantibodies.
Gluten-Containing Cereals, Nongluten-Containing Cereals, and Cereals Overall
Both HLA DR3/4 genotype and exposure to probiotics modified the association between early introduction of gluten-containing cereals and the outcomes (i.e., IA, GADA-first, and multiple autoantibodies) (Table 2). Children with the HLA DR3/4 genotype exposed to probiotics before the age of 52 weeks had an increased risk of IA and GADA-first if gluten-containing cereals were introduced between age 4 and 6 months compared with later (three-way interaction) (Fig. 1). However, among children with other HLA genotypes, early introduction of gluten-containing cereals was inversely associated with the risk of any IA if no probiotics were given before age of 52 weeks.
Country-Specific Analyses
There was an interaction between timing of rice introduction and country (P = 0.036) but not between timing of oat introduction and country. Only the U.S. and Sweden had a sufficient number of children in the subgroups to study the interaction. Timing of first rice cereal between age 4 and 6 months compared with later was associated with an increased risk of IA in the U.S. (hazard ratio [HR] 1.74; 95% CI 1.27, 2.38; P < 0.0005) but not in other countries (Table 3). U.S. children without probiotic exposure during the first 52 weeks, regardless of the HLA genotype, had an HR of 1.69 (1.22, 2.34; P = 0.0017) for the risk of any IA and 1.76 (1.10, 2.82; P = 0.019) for GADA-first when timing of rice introduction was between age 4 and 6 months compared with later.
Table 3.
Country-specific associations between timing of food introduction and IA
| U.S. | Finland | Germany | Sweden | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing of first food exposure (months) | Developed IA, n (%) | No IA, n (%) | HR (95% CI), P* | Developed IA, n (%) | No IA, n (%) | HR (95% CI), P* | Developed IA, n (%) | No IA, n (%) | HR (95% CI), P* | Developed IA, n (%) | No IA, n (%) | HR (95% CI), P* |
| Any solid food | ||||||||||||
| <4 | 112 (8.7) | 1,169 (91.3) | 1.78 (1.17, 2.69), 0.0066 | 82 (11.6) | 627 (88.4) | 0.67 (0.43, 1.03), 0.070 | 9 (6.3) | 133 (93.7) | 0.68 (0.31, 1.50), 0.340 | 101 (11.2) | 799 (88.8) | 0.75 (0.34, 1.62), 0.460 |
| 4 to <6 | 150 (10.3) | 1,301 (89.7) | 1.97 (1.32, 2.96), 0.001 | 100 (11.8) | 751 (88.2) | 0.64 (0.41, 0.98), 0.039 | 28 (12.4) | 197 (87.6) | 1.07 (0.61, 1.870, 0.813 | 184 (13.2) | 1,210 (86.8) | 0.82 (0.38, 1.76), 0.608 |
| ≥6 | 28 (5.8) | 452 (94.2) | 1 | 26 (19.0) | 111 (81.0) | 1 | 23 (13.9) | 142 (86.1) | 1 | 7 (20.0) | 28 (80.0) | 1 |
| Gluten-containing cereals | ||||||||||||
| <4 | 8 (6.2) | 122 (93.8) | 0.74 (0.36, 1.49), 0.392 | 3 (5.3) | 54 (94.7) | 0.42 (0.13, 1.31), 0.132 | 1 (2.6) | 38 (97.4) | 0.30 (0.04, 2.22), 0.240 | 24 (8.5) | 257 (91.5) | 0.73 (0.46, 1.16), 0.179 |
| 4 to <6 | 47 (8.4) | 512 (91.6) | 0.91 (0.67, 1.25), 0.565 | 71 (13.0) | 477 (87.0) | 1.07 (0.80, 1.42), 0.665 | 6 (6.7) | 83 (93.3) | 0.66 (0.28, 1.54), 0.331 | 198 (13.3) | 1,287 (86.7) | 1.05 (0.80, 1.38), 0.740 |
| ≥6 | 234 (9.6) | 2,204 (90.4) | 1 | 133 (12.5) | 935 (87.5) | 1 | 53 (13.5) | 340 (86.4) | 1 | 69 (12.6) | 480 (87.4) | 1 |
| Missing | 85 | 24 | 11 | 14 | ||||||||
| Nongluten-containing cereals | ||||||||||||
| <4 | 66 (7.7) | 787 (92.3) | 1.19 (0.82, 1.73), 0.363 | 40 (11.9) | 296 (88.1) | 0.86 (0.57, 1.31), 0.486 | 2 (4.3) | 45 (95.7) | 0.47 (0.11, 1.95) 0.298 | 54 (10.7) | 451 (89.3) | 0.89 (0.51, 1.56) 0.690 |
| 4 to <6 | 176 (10.4) | 1,523 (89.6) | 1.55 (1.13, 2.14), 0.007 | 118 (11.6) | 903 (88.4) | 0.78 (0.56, 1.09), 0.149 | 16 (9.4) | 154 (90.6) | 0.75 (0.42, 1.33), 0.323 | 221 13.0) | 1,478 (87.0) | 0.99 (0.59, 1.65), 0.962 |
| ≥6 | 48 (7.3) | 606 (92.7) | 1 | 50 (15.0) | 284 (85.0) | 1 | 42 (13.6) | 268 (86.5) | 1 | 16 (14.2) | 97 (85.8) | 1 |
| Missing | 6 | 6 | 5 | 12 | ||||||||
| Rice | ||||||||||||
| <4 | 61 (7.8) | 720 (92.2) | 1.29 (0.89, 1.87), 0.185 | 1 (2.2) | 44 (97.8) | 0.20 (0.03, 1.40), 0.104 | 1 (2.6) | 37 (97.4) | 0.26 (0.04, 1.90), 0.185 | 23 (9.0) | 233 (91.0) | 0.77 (0.49, 1.21), 0.259 |
| 4 to <6 | 178 (10.7) | 1,480 (89.3) | 1.74 (1.27, 2.38), 0.0005 | 89 (12.3) | 634 (87.7) | 0.97 (0.73, 1.28), 0.815 | 15 (10.3) | 131 (89.7) | 0.86 (0.48, 1.55), 0.614 | 176 (13.1) | 1,164 (86.9) | 1.03 (0.80, 1.33), 0.824 |
| ≥6 | 51 (6.8) | 705 (93.2) | 1 | 117 (13.2) | 772 (86.8) | 1 | 44 (13.2) | 289 (86.8) | 1 | 92 (12.9) | 620 (87.1) | 1 |
| Missing | 17 | 40 | 15 | 21 | ||||||||
| Oat | ||||||||||||
| <4 | 12 (6.9) | 163 (93.1) | 0.82 (0.46, 1.46), 0.494 | 4 (14.8) | 23 (85.2) | 1.31 (0.48, 3.59), 0.596 | 0 | 7 (100.0) | 0 | 20 (8.4) | 218 (91.6) | 0.78 (0.48, 1.28), 0.327 |
| 4 to <6 | 84 (9.0) | 849 (91.0) | 0.96 (0.74, 1.24), 0.736 | 103 (11.4) | 798 (88.6) | 0.89 (0.67, 1.17), 0.402 | 4 (8.5) | 43 (91.5) | 0.87 (0.31, 2.43), 0.988 | 197 (13.3) | 1,286 (86.7) | 1.04 (0.79, 1.35), 0.796 |
| ≥6 | 190 (9.5) | 1,816 (90.5) | 1 | 100 (13.4) | 648 (86.6) | 1 | 55 (12.7) | 378 (87.3) | 1 | 74 (12.6) | 513 (87.4) | 1 |
| Missing | 98 | 21 | 45 | 21 | ||||||||
| Fruits and berries** | ||||||||||||
| <4 | 59 (8.0) | 680 (92.0) | 1.16 (0.84, 1.61), 0.368 | 50 (11.4) | 389 (88.6) | 0.72 (0.48, 1.08), 0.1114 | 4 (5.1) | 75 (94.9) | 0.47 (0.17, 1.33), 0.157 | 40 (8.2) | 446 (91.8) | 0.61 (0.39, 0.95), 0.029 |
| 4 to <6 | 137 (10.5) | 1,161 (89.5) | 1.42 (1.09, 1.85), 0.0087 | 112 (11.4) | 874 (88.6) | 0.70 (0.49, 0.98), 0.040 | 15 (8.3) | 165 (91.7) | 0.64 (0.35, 1.17), 0.147 | 214 (13.4) | 1,387 (86.6) | 0.91 (0.64, 1.29), 0.597 |
| ≥6 | 93 (8.0) | 1,067 (92.0) | 1 | 45 (17.1) | 219 (82.9) | 1 | 41 (15.4) | 225 (84.6) | 1 | 37 15.7) | 198 (84.3) | 1 |
| Missing | 15 | 8 | 7 | 7 | ||||||||
Boldface indicates significance at P < 0.05.
Adjusted for first-degree family member with type 1 diabetes status, sex of the child, probiotic exposure during the 1st year of life (52 weeks), and high-risk genotype (HLA DR3/4).
Fruits and berries are often served together with baby porridge.
Conclusions
As published before, early introduction of gluten-containing cereals overall was linked to a decreased risk of IA in the geographically diverse population of TEDDY (8). We also confirmed that the risk of IA related to early introduction of any solid food among children with the highest level of HLA genetic risk (DR3/4) may be modified by probiotics, although the association was not as strong as previously observed in the younger cohort of TEDDY participants (9). A novel finding was that early exposure to egg (age <9 months) is associated with an increased risk of GADA-first only in those who were exposed to probiotics.
Immune or microbiota responses to gluten-containing cereals may depend on both the HLA genotype and probiotic exposure, and they could interact with each other. Molecular mechanisms that drive probiotic effects that may interact with genotype and food are not well understood (10). Nevertheless, gluten in cereals can act as a double-edged sword in its connection to the risk of type 1 diabetes (11,12). Gluten in wheat, barley, and rye are suggested to increase the risk of IA by promoting gut permeability and dysbiosis and to increase proinflammatory cytokines (13). Whole-grain wheat also contains several bioactive compounds promoting overall health, such as prebiotic oligosaccharides, which are linked to healthy gut microbiota (14).
The Infant Feeding Practices study (15) concluded that introduction of solid complementary foods before 4–6 months of age poses a greater risk to infant health than does infant formula. In our study, we noticed an increased risk of any IA and IAA-first with early introduction of any solid foods but only among those who were carrying the HLA DR3/4 (DR3) genotype and who did not have probiotic exposure.
The association between early timing of rice and increased risk of any IA in U.S. TEDDY children was intriguing. A somewhat toxic form of inorganic arsenic is found in relatively large quantities in rice of U.S. origin, especially if grown in southern states (16). Arsenic is a toxic trace element that can affect β-cell function and increase the risk of type 1 diabetes in youth (17) and may possibly interact with the gut microbiome (18). To decrease the potential of adverse health effects, the U.S. Food and Drug Administration has recently given guidelines for industry to reduce the arsenic content of infant rice cereals to the of level 100 parts per billion, which should be achievable under current good manufacturing practices (19). The association with the outcome was found with rice exposure between age 4 and 6 months but not earlier. During this time, children are introduced to larger quantities of solid foods. Therefore, the exposure effect of possible contaminants may be stronger than with small tastings provided earlier.
It will be important to investigate the function and immune responses of the host microbiome when studying early diet, including probiotic usage in children with a genetically increased risk of type 1 diabetes. Rice as an early food also requires further attention. The results of this study do not impose any changes in the current recommendations on infant feeding.
Article Information
Acknowledgments. The authors thank Sarah Austin-Gonzalez with the Health Informatics Institute at the University of South Florida for copyediting and graphical assistance.
Funding. The TEDDY study is funded by National Institute of Diabetes and Digestive and Kidney Diseases grants U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483, U01 DK124166, and U01 DK128847 and contract HHSN267200700014C and by the National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, Centers for Disease Control and Prevention, and JDRF. This work is supported in part by National Institutes of Health/National Center for Advancing Translational Sciences clinical and translational science awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR002535).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. U.U. contributed to the study design and the acquisition, analysis, and interpretation of data and drafted the manuscript. L.K.M. performed the statistical analysis and contributed to the interpretation of data and the drafting of the manuscript. C.A.A., K.V., J.Y., and S.H. contributed to the acquisition and interpretation of the data and critically reviewed the manuscript. Å.L., M.R., W.H., R.M., J.T., A.-G.Z., B.A., J.P.K., S.M.V., and J.M.N. contributed to the study concept and design and the acquisition and interpretation of data and critically reviewed the manuscript. U.U. and L.K.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 18th Congress of the Immunology of Diabetes Society virtual meeting, 1–4 November 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.23799882.
S.M.V. and J.M.N. share the last authorship.
The TEDDY Study Group members are listed in the supplementary material online.
References
- 1. Davies JL, Kawaguchi Y, Bennett ST, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature 1994;371:130–136 [DOI] [PubMed] [Google Scholar]
- 2. Knip M, Virtanen SM, Akerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr 2010;91(Suppl.):1506S–1513S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uusitalo U, Liu X, Yang J, et al.; TEDDY Study Group . Association of early exposure of probiotics and islet autoimmunity in the TEDDY Study. JAMA Pediatr 2016a;170:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 5. TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hagopian WA, Erlich H, Lernmark A, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uusitalo U, Lee HS, Andrén Aronsson C, et al.; TEDDY Study Group . Early infant diet and islet autoimmunity in the TEDDY study. Diabetes Care 2018;41:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uusitalo U, Lee HS, Andren Aronsson C, et al., TEDDY Study Group . Human leukocyte antigen (HLA) genotype and probiotics modify the association between early infant feeding and islet autoimmunity in the TEDDY study. Experimental Biology Meeting abstract. FASEB J 2016b;30:S1:lb269. [Google Scholar]
- 10. Lebeer S, Bron PA, Marco ML, et al. Identification of probiotic effector molecules: present state and future perspectives. Curr Opin Biotechnol 2018;49:217–223 [DOI] [PubMed] [Google Scholar]
- 11. Antvorskov JC, Josefsen K, Engkilde K, Funda DP, Buschard K. Dietary gluten and the development of type 1 diabetes. Diabetologia 2014;57:1770–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olivares M, Rodriguez J, Pötgens SA, et al. The Janus face of cereals: wheat-derived prebiotics counteract the detrimental effect of gluten on metabolic homeostasis in mice fed a high-fat/high-sucrose diet. Mol Nutr Food Res 2019;63:e1900632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haupt-Jorgensen M, Holm LJ, Josefsen K, Buschard K. Possible prevention of diabetes with gluten-free diet. Nutrients 2018;10:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jefferson A, Adolphus K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: a systematic review. Front Nutr 2019;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rippey PLF, Aravena F, Nyonator JP. Health impacts on early complementary food introduction between formula-fed and breastfed infants. J Pediatr Gastroenterol Nutr 2020;70:375–380 [DOI] [PubMed] [Google Scholar]
- 16. Potera C. U.S. rice serves up arsenic. Environ Health Perspect 2007;115:A296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grau-Pérez M, Kuo CC, Spratlen M, et al. The association of arsenic exposure and metabolism with type 1 and type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care 2017;40:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coryell M, Roggenbeck BA, Walk ST. The human gut microbiome’s influence on arsenic toxicity. Curr Pharmacol Rep 2019;5:491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration . Supporting document for action level for inorganic arsenic in rice cereals for infants. August 2020. Accessed 2 May 2023. Available from https://www.fda.gov/food/chemical-metals-natural-toxins-pesticides-guidance-documents-regulations/supporting-document-action-level-inorganic-arsenic-rice-cereals-infants