Abstract
OBJECTIVE
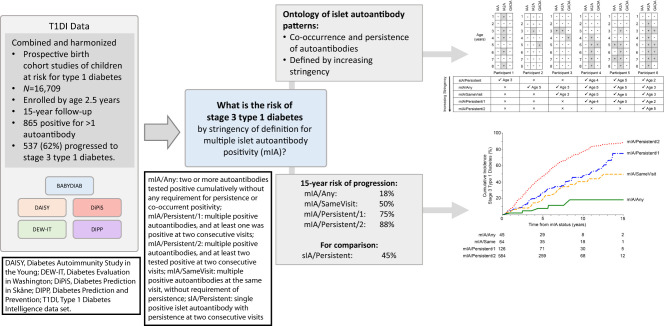
To estimate the risk of progression to stage 3 type 1 diabetes based on varying definitions of multiple islet autoantibody positivity (mIA).
RESEARCH DESIGN AND METHODS
Type 1 Diabetes Intelligence (T1DI) is a combined prospective data set of children from Finland, Germany, Sweden, and the U.S. who have an increased genetic risk for type 1 diabetes. Analysis included 16,709 infants-toddlers enrolled by age 2.5 years and comparison between groups using Kaplan-Meier survival analysis.
RESULTS
Of 865 (5%) children with mIA, 537 (62%) progressed to type 1 diabetes. The 15-year cumulative incidence of diabetes varied from the most stringent definition (mIA/Persistent/2: two or more islet autoantibodies positive at the same visit with two or more antibodies persistent at next visit; 88% [95% CI 85–92%]) to the least stringent (mIA/Any: positivity for two islet autoantibodies without co-occurring positivity or persistence; 18% [5–40%]). Progression in mIA/Persistent/2 was significantly higher than all other groups (P < 0.0001). Intermediate stringency definitions showed intermediate risk and were significantly different than mIA/Any (P < 0.05); however, differences waned over the 2-year follow-up among those who did not subsequently reach higher stringency. Among mIA/Persistent/2 individuals with three autoantibodies, loss of one autoantibody by the 2-year follow-up was associated with accelerated progression. Age was significantly associated with time from seroconversion to mIA/Persistent/2 status and mIA to stage 3 type 1 diabetes.
CONCLUSIONS
The 15-year risk of progression to type 1 diabetes risk varies markedly from 18 to 88% based on the stringency of mIA definition. While initial categorization identifies highest-risk individuals, short-term follow-up over 2 years may help stratify evolving risk, especially for those with less stringent definitions of mIA.
Graphical Abstract
Introduction
Type 1 diabetes results from chronic autoimmune destruction of pancreatic β-cells. Onset of clinical symptoms is typically preceded by a period of islet autoimmunity, characterized by development of one or more autoantibodies against islet autoantigens (insulin autoantibody [IAA], GAD autoantibody [GADA], insulinoma antigen-2 autoantibody [IA-2A], and zinc transporter type 8 autoantibody [ZnT8A]) (1). Islet autoantibody positivity is known to predict risk for type 1 diabetes (2). Previous prospective studies showed that multiple islet autoantibody positivity (mIA) strongly predicts progression to symptomatic type 1 diabetes (3–5), forming the basis of the staging of presymptomatic type 1 diabetes (6) guidelines adopted by the American Diabetes Association (ADA) and the International Society for Pediatric and Adolescent Diabetes (ISPAD) (7,8). Proper staging is important for accurate communication of risk to patients and families and for identification of individuals who may benefit from potential interventions, such as the recently approved teplizumab, as well as other promising candidates in the pipeline for clinical use (9,10). Further, studies exploring universal screening for islet autoantibodies present the potential for more routine identification of individuals at early-stage type 1 diabetes (11,12).
Although current staging definitions use a binary definition of autoantibody positivity (i.e., presence or absence), titers of islet autoantibodies can fluctuate, and individuals can occasionally revert to autoantibody negative (13–18). These fluctuations impact the magnitude of the predicted risk (15–17,19) and may stratify risk groups further. Differences in sampling frequency may also impact determination of mIA. Previous studies have typically defined mIA status as occurring in individuals who first meet the definition of seroconversion: at least one islet autoantibody positive on at least two consecutive visits. Multiple islet autoantibody status is typically defined as the visit at or following seroconversion with two or more positive islet autoantibodies that persist at the following visit. It is unclear whether the most stringent definitions of mIA are required to identify children at the highest risk (stage 1 type 1 diabetes), or whether more permissive definitions are adequate to define this high-risk group. As islet autoantibody testing moves from prospective studies to general population screening (11,12), a better understanding of the predictive characteristics of various definitions of mIA status will have significant clinical utility in the diagnosis of stage 1 type 1 diabetes. Less stringent definitions of islet autoantibody status may be sufficient to predict high-risk or intermediate-risk individuals. We hypothesized that both persistence and co-occurrence of more than one type of islet autoantibody would predict risk of stage 3 type 1 diabetes. We explore the impact of several definitions of mIA, incorporating co-occurrence and persistence over short-term follow-up (up to 2 years) in our large Type 1 Diabetes Intelligence (T1DI) study cohort (20) in order to inform further stratification of risk in individuals with mIA status.
Research Design and Methods
Study Population
The T1DI cohort encompasses data aggregated from 24,662 children in five prospective cohort studies from Finland (Diabetes Prediction and Prevention [DIPP]) (21), Germany (BABYDIAB and BABYDIET) (22), Sweden (Diabetes Prediction in Skåne [DiPiS]) (23), and the U.S. (Diabetes Autoimmunity Study in the Young [DAISY] [24] and Diabetes Evaluation in Washington [DEW-IT] [25]). The cohorts, various islet autoantibody assays used by each component study, and data harmonization are described briefly in the Supplementary Material and in detail elsewhere (20). For these analyses, we used a homogeneous infant-toddler subcohort (20) of 16,709 participants who were first tested for islet autoantibodies to insulin (IAA), GAD (GADA), and IA-2A at or before 2.5 years of age. These participants had a median of 12 visits and 10.4 years of follow-up, although the follow-up interval for assessment of islet autoantibodies varied among individual studies (range 3–36 months). Participants were followed for up to 15 years or until a diagnosis of stage 3 type 1 diabetes, whichever came first.
All individual study protocols were approved by local institutional review boards. The T1DI study cohort aggregation was performed in accordance with the Health Insurance Portability and Accountability Act and the General Data Protection Regulation regulations. IBM Research performed the analyses presented here with JDRF and its academic partners in the T1DI Study group.
Evaluating Type 1 Diabetes Using Ontology of Islet Autoantibody Positivity
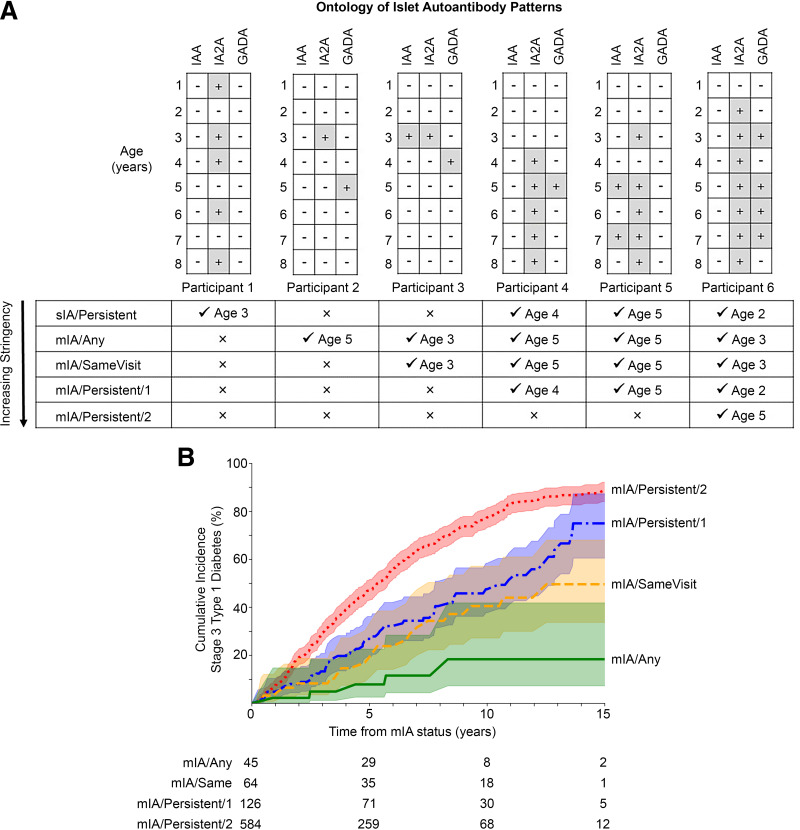
Stage 3 type 1 diabetes diagnosis is the primary end point in this study, defined according to ADA criteria (8). Islet autoantibody positivity is defined as the presence of one or more autoantibodies in a participant’s serum. We conceived an ontology for mIA as described in Fig. 1A. Participant visits in the T1DI data set were labeled according to the definitions they satisfied at that visit. Of note, a participant can satisfy more than one definition of islet autoantibody positivity, especially when considering variable duration of follow-up. Therefore, we define a concept of “stringency” for our analyses. We sought to capture the dynamic process of autoimmunity development beyond antibody type or number by a given time point (i.e., up to that follow-up duration). For example, if we consider the entire follow-up duration for participant 6 (Fig. 1A) then they achieved highest stringency of mIA/Persistent/2 by age 5 but had achieved mIA/Persistent/1 by age 2. The categories of stringency in increasing order are as follows:
Single islet autoantibody positivity (sIA)/Persistent: single positive islet autoantibody with persistence at two consecutive visits (Participants 1, 4–6).
mIA/Any: two or more autoantibodies tested positive cumulatively without any requirement for persistence or co-occurrent positivity. This is the baseline, least stringent mIA status (Participants 2–6).
mIA/SameVisit: multiple positive autoantibodies at the same visit, without requirement of persistence (Participants 3–6).
mIA/Persistent/1: multiple positive autoantibodies, and at least one was positive at two consecutive visits (Participants 4–6).
mIA/Persistent/2: multiple positive autoantibodies, and at least two tested positive at two consecutive visits (Participant 6).
Figure 1.
Ontologic analysis of islet autoantibody patterns. A: Diagram shows the ontology of islet autoantibody patterns as determined by persistence and number of co-occurring autoantibodies. Examples of serial autoantibody measurements with outcome for each islet autoantibody: +for positive, −for negative. Each visit is labeled with age in years, depicted as annual visit for illustration purposes. Definition chart is in order of increasing stringency. For each example participant, a checkmark indicates whether a definition was met and the age at which criteria were met. B: Cumulative incidence of type 1 diabetes by highest stringency of mIA definition. P < 0.005 by multivariate log-rank test for B. Shaded areas show the 95% CI. Pairwise comparisons are in Supplementary Table 2.
We also hypothesized that persistence (or loss) of antibodies within a clinically relevant time period (e.g., 2 years) in mIA/Persistent/2-positive participants may affect progression to stage 3 type 1 diabetes, based on a recent report (15). To evaluate this, we define an initial time point (t0) at which mIA/Persistent/2 status was reached and status at (t1) 2 ± 0.5 years later as follows (Supplementary Table 1):
M-Sustained: the mIA/Persistent/2 participant either retained the same number or gained an additional antibody at t1.
M3-M2: the mIA/Persistent/2 participant at t0 lost one antibody but remained multiple autoantibody positive at t1 (i.e., from three to two antibodies).
M-Single: the mIA/Persistent/2 participant at t0 lost one or more antibodies and was single antibody positive at t1.
Individuals who lost all antibodies or had no available data at t1 were excluded from analysis.
Multiple Autoantibodies Stringency Analyses
We analyzed mIA stringency at two analytic time points: 1) the “immediate next visit” and 2) the 2 ± 0.5-year follow-up. Median intervals were 0.27 years (interquartile range [IQR] 0.23–0.48) and 2.0 years (IQR 1.9–2.1), respectively. For both analyses, we stratified the cohort by the highest stringency of mIA achieved by the analytic time point (per ontology defined above). The rationale to consider the immediate next visit was that participants often develop antibody persistence subsequently, but some do not (Fig. 1A, participant 4 vs. 2); therefore, confirmation of mIA status needs at least one additional assessment. Thus, the next immediate visit qualifies stringency of mIA status in the surrounding period. The longer 2-year assessment qualifies stringency (or any change) during follow-up.
Multiple Autoantibodies Persistence (or Loss) Analyses
Persistence (or loss) of antibodies during 2-year follow-up was analyzed in mIA/Persistent/2 children, per definitions above (M-Sustained, M3-M2, M-Single). The rationale was that most children achieving this category continue to show some amount of persistence in the subsequent visits.
Age Analyses
We analyzed effect of age by quartiles at the immediate next visit on two intervals of progression: 1) from sIA/Persistent to mIA/Persistent/2 and 2) mIA/Persistent/2 to stage 3 type 1 diabetes.
Statistical Analyses
Kaplan-Meier survival analyses generated cumulative incidence (risk) estimates stratified by mIA stringency or age quartiles at the two analytic time points: immediate next visit and 2-year follow-up. Participants were categorized exclusively to the most stringent level at the analytic time point considered.
For all analyses of progression to stage 3 type 1 diabetes, event time is defined as time at the diagnosis of clinical diabetes or the last visit for those who did not progress. For all analyses, the first visit of achieving mIA status (or the first visit of stringent definition of interest) is used as the start (index) time for survival analyses, while the strata (for age, stringency, or persistence or loss) are defined based on the analytic time point.
Kaplan-Meier curves were plotted with 95% CIs and the multivariate log-rank test was used to confirm statistical differences. Pairwise statistical comparisons were made where relevant. We calculated the positive predictive value (PPV) and sensitivity (SENS) for onset of stage 3 type 1 diabetes in 15 years of follow-up from each analytic time point t1 based on stringency and persistence (or loss) of mIA status. Since not all participants were followed for the entire 15-year follow-up period, we used inverse probability of censoring weighting (26) to handle censored observations in order to calculate these weighted metrics. Using Python 3.6 software, all statistical significance was tested at P < 0.05 using the Bonferroni correction for multiple comparisons when appropriate (corrected threshold noted in individual analyses).
Results
Among 16,709 participants, 865 (5%) had developed more than one islet autoantibody at some point during follow-up (i.e., were mIA [encompassing all stringency levels]). Of the mIA participants, 46 (5%) were diagnosed at or before the next follow-up visit (Supplementary Fig. 1). Of the remaining 819 participants with mIA status, 491 participants (57%) were diagnosed with stage 3 type 1 diabetes at a median of 4.0 years (IQR 2.0–6.6) following mIA status.
When considering the entire group of individuals with mIA, the number of individuals per group increased with stringency, with 45 (6%) mIA/Any, 64 (10%) mIA/SameVisit, 126 (15%) mIA/Persistent/1, and 584 (71%) achieving the highest stringency of mIA/Persistent/2 (Table 1 and Supplementary Fig. 1).
Table 1.
Cohort characteristics of mIA definition (n = 819) at both immediate next visit and at 2 ± 5 years following first mIA status (n = 638)
| Stringency at definition | No. (% of all mIA) | Age at definition, years Median (IQR) | Stage 3 by 15 years n (%) | Years to stage 3 Median (IQR) | 5-year incidence % (95% CI) | 10-year incidence % (95% CI) | 15-year incidence % (95% CI) | PPV, 15 years % (95% CI) | SENS, 15 years % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| sIA/Persistent | 1,047 (N/A) | 4.8 (2.0–8.1) | 100 (10) | 4.3 (2.3–7.1) | 15 (12–17) | 32 (30–35) | 45 (40–52) | 53 (47–59) | 35 (33–38) |
| mIA/Any | 45 (6) | 8.5 (5.3–12.1) | 5 (11) | 4.4 (2.5–5.7) | 8 (2–22) | 18 (5–40) | 18 (5–40) | 38 (0–84) | 26 (6–46) |
| mIA/SameVisit | 64 (8) | 3.5 (2.0–5.3) | 20 (31) | 5.2 (3.0–7.1) | 18 (15–35) | 40 (22–50) | 50 (35–60) | 87 (69–100) | 47 (41–52) |
| mIA/Persistent/1 | 126 (15) | 5.1 (2.7–8.9) | 57 (45) | 4.8 (2.8–8.4) | 25 (18–35) | 45 (35–55) | 75 (60–85) | 72 (57–88) | 42 (37–47) |
| mIA/Persistent/2 | 584 (71) | 3.0 (1.8–5.9) | 409 (70) | 3.8 (1.9–6.3) | 48 (42–50) | 75 (70–80) | 88 (85–92) | 85 (80–91) | 46 (45–48) |
| Stringency at 2 years | No. (% of all mIA) | Age at follow-up, years Median (IQR) | Stage 3 by 15 years n (%) | Years to stage 3 Median (IQR) | Cumulative incidence, 5 years % (95% CI) | Cumulative incidence, 10 years % (95% CI) | Cumulative incidence, 15 years % (95% CI) | PPV, 15 years % (95% CI) | SENS, 15 years % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| mIA/Any | 32 (5) | 9.1 (5.3–12.1) | 3 (9) | 2.5 (1.7–5.4) | 6 (1–25) | 18 (3–55) | 18 (3–55) | ND | ND |
| mIA/SameVisit | 35 (6) | 3.5 (2.0–6.5) | 4 (11) | 2.7 (1.4–4.1) | 15 (5–25) | 12 (5–20) | 12 (5–20) | 61 (0–100) | 36 (12–59) |
| mIA/Persistent/1 | 60 (9) | 5.8 (3.0–10.5) | 14 (23) | 3.5 (2.8–7.4) | 16 (8–28) | 30 (20–50) | 40 (25–60) | 77 (41–100) | 43 (32–54) |
| mIA/Persistent/2 | 511 (80) | 3.3 (2.0–6.0) | 345 (68) | 5.2 (3.4–7.4) | 30 (28–32) | 70 (65–75) | 85 (80–90) | 83 (77–88) | 45 (43–47) |
Participants who were sIA/Persistent (n = 1,047) at seroconversion are included at the top of the table for comparison; this group includes participants who may later develop mIA. All incidences reported are cumulative incidence. Stringency at definition is at immediate next visit after achieving mIA status, and mIA groups are exclusive by the most stringent definition at the time of the initial mIA status. Stringency at 2 years is at visit 2 ± 0.5 years after achieving mIA status. Categories are listed by increasing stringency. Age, age at mIA status; N/A, not applicable as sIA/Persistent is not a subgroup of mIA definitions; ND, not determined due to insufficient n.
Characteristics of Cohort Based on Multiple Islet Autoantibody Definition
The median age of the cohort at the index visit when mIA was first noted was 3.6 years (IQR 2.0–7.2) and age at immediate next visit was 4.0 years (IQR 2.3–7.6). Survival analysis showed that mIA/Persistent/2 had a significantly higher progression to stage 3 type 1 diabetes than all other definitions of mIA status (P < 0.0001) (Fig. 1B and Supplementary Table 2). There was no significant difference between mIA/Persistent/1 and mIA/SameVisit (P = 0.15), but mIA/Persistent/1 was significantly more likely to progress than mIA/Any (P < 0.01). The difference between mIA/SameVisit and mIA/Any did not meet significance when corrected for multiple comparisons (P = 0.036). The 15-year cumulative incidence of developing stage 3 type 1 diabetes increased with increasing level of stringency at this visit and varied from 18 to 88% (Table 1). Cumulative incidence at the 5- and 10-year follow-up showed the same pattern. The weighted PPV for developing stage 3 type 1 diabetes within 15 years was highest for mIA/SameVisit, followed by mIA/Persistent/2, mIA/Persistent/1, and mIA/Any (87% [69–100%], 85% [80–91%], 72% [57–88%], and 38% [0–84%], respectively) (Table 1). Weighted sensitivity of the various definitions to identify individuals who would develop stage 3 type 1 diabetes showed a similar pattern (mIA/SameVisit: 47% [41–52%]; mIA/Persistent/2: 46% [45–48%]; mIA/Persistent/1: 42% [37–47%]; and mIA/Any: 26% [6–46%]). Of note, the cumulative incidence for sIA/Persistent at all three time points was between the incidence for mIA/Any and mIA/SameVisit; 15-year PPV and SENS showed a similar pattern (Table 1).
Age Effect on Progression
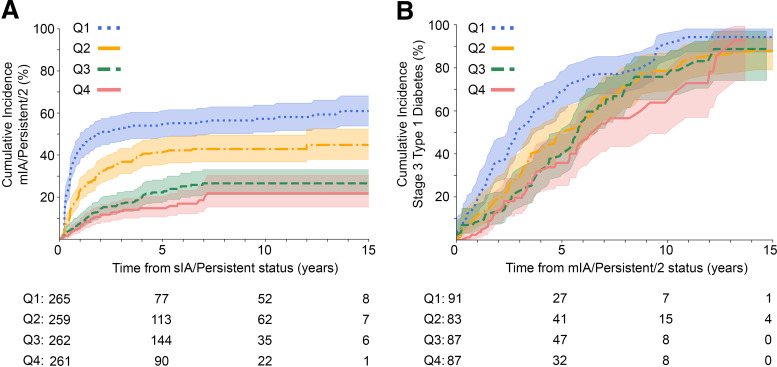
Age at achieving sIA/Persistent status was significantly related to the rate of progression to mIA/Persistent/2 status, with the youngest two quartiles differing from each other and the other two groups (P < 0.0001), while the oldest two quartiles were not significantly different from each other (Fig. 2A and Supplementary Table 3). Age at the immediate next visit after the mIA/Persistent/2 index visit was significantly related to the rate of progression to stage 3 type 1 diabetes, with the youngest age quartile differing from all others except quartile 2, which did not reach significance when adjusted for multiple comparisons (quartile 1 vs. quartile 2, P = 0.0093) (Fig. 2B and Supplementary Table 4).
Figure 2.
Impact of age on progression. A: Cumulative incidence of mIA/Persistent/2 stratified by age quartile (Q) at sIA/Persistent status (n = 1,047). B: Cumulative incidence of stage 3 type 1 diabetes stratified by age quartile at mIA/Persistent/2 status (n = 819). Age quartile intervals for both A and B are as follows: Q1 (0.0, 2.0), Q2 (2.0, 3.5), Q3 (3.5, 7.1), Q4 (7.1, 18.7); (parentheses indicate participant are older than lower age boundary, bracket indicates age quartile is inclusive of upper boundary age). P < 0.005 by multivariate log-rank test for both A and B. The shaded areas show the 95% CI. Pairwise comparisons are in Supplementary Tables 3 and 4, respectively.
Characteristics of Cohort Based on Index Visit Status and mIA Status 2 Years Later
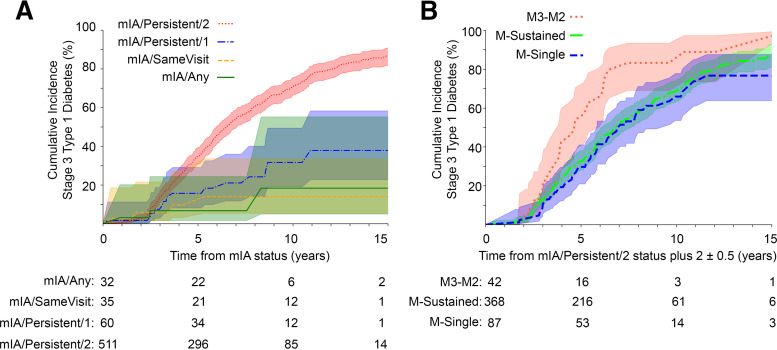
As antibody status can fluctuate over time, we examined the impact on progression of mIA status at the visit 2 years following the initial mIA index visit. Of mIA participants, 679 were still in the cohort 2 years after the index visit. Of these, 638 had follow-up data available at this time point (Supplementary Fig. 2), and 366 (57%) were later diagnosed with stage 3 type 1 diabetes in a median of 5.1 years (IQR 3.3–7.3). Most participants at the 2-year follow-up demonstrated the highest stringency of mIA/Persistent/2 at the index visit (n = 511 [80%]), followed by mIA/Persistent/1 (n = 60 [10%]) (Table 1). As before, the 15-year cumulative incidence of developing stage 3 type 1 diabetes increased with increasing stringency from 18 to 85% (Table 1) by this visit. However, survival analysis showed that while the highest stringency of mIA/Persistent/2 was significantly different from the other groups (P < 0.0001), the lesser three mIA stringency patterns no longer differed from each other (Fig. 3A and Supplementary Table 5). The weighted PPV and SENS to developing stage 3 type 1 diabetes in 15 years of follow-up was highest for mIA/Persistent/2, followed by mIA/Persistent/1 and mIA/SameVisit (Table 1). Of note, when examining risk of progression for those who had some level of persistence at baseline (mIA/Persistent/1 and mIA/Persistent/2), survival analysis showed a pronounced separation between those who had achieved mIA/Persistent/2 by the 2-year follow-up compared with those who remained mIA/Persistent/1 (P < 0.0001) (Supplementary Fig. 3).
Figure 3.
Impact of 2 ± 0.5 years of follow-up on cumulative incidence of stage 3 type 1 diabetes. A: Individuals who were mIA by any definition at baseline, stratified at follow-up by highest stringency of mIA definition achieved. B: Individuals who were mIA/Persistent/2 at baseline stratified at 2-year follow-up by persistence (or loss) of autoantibodies. Shaded areas show the 95% CI. P < 0.005 by multivariate log-rank test for A and B. Pairwise comparisons are in Supplementary Table 5 and Supplementary Table 6, respectively.
Further exploration of a 1- and 5-year follow-up interval showed a similar pattern of separation of the mIA/Persistent/2 group from the other categories (P < 0.01 and P < 0.001, respectively) (Supplementary Fig. 4A and B). The mIA/Persistent/1 group showed a significantly higher risk of progression than mIA/Any (P = 0.001) at the 1-year follow-up, but not at the 2- or 5-year follow-up (Fig. 3A and Supplementary Fig. 4A and B), indicating this intermediate category differentiates risk stratification over the short-term, but ultimately, this differentiation wanes.
Age Effect and Increasing mIA Stringency at 2 Years
Survival analysis of varying stringencies of mIA at 2 years of follow-up showed that age played an important role in risk stratification when looking at an inclusive definition of mIA (Supplementary Fig. 5A). In contrast, when the most stringent definition was used (mIA/Persistent/2), only the youngest quartile showed significantly faster progression than other age-groups (P < 0.01) (Supplementary Fig. 5B).
Characteristics of the Cohort Based on Antibody Persistence or Reversion 2 Years Later
There were 521 participants who had a stringency of mIA/Persistent/2 at baseline and had data available at a visit 2 years later (Supplementary Table 1). Among them, 368 (71%) remained multiple positive (M-Sustained) at follow-up (i.e., either with same number of multiple autoantibodies or gained additional antibody), 87 (17%) became M-Single, 42 (8%) became M3-M2, and 24 (5%) had no measurement or lost all autoantibodies by this visit. Figure 3B shows the Kaplan-Meier survival analysis of risk for progression to stage 3 type 1 diabetes based on persistence/reversion category. mIA/Persistent/2 individuals who became M-Sustained did not differ from those who became M-Single (P = 0.30). However, M3-M2 individuals progressed significantly faster than both M-Sustained and M-Single groups (P < 0.01) (Fig. 3B and Supplementary Table 6). The difference in rates of progression to stage 3 type 1 diabetes peaked at 5–6 years of follow-up from the initial mIA/Persistent/2 visit. The PPV of M3-M2, M-Sustained, and M-Single categories of persistence to developing stage 3 type 1 diabetes in 7 years of follow-up was as follows: 81% (95% CI 70–91), 52% (95% CI: 48–57), and 52% (95% CI 43–61), respectively. Of the 42 individuals in the M3-M2 subgroup, the majority (60% [n = 25]) had lost IAA, followed by GADA (33% [n = 14]). Only a few participants (7% [n = 3]) lost IA-2A by the 2-year follow-up.
Conclusions
The Endocrine Society, ADA, and JDRF define stage 1 type 1 diabetes based on the high risk of progression for individuals with mIA (6). Previous work supporting this staging by Ziegler et al. (3) included individuals who were followed prospectively from infancy and met their definition of “seroconversion” (i.e., at least one islet autoantibody positive with confirmation at the subsequent visit). This analysis stratified participants by the maximum number of islet autoantibodies positive over the duration of follow-up; those with two or more islet autoantibodies were noted to have a high risk of progression to symptomatic (stage 3) type 1 diabetes. Progression risk in this previous work was estimated from the time of seroconversion, which is often before an individual becomes mIA.
Population screening identifies individuals at a moment in time, without knowledge of prior history of islet autoantibody status. For clinical utility, it is helpful to define risk associated with diverse presentations of multiple autoantibody status and estimate risk based on the time of meeting the mIA definition. In this study, we refine risk stratification based on a time closer to an individual’s achieving mIA status.
Prospective studies have observed that antibody levels can vary over time and that individuals can acquire additional autoantibodies or previously positive autoantibodies can revert to negative (18). The current definition of stage 1 does not specify whether persistence or confirmation of mIA status is required in order to identify those at highest risk of progression to symptomatic disease. In that regard, our work expands previous findings (5,15,27,28) by examining a large prospective cohort with more granularity for progression risk. We further explore broader categories of mIA (i.e., both with and without persistence of one or more autoantibodies and with or without contemporaneous positivity of autoantibodies). We also examine the impact of persistence of antibodies over relatively short time windows of follow-up.
As the definition of positive autoantibody uses the 98th to 99th percentile for the control population, by definition, some positive results are spurious. In one previous report, 31% of sIA results were not confirmed upon subsequent testing (18). Thus, the least stringent groups, mIA/Any and mIA/SameVisit, which do not have confirmation (persistence) of any autoantibody, are most likely to represent a constellation of false-positive results. This analysis is the first to explore whether an additional, albeit unconfirmed, autoantibody substantially increases the likelihood of identifying truly at-risk individuals and whether the co-occurrence of the unconfirmed autoantibodies makes a difference in risk prediction. It is interesting to note that the 15-year incidence for stage 3 type 1 diabetes for sIA/Persistent is similar to that of mIA/SameVisit but higher than the incidence for mIA/Any. This mIA/Any group is the smallest group with the oldest age at definition of mIA status, suggesting that this category may represent either false-positive results or a transient, nonpathogenic process. It is also interesting to note that at initial definition, mIA/SameVisit, mIA/Persistent/1, and mIA/Persistent/2 all have similar weighted sensitivity and PPV for 15-year risk of stage 3 type 1 diabetes (Table 1). This suggests that while risk at the time of definition may differ between stringencies of mIA definition, less stringent definitions are still effective at identifying at-risk individuals.
Our work shows that the most stringent definition, requiring not only concurrent presence of two or more autoantibodies but also persistence of at least two of those autoantibodies, identifies children at the highest risk for progression. Definitions that do not require persistence of multiple autoantibodies but still include at least one visit with multiple positive autoantibodies at the same visit (mIA/SameVisit and mIA/Persistent/1) identify a moderate-risk group. Finally, individuals whose multiple antibodies are neither contemporaneous nor persistent (mIA/Any) have the lowest cumulative incidence.
Younger age at seroconversion is associated with faster progression to type 1 diabetes (20,17,29). We extend this observation, demonstrating that younger age at sIA/Persistent status (seroconversion) is associated with a higher likelihood of reaching mIA/Persistent/2 status. Progression from mIA/Persistent/2 status to stage 3 type 1 diabetes is fastest in the youngest quartile children and similarly slower in the three oldest quartiles.
We also examine the impact of persistence of antibodies over relatively short defined time windows of follow-up, particularly to further define the risk of those who are in intermediate categories at baseline. At 1 year of follow-up, those who are still mIA/SameVisit have moved to a lower-risk trajectory, while mIA/Persistent/1 has a risk between mIA/Persistent/2 and the other groups. By 2 years of follow-up, individuals who are still mIA/Persistent/1 no longer show significant differences in progression risk compared with the lowest stringency (mIA/Any). Thus, progression to a more stringent mIA definition over the short-term is an important predictor of risk. This parallels previous observations of initially sIA/Persistent individuals in this cohort, whose risk stratified substantially over the same 2-year window, with 15-year incidences of 12% (95% CI 10–25), 30% (95% CI 20–40), and 82% (95% CI 80–95) for those who lost positivity, remained sIA, or became mIA, respectively (20).
Analysis looking at persistence of multiple islet autoantibodies compared with those with reversion of one or more antibodies at 2 years shows that individuals who sustained mIA (no loss of autoantibodies) had similar progression to those who had lost all but one autoantibody at the 2-year follow-up. In contrast, individuals who were positive with three autoantibodies but had lost a single antibody (M3-M2) at the 2-year follow-up had a more rapid progression to type 1 diabetes, particularly in the 5 years following this loss. Previous studies have noted that loss of GADA in mIA children may predict faster progression (15,30–32); however, differences by the autoantibody lost did not reach significance in this analysis.
Strengths of this study include the large T1DI infant cohort from five countries that followed participants prospectively for a period of at least 15 years. Additionally, each contributing site participated in the Diabetes Autoantibody Standardization Program (DASP) (33) and its successor, the Islet Autoantibody Standardization Program (IASP) (34). Consistent participation in these proficiency workshops ensures standardized quality control procedures and accuracy of the assays leading to broadly comparable islet autoantibody outcomes data across the participating T1DI sites. A further strength includes the use of definitions that do not require extensive longitudinal antibody data. As the momentum for screening children in the general population grows, there will be an increasing need for predicting meaningful risk in the absence of information prior to screening (i.e., in a screening population rather than a prospective cohort).
Limitations include study factors that may impact accurate determination of autoantibody status. As there was variability in the time intervals between studies, transiently positive autoantibodies could be missed. Furthermore, we used the binary outcome (positive or negative) of autoantibody measurement in the T1DI cohort to be more applicable to commercial laboratories that do not participate in standardization programs. Autoantibody titer levels can be an important predictor of risk, as was explored in this cohort (19,36,37) and by others (3,35). Additionally, previous reports have explored the importance of individual autoantibodies and their pattern of appearance and disappearance on risk (3,35,38). In order to specifically address the criteria for stage 1 type 1 diabetes, for our ontology development and analyses we simplified observations to autoantibody number. Finally, not all studies assayed prospectively for ZnT8 or islet cytoplasmic antibodies; therefore, these were not included in the analysis.
In summary, our work indicates that individuals who meet an intermediate stringency of mIA (mIA/Persistent/1 or mIA/SameVisit) should be considered at intermediate risk, between persistent sIA/Persistent and those with the most stringent mIA definition. Follow-up over 2 years can differentiate which of these intermediate-risk individuals will enter the highest risk category and which will continue to remain lower risk, indicating that ongoing measurement of islet autoantibodies has clinical utility. Further, loss of a single autoantibody in those who are mIA/Persistent/2 and positive for all three autoantibodies confers a short-term increase in risk of progression. These findings may be helpful in further refining our definitions and stratification of early-stage type 1 diabetes. Future work must be done to confirm these findings in other prospective cohorts or in individuals identified through population screening. Additional information regarding autoantibody titer, genetic risk score, and other predictive factors may be helpful in further differentiating risk.
Article Information
Acknowledgments. The authors thank the participants of the DAISY, DiPiS, DIPP, DEW-IT, and BABYDIAB studies.
Funding. This work was supported by funding from JDRF (IBM: 1-RSC-2017-368-I-X, 1-IND-2019-717-I-X; DAISY: 1-SRA-2019-722-I-X, 1-RSC-2017-517-I-X, 5-ECR-2017-388-A-N; DiPiS: 1-SRA-2019-720-I-X, 1-RSC-2017-526-I-X; DIPP: 1-RSC-2018-555-I-X; DEW-IT: 1-SRA-2019-719-I-X, 1-RSC-2017-516-I-X); the National Institutes of Health; the National Institute of Diabetes and Digestive and Kidney Diseases (DAISY: DK032493, DK032083, DK104351, and DK116073; DiPiS: DK26190); and the Centers for Disease Control and Prevention (DEW-IT: UR6/CCU017247). The DIPP study was funded by JDRF (grants 1-SRA-2016-342-M-R, 1-SRA-2019-732-M-B), the European Union (grant BMH4-CT98-3314), the Novo Nordisk Foundation Center for Basic Metabolic Research, the Academy of Finland (Decision No 292538), the Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012–2017 (Decision No. 250114), Special Research Funds for University Hospitals in Finland, the Diabetes Research Foundation (Finland), and the Sigrid Juséliuksen Säätiö Foundation (Finland). The BABYDIAB study was funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) to the German Center for Diabetes Research (Deutsches Zentrum für Diabetesforschung). The DiPiS study was funded by the Swedish Research Council (grant no. 14064), the Swedish Childhood Diabetes Foundation, the Swedish Diabetes Association, the Nordisk Insulin Fund, SUS Funds, the Lions Clubs International Foundation, District 101-S, The Royal Physiographic Society (Kungliga Fysiografiska Sällskapet i Lund), Skåne County Council’s Foundation for Research and Development, as well as the Lund University Diabetes Centre Industrial Research Centre/ Excellence Of Diabetes Research in Sweden (EXODIAB) funding from the Swedish Foundation for Strategic Research (Stiftelsen för Strategisk Forskning) (Dnr IRC15-0067) and the Swedish Research Council (Dnr 2009-1039). Additional funding for DEW-IT was provided by the Hussman Foundation and by the Washington State Life Science Discovery Fund.
Duality of Interest. M.G., K.N., and V.A. are current employees of IBM. Y.L. is a former employee of IBM and performed this work while at IBM. J.L.D. performed this work as an employee of JDRF and is now an employee of Jansen Research and Development, LLC. O.L. is a current employee of JDRF. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. B.I.F. wrote the first and final draft of this manuscript. B.I.F., M.L., W.H., C.W., J.T., and R.V. are site primary and co-investigators and were responsible for study data. B.I.F., M.L., W.H., C.W., and R.V. are representatives of the data originating sites. M.G. helped with feature engineering and ontology development (mIA). Y.L. helped with subanalyses. K.N., J.L.D., and O.L. provided logistical support for the project and drafted revisions of the manuscript. O.L. is a representative of JDRF, the convener and funder of the overall initiative. V.A. is the primary investigator for the IBM Research study and takes responsibility for all analyses presented. V.A., as a representative of IBM, is the technical research lead for this project. All authors made substantial contributions to conception and design of the manuscript, participated in drafting the manuscript or revising it critically for important intellectual content, and gave final approval of the version to be submitted. V.A. the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019, and were published in Diabetes 2019;68(Suppl. 1):163-OR (https://doi.org/10.2337/db19-163-OR).
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21984812.
See accompanying article, p. 1747.
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air.
The full list of T1DI Study Group members can be found in the supplementary material.
Contributor Information
T1DI Study Group:
Anette G. Ziegler, Ezio Bonifacio, Peter Achenbach, Christiane Winkler, Marian Rewers, Brigitte I. Frohnert, Jill Norris, Andrea Steck, Kathleen Waugh, Liping Yu, William A. Hagopian, Michael Killian, Angela Wolf, Jocelyn Meyer, Claire Crouch, Jared Radtke, Åke Lernmark, Helena Elding Larsson, Markus Lundgren, Marlena Maziarz, Lampros Spiliopoulos, Josefin Jönsson, Riitta Veijola, Jorma Toppari, Jorma Ilonen, Mikael Knip, Vibha Anand, Mohamed Ghalwash, Kenney Ng, Zhiguo Li, B.C. Kwon, Harry Stravopolous, Eileen Koski, Ashwani Malhotra, Shelley Moore, Jianying Hu, Jessica Dunne, Bin Liu, Ying Li, Olivia Lou, and Frank Martin
References
- 1. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986;314:1360–1368 [DOI] [PubMed] [Google Scholar]
- 2. Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997;46:1701–1710 [DOI] [PubMed] [Google Scholar]
- 3. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Couper JJ, Haller MJ, Ziegler AG, Knip M, Ludvigsson J; International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014. Phases of type 1 diabetes in children and adolescents. Pediatr Diabetes 2014;15(Suppl. 20):18–25 [DOI] [PubMed] [Google Scholar]
- 8. ElSayed NA, Aleppo G, Aroda VR, et al.; on behalf of the American Diabetes Association . 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S19–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 2019;7:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziegler AG, Kick K, Bonifacio E, et al.; Fr1da Study Group . Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 2020;323:339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McQueen RB, Geno Rasmussen C, Waugh K, et al. Cost and cost-effectiveness of large-scale screening for type 1 diabetes in Colorado. Diabetes Care 2020;43:1496–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spencer KM, Tarn A, Dean BM, Lister J, Bottazzo GF. Fluctuating islet-cell autoimmunity in unaffected relatives of patients with insulin-dependent diabetes. Lancet 1984;1:764–766 [DOI] [PubMed] [Google Scholar]
- 14. Kimpimäki T, Kulmala P, Savola K, et al. Natural history of β-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab 2002;87:4572–4579 [DOI] [PubMed] [Google Scholar]
- 15. Vehik K, Lynch KF, Schatz DA, et al.; TEDDY Study Group . Reversion of β-cell autoimmunity changes risk of type 1 diabetes: TEDDY study. Diabetes Care 2016;39:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. So M, O’Rourke C, Bahnson HT, Greenbaum CJ, Speake C. Autoantibody reversion: changing risk categories in multiple-autoantibody-positive individuals. Diabetes Care 2020;43:913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frohnert BI, Ide L, Dong F, et al. Late-onset islet autoimmunity in childhood: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 2017;60:998–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 19. Ng K, Stavropoulos H, Anand V, et al.; T1DI Study Group . Islet autoantibody type-specific titer thresholds improve stratification of risk of progression to type 1 diabetes in children. Diabetes Care 2022;45:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand V, Li Y, Liu B, et al.; T1DI Study Group . Islet autoimmunity and HLA markers of presymptomatic and clinical type 1 diabetes: joint analyses of prospective cohort studies in Finland, Germany, Sweden, and the U.S. Diabetes Care 2021;44:2269–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kupila A, Muona P, Simell T, et al.; Juvenile Diabetes Research Foundation Centre for the Prevention of Type I Diabetes in Finland . Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 2001;44:290–297 [DOI] [PubMed] [Google Scholar]
- 22. Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999;48:460–468 [DOI] [PubMed] [Google Scholar]
- 23. Elding Larsson H. A Swedish approach to the prevention of type 1 diabetes. Pediatr Diabetes 2016;17(Suppl. 22):73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 25. Wion E, Brantley M, Stevens J, et al. Population-wide infant screening for HLA-based type 1 diabetes risk via dried blood spots from the public health infrastructure. Ann N Y Acad Sci 2003;1005:400–403 [DOI] [PubMed] [Google Scholar]
- 26. Vock DM, Wolfson J, Bandyopadhyay S, et al. Adapting machine learning techniques to censored time-to-event health record data: a general-purpose approach using inverse probability of censoring weighting. J Biomed Inform 2016;61:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Endesfelder D, Zu Castell W, Bonifacio E, et al.; TEDDY Study Group . Time-resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 2019;68:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Köhler M, Beyerlein A, Vehik K, et al.; TEDDY study group . Joint modeling of longitudinal autoantibody patterns and progression to type 1 diabetes: results from the TEDDY study. Acta Diabetol 2017;54:1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bauer W, Veijola R, Lempainen J, et al. Age at seroconversion, HLA genotype, and specificity of autoantibodies in progression of islet autoimmunity in childhood. J Clin Endocrinol Metab 2019;104:4521–4530 [DOI] [PubMed] [Google Scholar]
- 30. Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 2017;60:1370–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ziegler AG, Bonifacio E. Why is the presence of autoantibodies against GAD associated with a relatively slow progression to clinical diabetes? Diabetologia 2020;63:1665–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacobsen LM, Larsson HE, Tamura RN, et al.; TEDDY Study Group . Predicting progression to type 1 diabetes from ages 3 to 6 in islet autoantibody positive TEDDY children. Pediatr Diabetes 2019;20:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 2003;52:1128–1136 [DOI] [PubMed] [Google Scholar]
- 34. Lampasona V, Pittman DL, Williams AJ, et al.; Participating Laboratories . Islet Autoantibody Standardization Program 2018 Workshop: interlaboratory comparison of glutamic acid decarboxylase autoantibody assay performance. Clin Chem 2019;65:1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobsen LM, Bocchino L, Evans-Molina C, et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia 2020;63:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwon BC, Achenbach P, Anand V, et al. Islet autoantibody levels differentiate progression trajectories in individuals with presymptomatic type 1 diabetes. Diabetes 2022;71:2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng K, Anand V, Stavropoulos H, et al.; T1DI Study Group . Quantifying the utility of islet autoantibody levels in the prediction of type 1 diabetes in children. Diabetologia 2023;66:93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon BC, Anand V, Achenbach P, et al.; T1DI Study Group . Progression of type 1 diabetes from latency to symptomatic disease is predicted by distinct autoimmune trajectories. Nat Commun 2022;13:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]