Abstract
OBJECTIVE
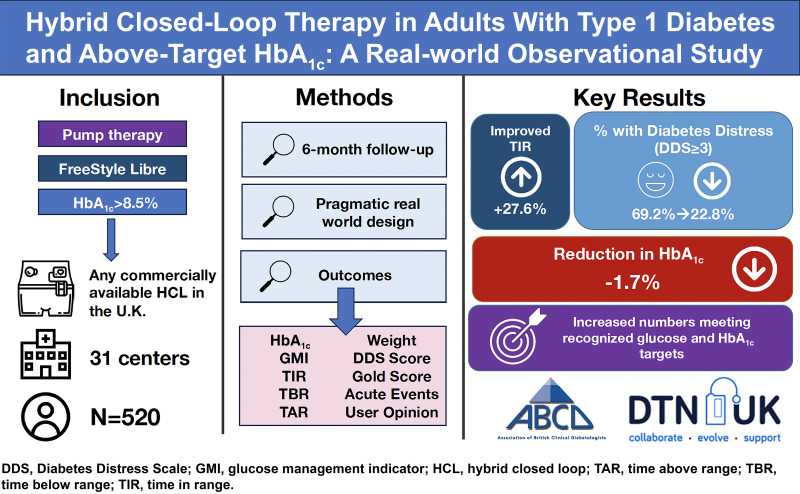
We explored longitudinal changes associated with switching to hybrid closed-loop (HCL) insulin delivery systems in adults with type 1 diabetes and elevated HbA1c levels despite the use of intermittently scanned continuous glucose monitoring (isCGM) and insulin pump therapy.
RESEARCH DESIGN AND METHODS
We undertook a pragmatic, preplanned observational study of participants included in the National Health Service England closed-loop pilot. Adults using isCGM and insulin pump across 31 diabetes centers in England with an HbA1c ≥8.5% who were willing to commence HCL therapy were included. Outcomes included change in HbA1c, sensor glucometrics, diabetes distress score, Gold score (hypoglycemia awareness), acute event rates, and user opinion of HCL.
RESULTS
In total, 570 HCL users were included (median age 40 [IQR 29–50] years, 67% female, and 85% White). Mean baseline HbA1c was 9.4 ± 0.9% (78.9 ± 9.1 mmol/mol) with a median follow-up of 5.1 (IQR 3.9–6.6) months. Of 520 users continuing HCL at follow-up, mean adjusted HbA1c reduced by 1.7% (95% CI 1.5, 1.8; P < 0.0001) (18.1 mmol/mol [95% CI 16.6, 19.6]; P < 0.0001). Time in range (70–180 mg/dL) increased from 34.2 to 61.9% (P < 0.001). Individuals with HbA1c of ≤58 mmol/mol rose from 0 to 39.4% (P < 0.0001), and those achieving ≥70% glucose time in range and <4% time below range increased from 0.8 to 28.2% (P < 0.0001). Almost all participants rated HCL therapy as having a positive impact on quality of life (94.7% [540 of 570]).
CONCLUSIONS
Use of HCL is associated with improvements in HbA1c, time in range, hypoglycemia, and diabetes-related distress and quality of life in people with type 1 diabetes in the real world.
Graphical Abstract
Introduction
There are currently >400,000 people living with type 1 diabetes in England and Wales, and the incidence is increasing (1,2). Type 1 diabetes is one of the most challenging long-term conditions to self-manage. It requires multiple daily glucose measurements, counting carbohydrates, and calculating and injecting insulin doses multiple times daily in the unrelenting endeavor to achieve normoglycemia. Unsurprisingly, glucose levels in many people living with type 1 diabetes remain above target, and according to the 2021/2022 UK National Diabetes Audit, only 12.7% in England achieved the recommended HbA1c target of ≤6.5% (48 mmol/mol), and only 36.3% achieved an HbA1c level ≤7.5% (58 mmol/mol) (1). While insulin pump therapy and structured education may increase the chance of achieving this target, evidence from the National Diabetes Pump Audit demonstrated similar numbers achieving an HbA1c ≤7.5% (58 mmol/mol) among people who were using and not using insulin pumps, with an average HbA1c among pump users of ∼8.0% (64 mmol/mol) (3). Furthermore, the risks of hypoglycemia remain a major barrier, while the constant vigilance over glucose levels and need for multiple self-care tasks can lead to diabetes burnout and distress (4).
Hybrid closed-loop (HCL) insulin delivery systems combine insulin pump therapies with continuous glucose monitoring (CGM) sensors to automate insulin delivery between meals and overnight to maintain glucose near a prespecified target level using algorithmic software contained within the insulin pump or a separate device (e.g., smartphone) (5). Currently available systems require a user-initiated meal bolus (a hybrid approach). In the U.K., the use of these systems has been limited by access to real-time CGM nationally and the need to meet criteria for insulin pump therapy as per the National Institute for Health and Care Excellence (NICE) technology appraisal guidance 151, “Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus” (6). While updates to NICE guideline 17, “Type 1 diabetes in adults,” may facilitate increased uptake of HCL through improved access to HCL-compatible real-time CGM, specific guidelines for U.K. use are not currently available (7).
In the available randomized controlled trials looking at single devices, HCL systems improve glycemic outcomes compared with insulin pump alone, multiple daily injections with CGM, and sensor-augmented pump therapy (8–12). However, the benefits demonstrated in these clinical trials reflect the outcomes in a group of people motivated to take part in research and often with HbA1c levels close to target at baseline with intensive protocolized follow-up. This limits the generalizability of these findings to the wider population with type 1 diabetes who will undergo standard follow-up, especially in a U.K. context where almost 66% of individuals have HbA1c levels above target (≥7.5% [58 mmol/mol]) (1).
Real-world evidence exists but is limited to single-system studies and, similar to the randomized controlled trial data, frequently included individuals who were at or near target HbA1c at baseline. Additionally, these studies often lacked data on HbA1c (instead reporting sensor-derived glucose data) and hospital admissions (13–16). In recognition of the disconnect between the evidence and observed clinical experience with HCL systems, National Health Service (NHS) England funded a real-world pilot scheme for the use of HCL systems in individuals with a high HbA1c level who were already using an insulin pump. The Association of British Clinical Diabetologists (ABCD) Closed-Loop Audit Program was designed to capture routine anonymized outcome data from adults who participated in this scheme. The aim was to assess the real-world effectiveness and safety of HCL systems commercially available in the U.K. in an NHS outpatient setting.
Research Design and Methods
The methodology for this observational study has been previously described (17). The population included in the NHS England adult HCL pilot were patients attending adult diabetes services with a diagnosis of type 1 diabetes managed with an insulin pump and intermittently scanned CGM (isCGM) with an HbA1c ≥8.5% (69 mmol/mol). Thirty-one adult diabetes centers across England were selected by NHS England to participate based on geography and pump experience. Patients were started on HCL between August and December 2021. Anonymized clinical outcome data were collected during routine clinical care, and clinical systems and electronic health records were reviewed and submitted via a secure online tool. This analysis reflects the data captured between 3 and 9 months of follow-up. The primary outcome was change in laboratory-derived HbA1c. Secondary outcomes included CGM metrics time in range (TIR) (70–180 mg/dL), time below range (TBR) (<70 mg/dL), glucose management indicator (GMI) (estimated HbA1c), Diabetes Distress Scale 2 (DDS2) score (18), Gold score (19), event rates, weight, BMI, and user opinion of HCL (using a 7-point Likert scale where 1 = strongly negative/would not recommend and 7 = strongly positive/would recommend). Sensor glucometrics were reported over 14 days per the international consensus guidelines and in keeping with routine clinical practice (20). Events of interest included hospital admission, paramedic callouts, and severe hypoglycemia (requiring third-party assistance to treat) and were reported by the clinical teams via the online tool. Sensor glucometrics were extracted from the relevant HCL system for the 14 days preceding follow-up. Follow-up frequency was determined by the responsible clinical teams purely on clinical need. The most recent available data for each patient, within the 3–9 month follow-up window, were used in this analysis. Only available paired data were included for each outcome of interest.
Sensitivity analysis was performed comparing the baseline characteristics of those with follow-up data compared with those who discontinued the system or in whom follow-up data were not available (i.e., not entered, failed to attend, lost to follow-up). Data were assessed for accuracy and completeness and analyzed using Stata SE 16 statistical software. The analysis was conducted by two individuals independently (T.S.J.C. and T.P.G.). Data were expressed as mean and SD for continuous outcomes with a parametric distribution and median and interquartile range (IQR) for nonparametrically distributed continuous outcomes (determined by tests of skew). Paired t tests were used for the analysis of continuous variables with a parametric distribution. Mann-Whitney U (Wilcoxon rank sum) tests were used to assess outcome variables with a nonparametric distribution. P < 0.01 was considered statistically significant to account for multiplicity. Change in HbA1c from baseline was adjusted for baseline characteristics and change in other covariates using a multivariable linear regression model with clustering by center to correct for key covariates determined a priori as follows: baseline HbA1c and weight, sex, age, duration of diabetes and pump use, deprivation level, HCL system, time in closed loop, ethnicity, and change in weight. Mann-Whitney U tests were used to assess changes in patterns of hospital admissions and paramedic callouts.
Patient and Public Engagement
The importance of closed-loop systems to people with diabetes has been emphasized repeatedly by groups on social media and in meetings with both U.K.-based charities (e.g., Diabetes UK) and NHS England. Many campaigns (e.g., #WeAreNotWaiting) aim to improve access to diabetes technology (21). Research into closed-loop systems has been established as a priority research area in diabetes by the James Lind Alliance (22). Acknowledging the importance of the lived experience of people with diabetes, simple questions were asked on the impact of these systems on quality of life and whether users would recommend these systems to others. Qualitative outcomes were explored in more depth in a dedicated study that will be reported at a later date. Two study authors live with type 1 diabetes.
Ethics
As a service evaluation and audit, this program only collects anonymized, routinely available clinical data. Tests not performed routinely were not required to be performed. As such, there was no requirement for approval by a research ethics committee. The ABCD nationwide audit program, which includes this audit, has Caldicott Guardian Approval and has also been approved by the Confidentiality Advisory Group (23).
Data and Resource Availability
Data sharing on key outcomes will be permitted on written application to the authors.
Results
Baseline data were available for 634 adults across the 31 centers, with follow-up data reported for 570 (89.9%), of whom 91.2% (520 of 570) continued to use HCL. Supplementary Material 1 contains the flow diagram for this analysis.
Baseline Characteristics
For the 520 individuals with baseline and follow-up data, the median age was 40 years (IQR 29–50); 67% (n = 348) were female; median diabetes duration was 21 years (IQR 15–30); 83% (n = 433) were White British; and 10% (50 of 500) were from the two most deprived quintiles, with a median index of multiple deprivation decile of 6 (IQR 3–8). The baseline characteristics of the cohort are summarized in Table 1. The HCL systems initiated in the NHS England pilot included Medtronic 780G (46%, n = 241), Tandem Control-IQ (37%, n = 193), CamAPS FX (5%, n = 27), Medtrum closed-loop system (4%, n = 19), and the Medtronic 670G (2%, n = 8); the system was not reported for 32 (6%) individuals.
Table 1.
Baseline characteristics
| Characteristic | Value (n = 520) |
|---|---|
| Age, years, median (IQR)† | 40 (29–51) |
| Diabetes duration, years, median (IQR)† | 21 (15–30) |
| Pump duration, years,† median (IQR) | 8 (5–11) |
| Weight, kg,* mean ± SD | 82 ± 18 |
| BMI, kg/m2,* mean ± SD | 29 ± 6 |
| Index of multiple deprivation decile,† median (IQR) | 6 (3–8) |
| Most deprived quintile of deprivation,‡ n (%) | 50 (10) |
| Sex‡ | |
| Female | 348 (67) |
| Male | 172 (33) |
| Ethnicity | |
| White, British | 433 (83) |
| White, other | 11 (2) |
| Mixed | 10 (2) |
| Asian | 14 (3) |
| Black | 6 (1) |
| Other | 4 (1) |
| Unknown | 42 (8) |
| HbA1c, mean ± SD | |
| % | 9.4 ± 0.9 |
| mmol/mol | 78.9 ± 9.1 |
| CGM metrics,* mean ± SD | |
| Total daily insulin dose, units | 51 ± 31 |
| GMI, mmol/mol | 72 ± 11 |
| TAR, level 2 (>250 mg/dL), % | 38 ± 19 |
| TAR, level 1 (181–250 mg/dL), % | 27 ± 11 |
| TIR (70–180 mg/dL), % | 34 ± 15 |
| TBR, level 1 (54–69 mg/dL), % | 2 ± 2 |
| TBR, level 2 (<54 mg/dL), % | 0 ± 1 |
| Scans per day at baseline, n | 7 ± 6 |
| Coefficient of variation | 38 ± 7 |
| Insulin pump/closed-loop system‡ | |
| Medtronic 780G | 241 (46) |
| Tandem Control-IQ | 193 (37) |
| CamAPS FX | 27 (5) |
| Medtrum | 19 (4) |
| Medtronic 670G | 8 (2) |
| Not recorded | 32 (6) |
TAR, time above range.
Data reported as mean ± standard deviation.
Data reported as median (interquartile range).
Data reported as number (percentage).
HbA1c and Sensor-Based Outcomes
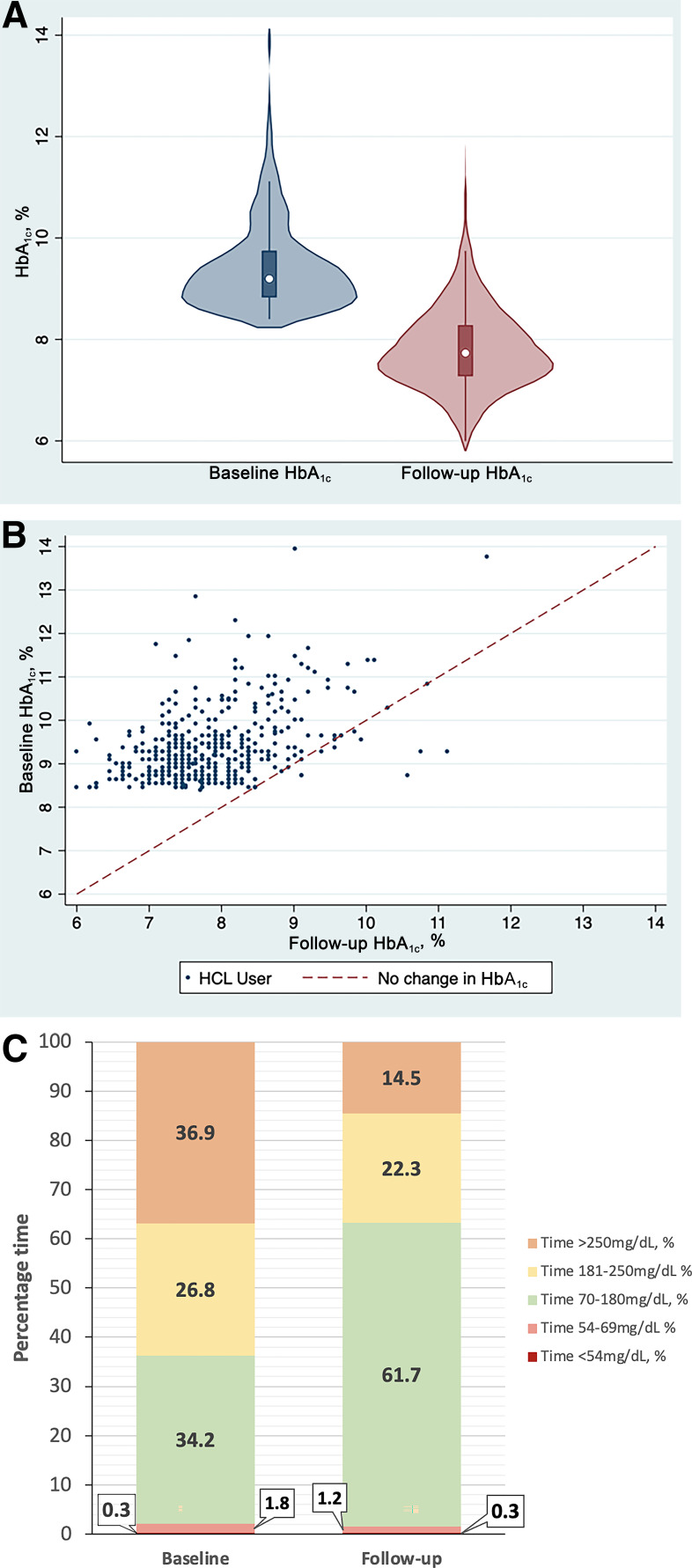
Across the population, HbA1c reduced from 9.4 ± 0.9% (78.9 ± 9.1 mmol/mol) at baseline to 7.8 ± 0.8% (62.1 ± 9.1 mmol/mol) over a median follow-up of 5.1 months (IQR 3.9–6.6). The mean unadjusted decrease in HbA1c was 1.5% (95% CI 1.4, 1.6; P < 0.001) (16.7 mmol/mol [95% CI 15.8, 17.6]; P < 0.001). Using a multivariable linear regression model to correct for key covariates, mean adjusted HbA1c reduced by 1.7% (95% CI 1.5, 1.8; P < 0.0001) (18.1 mmol/mol [95% CI 16.6, 19.6]; P < 0.0001). These results are demonstrated in the violin plot and scatter plots in Fig. 1A and B. There is no evidence for difference in HbA1c changes associated with HCL across baseline deprivation status or ethnicity (P = 0.999 for both) when assessed by pairwise comparisons within the regression model.
Figure 1.
A: Violin plot demonstrating HbA1c at baseline and follow-up (P < 0.0001). B: Scatter plot showing individual patient data for HbA1c at baseline, with the line representing the threshold for no change in HbA1c. C: Stacked bar chart demonstrating TIR at baseline and follow-up. Data are for individuals with complete sensor data only (n = 380).
The median time spent in closed-loop mode was 96% (IQR 92–99). The GMI decreased from 8.7 (71.6 mmol/mol) to 7.4% (57.3 mmol/mol) at follow-up (−1.3% [95% CI −1.2, −1.4]; P < 0.0001) (−15.4 mmol/mol [95% CI −13.7, −17.0]; P < 0.0001). TIR (70–180 mg/dL) increased from 34.2 to 61.9%, a mean increase of 27.8% (95% CI 26.2, 29.4; P < 0.001). TBR (<70 mg/dL) reduced from 2.1 to 1.6% (P = 0.01), with level 1 hypoglycemia (54–69 mg/dL) reducing from 1.8 to 1.3% (P < 0.001). There was no significant change in level 2 hypoglycemia (<54 mg/dL). The glucose coefficient of variation fell from 38.0 to 35.2% (−2.8% [95% CI −1.9, −3.6]; P < 0.0001). The changes in CGM-derived glucose metrics are displayed in Fig. 1C and Supplementary Material 2. The paired HbA1c and CGM-derived glucose metric outcomes are summarized in Table 2.
Table 2.
Baseline and follow-up HbA1c, CGM-derived glucose metrics (uncorrected changes), and patient-reported outcome measure scores
| n § | Baseline | Follow-up | Change (95% CI) | P * | |
|---|---|---|---|---|---|
| HbA1c | |||||
| mmol/mol | 436 | 78.9 ± 9.1 | 62.1 ± 9.1 | −16.7 (−15.8, −17.6) | <0.001 |
| % | 436 | 9.4 ± 0.8 | 7.8 ± 0.8 | −1.5 (−1.4, −1.6) | <0.001 |
| Glucose data | |||||
| TAR, level 2 (>250 mg/dL), % | 407 | 37.2 ± 19.1 | 14.9 ± 11.8 | −22.4 (−20.5, −24.2) | <0.001 |
| TAR, level 1 (181–250 mg/dL),# % | 381 | 26.8 ± 10.3 | 22.3 ± 11.7 | −4.5 (−3.0, −6.1) | <0.001 |
| TIR (70–180 mg/dL),# % | 417 | 34.2 ± 14.5 | 61.9 ± 13.1 | 27.8 (26.2, 29.4) | <0.001 |
| TBR (54–69 mg/dL),# % | 411 | 1.8 ± 2.4 | 1.3 ± 1.6 | −0.5 (0.2, 0.8) | <0.001 |
| TBR (<54 mg/dL),# % | 397 | 0.37 ± 1.00 | 0.35 ± 0.57 | −0.03 (−0.13, 0.18) | 0.729 |
| Coefficient of variation# | 325 | 38.0 ± 6.9 | 35.2 ± 6.7 | −2.8 (−1.9, −3.6) | <0.001 |
| Patient-reported outcome measures | |||||
| Gold score | 415 | 2.3 ± 1.4 | 1.9 ± 1.2 | −0.4 (−0.2, −0.5) | <0.001 |
| Diabetes distress score | 412 | 3.3 ± 1.3 | 2.2 ± 1.0 | −1.1 (−1.0, −1.3) | <0.001 |
| Diabetes distress score (average ≥3),† % (n) | 412 | 69.2 (285) | 22.8 (94) | −46.4 (−191) | <0.001 |
| Impaired awareness of hypoglycemia (Gold score ≥4), % (n) | 415 | 16.9 (70) | 9.4 (39) | −7.5 (−31) | <0.001 |
Data are mean ± SD unless otherwise indicated. TAR, time above range.
Statistical significance calculated using unpaired t tests for all covariates included in this table, following assessment for skew.
Statistical significance calculated using Chi-squared tests.
Number with available paired data at baseline and follow-up included in analysis for a given outcome; total cohort = 520.
Data derived from isCGM at baseline and real-time CGM at follow-up.
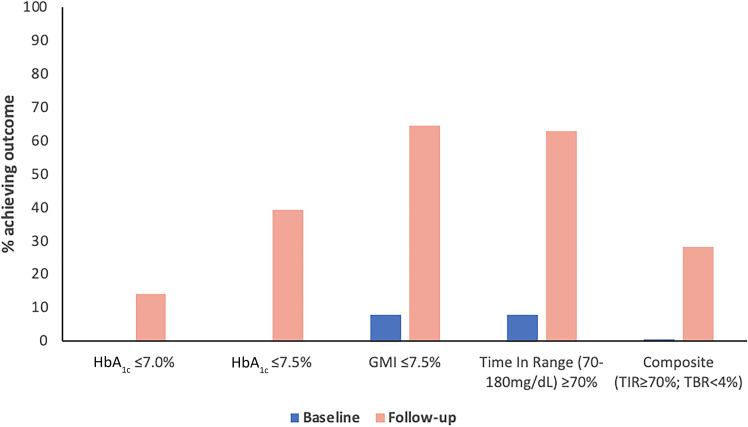
As per entry criteria, no individuals were achieving an HbA1c of ≤7.5% (58 mmol/mol) at baseline; 39.4% (176 of 447) of users met this HbA1c target at follow-up, and 14.1% (63 of 447) achieved HbA1c ≤7.0% (53 mmol/mol). At baseline, 0.8% met the internationally recommended targets of ≥70% TIR and <4% TBR, increasing to 28.2% (135 of 478) at follow-up (P < 0.0001). Figure 2 demonstrates the proportion of the cohort achieving recognized glycemic targets before and after closed-loop therapy. In total, 91.0% (n = 473) had an HbA1c drop ≥0.5%, with 79.6% (n = 414) achieving reductions ≥1.0%. Only 11 (2.1%) individuals using HCL experienced an increase in HbA1c.
Figure 2.
Proportion of individuals achieving targets for HbA1c, GMI, TIR, and a composite outcome of TIR and TBR at baseline and follow-up.
Weight, BMI, and Total Daily Dose of Insulin
Weight increased from a mean ± SD of 80.8 ± 18.1 kg at baseline to 82.7 ± 20.0 kg at follow-up, a mean increase of 1.9 kg (95% CI 0.9, 2.9; P < 0.001; n = 282). Among those with available data, the increase in BMI from 28.9 ± 6.9 kg/m2 at baseline to 29.2 ± 6.8 kg/m2 at follow-up was nonsignificant (P = 0.07). Total daily insulin doses increased from 49.4 ± 23.2 to 55.8 ± 1.7 units, an increase of 6.4 units (95% CI 4.6, 8.3; P < 0.001; n = 250).
Diabetes Distress, Gold Score, and User Satisfaction
Improvements in patient-reported outcome measures were observed with reduction in DDS2 score from 3.3 to 2.2, a mean reduction of −1.1 (95% CI −1.0, −1.3; P < 0.001). The proportion of individuals with high diabetes distress (DDS2 ≥3) reduced from 69.0% at baseline to 22.8% (P = 0.001). Gold score reduced from 2.3 to 1.9 (P < 0.001). These results are summarized in Table 2. Within the NHS England pilot (including those who discontinued using the system), 96.3% (549 of 570) would recommend HCL therapy to others with diabetes, and 94.7% (540 of 570) rated HCL therapy as having had a positive impact on their quality of life.
Acute and Adverse Events
Reported hospital admissions related to hypoglycemia and hyperglycemia/diabetic ketoacidosis and paramedic callouts (not resulting in admission) were low in this cohort, and no increase in events per 10 person-years were observed. These results are summarized in Supplementary Material 4. One death due to diabetic ketoacidosis occurred and was deemed unrelated to use of the HCL system.
A total of 37 adverse events were reported. The majority (24 of 37) of these adverse events were related to either pump or cannula issues (11 of 37 [29.7%]) or sensor failures, inaccuracies, and skin reactions (13 of 37 [35.1%]). A total of 50 users (8.8%) discontinued HCL therapy. Reasons for discontinuation were lack of trust in the system/anxiety (4 [8%]), erratic glucose levels (5 [10%]), issues with cannulas/skin site reactions (6 [12%]), early problems in adjusting to HCL (6 [12%]), and failing to attend follow-up appointments so discontinued by the clinical team (5 [10%]). Five individuals (10%) were moved off one HCL system because of technical issues and did not restart. No reasons were provided for discontinuation in the remaining individuals (19 [38%]).
Sensitivity Analysis
Sensitivity analysis comparing individuals with complete follow-up versus those who discontinued the HCL system or had missing follow-up data are shown in Supplementary Material 2. Those with absent follow-up higher time ≥250 mg/dL at baseline were more likely to be using ultrafast-acting insulin (P < 0.05 between groups for all). Baseline HbA1c between those with and without follow-up data was not significantly different (P = 0.25). All other baseline characteristics were similar between the groups.
Conclusions
This real-world evaluation of the NHS England pilot of HCL system use in people living with type 1 diabetes demonstrates substantial improvements in glycemic and patient-reported outcomes over 5 months of follow-up. The observed −1.7% (−18.1 mmol/mol) HbA1c change is greater than that reported in both randomized controlled trials and existing real-world studies, which reported HbA1c reductions between 0.4 and 0.7% between baseline and follow-up, although the average HbA1c of participants included in these studies was lower at baseline (8–10,15). Closer comparisons can be made to the Advanced Hybrid Closed Loop Study in Adult Population With Type 1 Diabetes (ADAPT) study, which randomized those with similar HbA1c levels (>8.0% [64 mmol/mol], mean baseline HbA1c of 9.0% [75 mmol/mol]) to either HCL or standard care (multiple daily injections with isCGM) (12). In the ADAPT study, a 1.5% HbA1c reduction was observed in the HCL arm at follow-up, with a relative difference of 1.4% between the intervention and control arms. Our results are similar but in a larger cohort already using insulin pump therapy at baseline and with a higher HbA1c. The reductions in HbA1c we report and the follow-up HbA1c levels achieved are likely to translate into significant reductions in complications in the long term. The proportion of people achieving HbA1c targets ≤7.5% (58 mmol/mol) in this real-world study increased from 0 to 39.4%. This exceeds the proportion of individuals already achieving this target within the National Diabetes Audit (36.3%) and National Diabetes Insulin Pump Audit (34.4% in 2017–2018) (1,3). Although follow-up glucose TIR (61.2% in 70–180 mg/dL) was lower than in many existing HCL studies, our observed population had much lower TIR at baseline (34.2%), and the change in TIR (an increase of 27.8%) was much greater (8–10,15,24,25). Of note, any change in GMI should be interpreted with caution, as sensors are limited in their ability to calculate this because of an inability to account for extremely high glucose values.
There was a 28% reduction in sensor-detected level 1 hypoglycemia and user-reported improvements in hypoglycemia awareness and diabetes distress. This finding may, however, be related to the switch from isCGM to real-time CGM and the provision of more advanced alarms, as has been previously demonstrated in trials (26). The majority of users reported a positive impact on quality of life and would recommend the use of HCL systems to other people living with diabetes. Some of these positive reports may be explained by the significant reductions in hyperglycemia, which have previously been linked to multiple quality-of-life factors, including mood, cognition, and even driving skills (27–30), and is consistent with existing qualitative evidence supporting the use of closed-loop insulin systems (31,32).
Although significant reductions in HbA1c and glucose levels were associated with HCL in this real-world study, >50% still had HbA1c levels above target. Achieving targets may further be improved in the future by improving the education, support, and training provided to users of HCL alongside further developments in HCL systems and is echoed by other studies, including the ADAPT study (33).
A small amount of weight gain (1.9 kg) was observed, which may be due to improved glucose levels and reductions in glycosuria and subsequent urinary calorie loss. This finding is comparable to those of randomized controlled trials (e.g., 2.1 kg in Tauschmann et al. [8]) (34).
These data also demonstrate that, despite the advances in technology and a generally positive response to HCL, the current generation of closed-loop systems is not for everyone. It should be noted that a small, but significant proportion of people (8.8%) discontinued use despite all users already being familiar with and established on stand-alone insulin pump therapy. The reasons for this are multifaceted, are not limited purely to technology or equipment issues, and should be explored further in future studies.
The main strength of our analysis is the real-world nature of the data captured, encompassing a large number of diverse HCL users across multiple different systems and across multiple centers. We know that in the longer term, despite the benefits of structured education, insulin pump therapy, and isCGM, a large proportion of people living with type 1 diabetes are unable to achieve target glucose levels and remain at high risk of complications (35). Within a U.K. context, we know that only one-third of insulin pump users achieve a target HbA1c ≤7.5% (58 mmol/mol) (3). The HbA1c reductions observed in this study are likely to significantly reduce future risk of complications with important health economic implications. By including data from multiple HCL systems, our observations are generalizable to a broad population of individuals with type 1 diabetes and HbA1c levels above target, irrespective of the system used. The findings observed are less generalizable to individuals with lower baseline HbA1c levels, though HCL use is supported in these individuals by existing randomized controlled trial evidence (8,9,11).
There are limitations to this real-world study, including the lack of a control group, the possibility of unmeasured confounders contributing to the changes observed, underreporting of adverse events, and missing data. Despite these limitations, there is novelty in assessing the use of these technologies outside a randomized controlled trial environment. Our findings mirror what has been reported in previous trials, particularly the ADAPT study, over a similar 5.1-month median follow-up period (1,33). Our data provide additional supportive evidence that benefits associated with HCL accrue in the real world, not just in a research setting. Only 7% of individuals were from a non-White ethnic background, limiting the generalizability of the findings to other racial and ethnic populations.
There are a few other specific limitations. The TIR analysis compared the data from isCGM (FreeStyle Libre) at baseline with real-time CGM (varied depending on the system used) at follow-up, and accuracy and data capture may differ between these devices. It should be noted that while the sites selected for inclusion were those with the greatest numbers of pump users and, therefore experience, in diabetes technology, many participants were initiated on HCL systems rapidly and during a period when face-to-face visits were less routine as services recovered from the COVID-19 pandemic. Other than initial support in commencing the technology, centers treated this cohort of HCL users via usual pathways. The expertise of the teams may have contributed to the results in part, and it will be vital to ensure that health care professionals from smaller services are supported in promoting, onboarding, and managing HCL systems in the future. A comprehensive best practice and service delivery guide has already been developed from our experiences in the pilot to support teams in the further rollout of HCL (5).
This work has informed the NICE technology appraisal of HCL technology. Ongoing surveillance to understand longer-term glycemic outcomes in this cohort remains of interest.
In conclusion, among adults with type 1 diabetes treated with insulin pump therapy and isCGM with a high HbA1c, commencement of HCL was associated with reductions in HbA1c levels, improved CGM-derived glucose outcomes, reduced diabetes-related distress, and improved quality of life. These findings mirror those reported in existing randomized controlled trial data, provide evidence that those findings translate into a less resourced real-world setting, and support wider access to HCL therapy in people living with type 1 diabetes.
Article Information
Acknowledgments. The authors extend a special thanks to Professor Partha Kar, and his team at NHS England, who led the delivery of the NHS England closed-loop pilot.
Funding. The Association of British Clinical Diabetologists Closed-Loop Audit Program is funded by Diabetes Care Trust. NHS England funded the provision of HCL systems as part of a real-world pilot scheme launched in 2021 (36). The independent collection of outcome data, including the secure anonymized online tool, was funded by the Association of British Clinical Diabetologists.
Duality of Interest. T.S.J.C. has received personal fees from Novo Nordisk, Sanofi, Insulet, Eli Lilly, Dexcom, and Abbott Diabetes Care. T.P.G. has received personal fees from Novo Nordisk, Dexcom, Sanofi Aventis, Mundipharma, AbbVie, and Eli Lilly. P.N. has received speaker and advisory board fees from Lilly Diabetes Care, Abbott Diabetes Care, and Sanofi. G.G. has received advisory and speaker fees from Abbott, Medtronic, Insulet, and Dexcom. N.F. has received speaker fees from Novo Nordisk and Sanofi. I.C. has received speaker and advisory fees from Abbott Diabetes Care, Eli Lilly, Novo Nordisk, Insulet, and Dexcom. C.P. has received speaker fees and support to attend conferences from Novo Nordisk. M.A.K. has received speaker and/or advisory board fees from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Sanofi, and Abbott Diabetes Care. S.K. has received speaker fees from Novo Nordisk, Sanofi Aventis, Eli Lilly, and Medtronic. J.E. has received speaker and advisory board fees from Abbott, Dexcom, Insulet, Eli Lilly, Novo Nordisk, Roche, and Sanofi. R.E.J.R. has received speaker fees, consultancy fees, and/or educational sponsorships from BioQuest, GI Dynamics, and Novo Nordisk. P.H. has received personal fees from Abbott Diabetes Care, Eli Lilly, Insulet, Medtronic, and Sanofi Aventis. A.L. has received personal fees from Insulet, Dexcom, Abbott Diabetes Care, Novo Nordisk, Sanofi, and Eli Lilly and institutional research support from Novo Nordisk and Abbott Diabetes Care. P.C. has received personal fees from Abbott Diabetes Care, Dexcom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, Sanofi Aventis, and Glooko. E.G.W. has received personal fees from Abbott Diabetes Care, AstraZeneca, Dexcom, Eli Lilly, Embecta, Glooko, Insulet, Medtronic, Novo Nordisk, Roche, Sanofi Aventis, and Ypsomed. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.S.J.C. wrote the manuscript. T.S.J.C. and T.P.G. directly accessed and analyzed the data independently of each other. T.S.J.C., T.P.G., Y.W.Y., P.N., G.G., N.F., I.C., A.Chak., C.P., M.A.K., S.S., S.K. E.G., A.Chap., S.H., J.E., L.L., R.E.J.R., P.H., A.L., P.C., and E.G.W. contributed to the data collection. T.S.J.C., T.P.G., R.E.J.R., P.H., A.L., P.C., and E.G.W. were involved in the design and setup of the audit program. T.P.G., R.E.J.R., P.H., A.L., P.C., E.G.W. made significant contributions to the manuscript. All authors had input into the review and editing of the manuscript. T.S.J.C. and T.P.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the ABCD Diabetes Technology Network UK Meeting 2022, Birmingham, U.K., 7 September 2022, the 16th International Conference on Advanced Technologies and Treatments for Diabetes, Berlin, Germany, 22–25 February 2023, and the Diabetes UK Conference, Liverpool, England, U.K., 26–28 April 2023.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.23780670.
P.C. and E.G.W. are joint senior authors.
A complete list of the ABCD DTN-UK Closed-Loop Audit Contributors can be found in the supplementary material online.
Contributor Information
The ABCD Closed-Loop Audit Contributors:
Mark Evans, Eleanor Gurnell, Sara Hartnell, Katy Davenport, Iona O’Reilly, Helen Brown, Shafie Kamaruddin, Sharon Pickering, Kamal Abouglia, Claire Wadham, Gerry Rayman, Sufyan Hussain, Anna Brackenridge, Siobhan Pender, Rosarie Atkinson, Melanie Bahadur, Hatem Eid, Janet Carling, Linzi Oldfield, Christopher Philbey, Peter Hammond, Sean Haywood, Geraldine Gallen, Helen Rodgers, Kaylee Lovie, Emma Whight, Georgia Nobel-Bell, Sophie Harris, Anne Cartwright, Ian Garnett, Jennifer Harvey, Frances McCulloch, Yew Wen Yap, Philip Weston, Alyson Chapman, Lynne Findlow, Lalantha Leelarathna, Sheetal Ohol, Hood Thabit, Andrea Urwin, Darron Cowlam, Sonia Thomas, Emma Hyland, Zoey Yearsley, Johnathan Schofield, Clare Soar, Laura Fenn, Budd Mendis, Zin Zin Htike, Elizabeth Cheyne, Julie Emsley, Elisabeth Jones, Vernon Parfitt, Lynn Sawyer, Santo Colosimo, Shani Apsara, Dilrukshi Mathara Diddhenipothage, Alistar Lumb, Katie Hards, Florence Edohen, Sue Beaden, Iain Cranston, Julie Taylor, Lisa Skinner, Zosanglura Bawlchhim, Bev Tuthill, Melissa Louise Cull, Robert E.J. Ryder, Sarah Mitchell, Jill Rimell, Clare Foley, Carla Gianfrancesco, Jackie Elliott, Sally Butter, Giorgio Carrieri, Isy Douek, Rhodri King, Paul Lambert, Paula Lionetti, Janet Cardwell, Niall Furlong, Suzanne Keigan, Rachael Milne, Philomena Wilkinson, Siobhan Ashton-Cleary, Sarah White, Karen Anthony, Stefania Ribul Mazzola, Stephanie Sweeney, Parth Narendran, Ali Karamat, Sanjay Saraf, Barbara Hudson, Manyee Li, Rebecca Skelding, Lisa Williams, Karen Bartha, Anna-Marie Jesson, Lynn Grandy, Deborah Brewer, Joanne Edwards, Nicola Sears, Manjit Shergil, Andy Baldwin, Thomas Crabtree, Isabelle Van Heeswijk, Linn Langeland, Nicola Taylor, Emma Wilmot, Sarah Owen, Rachel Taylor, Emma Robinson, Mohammed Bakhit, Amy Redfern, Cathy Kedge, Christine Kotonya, Haleema Hayat, Katy Gerrard, Nicci Pearson, Zara Redfern, Louise Curtis, Alison Galea, Melanie Weiss, Nikki Stacey, Helen Partridge, Pratik Choudhary, Tomás Griffin, Dawn Ackroyd, Liz Turrell, Ali J. Chakera, Vicki Lambert, Alison Suarez, Jesina Kirby, Nicola Lloyd, Eleni Karathenasi, Samantha McKinnon, Mindy Levitt, Raj Govindan, Becky Haskoll, Simon Saunders, Razak Kehinde, James Lee, Thomas Galliford, Alex Bickerton, Ruth Hammond, and Ruth Walker
References
- 1. NHS Digital . National Diabetes Audit Core report 1: care processes and treatment targets 2021-22, underlying data, 2023. Accessed 5 February 2023. Available from https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/nda-core-21-22/nda-core-21-22-data
- 2. Gregory GA, Robinson TIG, Linklater SE, et al.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group . Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 2022;10:741–760 [DOI] [PubMed] [Google Scholar]
- 3. NHS Digital . National Diabetes Insulin Pump Audit, 2017-18, 2018. Accessed 6 February 2023. Available from https://files.digital.nhs.uk/E0/030704/National%20Diabetes%20Insulin%20Pump%20Audit%202017-18%20Report%20v2.pdf
- 4. Chatwin H, Broadley M, Speight J, et al.; Hypo-RESOLVE Consortium . The impact of hypoglycaemia on quality of life outcomes among adults with type 1 diabetes: a systematic review. Diabetes Res Clin Pract 2021;174:108752. [DOI] [PubMed] [Google Scholar]
- 5. Griffin TP, Gallen G, Hartnell S, et al. UK’s Association of British Clinical Diabetologist’s Diabetes Technology Network (ABCD-DTN): best practice guide for hybrid closed-loop therapy. Diabet Med 2023;40:e15078. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence . Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus: technology appraisal guidance [TA151], 2008 Accessed 1 February 2023. Available from https://www.nice.org.uk/guidance/ta151
- 7. National Institute for Health and Care Excellence . Type 1 diabetes in adults: diagnosis and management, 2022. Accessed 1 February 2023. Available from https://www.nice.org.uk/guidance/ng17 [PubMed]
- 8. Tauschmann M, Thabit H, Bally L, et al.; APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boughton CK, Hartnell S, Thabit H, et al. Hybrid closed-loop glucose control compared with sensor augmented pump therapy in older adults with type 1 diabetes: an open-label multicentre, multinational, randomised, crossover study. Lancet Healthy Longev 2022;3:e135–e142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell SJ, Beck RW, Damiano ER, et al.; Bionic Pancreas Research Group . Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N Engl J Med 2022;387:1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choudhary P, Kolassa R, Keuthage W, et al.; ADAPT study Group . Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol 2022;10:720–731 [DOI] [PubMed] [Google Scholar]
- 13. Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Usoh CO, Johnson CP, Speiser JL, Bundy R, Dharod A, Aloi JA. Real-world efficacy of the hybrid closed-loop system. J Diabetes Sci Technol 2021;16:659–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the MiniMed advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24:178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knoll C, Peacock S, Wäldchen M, et al. Real-world evidence on clinical outcomes of people with type 1 diabetes using open-source and commercial automated insulin dosing systems: a systematic review. Diabet Med 2021;39:e14741. [DOI] [PubMed] [Google Scholar]
- 17. Crabtree TSJ, Griffin TP, Lumb A, et al. Protocol for the Diabetes Technology Network UK and Association of British Clinical Diabetologists’ closed-loop insulin delivery audit programme. Br J Diabetes 2022;22:9–13 [Google Scholar]
- 18. Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 20. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis D. History and perspective on DIY closed looping. J Diabetes Sci Technol 2019;13:790–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James Lind Alliance Priority Setting Partnerships . Diabetes (type 1) top 10, 2023. Accessed 12 February 2023. Available from https://www.jla.nihr.ac.uk/priority-setting-partnerships/diabetes-type-1/top-10-priorities
- 23. NHS Health Research Authority. Confidentiality Advisory Group , 2021. Accessed 1 October 2022. Available from https://www.hra.nhs.uk/approvals-amendments/what-approvals-do-i-need/confidentiality-advisory-group
- 24. Bergenstal RM, Nimri R, Beck RW, et al.; FLAIR Study Group . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021;397:208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hásková A, Radovnická L, Petruželková L, et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care 2020;43:2744–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polonsky WH, Fortmann AL. The influence of time in range on daily mood in adults with type 1 diabetes. J Diabetes Complications 2020;34:107746. [DOI] [PubMed] [Google Scholar]
- 28. Gonder-Frederick LA, Zrebiec JF, Bauchowitz AU, et al. Cognitive function is disrupted by both hypo- and hyperglycemia in school-aged children with type 1 diabetes: a field study. Diabetes Care 2009;32:1001–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heller S, Meneghini L, Nikolajsen A, et al. Towards a better understanding of postprandial hyperglycemic episodes in people with diabetes: impact on daily functioning. Curr Med Res Opin 2019;35:525–533 [DOI] [PubMed] [Google Scholar]
- 30. Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care 2004;27:2335–2340 [DOI] [PubMed] [Google Scholar]
- 31. McAuley SA, Lee MH, Paldus B, et al.; Australian JDRF Closed-Loop Research Group . Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care 2020;43:3024–3033 [DOI] [PubMed] [Google Scholar]
- 32. Beato-Víbora PI, Gallego-Gamero F, Lázaro-Martín L, Romero-Pérez MDM, Arroyo-Díez FJ. Prospective analysis of the impact of commercialized hybrid closed-loop system on glycemic control, glycemic variability, and patient-related outcomes in children and adults: a focus on superiority over predictive low-glucose suspend technology. Diabetes Technol Ther 2020;22:912–919 [DOI] [PubMed] [Google Scholar]
- 33. Choudhary P, Kolassa R, Keuthage W, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol 2022;10:720–731 [DOI] [PubMed] [Google Scholar]
- 34. Lawton J, Blackburn M, Rankin D, et al.; APCam11 Consortium . The impact of using a closed-loop system on food choices and eating practices among people with type 1 diabetes: a qualitative study involving adults, teenagers and parents. Diabet Med 2019;36:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Renard E, Ikegami H, Daher Vianna AG, et al. The SAGE study: global observational analysis of glycaemic control, hypoglycaemia and diabetes management in T1DM. Diabetes Metab Res Rev 2021;37:e3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. NHS England . Patients with type 1 diabetes to get artificial pancreas on the NHS, 2021. Accessed 10 December 2022. Available from https://www.england.nhs.uk/2021/06/patients-with-type-1-diabetes-to-get-artificial-pancreas-on-the-nhs