Abstract
In this review, we describe the pathophysiology, diagnosis, and treatment of spinal dorsal intradural arteriovenous fistulas (DI-AVFs), focusing on novel research areas. DI-AVFs compose the most common subgroup of spinal arteriovenous lesions and most commonly involve the thoracic spine, followed by lumbar and sacral segments. The pathogenesis underlying DI-AVFs is an area of emerging understanding, thought to be attributable to venous congestion and hypertension that precipitate ascending myelopathy. Patients with DI-AVFs typically present with motor, sensory, or urinary dysfunction, although a wide swath of other less common symptoms has been reported. DI-AVFs can be subdivided by spinal region, which in turn is associated with 4 distinct clinical phenotypes: craniocervical junction (CCJ), subaxial cervical, thoracic, and lumbosacral. Patients with CCJ and lumbosacral DI-AVFs have particularly interesting presentations and treatment considerations. High-value diagnostic findings on MRI include flow voids, missing-piece sign, and T2-weighted intramedullary hyperintensity. However, digital subtraction angiography is the gold standard for diagnosis and localization of DI-AVFs and for definitive treatment planning. Surgical disconnection of DI-AVFs is almost universally curative and frontline treatment, especially for CCJ and lumbosacral DI-AVFs. Endovascular techniques evolve in promising ways, such as improved visualization, distal access, and liquid embolic techniques. The pathophysiology of DI-AVFs is better understood using newly identified radiologic diagnostic markers. Despite new techniques and devices introduced in the endovascular field, surgery remains the gold-standard treatment for DI-AVFs.
Introduction
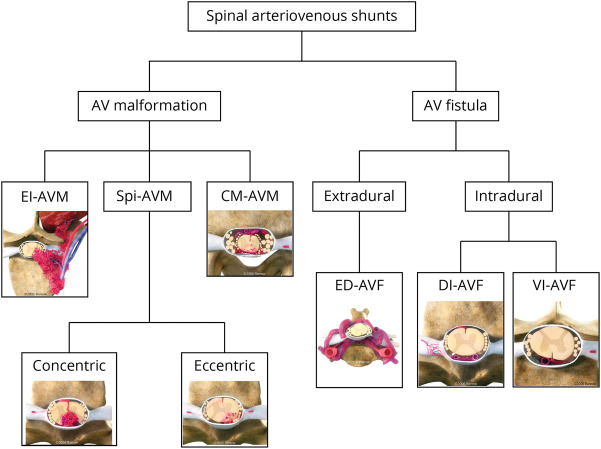
Arteriovenous shunting lesions are the most common vascular disorders of the spinal cord but are nonetheless rare, with an overall incidence of 1 or 2 cases per million per year.1 The Spetzler et al.2 spinal vascular classification grouped spinal arteriovenous shunts primarily into arteriovenous malformations (AVMs) and fistulas (AVFs), with discrete subtypes for both AVMs and AVFs (Figure 1).3 It is well known that AVMs are congenital in nature, but the pathogenesis of AVFs is debatable, and current evidence more likely supports an acquired etiology.4 Based on a large experience with 155 spinal vascular malformations from Bicêtre, a genetics-based classification was proposed in which focal lesions of the nerve root and filum terminale were generally not genetically associated.5 Spinal AVFs were dichotomized by Kim and Spetzler3 as intradural or extradural (Figure 1). Within the intradural group, dorsal intradural AVFs (DI-AVFs) were the most prevalent, accounting for 60%–70% of all spinal vascular lesions.6,7 Herein, we review the natural history, pathophysiology, diagnosis, and management of spinal DI-AVFs, with particular attention to differential aspects of care influenced by their neuroanatomic location, which includes craniocervical junction (CCJ) DI-AVFs (C1-C2, foramen magnum region, and the marginal sinus), subaxial cervical DI-AVFs (C3-C8), thoracic DI-AVFs, and lumbosacral DI-AVFs. Because CCJ AVFs represent the rostral extreme of the same stereotypical anatomy found in more caudal locations, they are included in this review, although they are not classically part of the Spetzler classification.
Figure 1. Classification of Spinal Vascular Shunts.
Kim and Spetzler's 2006 modified classification of spinal vascular shunts categorizes lesions as AVMs or AVFs with distinct subtypes based on variations in their anatomy, pathophysiology, and management.3 AVF = arteriovenous fistula; AVM = arteriovenous malformation; CM-AVM = conus medullaris AVM; DI-AVF = dorsal intradural AVF; ED-AVF = extradural AVF; El-AVM = extradural-intradural AVM; Spi-AVM = intramedullary AVM; VI-AVF = ventral intradural AVF. Used with permission from Barrow Neurological Institute, Phoenix, AZ.
Methods
The literature was searched on PubMed for articles in English evaluating the natural history, imaging, and management of spinal DI-AVFs from inception through February 2022. Search strategies were created using a combination of keywords and standardized index terms that included “arteriovenous fistula,” “spine,” “dorsal,” “shunt,” “dural,” “imaging,” “angiography,” “MRI,” “endovascular,” “clipping,” and “conservative.” The vast majority of articles published about spinal DI-AVFs are of a retrospective nature, with concerns about selection and publication bias among the available evidence, as demonstrated in previous meta-analyses.8,9 Although very few multicenter cohort studies have been published recently,10,11 an updated systematic review compatible with Preferred Reporting Items for Systematic Reviews and Meta-Analysis criteria was expected to be associated with several limitations. Therefore, the decision was made for a narrative review of the available evidence, which was summarized under 3 categories: natural history and pathophysiology, diagnosis and management, and areas in need of research.
Natural History and Pathophysiology
Natural History and Presentation
Patients with DI-AVFs are predominantly male and generally receive the diagnosis in their seventh or eighth decade of life.6,7 Delayed diagnosis of DI-AVFs is common because they are frequently underdiagnosed or misdiagnosed as other inflammatory, degenerative, or oncologic spine disorders in about 22% of patients.12,13 Once diagnosed, DI-AVFs are disproportionately found to involve the thoracic spine, followed by the lumbar, sacral, and cervical regions.4,13,14 Large DI-AVFs may uncommonly involve multiple spinal levels or regions, and rare instances of multiple concomitant DI-AVFs have been reported.15 Most patients who present with DI-AVFs have gradual symptomatic progression over periods as long as 10 years, although about 10% demonstrate acute neurologic changes, and there are infrequent cases of incidental diagnosis.7,12,16,17
Patients with DI-AVFs typically present with a wide spectrum of myelopathic symptoms arising from increased pressure within the spinal venous drainage or spinal cord dysfunction, very rarely from spinal cord compression. Radiculopathy is less common (5%–10%).13 Predictably, symptoms are influenced by the level of the arteriovenous shunt location and the extent of associated secondary spinal cord changes. Common index symptoms include motor weakness, sensory deficits, bowel and bladder dysfunction, neuropathic pain, and reflex abnormalities; rare presentations include respiratory failure or spinal subarachnoid hemorrhage (SAH).18 Spinal cord infarction is also an underlying cause of presenting symptoms of DI-AVFs. Zalewski et al.19 identified DI-AVFs as the most common cause of spinal cord infarction, accounting for 30% of 280 patients.
CCJ and Subaxial Cervical DI-AVFs
Cervical DI-AVFs account for only 2%–2.5% of all DI-AVFs.17 In women, the prevalence of CCJ DI-AVFs is higher compared with that of subaxial cervical and thoracic DI-AVFs, which predominantly affect men.6,15 Patients with cervical DI-AVFs are diagnosed in their 50s, but cases with younger presenting ages have been reported for CCJ lesions.20 Patients with CCJ DI-AVFs often present with intracranial hypertension and SAH thought to be attributable to very common cortical reflux from the spinal shunt, with an incidence of SAH of 43% reported in a previous systematic review.21,22 SAH associated with CCJ DI-AVFs is predominantly mildly symptomatic at presentation, with Hunt and Hess grades of I-II in approximately 80% of patients presenting with SAH of CCJ DI-AVFs.22 In a few case series and systematic reviews that included a cumulative sample of different types of CCJ arteriovenous shunts,22-24 factors such as the presence of varix, intracranial drainage, aneurysmal dilation of the feeder artery, and specific feeders (e.g., anterior spinal artery) were reported to be significantly associated with the hemorrhagic presentation of CCJ arteriovenous shunts. Nevertheless, analysis of the association of these factors with the hemorrhagic presentation of only CCJ DI-AVFs is not available. More caudal presentations of CCJ DI-AVFs with thoracic myelopathy have also been reported.15 This feature of CCJ DI-AVFs often prompts earlier diagnosis, resulting in a younger (aged in the 50s) patient population compared with that for DI-AVFs overall. However, because of the relative rarity of CCJ DI-AVFs, many investigators have not reported them discretely from other cervical DI-AVFs or from overall CCJ arteriovenous shunts.
Thoracic and Lumbosacral DI-AVFs
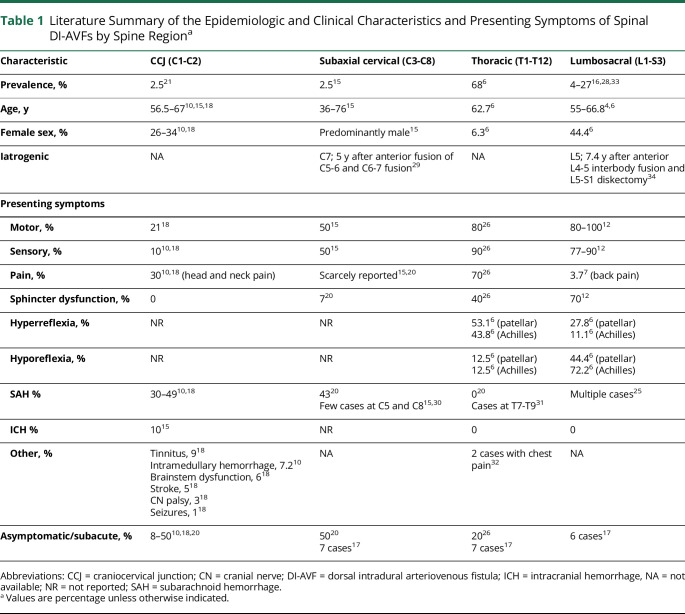
Thoracic and lumbar levels are the most common sites of DI-AVFs. Lumbosacral DI-AVFs have the highest female prevalence among all DI-AVFs.6,15 Patients with thoracic and lumbosacral DI-AVFs often present with motor and sensory symptoms and rarely present with SAH.25 Patients with thoracic lesions also exhibit hyperreflexia because of long tract dysfunction.26 However, hyporeflexia is commonly observed in patients with lumbosacral DI-AVFs, particularly of the Achilles tendon reflex, possibly due to conus and epiconus syndromes.6,13 Micturition disorders and sphincter disturbances have been reported in patients with lower thoracic and lumbosacral DI-AVFs, and those with thoracic lesions have a worse course. The thoracic segment is commonly divided into upper, middle, and lower segments. Because of the different internal diameters and variations in the vasculature of the thoracic spinal canal over the 3 segments, they can be associated with different outcomes. Midthoracic segments of the spinal cord are vulnerable, and patients with AVFs in this region may have worse outcomes, particularly in the wake of spinal hemorrhage due to their weak vasculature and the relatively small size of the spinal cord at these levels compared with upper and lower thoracic levels.14,27 Lumbosacral DI-AVFs, particularly sacral lesions, constitute a unique entity among spinal arteriovenous shunts because of their unique anatomy and the presenting symptoms they cause.20,28 Some investigators have reported that patients with lumbosacral DI-AVFs have more severe disability on presentation,20 whereas others have found that the severity of disability at presentation did not correlate with fistula level, T2 signal changes, or clinical presentation.13 Table 1 summarizes the reported literature on the epidemiologic and clinical characteristics and presenting symptoms of DI-AVFs by the involved spine region.4,6,7,10,12,15-18,20,21,25,26,28-34 Figure 2 demonstrates the prevalence of DI-AVFs over spinal regions35; data are for 59 cases, obtained as part of a retrospective analysis of 146 surgically managed intramedullary spinal cord cavernous malformations treated at our institution.4,6,7,10,12,15-18,20,21,26,28-34
Table 1.
Literature Summary of the Epidemiologic and Clinical Characteristics and Presenting Symptoms of Spinal DI-AVFs by Spine Regiona
Figure 2. DI-AVFs Prevalence by Spinal Region.
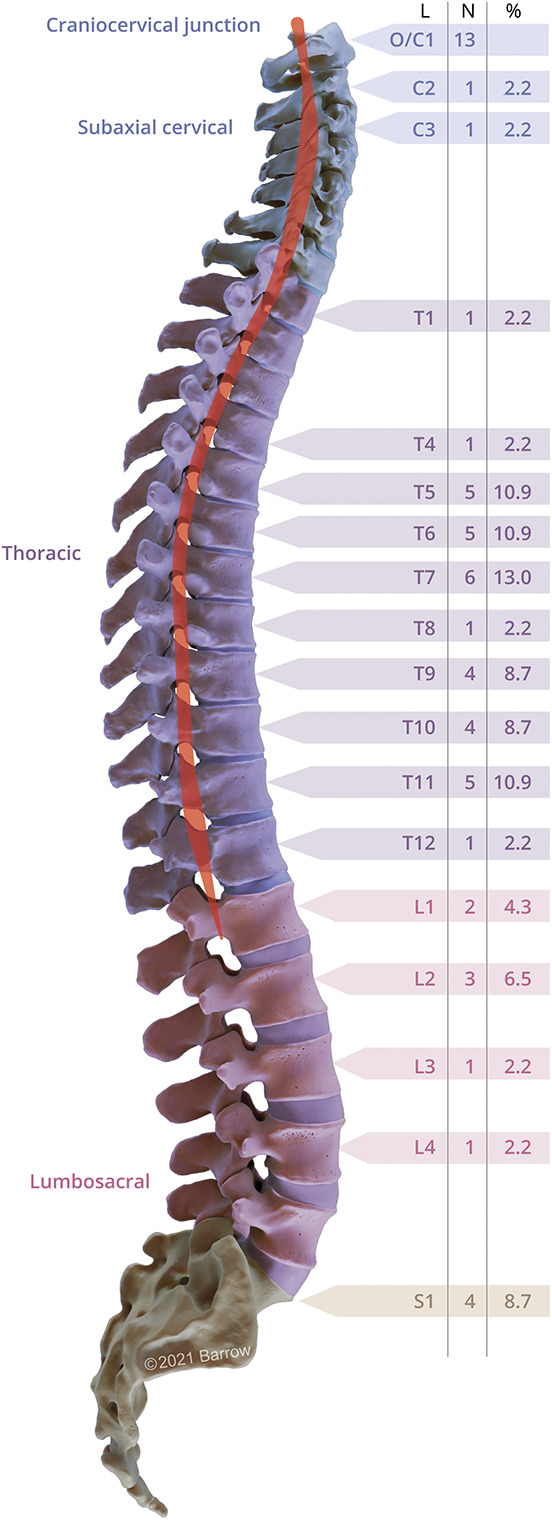
Prevalence of DI-AVFs over spinal regions. The artist's illustration demonstrates the prevalence of spinal DI-AVFs over the spinal cord (red highlight) segments based on an institutional series of 59 patients. Data were obtained as part of a retrospective analysis of 146 surgically managed intramedullary spinal cord cavernous malformations treated at our institution.35 The figure shows the distribution of the dorsal dural fistulas by the 4 spinal regions: (1) craniocervical junction (n = 13 patients, 22%), (2) subaxial cervical (n = 2 patients, 3%), (3) thoracic (n = 33 patients, 55.9%), and (4) lumbosacral (n = 11 patients, 19%). DI-AVF = dorsal intradural arteriovenous fistula; L = spinal level; O/C = occipital-cervical. Used with permission from Barrow Neurological Institute, Phoenix, AZ.
Pathogenesis and Pathophysiology
The pathogenesis of DI-AVFs is incompletely understood, with contemporary theories focusing on venous hypertension as the proximal driver of ascending myelopathy.4,14,36 Perimedullary veins are valveless, so in the presence of an arteriovenous shunt, arterial pressure is transmitted to the venous plexus, which leads to arterialization of the draining veins (Figure 3, A and B) and subsequently to progressive worsening of the arteriovenous shunt and venous congestion in the spinal cord. Mounting venous pressure precipitates edema and a congested state that reduces arterial perfusion and leads to hypoxia, myelopathy, and, potentially, cord infarction.37 Although the pathology is focal, the continuous vasculature of the spinal cord transmits this hypertensive state to multiple spinal segments above and below the lesion, which may compromise the whole spinal cord. The flowchart in Figure 3C summarizes the pathophysiologic cascade of spinal DI-AVFs, which is relatively conserved across spinal regions.
Figure 3. Pathophysiologic Cascade and a Proposed Diagnosis Protocol of DI-AVFs.
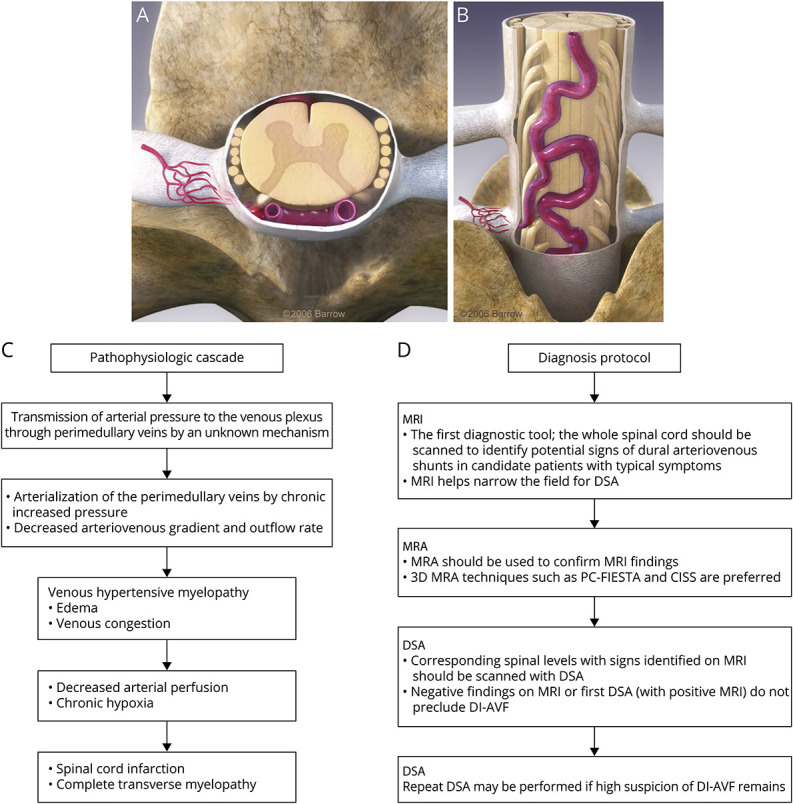
(A and B) Three-dimensional figures illustrate the arterialized vein (A, axial view; B, posterior view). (C) Flowchart of the pathophysiologic cascade of spinal DI-AVFs. (D) A proposed diagnosis protocol of DI-AVFs based on our institutional experience and evidence in the neurosurgery literature. 3D = 3 dimensional; CISS = constructive interference in steady state; DI-AVF = dorsal intradural arteriovenous fistula; DSA = digital subtraction angiography; MRA = magnetic resonance angiography; PC-FIESTA = phase-cycled fast imaging employing steady-state acquisition. Used with permission from Barrow Neurological Institute, Phoenix, AZ.
The origin of the shunting lesion observed in DI-AVFs is debatable, with no definitive evidence regarding an acquired, congenital, or multifactorial mechanism. Various iatrogenic causes of DI-AVF formation include diskectomy, laminectomy, and interbody fusion.29,34 In tandem, genetic diseases associated with spinal AVMs have been implicated that include hereditary hemorrhagic telangiectasia, Cobb syndrome, Klippel-Trénaunay syndrome, and Parkes Weber syndrome.5 Nevertheless, no strong evidence is available on the genetic origin of spinal DI-AVFs. Therefore, the acquired origin of spinal DI-AVFs is more widely accepted, supported by their presentation at late life stages.
The shunt of the DI-AVF forms between the radiculomeningeal artery (the feeder artery) that passes along with the nerve root sleeve and the radiculomedullary vein. The main supplying artery differs by spinal region and level of vertebra.
CCJ and Cervical DI-AVFs
In CCJ AVFs, the dural vessels around the vertebral artery cuff are the corollary of the nerve root sleeve and associated arteries; these vessels include branches of the posterior meningeal artery or the neuromeningeal trunk of the ascending pharyngeal artery.21 The veins in this region include the marginal or occipital sinus or both.24,38 In the rest of the cervical spine, DI-AVFs follow the pattern of the nerve root dural supply (radiculomeningeal artery) and the intradural radiculomedullary vein. Cervical DI-AVFs are often supplied by small, tortuous branches of dural arteries in this region, including the posterior meningeal artery or other branches of the vertebral arteries.
Thoracic and Upper Lumbar DI-AVFs
Fistulas at the thoracic and upper lumbar levels receive feeders from intercostal arteries, which may also give rise to the artery of Adamkiewicz.6,36,39 Upper and middle thoracic spinal segments are supplied by posterior spinal arteries, which are pairs. Segments between T9 and L2 are commonly supplied by branches of the artery of Adamkiewicz, which is single. Identification of the artery of Adamkiewicz is particularly critical for treatment planning. Apart from the treatment of DI-AVF, preservation of the spinal vasculature is crucial. Devastating outcomes related to iatrogenic occlusion of the artery of Adamkiewicz can occur after endovascular embolization of lower thoracic levels, and microsurgical clipping was therefore recommended in published series.40,41
Lumbosacral DI-AVFs
Middle or lateral sacral arteries and, less frequently, iliolumbar arteries are suppliers of lumbosacral DI-AVFs.6,28 These small arteries play a crucial role in the selection of microsurgery vs endovascular treatment. In some lumbosacral DI-AVF cases, endovascular access to the tiny feeders might not be feasible, and microsurgical resection would be the sole curative treatment.
Diagnosis and Management
Clinical and Radiographic Diagnosis
Although swift diagnosis improves the likelihood of faster treatment and better symptom resolution after treatment, spinal DI-AVFs are frequently missed because of their rarity and heterogeneous pattern of presentation. Classic features include ascending motor paresis and sensory loss with urinary dysfunction, but essentially any symptom localizing to the spinal cord may be associated with a DI-AVF. A common misdiagnosis is autoimmune disease, such as transverse myelitis42 or neuromyelitis optica. These patients may be mistakenly referred for biopsy. In this setting, the history of symptomatic worsening of motor functions or acute onset motor disabilities (e.g., paraplegia) after corticosteroid therapy is relatively specific for DI-AVF and should prompt dedicated vascular imaging in patients with suspected AV shunt.43 Catastrophic results can be avoided by appropriate diagnosis.
Various imaging modalities that are useful include CT, MRI, magnetic resonance angiography (MRA), and digital subtraction angiography (DSA). A dilated draining vein is commonly identified on multidetector CT images of patients with DI-AVFs, but this finding has a low specificity and requires higher-resolution follow-up studies.44,45
MRI
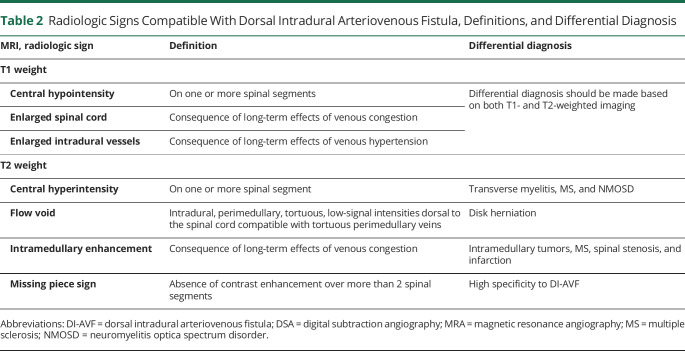
MRI should be the initial diagnostic tool used on suspicion of spinal DI-AVF. Key features on MRI include spinal cord edema, T2-hyperintensity in the spinal cord, intramedullary enhancement, flow voids, and prominent perimedullary vessels.16,28,44-46 The MRI finding with perhaps the highest specificity is the recently described missing-piece sign, in which contrast enhancement is absent over more than 2 spinal segments, which indirectly highlights the uneven venous drainage pattern induced by the DI-AVF.47 Other reports have identified similar, less rigidly defined MRI features of DI-AVFs, such as patchy or diffuse enhancement of the spinal cord.48 MRI can be used to distinguish DI-AVFs from mimics such as myelitis.49 Table 2 summarizes the most common radiologic signs of MRI compatible with spinal DI-AVFs and the differential diagnosis.
Table 2.
Radiologic Signs Compatible With Dorsal Intradural Arteriovenous Fistula, Definitions, and Differential Diagnosis
MRA
Although there are few MRI signs of high diagnostic value for DI-AVFs, further radiologic investigation is required to detect the exact location of the fistulous connection before treatment. Thus, selective catheterization of all feeders of spinal levels might be required before the procedure, which is associated with a high amount of radiation. New 3-dimensional (3D) MRA techniques were introduced that showed high fistula detection rates of up to approximately 90% in previous imaging studies,13,50-52 such as 3D time-of-flight MRA, different flip-angle evolutions, 3D phase-cycled fast imaging employing steady-state acquisition, and a 3D constructive interference steady-state technique. To facilitate identifying the precise fistula location and decrease DSA attempts, 3D MRA should be performed before DSA, which is used for confirmative fistula localization and treatment planning.
DSA
DSA is the gold standard of diagnostic imaging for suspected DI-AVFs. In addition, it is important for treatment planning as the optimal way to visualize the access pathway and spinal vasculature. Although multiple scan attempts might be needed, several studies reported an absolute diagnostic efficacy of DSA for DI-AVFs.4,6,7,10,12,15-18,20,21,26,28-34
Understanding the vascular anatomy of the spinal cord is crucial to making the right radiographic diagnosis. Selective angiography can help achieve optimal visualization of the dural shunt, given the variations of feeders by spine region (e.g., internal iliac arteries for lumbosacral DI-AVFs).53 DI-AVFs are differentiated from other vascular lesions by arteriovenous shunting in the presence of an arterialized draining vein with retrograde intradural drainage. Typically, the vein is located adjacent to the ipsilateral corresponding pedicle. Identification of venous reflux is the critical factor distinguishing a DI-AVF from an extradural AVF and other spinal shunts, with major implications for treatment pathways.7 Figure 3D delineates a protocol for diagnosis of DI-AVFs based on our institutional experience and a review of evidence in the neurosurgery literature.46
Treatment Paradigms
Three major clinical pathways have been defined for DI-AVFs, each with variations based on institutional protocols. Conservative management is seldom recommended for patients with symptomatic lesions. For DI-AVFs that require treatment, the treatment options include microsurgical occlusion and endovascular embolization.7,12,13,15,17,28
Conservative Management
Given the potential for neurologic decline in patients with a treatable lesion, there is sparse evidence in support of conservative management for DI-AVFs. Observation may be appropriate for patients with asymptomatic lesions that were identified incidentally and for patients with lesions that resolve spontaneously after presentation. Shimizu et al.17 described 20 cases of asymptomatic DI-AVFs that were observed: 4 (20%) became symptomatic and were subsequently recommended for treatment, 1 spontaneously involuted, and the rest were clinically quiescent over a median follow-up of 1.5 years (follow-up available for 10 of 20 cases). Although anecdotal, this finding provides some evidence in support of observing patients who are averse to intervention or who are poor surgical candidates for unrelated medical reasons. Notably, the midthoracic location was associated with lower rates of spontaneous recovery and an unfavorable clinical course, with higher rates of subsequent treatment. The patients in this subgroup may benefit from a more aggressive posture toward early intervention.6,13,14 These findings helped motivate the development of treatment criteria for DI-AVFs described by Yu et al.,14 which includes age older than 28 years at onset, initial modified Aminoff and Logue disability scale score greater than 3 (e.g., severe symptoms), and midthoracic localization. Sato et al., in a small case series, compared the characteristics of 8 patients with thoracic DI-AVFs, which were symptomatic at presentation, with 2 asymptomatic DI-AVFs.54 The authors noted that patients with asymptomatic fistulas were 10 years younger than those with symptomatic lesions. In addition, no signs of spinal cord edema were observed on MRI of asymptomatic DI-AVFs, unlike the symptomatic lesions. However, the authors demonstrated early signs of venous hypertension in asymptomatic fistulas represented by the radicular venous outflow from arterialized perimedullary veins to the extradural venous plexus. Although observation might be appropriate for asymptomatic DI-AVFs, particularly in patients with several comorbidities, many conservatively treated asymptomatic DI-AVFs might become symptomatic in later stages due to an increase in intravenous pressure and associated pathologic mechanisms over the time of observation. Therefore, early treatment of asymptomatic DI-AVFs in young patients to avoid delayed progression might be substantial. Nevertheless, the current literature evidence is unfortunately insufficient to answer this question, and further longitudinal studies on asymptomatic DI-AVFs are warranted.
Microsurgical Treatment
Based on strong evidence in the literature, microsurgical clip occlusion of DI-AVFs has consistently demonstrated the highest rates of treatment efficacy and safety, and it is correspondingly considered the contemporary standard of care.55 It is particularly preferred for CCJ and lumbosacral locations, which are more prone to treatment failure and higher rates of procedural complications than thoracic or subaxial cervical lesions.11,15,21,28
Microsurgical clip occlusion of DI-AVFs is a simple process that relies on precise anatomical localization with preoperative DSA. Routine neurosurgical techniques are used for soft tissue exposure, wide laminectomy at the level, midline durotomy, arachnoid dissection to the arterialized draining vein, and clip occlusion. The proximal segment of the arterialized vein is carefully isolated and clip ligated, yielding a change in the color of blood on indocyanine green videoangiography (ICG-VA) from bright red to dark purple. Thus, our experience, supported by evidence in the literature, showed that ICG-VA may be used both to demonstrate the fistula and to confirm its obliteration.56,57 If there is concern that critical spinal perfusion may depend in part on the target vessel, a period of trial clipping with neurophysiologic monitoring may provide guidance before permanent cauterization and division of the draining vein.
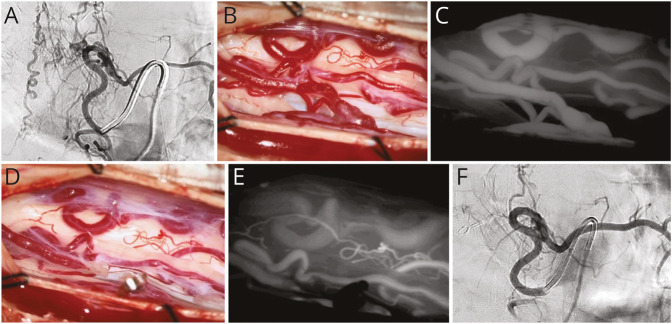
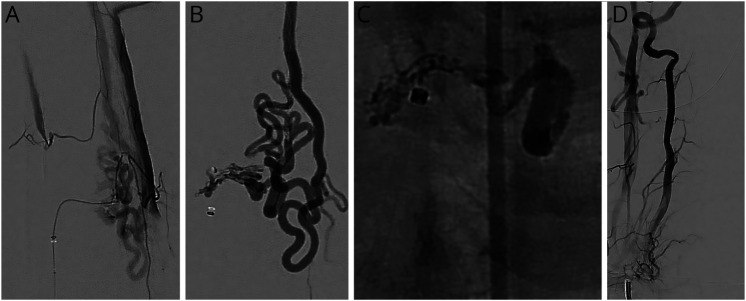
Surgical treatment of lumbosacral DI-AVFs typically yields favorable neurologic outcomes.6,58 Motor symptoms frequently resolve the earliest and most reliably, with most patients recovering completely.27,58 Sensory symptoms improve in a more delayed fashion and are less likely to fully resolve after treatment, whereas micturition dysfunction lags and has the lowest probability of long-term recovery among common presenting symptoms. Postoperative radiographic changes may be difficult to interpret, and they are often poorly correlated with clinical findings. There is evidence that the persistence of intramedullary hyperintensity on T2-weighted MRI sequences is concerning for residual or recurrent fistula, particularly in the absence of clinical improvement.28,48,59,60,e1 DSA remains the gold standard diagnostic imaging modality for ruling out residual or recurrent AV shunting; however, intraoperative ICG-VA is reported to have a 100% negative predictive value for demonstrating fistula obliteration and is an accepted practice for initial confirmation of treatment success.57 Figure 4 shows pre-, intra-, and postoperative images of a previously published case of a male patient in his early 40s who was admitted with progressive weakness and paresthesia of the lower limbs, particularly on the right side; a thoracic DI-AVF was diagnosed, which was managed microsurgically.e2,e3
Figure 4. Microsurgical Disconnection of a Thoracic DI-AVF.
Microsurgical disconnection of a thoracic DI-AVF.e2 (A) A left DI-AVF at T7 was detected and diagnosed using DSA. (B) The posterior spinal artery and arterialized draining vein in the left lateral recess were exposed. (C) The shunt was confirmed via intraoperative ICG-VA. (D) The fistulous connection was clipped, and cauterization and division of the arterialized vein were subsequently performed. (E) Complete obliteration of the arteriovenous shunt was demonstrated on the second ICG-VA. (F) Fistula obliteration with no residual was confirmed via postoperative DSA. DI-AVF = dorsal intradural arteriovenous fistula; DSA = digital subtraction angiography; ICG-VA = indocyanine green videoangiography. Used with permission from Barrow Neurological Institute, Phoenix, AZ.
Endovascular Embolization
Endovascular embolization gained popularity as a new treatment for spinal arteriovenous shunts over the past decade, particularly for lesions that are difficult to reach by surgery, such as ventral spinal AVFs.8 Endovascular embolization also offers an alternative strategy to treat DI-AVFs that may be preferable for certain lesions or specific patients, although resection is still considered the definitive treatment. The goal of any treatment for DI-AVFs is to occlude or interrupt the flow within the proximal segment of the arterialized intradural vein, which may be difficult to achieve using current embolization techniques—particularly in the more anatomically challenging CCJ and sacral lesions.10,e4
Liquid embolic agents (e.g., Onyx [Medtronic, plc, Dublin, Ireland] and n-butyl cyanoacrylate [n-BCA, Trufill, Cordis Neurovascular, Baar, Switzerland]), as demonstrated widely in the neurointerventional literature, are relatively safe, although they do carry some risk of thromboembolic ischemia.e5,e6 Few case series reported a substantial efficacy of anticoagulants such as heparin and vitamin K anticoagulants for the management of ischemic events (e.g., venous thrombosis) after the embolization of spinal DI-AVFs using liquid agents. The authors highlighted the importance of prophylactic anticoagulation before embolization.e7-e9 Nevertheless, there are durability concerns about these agents and the predisposition of embolized DI-AVFs to recur.28 Prior series have reported a wide range of treatment failure rates, with the need for follow-up surgical intervention in 7.7%–64.3% of patients.28,e7,e10 The adhesive agent n-BCA has been used to interrupt DI-AVFs with good clinical and angiographic outcomes, which is promising. Gioppo et al.28 reported a reduction in intramedullary enhancement in 91%, gait improvement in 73%, and an ischemic complication rate of 10% in 10 patients with DI-AVFs embolized with n-BCA. These authors preferred the superior efficacy of n-BCA over other liquid embolic agents because of its adhesive character, sclerosing function, and ability to penetrate through the nidus with custom dilution.21,28,e4
Sasamori et al.e4 compared treatment outcomes for a cohort of 50 patients with 50 DI-AVFs, treated either with n-BCA embolization or surgical disconnection. The authors reported obliteration of DI-AVFs in 22 of 31 patients (71%) in the embolization group compared with 18 of 19 patients (95%) in the surgical group. Additional treatments for recurrent or incompletely occluded lesions were required for 9 of 31 patients (29%) with embolized fistulas compared with only 1 of 19 patients (5%) with surgically clipped lesions. Gross et al.e3 found similar results and noted no significant difference in complication rates between microsurgery and embolization groups. They concluded that embolization might initially be used safely as a less invasive treatment modality for DI-AVFs. Nevertheless, there was still a high rate (46%, 13 of 28) of microsurgical bailout.
Clinical improvement in motor function has been reported in 80%–90% of patients who received embolization for DI-AVFs, whereas only 20%–50% have reported modest improvement in micturition.28,e3 Symptomatic relapse after embolization has been reported to be low (0%–6%) in many series,28,e3,e11 but high (up to 90%) in others.21,e10 In the absence of uniform evidence, expert consensus links this discrepancy to the variety of embolization materials in use and varying treatment protocols and fistula locations.
Despite rapid improvement in endovascular embolization materials and techniques, the available evidence shows that radiographic recurrence is common in embolized fistulas.28,e3,e6,e7,e10,e12 Lee et al.e12 reported that 8 (22%) of 37 patients with DI-AVFs that were embolized using n-BCA had radiographic recurrence at a median follow-up of 2 years (IQR 10.5–42 months). Embolized DI-AVFs present a particular challenge in follow-up assessment, given the increased ambiguity in determining whether a persistent clinical syndrome is referrable to active shunting, treatment complications, or severe disease despite successful treatment—a question that generally requires repeat MRI and DSA for definitive discrimination.28,e10
With these considerations in mind, it is well accepted that microsurgery remains the first-line treatment for most patients with DI-AVFs, given its overall advantages in terms of treatment efficacy, durability, and safety. Nevertheless, as demonstrated in previous literature,e3 we believe that approximately 80% of spinal DI-AVFs can be candidates for embolization, which might be attempted during the diagnostic angiography. Based on our experience, we observed that complete obliteration of the fistulous connection can be achieved in approximately half of DI-AVFs, mainly with occlusion of the draining vein. Furthermore, if occlusion of the draining vein could not be achieved, our practice is to not consider angiographic nonopacification as adequate occlusion, and because of the high recurrence rate of DI-AVFs, surgical disconnection should be performed. Although we observed higher obliteration rates using specific embolization agents, this might differ significantly by the agent characteristics and the experience of the neurointerventionalist with that agent. Finally, when the complication rate is thought to be comparable between the 2 treatment modalities (embolization vs microsurgery) for a specific case, embolization might be attempted during the diagnostic DSA; however, an option for the definitive treatment should be made for the modality associated with the higher obliteration rate (microsurgery). Figure 5 illustrates a previously published case of a patient with a cervical DI-AVF that was embolized using n-BCA, resulting in an uncomplicated postintervention course and demonstrated fistula obliteration.e3
Figure 5. Endovascular Disconnection of a Cervical DI-AVF.
Endovascular disconnection of a cervical DI-AVF.e3 (A) Opacification of the corresponding fistula, the anterior spinal artery, and the bilateral vertebral arteries was detected via superselective anteroposterior angiography after catheterization of the radicular feeder of the cervical lesion. (B) The microcatheter was adjusted into another branch of the radicular feeder, where a second superselective anteroposterior angiogram demonstrated opacification of only the fistula and no pial branches. (C) An anteroposterior unsubtracted angiogram demonstrated embolization of the fistula with n-butyl cyanoacrylate after an uncomplicated procedure, with penetration of the draining vein. (D) A follow-up anteroposterior angiogram performed by injecting the right subclavian artery showed no residual arteriovenous shunting. DI-AVF = dorsal intradural arteriovenous fistula. Used with permission from Barrow Neurological Institute, Phoenix, AZ.
Effect of Fistula Location on Treatment Outcomes
Despite the fact that microsurgical and endovascular approaches to DI-AVFs are similar regardless of fistula location, posttreatment outcomes appear to show distinct differences by lesion level because of multiple factors, including variable arterial supply and clinical presentation. Takai et al.10 examined a multi-institutional cohort of 97 patients with CCJ DI-AVFs and found that endovascular treatment (n = 19) compared with microsurgical treatment (n = 78) was correlated with higher rates of ischemic complications (26% vs 7.7%; p = 0.04) and retreatment (63% vs 2.6%; p < 0.001) (at a median 23-month follow-up). Cenzato et al.27 reported a significant (p = 0.004) association of midthoracic DI-AVFs with worse posttreatment clinical outcomes at 3-year follow-up compared with outcomes for lower thoracic (T9-T12) and lumbosacral lesions independent of applied treatment (endovascular or surgical), linking this to their weak vascularization. Lee et al.e12 found that cervical DI-AVFs are associated with the highest rate of incomplete occlusion after endovascular treatment, followed by thoracic and lumbosacral lesions (p = 0.04). However, clinical outcomes were not correlated with fistula location. Furthermore, fistulas with multiple feeders were significantly associated with higher rates of incomplete occlusion (73.7%, 14 of 24; p < 0.001).
Appropriate treatment should be selected on the basis of both the patient's clinical status and the anatomic characteristics of the fistula. Regardless of spine level, from a neurosurgical perspective, microsurgery remains the preferred initial treatment for cervical and midthoracic DI-AVFs and lesions with multiple feeders. Endovascular techniques have the advantage of simultaneous diagnosis and management during a single intervention. Thus, they may be a viable alternative for patients with lower thoracic and lumbar DI-AVFs that do not involve critical spinal cord arterial supply.
Areas in Need of Research
DI-AVFs are an important niche for future study because of their common prevalence among spinal AVMs, unique pathophysiology, difficult and delayed diagnosis, and morbid consequences. More investigation into the presentation (identifying more specific presentation symptoms), natural history (understanding the molecular basis of the AV shunt to possibly halt the fistulous connection at earlier stages), treatment (inventing new minimally invasive techniques), and outcomes of patients with DI-AVFs should facilitate more rapid diagnosis and more appropriate treatment before neurologic progression. Additional research results should also increase the awareness of DI-AVFs among frontline clinicians and should improve diagnostic accuracy in common imaging modalities such as CT and MRI. Artificial intelligence (AI) still demonstrates promise in the diagnosis of cerebrovascular lesions, with comparable accuracy to the DSA reading of cerebral aneurysms, AVMs, and dural AVFs by 2 independent neuroradiologists, as demonstrated in a recent publication.e13 AI-based DSA imaging might also help reduce the need for multiple DSA attempts to obtain 3D DSA imaging and reduce the consequential radiation to which patients would be exposed. The increasing utilization of AI in the diagnostic radiology paradigm may be particularly helpful in alerting radiologists to subtle indications of disease, thereby increasing the probability of correctly diagnosing these rare lesions when they are captured on routine imaging studies.
Many patients with DI-AVFs require long-term rehabilitation for persistent neurologic deficits, whether referable to disease progression, treatment, or both.e14 In addition to an accelerated diagnostic pipeline, better rehabilitation modalities and wider, more long-term access to rehabilitation medicine would substantially affect functional and quality-of-life outcomes for patients with DI-AVF. In particular, neurostimulation and assistive robotics may help address deficits in pain, sensorimotor, and autonomic domains.e14 Assistive robotics, in addition to their high patient satisfaction and patient acceptance rates, have demonstrated efficiency in decreasing errors in task performance and application of repeated movements related to human nature or lack of experience among rehabilitation assistants, as reported among patients with traumatic brain injury.e15 Therefore, further implementation of these devices in rehabilitation might reflect positively on the outcomes of patients with neurologic injury.
From the perspective of basic science, understanding the disease mechanisms underlying the development and progression of DI-AVFs at a cellular level remains incomplete, and further research may facilitate the development of adjuvant medical interventions with the potential to reverse these pathophysiologic changes in the spinal cord.e16 Other treatments can also be imagined that would target and repair dysfunctional pathways, such as those coordinating urinary function—perhaps the most common and frustrating source of disease and treatment morbidity for patients with DI-AVF.
Finally, as discussed earlier, current evidence supports the association of endovascular embolization with high rates of treatment failure, often requiring a shift to microsurgical treatment.28,e3,e7,e10 Improvements in endovascular techniques would make embolization a more attractive treatment option, with a lower risk of complications or treatment failure than that reported with contemporary modalities and currently available embolization agents. For example, relatively recent studies have reported much sharper imaging using radiographic microscopes with high-definition zoom mode and high resolution.e17-e19 Such techniques and devices like high-definition microangiographic fluoroscopy may improve visualization of DI-AVFs, their microscopic feeders, and deployed embolization materials, leading in turn to better obliteration rates.e18,e20 Similarly, the use of small microballoons is one area of recent technologic development that is anticipated to markedly enhance the endovascular management of lesions with small feeders, which are commonly encountered in DI-AVFs.e21 Initial experiences with microballoons, such as Scepter mini balloons, are being published; microballoons have demonstrated promise in arresting flow within distal and small vessels and have a high safety profile.e21-e23 Nevertheless, further studies are needed to identify the benefits and uses of these devices and explore more techniques and interventions useful for the management of DI-AVFs and other neurovascular disorders.
Spinal DI-AVFs cause venous hypertension, venous congestion, and ascending myelopathy, but their pathogenesis is not well known. Thoracic segments are the segments that are most commonly involved, and they are associated with worse outcomes. CCJ and sacral DI-AVFs are unique subtypes representing the most cranial and caudal extensions of the same pathology. Valuable MRI findings have been described, but DSA remains the gold standard for diagnosing DI-AVFs. Based on published literature, expert consensus is that microsurgical disconnection is the most effective and durable treatment and should be considered the first-line treatment for most patients. Endovascular techniques continue to evolve and offer an alternative to microsurgical treatment in select patients.
Acknowledgment
The authors thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation, of which no members qualify for authorship.
Glossary
- 3D
3 dimensional
- AI
artificial intelligence
- AVF
arteriovenous fistula
- AVM
arteriovenous malformation
- CCJ
craniocervical junction
- DI-AVF
dorsal intradural arteriovenous fistula
- DSA
digital subtraction angiography
- ICG-VA
indocyanine green videoangiography
- MRA
magnetic resonance angiography
- n-BCA
n-butyl cyanoacrylate
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Thron A. Spinal dural arteriovenous fistulas [in German]. Radiologe. 2001;41(11):955-960. [DOI] [PubMed] [Google Scholar]
- 2.Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002;96(2):145-156. [DOI] [PubMed] [Google Scholar]
- 3.Kim LJ, Spetzler RF. Classification and surgical management of spinal arteriovenous lesions: arteriovenous fistulae and arteriovenous malformations. Neurosurgery. 2006;59(suppl 5):S3-195-S3-201; discussion S193-S113. [DOI] [PubMed] [Google Scholar]
- 4.Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129(12):3150-3164. [DOI] [PubMed] [Google Scholar]
- 5.Rodesch G, Hurth M, Alvarez H, Ducot B, Tadie M, Lasjaunias P. Angio-architecture of spinal cord arteriovenous shunts at presentation. Clinical correlations in adults and children. The Bicetre experience on 155 consecutive patients seen between 1981-1999. Acta Neurochir (Wien). 2004;146(3):217-226; discussion 226-217. [DOI] [PubMed] [Google Scholar]
- 6.Endo T, Kajitani T, Inoue T, et al. Clinical characteristics of lumbosacral spinal dural arteriovenous fistula (DAVF)-comparison with thoracic DAVF. World Neurosurg. 2018;110:e383-e388. [DOI] [PubMed] [Google Scholar]
- 7.Kiyosue H, Matsumaru Y, Niimi Y, et al. Angiographic and clinical characteristics of thoracolumbar spinal epidural and dural arteriovenous fistulas. Stroke. 2017;48(12):3215-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal A, Cesare J, Lu VM, et al. Outcomes following surgical versus endovascular treatment of spinal dural arteriovenous fistula: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2019;90(10):1139-1146. [DOI] [PubMed] [Google Scholar]
- 9.Bakker NA, Uyttenboogaart M, Luijckx GJ, et al. Recurrence rates after surgical or endovascular treatment of spinal dural arteriovenous fistulas: a meta-analysis. Neurosurgery. 2015;77(1):137-144; discussion 144. [DOI] [PubMed] [Google Scholar]
- 10.Takai K, Endo T, Seki T, et al. Neurosurgical versus endovascular treatment of craniocervical junction arteriovenous fistulas: a multicenter cohort study of 97 patients. J Neurosurg. 2021:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Takai K, Endo T, Yasuhara T, et al. Neurosurgical versus endovascular treatment of spinal dural arteriovenous fistulas: a multicenter study of 195 patients. J Neurosurg Spine. 2020:1-8. [DOI] [PubMed] [Google Scholar]
- 12.Jablawi F, Nikoubashman O, Schubert GA, Dafotakis M, Hans FJ, Mull M. Clinical and radiologic characteristics of deep lumbosacral dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2018;39(2):392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muralidharan R, Saladino A, Lanzino G, Atkinson JL, Rabinstein AA. The clinical and radiological presentation of spinal dural arteriovenous fistula. Spine (Phila Pa 1976). 2011;36(25):E1641-E1647. [DOI] [PubMed] [Google Scholar]
- 14.Yu JX, Hong T, Krings T, et al. Natural history of spinal cord arteriovenous shunts: an observational study. Brain. 2019;142(8):2265-2275. [DOI] [PubMed] [Google Scholar]
- 15.Kim DJ, Willinsky R, Geibprasert S, et al. Angiographic characteristics and treatment of cervical spinal dural arteriovenous shunts. AJNR Am J Neuroradiol. 2010;31(8):1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinjikji W, Nasr DM, Morris JM, Rabinstein AA, Lanzino G. Clinical outcomes of patients with delayed diagnosis of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2016;37(2):380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu K, Takeda M, Mitsuhara T, et al. Asymptomatic spinal dural arteriovenous fistula: case series and systematic review. J Neurosurg Spine. 2019;31(5):733-741. [DOI] [PubMed] [Google Scholar]
- 18.Do AS, Kapurch J, Kumar R, Port J, Miller JW, Van Gompel JJ. The long and winding road: thoracic myelopathy associated with occipitocervical dural arteriovenous fistula. World Neurosurg. 2017;108:998.e7-998.e16. [DOI] [PubMed] [Google Scholar]
- 19.Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76(1):56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinoyama M, Endo T, Takahash T, et al. Long-term outcome of cervical and thoracolumbar dural arteriovenous fistulas with emphasis on sensory disturbance and neuropathic pain. World Neurosurg. 2010;73(4):401-408. [DOI] [PubMed] [Google Scholar]
- 21.Jiang P, Lv X, Wu Z, Li Y. Dural arteriovenous fistula of crianiocervical junction: four case reports. Neurol India. 2012;60(1):94-95. [DOI] [PubMed] [Google Scholar]
- 22.Wang JY, Molenda J, Bydon A, et al. Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci. 2015;22(11):1701-1707. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction: a systematic review. J Neurointerv Surg. 2016;8(6):648-653. [DOI] [PubMed] [Google Scholar]
- 24.Hiramatsu M, Sugiu K, Ishiguro T, et al. Angioarchitecture of arteriovenous fistulas at the craniocervical junction: a multicenter cohort study of 54 patients. J Neurosurg. 2018;128(6):1839-1849. [DOI] [PubMed] [Google Scholar]
- 25.Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg. 2004;100(4):385-391. [DOI] [PubMed] [Google Scholar]
- 26.Fox S, Hnenny L, Ahmed U, Meguro K, Kelly ME. Spinal dural arteriovenous fistula: a case series and review of imaging findings. Spinal Cord Ser Cases. 2017;3(1):17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cenzato M, Debernardi A, Stefini R, et al. Spinal dural arteriovenous fistulas: outcome and prognostic factors. Neurosurg Focus. 2012;32(5):E11. [DOI] [PubMed] [Google Scholar]
- 28.Gioppo A, Farago G, Giannitto C, et al. Sacral dural arteriovenous fistulas: a diagnostic and therapeutic challenge: single-centre experience of 13 cases and review of the literature. J Neurointerv Surg. 2018;10(4):415-421. [DOI] [PubMed] [Google Scholar]
- 29.Kanematsu R, Hanakita J, Takahashi T, Tomita Y, Minami M. An acquired cervical dural arteriovenous fistula after cervical anterior fusion: case report and literature review. World Neurosurg. 2019;128:50-54. [DOI] [PubMed] [Google Scholar]
- 30.Gao P, Du S, Ren J, Li G, Zhang H. Teaching neuroimages: lower cervical spine dural arteriovenous fistula presenting as subarachnoid hemorrhage. Neurology. 2019;92(15):e1798-e1800. [DOI] [PubMed] [Google Scholar]
- 31.Minami M, Hanakita J, Takahashi T, et al. Spinal dural arteriovenous fistula with hematomyelia caused by intraparenchymal varix of draining vein. Spine J. 2009;9(4):e15-e19. [DOI] [PubMed] [Google Scholar]
- 32.Bioh G, Bogle R. Spinal dural AV fistula: an unusual cause of chest pain. BMJ Case Rep. 2014;2014(2):bcr2013202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safaee MM, Clark AJ, Burkhardt JK, Winkler EA, Lawton MT. Timing, severity of deficits, and clinical improvement after surgery for spinal dural arteriovenous fistulas. J Neurosurg Spine. 2018;29(1):85-91. [DOI] [PubMed] [Google Scholar]
- 34.Yoshino O, Matsui H, Hirano N, Tsuji H. Acquired dural arteriovenous malformations of the lumbar spine: case report. Neurosurgery. 1998;42(6):1387-1389. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan VM, Karahalios K, Shlobin NA, et al. Residual and recurrent spinal cord cavernous malformations: outcomes and techniques to optimize resection and a systemic review of the literature. Oper Neurosurg (Hagerstown). 2023;24(1):44-54. [DOI] [PubMed] [Google Scholar]
- 36.Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A. Vascular anatomy of the spinal cord. J Neurointerv Surg. 2012;4(1):67-74. [DOI] [PubMed] [Google Scholar]
- 37.Gobin YP, Rogopoulos A, Aymard A, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with spinal perimedullary venous drainage. J Neurosurg. 1992;77(5):718-723. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan VM, Catapano JS, Frisoli FA, Mooney MA, Lawton MT. Microsurgical management of a marginal sinus dural arteriovenous fistula: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2021;21(5):E447-E448. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Ishii K, Nakamura M, et al. The validity of intraoperative angiography for the treatment of spinal arteriovenous fistula. J Spinal Disord Tech. 2007;20(6):442-448. [DOI] [PubMed] [Google Scholar]
- 40.Eneling J, Karlsson PM, Rossitti S. A treatment-refractory spinal dural arteriovenous fistula sharing arterial origin with the artery of Adamkiewicz: repeated endovascular treatment after failed microsurgery. Surg Neurol Int. 2014;5:S165-S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shedid D, Podichetty VK. Common origin of the artery of Adamkiewicz and a posterior spinal artery with a spinal dural arteriovenous fistula: a case report. Br J Neurosurg. 2009;23(6):630-633. [DOI] [PubMed] [Google Scholar]
- 42.Sechi E, Flanagan EP. Spinal arteriovenous fistula's often misdiagnosed as myelitis; can we stem the flow? J Neurol Sci. 2020;413:116868. [DOI] [PubMed] [Google Scholar]
- 43.Nasr DM, Brinjikji W, Rabinstein AA, Lanzino G. Clinical outcomes following corticosteroid administration in patients with delayed diagnosis of spinal arteriovenous fistulas. J Neurointerv Surg. 2017;9(6):607-610. [DOI] [PubMed] [Google Scholar]
- 44.Sakai Y, Matsuyama Y, Imagama S, Ito Z, Wakao N, Ishiguro N. Clinical utility of multidetector row computed tomography for diagnosing spinal dural arteriovenous fistulas undiagnosed by magnetic resonance imaging. Geriatr Gerontol Int. 2010;10(3):255-263. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi S, Eguchi K, Kiura Y, et al. Multi-detector-row CT angiography as a preoperative evaluation for spinal arteriovenous fistulae. Neurosurg Rev. 2007;30(4):321-326; discussion 327. [DOI] [PubMed] [Google Scholar]
- 46.Morris JM. Imaging of dural arteriovenous fistula. Radiol Clin North Am. 2012;50(4):823-839. [DOI] [PubMed] [Google Scholar]
- 47.Zalewski NL, Rabinstein AA, Brinjikji W, et al. Unique gadolinium enhancement pattern in spinal dural arteriovenous fistulas. JAMA Neurol. 2018;75(12):1542-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horikoshi T, Hida K, Iwasaki Y, Abe H, Akino M. Chronological changes in MRI findings of spinal dural arteriovenous fistula. Surg Neurol. 2000;53(3):243-249. [DOI] [PubMed] [Google Scholar]
- 49.Mustafa R, Passe TJ, Lopez-Chiriboga AS, et al. Utility of MRI enhancement pattern in myelopathies with longitudinally extensive T2 lesions. Neurol Clin Pract. 2021;11(5):e601-e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luetmer PH, Lane JI, Gilbertson JR, Bernstein MA, Huston J III, Atkinson JL. Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol. 2005;26(4):711-718. [PMC free article] [PubMed] [Google Scholar]
- 51.Meckel S, Maier M, Ruiz DS, et al. MR angiography of dural arteriovenous fistulas: diagnosis and follow-up after treatment using a time-resolved 3D contrast-enhanced technique. AJNR Am J Neuroradiol. 2007;28(5):877-884. [PMC free article] [PubMed] [Google Scholar]
- 52.Kannath SK, Alampath P, Enakshy Rajan J, Thomas B, Sankara Sarma P, Tirur Raman K. Utility of 3D SPACE T2-weighted volumetric sequence in the localization of spinal dural arteriovenous fistula. J Neurosurg Spine. 2016;25(1):125-132. [DOI] [PubMed] [Google Scholar]
- 53.Larsen DW, Halbach VV, Teitelbaum GP, et al. Spinal dural arteriovenous fistulas supplied by branches of the internal iliac arteries. Surg Neurol. 1995;43(1):35-40; discussion 40-41. [DOI] [PubMed] [Google Scholar]
- 54.Sato K, Terbrugge KG, Krings T. Asymptomatic spinal dural arteriovenous fistulas: pathomechanical considerations. J Neurosurg Spine. 2012;16(5):441-446. [DOI] [PubMed] [Google Scholar]
- 55.Rangel-Castilla L, Russin JJ, Zaidi HA, et al. Contemporary management of spinal AVFs and AVMs: lessons learned from 110 cases. Neurosurg Focus. 2014;37(3):E14. [DOI] [PubMed] [Google Scholar]
- 56.Oh JK, Shin HC, Kim TY, et al. Intraoperative indocyanine green video-angiography: spinal dural arteriovenous fistula. Spine (Phila Pa 1976). 2011;36(24):E1578-E1580. [DOI] [PubMed] [Google Scholar]
- 57.Schuette AJ, Cawley CM, Barrow DL. Indocyanine green videoangiography in the management of dural arteriovenous fistulae. Neurosurgery. 2010;67(3):658-662; discussion 662. [DOI] [PubMed] [Google Scholar]
- 58.Cenzato M, Versari P, Righi C, Simionato F, Casali C, Giovanelli M. Spinal dural arteriovenous fistulae: analysis of outcome in relation to pretreatment indicators. Neurosurgery. 2004;55(4):815-822; discussion 822-823. [DOI] [PubMed] [Google Scholar]
- 59.Willinsky RA, terBrugge K, Montanera W, Mikulis D, Wallace MC. Posttreatment MR findings in spinal dural arteriovenous malformations. AJNR Am J Neuroradiol. 1995;16(10):2063-2071. [PMC free article] [PubMed] [Google Scholar]
- 60.Dormont D, Gelbert F, Assouline E, et al. MR imaging of spinal cord arteriovenous malformations at 0.5 T: study of 34 cases. AJNR Am J Neuroradiol. 1988;9(5):833-838. [PMC free article] [PubMed] [Google Scholar]
- eReferences are listed at; links.lww.com/WNL/C783.