Abstract
Background and Objectives
Medulloblastomas are embryonal tumors predominantly affecting children. Recognition of molecularly defined subgroups has advanced management. Factors influencing the management and prognosis of adult patients with medulloblastoma remains poorly understood.
Methods
We examined the management, prognostic factors, and, when possible, molecular subgroup differences (subset) in adult patients (aged 18 years or older) with medulloblastoma from our center (specialty Neuro-Oncology clinic within a large academic practice) diagnosed between 1992 and 2020. Molecular subtyping corresponding to the 2021 WHO Classification was performed. Kaplan-Meier estimates (with log-rank test) were performed for univariate survival analysis with Cox regression used for multivariate analyses.
Results
We included 76 adult patients with medulloblastoma (62% male), with a median age of 32 years at diagnosis (range: 18–66) and median follow-up of 7.7 years (range: 0.6–27). A subset of 58 patients had molecular subgroup characterization—37 SHH-activated, 12 non-WNT/non-SHH, and 9 WNT-activated. Approximately 67% underwent gross total resection, 75% received chemotherapy at diagnosis, and 97% received craniospinal irradiation with boost. The median overall survival (OS) for the whole cohort was 14.8 years. The 2-, 5-, and 10-year OS rates were 93% (95% CI 88–99), 86% (78–94), and 64% (53–78), respectively. Survival was longer for younger patients (aged 30 years or older: 9.9 years; younger than 30 years: estimated >15.4 years; log-rank p < 0.001). There was no survival difference by molecular subgroup or extent of resection. Only age at diagnosis remained significant in multivariate survival analyses.
Discussion
We report one of the largest retrospective cohorts in adult patients with medulloblastoma with molecular subtyping. Survival and molecular subgroup frequencies were similar to prior reports. Survival was better for adult patients younger than 30 years at diagnosis and was not significantly different by molecular subgroup or management characteristics (extent of resection, RT characteristics, or chemotherapy timing or regimen).
Introduction
Medulloblastoma is a primary malignant, high-grade embryonal CNS tumor.1,2 Incidence is highly variable based on age, occurring at a rate of 0.53–0.58/100,000 in those aged 0–9 years and 0.02/100,000 in those older than 40 years.3,4 Given the rarity of this diagnosis in adult patients, there is limited evidence regarding the optimal management strategy and long-term clinical outcomes in patients diagnosed in adulthood. The clinical management strategy in adult patients is frequently extrapolated from the pediatric experience. However, clinical features and prognosis seem variable between adult and pediatric patients,1,5 which may support management differences depending on age. Prior prospective and retrospective reports in adults have used various techniques such as radiation therapy (RT) type/dose, chemotherapy, or a combination of therapy after surgical resection.6-10
Recently, the molecular characterization of medulloblastoma elucidated 4 distinct molecular subgroups—sonic-hedgehog (SHH or SHH-activated), wingless (WNT or WNT-activated), and non-WNT/non-SHH (group 3 and group 4).1,11 SHH-activated is further categorized based on TP53 status as SHH-TP53 wild-type (wt) or SHH-TP53 mutant (mut), with a worse prognosis in the latter.12 Medulloblastoma molecular subtyping has been further subclassified into 4 subgroups of SHH-activated and 8 subgroups of non-WNT/non-SHH in the updated 2021 WHO Classification of Tumors of the CNS.13 Despite improved understanding of these molecular drivers, there is a paucity of subgroup-specific management and long-term outcomes in adults.
To aid in our clinical approach to adult patients diagnosed with medulloblastoma, we examined management, prognostic factors, and molecular subgroup differences (subset) in adult patients with medulloblastoma from our center (specialty neuro-oncology clinic within a large academic practice) diagnosed between 1992 and 2020.
Methods
Demographics, Diagnosis, and Management
After approval from the Mayo Clinic Rochester Institutional Review Board, patients were identified through search of our electronic medical record database search tool for the diagnosis of “medulloblastoma” and/or through an established Radiation Oncology dataset of patients with medulloblastoma. All included patients had a pathologically confirmed diagnosis of medulloblastoma at our institution. All patients were 18 years of age or older during initial diagnosis. Patients without comprehensive clinical records and/or follow-up were excluded from analyses. Included patients were diagnosed from January 1992 to October 2020. The last review date for follow-up was May 2022.
Information collected included demographics, medical history, details of initial presentation, diagnostic information including pathology (histopathologic diagnosis, immunohistochemistry, cytogenetics, etc), modified Chang stage (M0-M4), management (extent of surgical resection, RT details, chemotherapy type and frequency), autologous stem cell transplant (ASCT) status, and survival outcomes. Molecular subgroup classification was determined by immunohistochemistry in 30 patients during diagnosis and in 28 patients retrospectively for this analysis.
Statistical Analyses
We performed descriptive statistics of the whole sample and assessed differences by molecular subgroup, age category, gender, and ASCT status using t tests or analysis of variance (ANOVA) for continuous variables and X2 analyses for categorical variables. Disseminated disease (M+) at diagnosis was defined by cytologic confirmation on CSF sample and/or imaging findings consistent with disseminated disease (i.e., leptomeningeal contrast enhancement in the neuroaxis).
Overall survival (OS) was calculated as time from initial diagnosis to death. Progression-free survival (PFS) was calculated as time from initial diagnosis to first clinically determined progression (i.e., new symptoms) with radiographic evidence supporting disease progression and, in most cases, prompting new treatment recommendations or supportive care or death. We calculated OS and PFS using the Kaplan-Meier method for univariate survival analyses with log-rank used to assess for group differences. Cox regression analysis was used for multivariate survival analyses adjusted for age at diagnosis and gender.
We performed survival analyses to assess for differences in survival by age at diagnosis (younger than 30 years, 30 years or older), molecular subgroup (SHH, TP53-wt; WNT; non-WNT/non-SHH), ASCT (yes or no) and ASCT timing (initial diagnosis or recurrence), presence of disseminated disease at diagnosis (yes or no), extent of initial surgery (biopsy/subtotal resection [STR] or gross total resection [GTR]), decade of diagnosis (1990s, 2000s, 2010s, or 2020s), time of diagnosis before or after routine molecular subgroup analysis (before or after 2012), time of subgroup characterization (at diagnosis or retrospective), location (midline or lateral/hemispheric), initial chemotherapy type (platinum-containing regimen, vincristine-containing regimen, a or combination of cisplatin, etoposide, and cyclophosphamide [CisP+E+Cyclo]), neoadjuvant vs adjuvant therapy at initial diagnosis, boost type (posterior fossa [PF] or tumor bed [TB]), craniospinal irradiation (CSI) dose (less or greater than/equal to the median, 36 Gy), total radiation dose (CSI + boost, lesser or greater than the median, 54.8 Gy), or boost dose (less of greater than the median, 19.8 Gy).
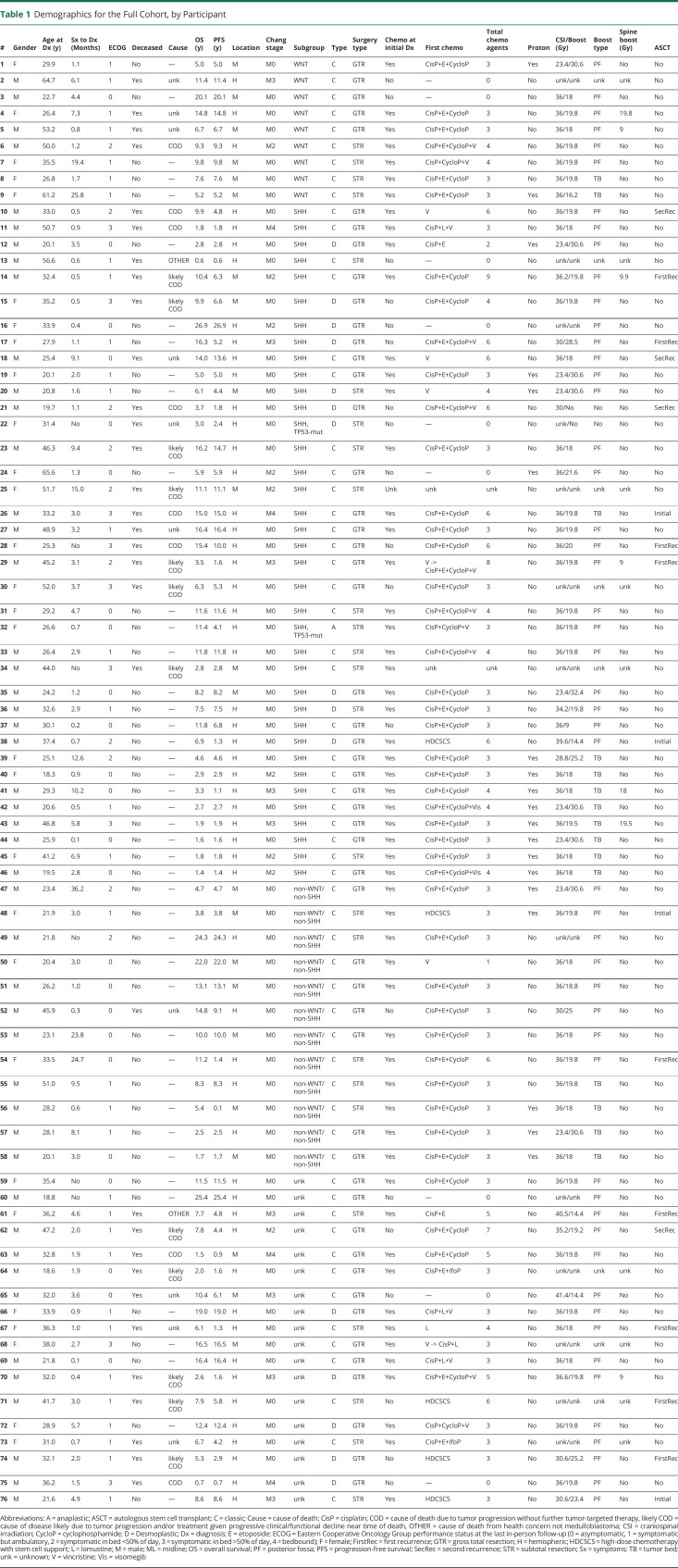
Thirty years was selected as the cutoff for “younger” vs “older” adults in our cohort providing near-equal group distribution (younger than 30 years, n = 35, 30 years or older, n = 41). We assessed outcomes for age continuously in Cox regression analyses. GTR was defined as <1.5 cm2 residual tumor during routine clinical cares based on assessment of postoperative imaging. All analyses were performed on BlueSky Statistics (Commercial Server Edition, version 7.40). Note that statistical analyses were not corrected for multiple comparisons and that any results with p > 0.01 (i.e., log-rank, X2, etc.) should be interpreted with caution. Individual patient-level data are included in Table 1. Anonymized data not published within this article will be made available on request.
Table 1.
Demographics for the Full Cohort, by Participant
Results
Patient Characteristics
A total of 76 adult patients were identified with histopathologic diagnosis of medulloblastoma and sufficient clinical characterization and follow-up for study inclusion. All cases were confirmed by neuropathologists at Mayo Clinic Rochester (Minnesota). Decade of diagnosis included the 1990s (22%), 2000s (32%), 2010s (40%), and 2020s (6%); with 36% (n = 27) being diagnosed after 2012, when molecular subgroup analyses became more integrated into clinical practice. The median age at diagnosis was 32 years (range: 18–66 years). We dichotomized by age into groups of those younger than 30 years and 30 years or older, with 35 patients younger than 30 years (46%) and 41 patients 30 years or older (54%). The median follow-up time was 7.7 years (range: 0.6–27). The sample was predominantly male individuals (male: n = 47, female: n = 29). The median time from initial symptoms to diagnosis was 2.4 months (range: 0.1–36), with the most common initial symptoms being headache (66%) and gait imbalance (32%). In total, 51% of patients had disease recurrence, and 42% had died at last follow-up. See Table 1 for demographic and management details.
Tumor Characteristics
Most tumors (71%) were hemispheric (left 59%, right 41%) rather than midline (29%) at presentation. Histologically, most were classic (79%), followed by desmoplastic or nodular (20%) and anaplastic (1%). During initial histopathologic diagnosis, disseminated disease (M+) was present in 29% of patients.
Molecular Subgroups
Of all 76 patients, 58 had molecular subgroup characterization performed at initial diagnosis (n = 30) or retrospectively (n = 28). The most common subgroup was SHH-activated (n = 37, 64%), followed by non-WNT/non-SHH (n = 12, 21%) and WNT-activated (n = 9, 15%). Of the 34 patients with TP53 immunohistochemistry performed, only 2 (6%) had a TP53 mutation (TP53-mut)—both of which were within in the SHH-activated subgroup, and histologically, one was anaplastic and the other desmoplastic.
Molecular Subtypes
Twenty-three patients had testing for copy number alterations—specifically assessing for monosomy 6, MYCN or MYC gene amplification. One patient (#1, aged 30 years at diagnosis) within the WNT-activated subgroup had monosomy 6 and thus WNTα subtype—generally seen in younger patients than with WNTβ subtype and comprises much of the WNT-activated subgroup (approximately 70%). One patient (#56, aged 28 years at diagnosis) within the non-WNT/non-SHH subgroup was found to have MYCN gene amplification and thus classified as group 4α subtype.14
Management
At initial diagnosis, 51 patients (67%) had GTR with the remainder having STR. All patients underwent RT as part of treatment at initial diagnosis, and 25.0% (n = 19) received proton beam radiotherapy. Of the 68 patients with RT treatment details, all but 2 (3%) underwent CSI with a boost either to the posterior fossa (PF, 75%) or to the tumor bed (TB, 22%). Spine boosts were performed in 10% of patients. The median dose of administered CSI was 36 Gy (range: 23.4–41.4, 84% with CSI dose >29 Gy), median PF/TB boost was 19.8 Gy (range: 9–32.4), median RT total dose (CSI + boost) was 54.8 Gy (range: 30–58.5, 97% with RT dose >50 Gy), and spine boost was 9.9 Gy (range: 9–19.8). Boost type was different by decade of diagnosis (X2 (6) = 32.8, p < 0.0001), with TB boost being used more frequently starting in the 2010s (TB use by decade—1990s: 0%, 2000s: 5%, 2010s: 32%, 2020s: 100%). There was a decrease in hematologic laboratory values (hemoglobin, platelets, and leukocytes) after RT (eTable, links.lww.com/WNL/D15). Transfusion was not necessary secondary to RT toxicity. Proton RT tended to lead to a lower drop in platelets than photon RT (proton: −36 ×109/L, photon: −87 ×109/L; t = −1.79(33), p = 0.08), although interpretation is limited by incomplete laboratory data.
Most of the patients (75%) received chemotherapy at initial diagnosis, with 88% receiving chemotherapy at some point in their care. Patients not receiving chemotherapy (n = 9) tended to be older at diagnosis (no chemotherapy: mean age 40 years, chemotherapy: mean age 32 years; t = 1.97(73), p = 0.05) and tended to be more remotely diagnosed (% of diagnoses by decade—no chemotherapy: 1990s: 56%, 2000s: 22%, 2010s: 22%, 2020s: 0%); however, other features were similar between groups. The most common administration timing was adjuvant after radiotherapy (70%), followed by neoadjuvant (15%) and first chemotherapy at recurrence (15%). Platinum-containing regimens were the most common initial choice (n = 60, 92%) with the combination of cisplatin, etoposide, and cyclophosphamide (CisP+E+Cyclo) used as the first regimen in 68% of patients (n = 44). Vincristine was used in 24% of patients (n = 18), alone concurrent with RT (n = 6, 9%) or vincristine in combination with platinum-based treatment after RT (n = 12, 18%). In total, 16 different chemotherapeutic agents were used at some point in the care of patients in our cohort, with a median of 3 agents.
Survival
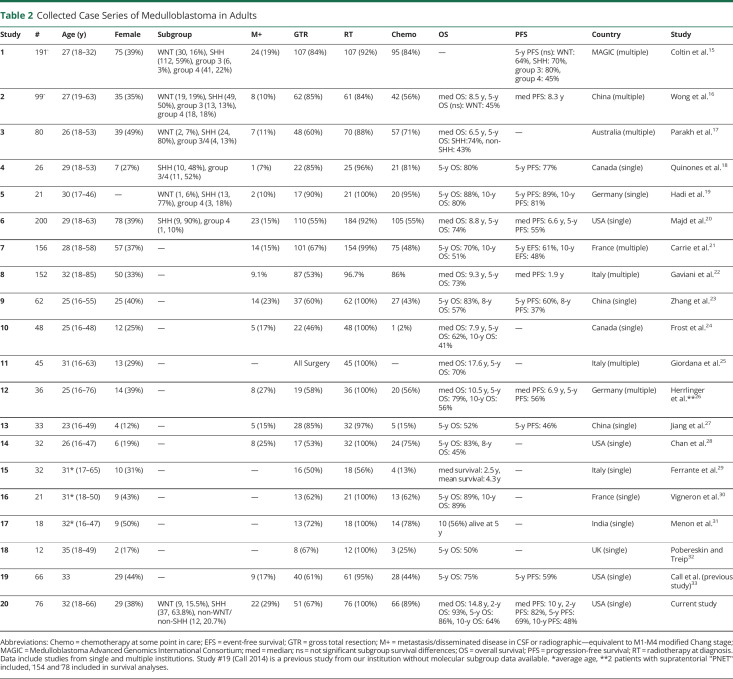
The median OS (mOS) for the whole sample was 14.8 years; with 2-, 5-, and 10-year OS rates of 93% (95% CI 88–99), 86% (78%–94%), and 64% (53%–78%), respectively. The median PFS (mPFS) for the whole sample was 10 years; with 2-, 5-, and 10-year PFS rates of 82% (75%–92%), 69% (59%–81%), and 48% (36%–63%), respectively. We have included a compilation of published case series of adults with medulloblastoma in Table 2 for comparison with our cohort.
Table 2.
Collected Case Series of Medulloblastoma in Adults
In univariate survival analyses, survival was better for younger patients ([mOS: 30 years or older: 9.9 years, younger than 30 years: estimated >15.4 years—median not met; log-rank p < 0.001], [mPFS: 30 years or older: 6.3 years, younger than 30 years: 14.8 years; log-rank p < 0.005]). Survival was worse in those with disseminated disease (M+) at diagnosis (mOS: yes: 10.4 years, no: 15.4 years; log-rank p = 0.02). Male patients had a trend toward worse mOS than female patients (male: 11.4 years, female: 15.4 years; log-rank p = 0.07). There was also a trend for those receiving chemotherapy at initial diagnosis having better mPFS (yes: 14.7 years, no: 6.1 years; log-rank p = 0.07).
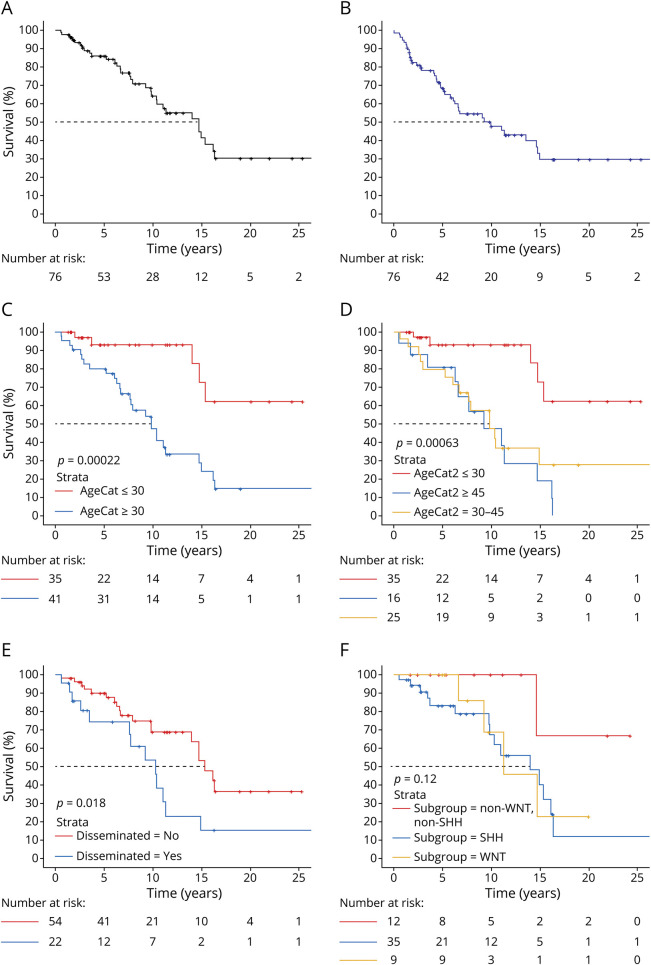
There were no survival (OS or PFS) differences by location of primary tumor (hemispheric (left, right) or midline), histologic type (classic or desmoplastic), type of initial surgery (STR or GTR), decade of diagnosis (1990s, 2000s, 2010s, or 2020s), diagnosis year (before or after 2012), initial chemotherapy type (platinum-containing regimen, vincristine-containing regimen, or a combination of CisP+E+Cyclo), neoadjuvant vs adjuvant therapy at initial diagnosis, boost type (PF or TB), CSI dose (less or greater than the median, 36 Gy), total radiation dose (CSI + boost, lesser or greater than the median, 54.8 Gy), or boost dose (less of greater than the median, 19.8 Gy) or time of subgroup characterization (at diagnosis or retrospective). See Figure for Kaplan-Meier plots for the whole sample and by age at diagnosis, molecular subgroup, and extent of disease.
Figure. Kaplan-Meier Plots.
(A) mOS for the full cohort was 14.8 y with 2-, 5-, and 10-y OS rates of 93%, 86%, and 64%, respectively, (B) mPFS for the full cohort was 10 y with 2-, 5-, and 10-y PFS rates of 82%, 69%, and 48%, respectively, (C) mOS (30 y or older: 9.9 y, younger than 30 y: estimated >15.4 y—median not met; log-rank p < 0.001) and (D) (older than 45 y, 9.3 y, aged 30–45 y, 9.9 y, younger than 30 y: estimated >15.4 y—median not met; log-rank p < 0.001) by age category showing that younger adults had better longevity, (E) patients with disseminated disease (M+) at diagnosis had worse survival (mOS: yes: 10.4 y, no: 15.4 y; log-rank p = 0.02), and (F) there were no survival differences by molecular subgroup (mOS: SHH, TP53-wt: 14 y, WNT-activated: 11.4 y, non-WNT/non-SHH: estimated >14.8 y—median not met; log-rank p = 0.12). PFS = progression-free survival.
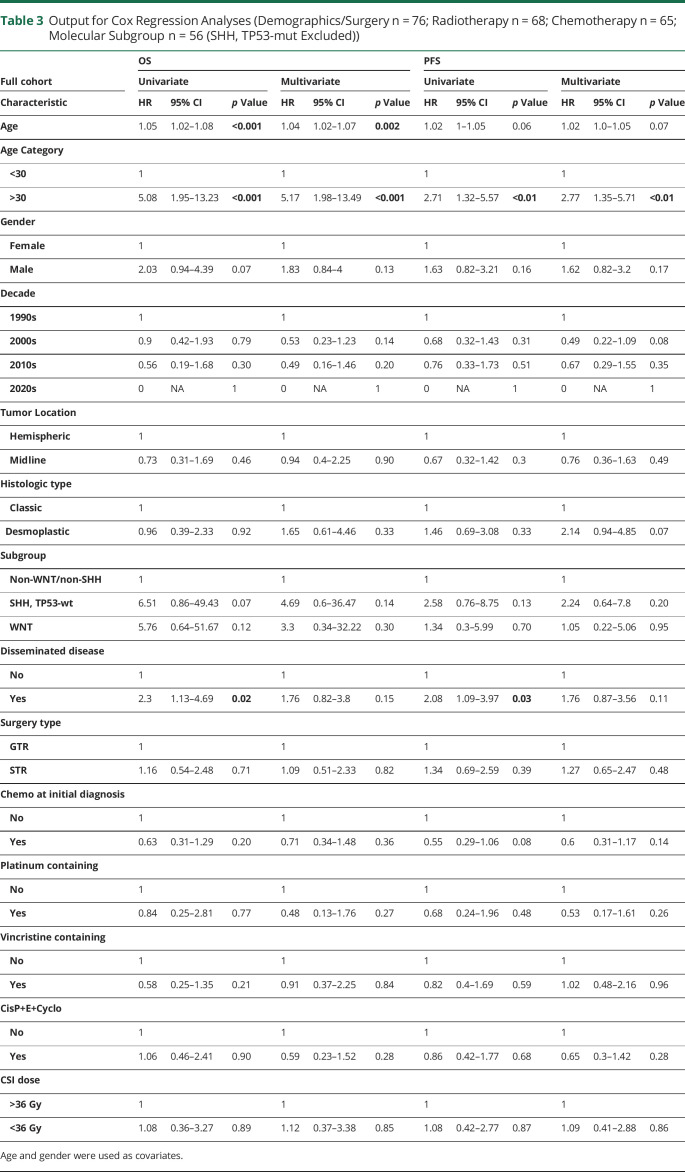
In multivariate Cox regression analyses including the whole cohort controlling for age and gender (Table 3), only age at diagnosis continued to be significantly related to prognosis (OS and PFS). We found 32 patients were dead at the last follow-up, with cause of death primarily directly related to tumor progression without further disease-targeted therapy (n = 8) or cause of death likely due to tumor progression and/or treatment, given progressive clinical/functional decline near time of death (n = 20). Of the deceased patients, 21 (66%) had radiographic and/or clinical progression before death. This compares with only 18.2% (n = 8) of the 44 patients who were alive at the last follow-up (X2 (1, n = 76) = 17.7, p < 0.0001). Patients with clear radiographic or clinical progression were likely to have worse survival (mOS for those with disease progression was 9.9 months vs estimate >15 years (median not reached) for those without progressive disease at the last follow-up, log-rank p < 0.001).
Table 3.
Output for Cox Regression Analyses (Demographics/Surgery n = 76; Radiotherapy n = 68; Chemotherapy n = 65; Molecular Subgroup n = 56 (SHH, TP53-mut Excluded))
Molecular Subgroup
We divided the SHH-activated subgroup by TP53 status because the TP53-mut is related to worse survival relative to TP53-wt12—2 patients with SHH, TP53-mut were excluded to ensure a more homogenous SHH-activated group for interpretation. The mean age at diagnosis by subgroup was 34 years for SHH-activated, TP53-wt, 41 years for WNT-activated, and 29 years for non-WNT/non-SHH (F(2,53) = 2.53, p = 0.09). There was no difference in gender between the subgroups (% female: SHH, TP53-wt: 34%, WNT-activated: 56%, non-WNT/non-SHH: 25%; X2 (2) = 2.17, p = 0.3). Tumor location was different by subgroup with SHH, TP53-wt more commonly being hemispheric at 83%, while WNT-activated and non-WNT/non-SHH were more commonly midline at 67% and 58%, respectively (X2 (2) = 11.9, p < 0.005). There was less disseminated disease at diagnosis within the non-WNT/non-SHH subgroup (0%), relative to the SHH, TP53-wt (37%) and WNT-activated (22%) subgroups (X2 (2) = 6.40, p = 0.04). There were no subgroup differences in the extent of surgical resection, chemotherapy, or RT characteristics.
There was no significant survival difference by molecular subgroup in univariate analysis ([mOS: SHH, TP53-wt: 14 years, WNT-activated: 11.4 years, non-WNT/non-SHH: estimated >14.8 years—median not met; log-rank p = 0.12; Figure] [mPFS: SHH, TP53-wt: 6.8 years, WNT-activated: 11.4 years, non-WNT/non-SHH: estimated >9.1 years—median not met; log-rank p = 0.18]). OS at the last follow-up for the 2 patients with SHH, TP53-mut was 3 years (deceased) and 11.4 years (censored), respectively. There were no survival differences by molecular subgroup when incorporating all patients with subgroup data ([mOS: SHH-activated: 14 years, WNT-activated: 11.4 years, non-WNT/non-SHH: estimated >14.8 years—median not met; log-rank, p = 0.11] [mPFS: SHH-activated: 6.8 years, WNT-activated: 11.4 years, non-WNT/non-SHH: estimated >9.1 years—median not met; log-rank, p = 0.12]). There were no survival (OS or PFS) differences by molecular subgroup in multivariate analyses, although the small size of subgroups limited the ability of this study to detect intergroup differences (Table 3).
Age
Given the apparent impact of age in our cohort, we further assessed for differences in patients younger than 30 years or 30 years or older during diagnosis. Older patients were more likely to have disseminated disease at diagnosis relative to younger patients (42% vs 14%, X2 (1) = 6.78, p < 0.01; mean age with disseminated disease 39 years, mean age without disseminated disease, 31 years, F(1,74) = 6.32, p = 0.01). There was no difference in surgery type, number having received chemotherapy, or chemotherapy characteristics (initial type or timing) by age. However, younger patients were more likely to receive chemotherapy during initial diagnosis (86% vs 65%, X2 (1) = 4.23, p = 0.04) and more often received proton therapy (43% vs 10%, X2 (1) = 11.03, p < 0.001).
Multivariate survival analyses controlling for disseminated disease, chemotherapy at initial diagnosis, proton therapy, and year of diagnosis continued to demonstrate worse survival in adults aged 30 years or older (OS: HR 4.07, 95% CI 1.5–11, p < 0.01; PFS: HR 2.32, 95% CI 1.1–5.1, p < 0.05).
Gender
There were no differences in patient, tumor, or treatment characteristics by gender. In survival analyses, we found female patients had worse PFS with STR relative to GTR (HR = 4.04, 95% CI 1.1–14.5, p = 0.03), which was not found in either male patients or the full cohort. Male patients were more likely to have worse prognosis with desmoplastic vs classic histology (PFS: HR = 3.42, 95% CI 1.2–9.6, p = 0.02) and disseminated disease at diagnosis relative to nondisseminated disease (OS: HR = 2.64, 95% CI 1–6.7, p = 0.04).
ASCT
Seventeen patients (22%) underwent ASCT therapy as part of their treatment course, preceded by a high-dose chemotherapy regimen (generally including a combination of thiotepa, carboplatin, ifosfamide, and carmustine). Timing of ASCT was more common at recurrence (first: 53%, second: 24%) than at initial diagnosis (24%). Patients receiving ASCT were younger at diagnosis (yes: 26 years, no: 36 years, t = 2.95(74), p < 0.005). Molecular subgroup data were comparable with those of the full cohort, with SHH-activated (62%), followed by non-WNT/non-SHH (23%) and WNT-activated (15%). Presence of disseminated disease at diagnosis was not more common in patients receiving ASCT (yes ASCT: 18%, no ASCT: 32%, X2 (1) = 1.36, p = 0.2). In univariate and multivariate (including age, gender, +/- disseminated disease at diagnosis) analyses, there were no survival differences by ASCT status or time of ASCT administration (initial diagnosis or recurrence).
Discussion
This is one of the largest series in adult patients with medulloblastoma (Table 2), where we report several key findings that support and expand on the current knowledge in this field. Taken together, our results show that age may be an important prognostic consideration in adults with medulloblastoma, where patients younger than 30 years at diagnosis had better prognosis. This finding has not been previously well recognized within the adult patient population. In addition, our data support previous studies with molecular subgroup data, showing that SHH-activated is the largest molecular driver (64%) and that there are no apparent survival differences by subgroup in our cohort, contrary to the childhood population.
The hemispheric preponderance of medulloblastoma is well reported in adults and is likely influenced by the molecular subgroup because SHH-activated tumors are more commonly hemispheric and have a higher incidence in adults.1,34 This contrasts what is seen in childhood medulloblastoma, where fourth ventricular non-WNT/non-SHH and WNT-activated tumors predominate.1
We found a median OS of approximately 15 years and median PFS of approximately 10 years. Survival in our cohort is similar, if not superior to most reports. The median mOS of series was 8.8 years (our cohort was 14.8 years) with only 1 other report describing mOS >10 years; and a median 5-year overall survival of 75% (our cohort was 86%).7,21,33,35-37 A population-based study using SEER data from 1992 to 2013 showed similar estimates of 2-, 5-, and 10-year overall survival rates for adult medulloblastoma of 85%, 74%, and 67%, respectively.38 Although medulloblastoma is an uncommon diagnosis in adults, it is apparent that the prognosis is better than many other primary CNS malignancies.4 Adults generally have similar survival as seen in children,1,5,39 which may be affected by patient and tumor characteristics.
We found that patients younger than 30 years had better survival than adults aged 30 years or older at diagnosis, with a median OS difference of over 5 years. Worse prognosis with older age has been infrequently reported in adult cohorts, with only 1 known report showing worse prognosis in adults older than 37 years.40 We initially divided patients into 3 age groups: younger than 30 years, 30–45 years, older than 45 years. The OS for the 30–45 years group was no different than that for older than 45 years group, so we merged the 2 for further analyses. Possible contributing factors in our cohort include that older adults were more likely to have disseminated disease at diagnosis, while younger patients were more likely to receive chemotherapy during initial diagnosis. However, older age remained related to worse survival even when controlling for these factors in multivariate survival analyses.
Unlike in the pediatric population, we found no apparent survival benefit based on extent of surgery nor with minor variability in RT or significant variability in chemotherapy treatment characteristics for the whole cohort. Last, there were no survival differences by time of subgroup characterization (during diagnosis or retrospective) or year of diagnosis, suggesting that any value from having subgroup information upfront was not related to survival benefit and that any global management improvements have either not been realized due to length of follow-up in censored patients (proton RT, RT to the tumor bed rather than PF) or have not made a significant impact on prognosis. Future analyses of this cohort may prove helpful in this assessment.
There were no survival differences by molecular subgroup, which is in keeping with prior adult cohorts with sufficient molecular subgroup characterization.15,16,41 In addition, we did not find the survival advantage seen in children with WNT-activated tumors, supporting previous reports in adults.15,41 In our cohort, this may partly be due to a trend toward WNT-activated patients being older, which was associated with worse prognosis (in children), potentially mitigating any survival benefit in these tumors. However, it is more likely that WNT-activated tumors are distinct between pediatric and adult patients supported by replication of findings in several adult cohorts. Further molecular classification in medulloblastoma now recognizes several subtypes within the broader molecular subgroups, as highlighted in the 2021 WHO Classification of Tumors of the CNS.13
Most of our patients underwent GTR at initial diagnosis (67%), with the remainder receiving STR, largely due to tumor location. We found that prognosis was not affected by EOR in our cohort. However, STR was a poor prognostic feature of PFS in female patients. We would not expect any gender-specific differences due to EOR. Thus, this may be more indicative of the male patients in our cohort where there was a trend of worse prognosis. Notably, recent studies assessing the EOR in medulloblastoma support a maximal safe surgical resection in both the pediatric and adult populations, with an approach favoring acceptance of small residual areas of tumor if the likelihood of neurologic morbidity is high. This is backed by a large retrospective international study (n = 787) in children showing no clear survival benefit depending on the extent of resection when controlling for molecular subgroup,42 helping to clarify survival benefit in prior studies with conflicting findings in the premolecular era.
RT remains a mainstay of medulloblastoma therapy,1,2 and all patients in our cohort underwent RT at initial diagnosis, with most of them receiving CSI and boost (97%). Two signs of advancements in RT during our study interval include focus on boost to the TB rather than PF and use of proton therapy, both of which have become more common in our institutional experience since the mid-2010s. We suspect that the benefit of these management practices could not be adequately assessed given relatively minimal duration from time of diagnosis. Future studies will provide insight into possible survival impact with less long-term morbidity, including the Alliance AMBUSH Trial43 and PersoMed-I trial (NCT04402073). A large phase 3 trial in children (ACNS0331; NCT00085735) showed that tumor bed (involved field) was noninferior (event-free survival) to PF boost RT, supporting the use of a more targeted boost that may limit therapy-related comorbidities.44
Chemotherapy is part of standard care in medulloblastoma after surgery and RT, commonly with the use of multiagent regimens.1 In our cohort, most patients received adjuvant chemotherapy (70%). We focused on initial chemotherapy plan, although a total of 16 chemotherapeutic agents were administered at any point in patient care. Platinum-containing therapy was by far most common (92%), with the CisP+E+Cyclo being frequently used (68%), followed by vincristine-containing regimen (24%). This is in line with the most widely used chemotherapies in children, from which much of the adult regimens have been derived.1 While uncertainties remain, the available evidence from clinical trials and large retrospective cohorts in adults with medulloblastoma supports RT with adjuvant chemotherapy (after maximal safe resection), with indeterminate benefit of neoadjuvant therapy. Many guiding studies were published before widespread molecular subgroup classification in clinical practice, which will be important to incorporate into future clinical trials.10,11,20,45,46
The main strength of our study is a large and well-characterized sample, given the rarity of this diagnosis. We provide patient-specific demographic, management, and outcome data with known molecular subgroup classification in a majority of the cohort. A chief limitation is the retrospective nature of our study. While the strongest evidence regarding management and prognosis is from prospective trials, medulloblastoma remains a rare diagnosis in adults, which serves as a prominent barrier in performing these studies. Moreover, because this is a single-institutional experience, management results should be interpreted with caution because this may be more representative of treatment practices at our institution. An additional limitation is incomplete management data for a small fraction of patients. We worked to include only patients with an extended follow-up, but because some patients were provided additional care outside of our institution, there were a few gaps in management. It is important to note that exclusion of patients without extended follow-up may bias our sample for overestimation of survival in that only patients healthy enough to continue with treatment and surveillance were observed. However, every effort was made to eliminate bias in patient selection. Most of the initially identified pathology-confirmed patients that were not included in analyses were only pathology reviewed (i.e., external pathology consultation), and thus, clinical information was not available for this article. Last, we would ideally have subgroup data for all patients in our cohort; however, because our cohort included patients diagnosed in the 1990s, this was not yet the standard practice.
We report one of the largest retrospective cohorts in adult patients with medulloblastoma with molecular subtyping. Survival and molecular subgroup frequencies were similar to previous reports. Survival was longer for patients younger than 30 years and was not significantly different by molecular subgroup or management characteristics (extent of resection, RT characteristics, or chemotherapy timing or regimen).
Acknowledgment
The authors thank all their patients for helping them to understand the management and outcomes of medulloblastoma.
Glossary
- ASCT
autologous stem cell transplant
- CSI
craniospinal irradiation
- GTR
gross total resection
- OS
overall survival
- PF
posterior fossa
- PFS
progression-free survival
- RT
radiation therapy
- STR
subtotal resection
- TB
tumor bed
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Northcott PA, Robinson GW, Kratz CP, et al. . Medulloblastoma. Nat Rev Dis Primers. 2019;5(1):11. doi: 10.1038/s41572-019-0063-6 [DOI] [PubMed] [Google Scholar]
- 2.Majd N, Penas-Prado M. Updates on management of adult medulloblastoma. Curr Treat Options Oncol. 2019;20(8):64. doi: 10.1007/s11864-019-0663-0 [DOI] [PubMed] [Google Scholar]
- 3.Orphanet. Medulloblastoma [online]. Accessed July 25, 2022. orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=616.
- 4.Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021;23(12 suppl 2):iii1-iii105. doi: 10.1093/neuonc/noab200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greuter L, Guzman R, Soleman J. Typical pediatric brain tumors occurring in adults-differences in management and outcome. Biomedicines. 2021;9(4):356. doi: 10.3390/biomedicines9040356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moots PL, O'Neill A, Londer H, et al. . Preradiation chemotherapy for adult high-risk medulloblastoma: a trial of the ECOG-ACRIN Cancer Research Group (E4397). Am J Clin Oncol. 2018;41(6):588-594. doi: 10.1097/coc.0000000000000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De B, Beal K, De Braganca KC, et al. . Long-term outcomes of adult medulloblastoma patients treated with radiotherapy. J Neurooncol. 2018;136(1):95-104. doi: 10.1007/s11060-017-2627-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110(9):2035-2041. doi: 10.1002/cncr.23003 [DOI] [PubMed] [Google Scholar]
- 9.Brandes AA, Paris MK, Basso U. Medulloblastomas: do molecular and biologic markers indicate different prognoses and treatments? Expert Rev Anticancer Ther. 2003;3(5):615-620. doi: 10.1586/14737140.3.5.615 [DOI] [PubMed] [Google Scholar]
- 10.Beier D, Proescholdt M, Reinert C, et al. . Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro Oncol. 2018;20(3):400-410. doi: 10.1093/neuonc/nox155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juraschka K, Taylor MD. Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr. 2019;24(4):353-363. doi: 10.3171/2019.5.peds18381 [DOI] [PubMed] [Google Scholar]
- 12.Ramaswamy V, Remke M, Bouffet E, et al. . Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821-831. doi: 10.1007/s00401-016-1569-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis DN, Perry A, Wesseling P, et al. . The 2021 WHO classification of tumors of the central nervous system: a summary. Neurooncol. 2021;23(8):1231-1251. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalli FMG, Remke M, Rampasek L, et al. . Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31(6):737-754.e6. doi: 10.1016/j.ccell.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coltin H, Sundaresan L, Smith KS, et al. . Subgroup and subtype-specific outcomes in adult medulloblastoma. Acta Neuropathol. 2021;142(5):859-871. doi: 10.1007/s00401-021-02358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong GC-H, Li KK-W, Wang W-W, et al. . Clinical and mutational profiles of adult medulloblastoma groups. Acta Neuropathologica Commun. 2020;8(1):191. doi: 10.1186/s40478-020-01066-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parakh S, Davies A, Westcott K, et al. . Adult medulloblastoma in an Australian population. J Clin Neurosci. 2022;102:65-70. doi: 10.1016/j.jocn.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 18.Quinones MC, Bélanger K, Lemieux Blanchard É, et al. . Adult medulloblastoma demographic, tumor and treatment impact since 2006: a Canadian University Experience. Curr Oncol. 2021;28(4):3104-3114. doi: 10.3390/curroncol28040271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadi I, Roengvoraphoj O, Niyazi M, et al. . Medulloblastoma in adults: a retrospective single institution analysis. Strahlenther Onkol 2018;194(3):225-234. doi: 10.1007/s00066-017-1235-5 [DOI] [PubMed] [Google Scholar]
- 20.Majd NK, Mastall M, Lin H, et al. . Clinical characterization of adult medulloblastoma and the effect of first-line therapies on outcome; the MD Anderson Cancer Center experience. Neurooncol Adv. 2021;3(1):vdab079. doi: 10.1093/noajnl/vdab079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrie C, Lasset C, Alapetite C, et al. . Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994;74(8):2352-2360. doi: [DOI] [PubMed] [Google Scholar]
- 22.Gaviani P, Simonetti G, Rudà R, et al. . Medulloblastoma of the adult: results from a multicenter retrospective study by AINO (Italian Association of Neuro-Oncology) and SIN (Italian Society of Neurology). Neurol Sci. 2021;42(2):665-671. doi: 10.1007/s10072-020-04556-6 [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, Ouyang T, Kang H, Long W, Thomas B, Zhu S. Adult medulloblastoma: clinical characters, prognostic factors, outcomes and patterns of relapse. J Neurooncol. 2015;124(2):255-264. doi: 10.1007/s11060-015-1833-y [DOI] [PubMed] [Google Scholar]
- 24.Frost PJ, Laperriere NJ, Wong CS, Milosevic MF, Simpson WJ, Pintilie M. Medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1995;32(4):951-957. doi: 10.1016/0360-3016(94)00612-o [DOI] [PubMed] [Google Scholar]
- 25.Giordana MT, Schiffer P, Lanotte M, Girardi P, Chio A. Epidemiology of adult medulloblastoma. Int J Cancer. 1999;80(5):689-692. doi: [DOI] [PubMed] [Google Scholar]
- 26.Herrlinger U, Steinbrecher A, Rieger J, et al. . Adult medulloblastoma: prognostic factors and response to therapy at diagnosis and at relapse. J Neurol. 2005;252(3):291-299. doi: 10.1007/s00415-005-0560-2 [DOI] [PubMed] [Google Scholar]
- 27.Jiang T, Zhu J, Dong J, et al. . Clinical outcomes of adult medulloblastoma: a retrospective analysis at a single Institute. Translational Neurosci Clin. 2015;1:17-24. doi: 10.18679/cn11-6030/r.2015.004 [DOI] [Google Scholar]
- 28.Chan AW, Tarbell NJ, Black PM, et al. . Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery 2000;47(3):623-631;discussion 631-622. doi: 10.1097/00006123-200009000-00018 [DOI] [PubMed] [Google Scholar]
- 29.Ferrante L, Mastronardi L, Celli P, Acqui M, Cervoni L, Fortuna A. Medulloblastoma in adulthood. J Neurosurg Sci. 1991;35(1):23-30. [PubMed] [Google Scholar]
- 30.Vigneron C, Antoni D, Coca A, et al. . Adult medulloblastoma: retrospective series of 21 patients. Cancer Radiother. 2016;20(1):14-17. doi: 10.1016/j.canrad.2015.07.156 [DOI] [PubMed] [Google Scholar]
- 31.Menon G, Krishnakumar K, Nair S. Adult medulloblastoma: clinical profile and treatment results of 18 patients. J Clin Neurosci. 2008;15(2):122-126. doi: 10.1016/j.jocn.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 32.Pobereskin L, Treip C. Adult medulloblastoma. J Neurol Neurosurg Psychiatry. 1986;49(1):39-42. doi: 10.1136/jnnp.49.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Call JA, Naik M, Rodriguez FJ, et al. . Long-term outcomes and role of chemotherapy in adults with newly diagnosed medulloblastoma. Am J Clin Oncol. 2014;37:1-7. doi: 10.1097/coc.0b013e31826b9cf0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remke M, Hielscher T, Northcott PA, et al. . Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717-2723. doi: 10.1200/jco.2011.34.9373 [DOI] [PubMed] [Google Scholar]
- 35.Ang C, Hauerstock D, Guiot MC, et al. . Characteristics and outcomes of medulloblastoma in adults. Pediatr Blood Cancer. 2008;51(5):603-607. doi: 10.1002/pbc.21588 [DOI] [PubMed] [Google Scholar]
- 36.Atalar B, Ozsahin M, Call J, et al. . Treatment outcome and prognostic factors for adult patients with medulloblastoma: the Rare Cancer Network (RCN) experience. Radiother Oncol. 2018;127(1):96-102. doi: 10.1016/j.radonc.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 37.Balducci M, Chiesa S, Chieffo D, et al. . The role of radiotherapy in adult medulloblastoma: long-term single-institution experience and a review of the literature. J Neurooncol. 2012;106(2):315-323. doi: 10.1007/s11060-011-0665-7 [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Dai Z, Cao Y, Wang L. Comparing children and adults with medulloblastoma: a SEER based analysis. Oncotarget. 2018;9(53):30189-30198. doi: 10.18632/oncotarget.23773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moisander-Joyce H, Sinha A, Fernandez-Ledon S, et al. . Survival in Adult and Pediatric Patients with Medulloblastoma: A 2018 SEER-Based Analysis. American Society of Clinical Oncology; 2020. [Google Scholar]
- 40.Giordana MT, Cavalla P, Chiò A, et al. . Prognostic factors in adult medulloblastoma. A clinico-pathologic study. Tumori. 1995;81(5):338-346. doi: 10.1177/030089169508100507 [DOI] [PubMed] [Google Scholar]
- 41.Goschzik T, Zur Muehlen A, Doerner E, et al. . Medulloblastoma in adults: cytogenetic phenotypes identify prognostic subgroups. J Neuropathol Exp Neurol. 2021;80(5):419-430. doi: 10.1093/jnen/nlab020 [DOI] [PubMed] [Google Scholar]
- 42.Thompson EM, Hielscher T, Bouffet E, et al. . Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484-495. doi: 10.1016/s1470-2045(15)00581-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahajan A, Shih H, Penas-Prado M, et al. . The alliance AMBUSH trial: rationale and design. Cancers (Basel). 2022;14(2):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalski JM, Janss AJ, Vezina LG, et al. . Children's Oncology Group phase III trial of reduced-dose and reduced-volume radiotherapy with chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2021;39(24):2685-2697. doi: 10.1200/jco.20.02730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franceschi E, Minichillo S, Mura A, et al. . Adjuvant chemotherapy in average-risk adult medulloblastoma patients improves survival: a long term study. BMC Cancer. 2020;20(1):755. doi: 10.1186/s12885-020-07237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kann BH, Lester-Coll NH, Park HS, et al. . Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2017;19(2):259-269. doi: 10.1093/neuonc/now150 [DOI] [PMC free article] [PubMed] [Google Scholar]