Abstract
Background and Objectives
Progressive nigrostriatal pathway degeneration occurs in individuals with dementia with Lewy bodies (LB). Our objective was to investigate whether repeat 123[I]-N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane (FP-CIT) single photon emission computed tomography (SPECT) can identify progressive dopaminergic loss in mild cognitive impairment (MCI) with Lewy bodies (MCI-LB).
Methods
Individuals with MCI-LB and MCI due to Alzheimer disease (MCI-AD) underwent comprehensive clinical assessment, 123[I]-FP-CIT SPECT at baseline and annual reviews, and baseline cardiac 123 iodine metaiodobenzylguanidine (I-MIBG). Mixed-effects models were used to investigate changes in 123[I]-FP-CIT specific binding ratio (SBR) in the striatum for each diagnostic group compared with controls. The time interval to the development of a quantitatively abnormal 123[I]-FP-CIT SPECT in the possible and probable MCI-LB groups was determined as the time it took for these groups to reach a striatal uptake 2 SDs below aged-matched controls. Test-retest variation was assessed using baseline and repeat scans in controls.
Results
We recruited 20 individuals with MCI-AD, 11 with possible MCI-LB, 25 with probable MCI-LB, and 29 age-matched controls. The mean time between baseline and the final image was 1.6 years (SD = 0.9, range 1.0–4.3). The annual estimated change in SBR was 0.23 for controls (95% CI −0.07 to 0.53), −0.09 (−0.55 to 0.36) for MCI-AD, −0.50 (−1.03 to 0.04) for possible MCI-LB, and −0.48 (−0.89 to −0.06) for probable MCI-LB. The median annual percentage change in SBR in MCI-LB was −5.6% (95% CI −8.2% to −2.9%) and 2.1% (−3.5% to 8.0%) for MCI-AD. The extrapolated time for a normal scan to become abnormal was 6 years. Controls and MCI-AD showed no significant change in dopaminergic binding over time. The mean test-retest variation in controls was 12% (SD 5.5%), which cautions against overinterpretation of small changes on repeat scanning.
Discussion
Progressive dopaminergic loss in the striatum is detectable using 123[I]-FP-CIT SPECT in MCI-LB at a group level. In clinical practice, individual change in striatal 123[I]-FP-CIT uptake seems to be of limited diagnostic value because of high test-retest variation.
Classification of Evidence
This study provides Class II evidence that longitudinal declines in striatal uptake measured using 123[I]-FP-CIT SPECT are associated with MCI due to Lewy body disease but not MCI due to Alzheimer disease.
Introduction
Dementia with Lewy bodies (DLB) is associated with progressive degeneration of the dopaminergic nigrostriatal pathway1 and is present in approximately 90% cases of DLB at postmortem.2 Striatal dopaminergic deficits can be detected several years ahead of clinical parkinsonism in other prodromal ɑ-synucleinopathies such as idiopathic REM sleep behavior disorder.3,4 It is known that dopaminergic deficits are present in some individuals with mild cognitive impairment with Lewy bodies (MCI-LB),5 but not whether degeneration over time can be detected at this stage.
The functional integrity of the dopaminergic nigrostriatal pathway can be studied in vivo using 123[I]-N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane (FP-CIT) single photon emission computed tomography (SPECT), a radiopharmaceutical that binds to the dopamine transporter. Degeneration of dopaminergic nigrostriatal neurons leads to reduced binding of striatal 123[I]-FP-CIT, and the decreased uptake of 123[I]-FP-CIT correlates with dopamine transporter density.6 123[I]-FP-CIT SPECT is a well-established imaging modality first used in the diagnosis of Parkinson disease (PD) and aids in differentiating between dementia subtypes.7
Serial 123[I]-FP-CIT SPECT imaging is an understudied topic in Lewy body disorders, but has previously demonstrated merit in early DLB to identify disease evolution in vivo. For instance, a 2016 study8 found that 10% of patients with probable DLB had an initial normal 123[I]-FP-CIT SPECT, which all became abnormal on repeat imaging an average of 1.5 years later.
The objective of this study was to determine whether repeated dopamine transporter imaging can detect longitudinal change in striatal tracer uptake in MCI-LB as compared with MCI-Alzheimer disease (AD) or age-matched controls. Our primary research question was “does longitudinal decline in striatal uptake measured using 123[I]-FP-CIT SPECT occur in patients with MCI-LB?” We hypothesized that the MCI-LB group would show significant decline over time, whereas the MCI-AD group and controls would show no significant change. As a secondary analysis, we estimated the time from baseline for the MCI-LB subset with normal baseline imaging to develop a quantitatively abnormal 123[I]-FP-CIT SPECT.
Methods
Participant Recruitment
Participants with MCI were recruited prospectively from memory clinics in the North-East of England between 2013 and 2015 (study 1) and 2016 and 2019 (study 2). Detailed inclusion and exclusion criteria are given in our previous publications.5,9 Healthy controls were 60 years or older with no MCI,10 dementia, or other suspected brain pathology. They had a normal brain MRI and Mini Mental State Examination [MMSE] score ≥26.
Clinical assessment included the following: the Movement Disorders Society-Unified Parkinson's Disease Rating Scale motor examination,11 Epworth Sleepiness Scale,12 Cumulative Illness Rating Scale for Geriatrics,13 and Geriatric Depression Scale.14
Standard Protocol Approvals, Registrations, and Patient Consents
The study received ethical approval (Research Ethics Committee Identification Number 15/NE/0420), and all participants gave written informed consent.
123[I]-FP-CIT SPECT Imaging
Participants were scanned 3–6 hours after a bolus intravenous injection of 185 MBq of 123[I]-FP-CIT (DaTSCAN; GE Healthcare, UK) was administered using a dual-headed gamma camera (Siemens Symbia S or Siemens Intevo) fitted with low-energy high-resolution parallel hole collimators. One hundred twenty 25-second views over a 360° orbit (60 views per detector) were acquired on a 128 × 128 matrix with a zoom of 1.23 × giving a pixel size of 3.9 × 3.9 mm. Overall image acquisition time was 25 minutes. Participants also had a cardiac 123I-MIBG scan as detailed in our previous publication.15
All participants in our first study (2013–2015) were invited to join our second study (2016–2021), where they had a repeat 123[I]-FP-CIT SPECT scan 1–3 years after baseline imaging and were invited to undergo a further repeat scan at least 1 year after that. We also recruited new participants to have baseline and repeat scans within the second study. Therefore, all participants included in this analysis had either 2 or 3 123[I]-FP-CIT SPECT scans, all using the same scanner.
123[I]-FP-CIT Specific Binding Ratio Analysis
123[I]-FP-CIT uptake was measured using specific binding ratios (SBRs) obtained from DaTQUANT v2.0 software (GE Healthcare, Chalfont St. Giles, UK).16 The software registers the scan to a normal database and overlays volumes of interest over the whole striatum, caudate, and putamen as well as a reference region in the occipital lobe representing nonspecific, displaceable activity. The SBR is defined as the ratio between the background subtracted count density in the striatal volume and the count density in the background volume. We did not move any striatal volumes, but did move the background volume anteriorly if the signal was reduced because of atrophy, because this would otherwise make the SBRs artificially high. Left and right-sided SBRs were recorded for the striatum, caudate, and putamen.
Diagnosis
An expert 3-person consensus panel of experienced old-aged psychiatrists (A.J.T., P.C.D., J.-P.T.) allocated participants with MCI to one of 3 clinical diagnoses, following National Institute on Aging and the Alzheimer's Association criteria10 and core features for DLB1 (later incorporated into research criteria for MCI-LB17). The 4 core features are parkinsonism, visual hallucinations, cognitive fluctuations, and REM sleep behavior disorder. The 3 categories are possible MCI-LB (MCI plus one core symptom of DLB or MCI with none of the 4 core symptoms of DLB but having an abnormal cardiac MIBG), probable MCI-LB (MCI plus 2 or more of the 4 core symptoms of DLB or MCI plus one core symptom of DLB and an abnormal cardiac MIBG), and MCI-AD (fulfilling criteria for MCI-AD10 with none of the 4 core symptoms of DLB and a normal cardiac MIBG).
The presence of parkinsonism was based on clinical judgment as to whether the participant exhibited “one or more spontaneous cardinal features of parkinsonism” defined in the MCI-LB research criteria17 as “bradykinesia (defined as slowness of movement and decrement in amplitude or speed), rest tremor, or rigidity.” MCI due to PD (PD-MCI) was excluded using the “1-year rule”—if parkinsonism was present more than 1 year before the onset of cognitive impairment, participants were classified as having preexisting PD and excluded.
Statistical Analysis
Mixed models were used to investigate differential changes in total striatal 123[I]-FP-CIT uptake primarily, and secondarily for the caudate and putamen separately, for each diagnostic group compared with controls. SBRs for both sides were input into the model, with side a random effect (as opposed to taking the mean value between left and right for each scan). All analyses were conducted using R statistical software (Version 3.5.2; R Foundation for Statistical Computing, Vienna, Austria). Fixed effects included were age (centered at 60 years), time in years from baseline imaging, sex, and diagnostic group (MCI-AD, possible MCI-LB, and probable MCI-LB, reference: controls). Random intercept and slopes were included for each hemisphere nested within each subject. We also ran an analysis with “most affected hemisphere” as a fixed effect, where the most affected hemisphere (right or left) was defined as the side with the largest decrease in whole striatal SBR between baseline and the most recent scan. This analysis enabled us to determine whether including only the most affected side in the model would yield a significantly greater rate of change than that obtained with the default of both sides included. The correlation between striatal SBR and markers of cognition (Addenbrooke's Cognitive Examination-Revised [ACE-R]) and parkinsonism (Unified Parkinson's Disease Rating Scale [UPDRS]) was examined at baseline and over time.
As a secondary analysis, we assessed how long it would take on average for participants with MCI-LB with a normal scan z-score at baseline (defined as striatal SBR within 2 SDs of control mean) to reach an abnormal z-score of −2 or lower.
A subgroup of participants with MCI-LB was identified with quantitatively normal striatal SBR z-scores at baseline (above −2). Their average rate of SBR change was assessed with mixed models as above. Estimated marginal means for SBR uptake for the subgroup were calculated at 1-month intervals from baseline to 5 years initially to calculate the expected time until the average 123[I]-FP-CIT uptake fell below 2 z-scores in the striatum or putamen, as an indicative marker of the development of abnormal 123[I]-FP-CIT uptake.
The annual percentage change in SBR was calculated for each participant in the possible and probable MCI-LB groups. The median annual rate of change in SBR for the striatum, caudate, and putamen and the interquartile range were derived from linear interpolation for each diagnostic group.
Test-retest variation in 123[I]-FP-CIT SPECT uptake (SBR) was assessed using the baseline and repeat scans for the healthy controls. The expected decline in uptake due to aging over 1 year is less than 1%,18 so differences above this level can be attributed to variation from scan to scan. The change in SBR between baseline and the repeat scan as a percentage of baseline value was calculated for each control for the striatal, caudate, and putamen regions. The mean and maximum percentage change in SBR was calculated for all regions for each side. Change in SBR was expressed as the absolute difference, that is, the modulus of the difference was used, so that all percentage difference results were positive.
Data Availability
Anonymized data not published within this article will be made available on request to any qualified investigator.
Results
Exclusion of Participants
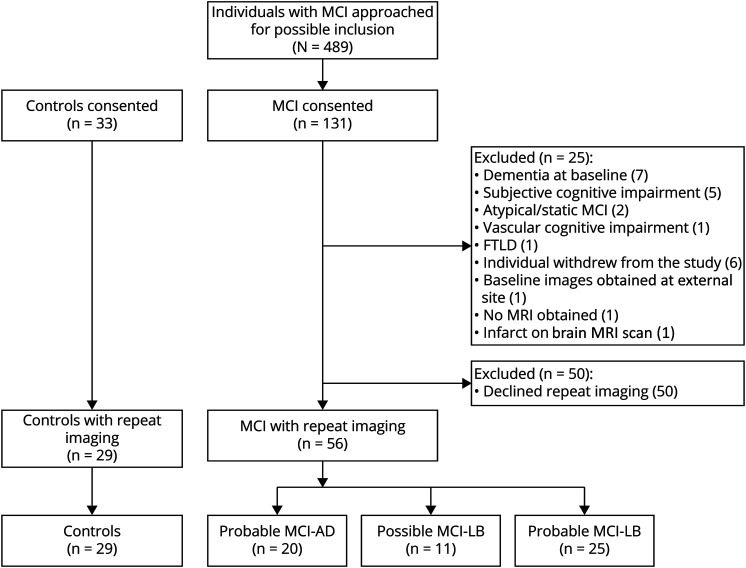
A total of 131 participants with MCI and 33 controls were consented and enrolled in this study, after confirming eligibility at the baseline visit (Figure 1). Twenty-five participants with MCI were excluded from the study for the reasons detailed in Figure 1. Fifty participants with MCI consented to baseline imaging only. Eighty-five participants (MCI: n = 56 and controls: n = 29) were included in the repeat 123[I]-FP-CIT SPECT analysis.
Figure 1. Participant Flowchart Showing Study Recruitment and Inclusion in This Repeat 123[I]-FP-CIT SPECT Analysis.
Timing of Repeat 123[I]-FP-CIT SPECT
Of the 85 individuals with MCI included, 74 underwent the first repeat scan between 12 and 18 months after baseline and 11 underwent the first repeat 123[I]-FP-CIT SPECT 19–36 months after baseline. Twenty-two had a third 123[I]-FP-CIT SPECT between 12 and 20 months after the second 123[I]-FP-CIT SPECT. There was a mean of 1.6 years (SD = 0.9) between the first and last images (range 1.0–4.3 years).
Demographics and Clinical Characteristics
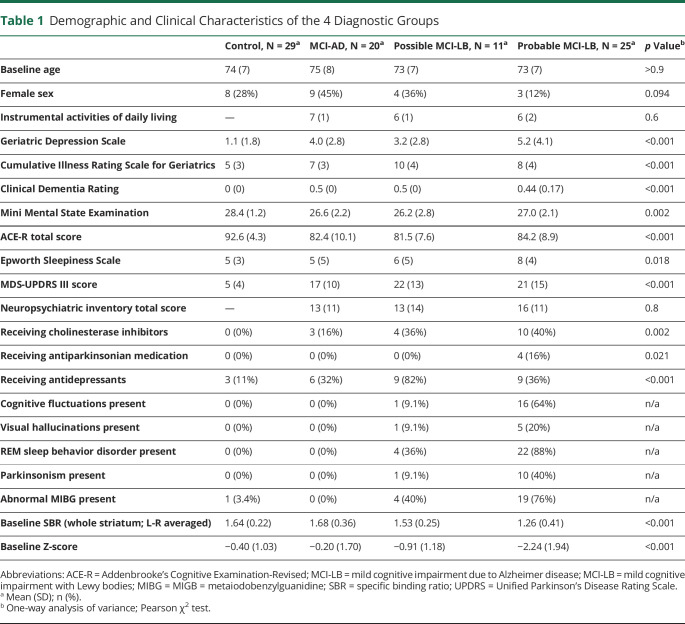
The baseline demographic and clinical characteristics of the different diagnostic groups are summarized in Table 1. All 4 groups (controls, MCI-AD, possible MCI-LB, and probable MCI-LB) were similar in age. There were no significant differences between global cognitive scores (MMSE, ACE-R) of MCI groups, but controls had higher cognitive scores than MCI groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of the 4 Diagnostic Groups
Assessment of Change in 123[I]-FP-CIT SPECT Uptake Over Time
Nonlinear terms were not supported for any continuous effect. Therefore, a linear mixed-effects model was used to assess the association between change in 123[I]-FP-CIT striatal uptake over time and diagnostic group.
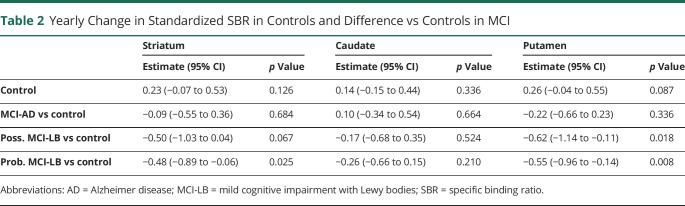
Significant predictors of longitudinal decline in striatal uptake are listed in Table 2. Probable MCI-LB was a significant predictor of longitudinal decline over time in 123[I]-FP-CIT uptake in the striatum (p = 0.025), and this was due to significant decline in putamen uptake (p = 0.008), but not caudate (p = 0.21). Possible MCI-LB was a predictor of decline in 123[I]-FP-CIT uptake in the putamen only (p = 0.018). MCI-AD was not a significant predictor of longitudinal striatal change (p = 0.68). Plots of the SBR results for longitudinal change over time in the whole striatum, caudate, and putamen for all diagnostic groups (controls, MCI-AD, possible MCI-LB and probable MCI-LB) are shown in Figure 2. We repeated the analysis with “most affected hemisphere” as a fixed effect in the mixed-effects model and found no significant interaction (time × MCI-LB × most affected hemisphere interaction: p = 0.93, whole striatum; p = 0.55, caudate; p = 0.74, putamen). This demonstrates that although, by definition, the most affected hemisphere declines in MCI-LB more than the least affected hemisphere, the additional decline compared with controls is not statistically significant. The plots of the average decline for all diagnostic groups for the most and least affected hemispheres are shown in eFigure 1, links.lww.com/WNL/D16. The baseline SBR is slightly higher for the most affected hemisphere than the least affected hemisphere in MCI-LB (striatum β = +0.30, p = 0.047).
Table 2.
Yearly Change in Standardized SBR in Controls and Difference vs Controls in MCI
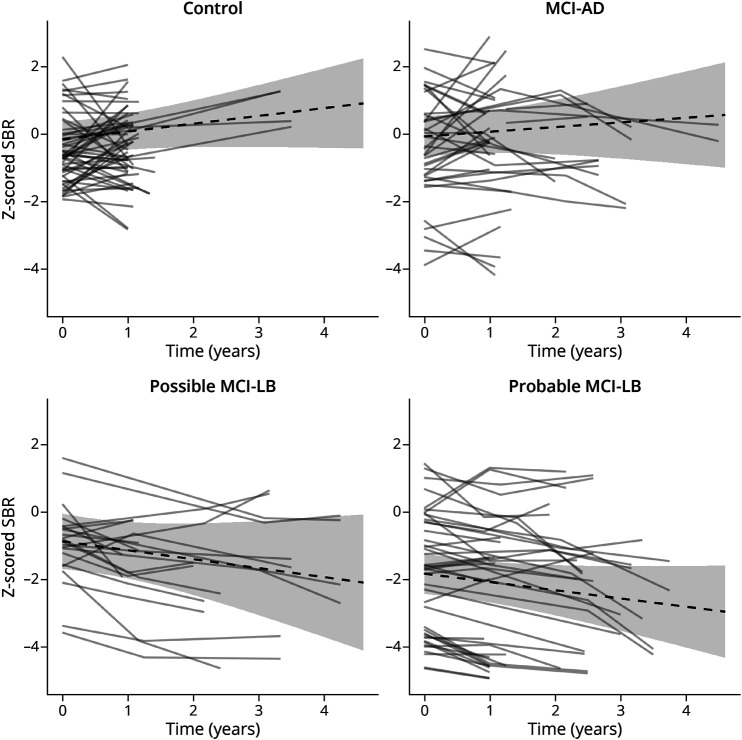
Figure 2. Standardized Trajectories of FPCIT Uptake to the Striatum in Control and MCI Groups (Dashed) and Individual Trajectories for the Left and Right Hemispheres (Solid; One Line for Left and One Line for Right).
MCI = mild cognitive impairment.
At baseline, there is no significant relationship between striatal SBR and ACE-R (r = 0.20, p = 0.251) or UPDRS (r = −0.29, p = 0.083) in the MCI-LB group. There are weak correlations with ACE-R (r = 0.15, p = 0.021) and UPDRS (r = −0.14, p = 0.020) over time for the MCI-LB group.
Annual Percentage Change in 123[I]-FP-CIT Uptake for MCI-LB Groups
Annual percentage change in 123[I]-FP-CIT uptake was determined using a log-linear model. There was no significant difference between the possible and probable MCI-LB groups in relation to annual percentage change in 123[I]-FP-CIT uptake in the striatum or striatal subregions. The median annual change in striatal 123[I]-FP-CIT uptake in the MCI-LB group was −5.6% for the striatum (95% CI −8.2% to −2.9%), −3.3% for the caudate (95% CI −5.8% to −0.6%), and −7.0% for the putamen (−10.3% to – 3.6%). The median annual decline in 123[I]-FP-CIT uptake was greater in the putamen than in the caudate for the MCI-LB groups. The median change in SBR in MCI-AD was 2.1% (−3.5% to 8.0%) for the striatum, 4.7% (−1.3% to 11.1%) for the caudate, and 0.1% (−5.7% to 6.2%) for the putamen.
Time Until Expected Uptake Is 2 SDs Below Mean
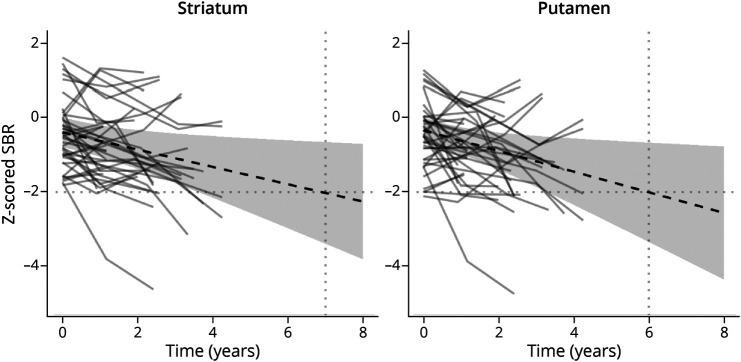
Six individuals with MCI-LB showed a decline from normal to a clearly abnormal quantified uptake during the study of 23 participants with possible MCI-LB with baseline z-scores above −2 (Figure 3). When considered as a group, the marginal estimate did not reach a z-score of −2 within the timescale of the study. Projecting the estimate forward and assuming a linear progression, the average MCI-LB case with baseline normal uptake would be expected to reach an abnormal uptake to the putamen or striatum 6 years after baseline.
Figure 3. Estimated Time Taken for Participants With MCI-LB With Baseline Striatal SBR Z-scores of > −2 to Reach ≤ −2 SDs Below the Control Mean.
MCI-LB = mild cognitive impairment with Lewy bodies; SBR = specific binding ratio.
Test-Retest Variation
The average percentage change in SBR between the control baseline and repeat scans was 11.3% (SD 5.3%), 12.4% (SD 5.7%), and 12.3% (SD 6.0%) for the right striatum, putamen, and caudate regions and 10.5% (SD 5.7%), 12.1% (SD 6.2%), and 13.1% (SD 5.2%) for the left striatum, putamen, and caudate regions, respectively. The maximum percentage changes were 33.4% for the left striatum, 35.4% for the left putamen, and 48.0% for the right caudate.
Classification of Evidence
This study provides Class II evidence that longitudinal declines in striatal uptake measured using 123[I]-FP-CIT SPECT are associated with MCI-LB but not MCI-AD.
Discussion
We aimed to assess whether a decrease in 123l-FP-CIT SPECT uptake over time was detectable in MCI-LB and MCI-AD groups compared with age-matched controls. Our results show a significantly greater decline in the striatal uptake of 123[I]-FP-CIT over time in the possible and probable MCI-LB groups, but a nonsignificant rate of change in striatal uptake in the MCI-AD group. The dopaminergic loss predominantly affects the putamen in both MCI-LB groups.
The rationale for repeat 123[I]-FP-CIT SPECT imaging is based on the observations that 10% of patients with DLB have only neocortical Lewy body pathology, sparing the midbrain2, and that 20% of people with DLB and 50% of people with MCI-LB have normal 123[I]-FP-CIT scans.5,9,19 Such findings are consistent with a view that as time progresses, Lewy body disease causes greater involvement of the substantia nigra and thus a higher proportion of abnormal scans. A study in patients with probable DLB demonstrated that those with a normal scan eventually developed an abnormal 123[I]-FP-CIT SPECT over time, with a median time between scans of 18 months.8 Our study indicates that progressive nigrostriatal pathway degeneration is already detectable at the prodromal stage of DLB. Using a linear mixed-effects model assessing rates of decline of 123[I]-FP-CIT uptake, we found a significant striatal decline of dopamine transporter availability in the putamen in both possible MCI-LB (p = 0.018) and probable MCI-LB (p = 0.008) groups over time. Decline in the whole striatum was significant in the probable MCI-LB group (p = 0.025), but not in the possible MCI-LB group (p = 0.067). The MCI-AD group did not have any significant decline in the whole striatum or its subregions, consistent with the lack of involvement of the nigrostriatal pathway in AD pathology.
On average, our model showed that an individual with MCI-LB normal baseline uptake would be expected to reach an abnormal uptake to the putamen or striatum around 6 years later, although this will depend on the level at baseline. Because this forecast lies outside the range of data in this study, this should be interpreted with caution. The model suggests that individuals with MCI-LB are unlikely to develop abnormal SBRs within a few years of a normal baseline scan, although this was seen in 6 of 23 participants in our study.
A previous study in idiopathic REM behavioral disorder (IRBD) found that a baseline reduction of 123[I]-FP-CIT uptake in the putamen greater than 25% from the mean of normal control values predicted those who developed a ɑ-synucleinopathy after 3 years of follow-up.20 Future longitudinal follow-up of our cohort will inform whether the extent of initial dopaminergic deficit on baseline 123[I]-FP-CIT imaging predicts time to conversion to DLB.
Our study found a median annual decline in the striatum of −6% in both the probable MCI-LB and possible MCI-LB groups. In both MCI-LB groups, the median loss in the striatum was predominately due to loss in the putamen (−7%) rather than the caudate (−3%). Therefore, our results suggest that dopaminergic loss in prodromal DLB occurs predominantly in the putamen, in contrast to early DLB.21-24 Only one study in DLB examined annual percentage loss in striatal 123[I]-FP-CIT uptake, and this showed a more uniform loss throughout the striatum and a loss far in excess of values in our study, with an annual decline in caudate uptake of −12.7% and −13.0% in the putamen.25 Our findings are more akin to IRBD and PD studies, which found greater loss in the putamen than the caudate. In IRBD, the annual rate of decline in the putamen was −5.8% and −3.0% in the caudate.26 Studies in PD have shown similar declines in the caudate (−4.6% to −11.0%) and in the putamen (−6.0% to −10.0%).27-29 In the IRBD study, those who converted to PD had a more rapid decline in the putamen of approximately 10% per year.26
Our repeat scans in controls showed that variation in SBR between scans is around 11% on average. This suggests that when evaluating a follow-up scan in a patient with MCI-LB, the SBR would need to drop substantially to be confident that the decline was not due to chance. The first repeat study in 6 healthy controls scanned 3–6 weeks apart reported a similar mean test-retest variation of 7.4% (range 2.1%–23.0%).30 The median annual change in our MCI-LB groups was −6%, so a follow-up scan performed a year or two after normal or equivocal baseline is unlikely to detect progressive dopaminergic loss—although this was seen in 6 individuals in our study.
This study has several strengths: (1) a prospective longitudinal design in a cohort of individuals with MCI-LB, using serial functional imaging at various time points; (2) the inclusion of matched controls to establish whether the process of aging alone could account for the decline in striatal 123[I]-FP-CIT uptake and estimate the test-retest variation; (3) the confirmation of clinical core features of LB disease by a consensus panel blinded to the imaging results; and (4) the use of mixed-effects models that make the best use of data by incorporating both left and right-sided SBR measures.
Limitations of the study include possible diagnostic misattribution, especially as many individuals are early in their disease trajectory. Future pathologic examination may lead to diagnostic reassignment. However, much effort was made to minimize this problem with the incorporation of cardiac MIBG into to the diagnostic process. Another limitation is relatively short follow-up with a variable time between baseline and repeat 123[I]-FP-CIT imaging. We have shown that in people with MCI, 12–18 months is typically too short a timescale to detect a decrease in striatal 123[I]-FP-CIT uptake with confidence. Future studies would benefit from a longer period of follow-up. Most of the participants (75/85) were on the same medication throughout the study, so the effect of medication on uptake results is likely to be minimal. However, 4 participants with probable MCI-LB, 4 with possible MCI-LB, one with MCI-AD, and one control reported a change in medication or dose between baseline and follow-up scans.
In conclusion, progressive decline in 123[I]-FP-CIT SPECT uptake over time is present in both possible and probable MCI-LB at a group level. Follow-up 123[I]-FP-CIT SPECT may be of value in patients with MCI-LB who have initial normal imaging. However, the variation in our control subjects on repeat scanning suggests an interval of several years from baseline may be required for a quantitative change in dopaminergic function to be both detectable and clinically meaningful.
Acknowledgment
We acknowledge support for this investigator-led study from GE Healthcare who provided ligand for the FP-CIT scans. Infrastructure and support was provided to the authors based at Newcastle by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre, a partnership between Newcastle Upon Tyne Hospitals NHS Foundation Trust and Newcastle University.
Glossary
- ACE-R
Addenbrooke's Cognitive Examination-Revised
- AD
Alzheimer disease
- DLB
dementia with Lewy bodies
- FP-CIT
N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane
- I
iodine
- IRBD
idiopathic REM behavioral disorder
- MCI-LB
mild cognitive impairment with Lewy bodies
- MIGB
metaiodobenzylguanidine
- MMSE
Mini Mental State Examination
- PD
Parkinson disease
- SBR
specific binding ratio
- SPECT
single photon emission computed tomography
Appendix. Authors
Footnotes
Editorial, page 505
Class of Evidence: NPub.org/coe
Study Funding
This study was funded by a major project research grant from Alzheimer's Research UK (ARUK-PG2015-13) and by the NIHR Newcastle Biomedical Research Centre.
Disclosure
R. Durcan reports no conflicts in relation to this work. J.T. O'Brien has acted as a consultant, has been a recipient of grant support, and received honoraria for talks for GE Healthcare. J.T. O'Brien is supported by the NIHR Cambridge Biomedical Research Centre. Outside of this work, J.T. O'Brien has acted as a consultant for TauRx, Axon, Eisai, Roche, and GE Healthcare and received grant funding from Alliance Medical and Merck. G. Roberts and G.S. Petrides have received honoraria from GE Healthcare for delivering educational workshops on FP-CIT imaging. J.-P. Taylor has received honoraria from GE Healthcare for delivering educational presentations on Lewy body disease. Outside of this work, J.-P. Taylor has acted as a consultant for Kyowa Kirin and Heptares Sosei and received grant funding from Heptares Sosei. P.C. Donaghy has received grant funding from Alzheimer's Research UK, Alzheimer's Society, Lewy Body Society, Weston Brain Institute, and the Medical Research Council. A.J. Thomas has received support for investigator-led studies and honoraria from GE Healthcare. C.A. Hamilton has received remuneration from Roche for public educational materials unrelated to this work. L.M. Allan is supported by the National Institute for Health Research Applied Research Collaboration South West Peninsula. S.J. Colloby, M. Firbank, and S. Lawley declare that they have no conflicts of interest to disclose. Go to Neurology.org/N for full disclosures.
References
- 1.McKeith IG, Boeve BF, Dickson DW, et al. . Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi. 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas AJ, Attems J, Colloby SJ, et al. . Autopsy validation of 123[I]-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017;88(3):276-283. doi. 10.1212/wnl.0000000000003512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iranzo A, Lomeña F, Stockner H, et al. . Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2010;9(11):1070-1077. doi. 10.1016/s1474-4422(10)70216-7 [DOI] [PubMed] [Google Scholar]
- 4.Boeve BF, Silber M, Ferman T, et al. . Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14(8):754-762. doi. 10.1016/j.sleep.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts G, Donaghy PC, Lloyd J, et al. Accuracy of dopaminergic imaging as a biomarker for mild cognitive impairment with Lewy bodies. Br J Psychiatry. 2021;218(5):276-282. doi. 10.1192/bjp.2020.234 [DOI] [PubMed] [Google Scholar]
- 6.Ba F, Martin WW. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord. 2015;21(2):87-94. doi. 10.1016/j.parkreldis.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 7.McKeith I, O'Brien J, Walker Z, et al. . Sensitivity and specificity of dopamine transporter imaging with 123[I]-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313. doi. 10.1016/s1474-4422(07)70057-1 [DOI] [PubMed] [Google Scholar]
- 8.van der Zande JJ, Booij J, Scheltens P, Raijmakers PGHM, Lemstra AW. [(123)]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2016;43(6):1060-1066. doi. 10.1007/s00259-016-3312-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas AJ, Donaghy P, Roberts G, et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med. 2019;49(3):396-402. doi. 10.1017/S0033291718000995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. doi. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz CG, Tilley BC, Shaftman SR, et al. . Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. doi. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. doi. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 13.Miller MD, Paradis CF, Houck PR, et al. . Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237-248. doi. 10.1016/0165-1781(92)90005-n [DOI] [PubMed] [Google Scholar]
- 14.D'Ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: the acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract. 1994;11(3):260-266. doi. 10.1093/fampra/11.3.260 [DOI] [PubMed] [Google Scholar]
- 15.Roberts G, Durcan R, Donaghy PC, et al. Accuracy of cardiac innervation scintigraphy for mild cognitive impairment with Lewy bodies. Neurology. 2021;96(23):e2801-e2811. doi. 10.1212/WNL.0000000000012060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neill M, Fisher JM, Brand C, et al. Practical application of DaTQUANT with optimal threshold for diagnostic accuracy of dopamine transporter SPECT. Tomography. 2021:7(4):980-989. doi. 10.3390/tomography7040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeith IG, Ferman TJ, Thomas AJ, et al. . Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94(17):743-755. doi. 10.1212/wnl.0000000000009323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varrone A, Dickson JC, Tossici-Bolt L, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40(2):213-227. doi. 10.1007/s00259-012-2276-8 [DOI] [PubMed] [Google Scholar]
- 19.O'Brien JT. Role of imaging techniques in the diagnosis of dementia. Br J Radiol. 2007;80(special issue 2):S71-S77. doi. 10.1259/bjr/33117326 [DOI] [PubMed] [Google Scholar]
- 20.Iranzo A, Santamaría J, Valldeoriola F, et al. . Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2017;82(3):419-428. doi. 10.1002/ana.25026 [DOI] [PubMed] [Google Scholar]
- 21.Ziebell M, Andersen BB, Pinborg LH, et al. . Striatal dopamine transporter binding does not correlate with clinical severity in dementia with Lewy bodies. J Nucl Med. 2013;54(7):1072-1076. doi. 10.2967/jnumed.112.114025 [DOI] [PubMed] [Google Scholar]
- 22.Ziebell M, Andersen BB, Thomsen G, et al. . Predictive value of dopamine transporter SPECT imaging with [(1)(2)(3)I]PE2I in patients with subtle parkinsonian symptoms. Eur J Nucl Med Mol Imaging. 2012;39(2):242-250. doi. 10.1007/s00259-011-1976-9 [DOI] [PubMed] [Google Scholar]
- 23.Walker Z, Costa DC, Walker RW, et al. . Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology. 2004;62(9):1568-1572. doi. 10.1212/01.wnl.0000123248.39847.1d [DOI] [PubMed] [Google Scholar]
- 24.O'Brien JT, Colloby S, Fenwick J, et al. . Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61(6):919-925. doi. 10.1001/archneur.61.6.919 [DOI] [PubMed] [Google Scholar]
- 25.Colloby SJ, Williams ED, Burn DJ, Lloyd JJ, McKeith IG, O'Brien JT. Progression of dopaminergic degeneration in dementia with Lewy bodies and Parkinson's disease with and without dementia assessed using 123[I]-FP-CIT SPECT. Eur J Nucl Med Mol Imaging. 2005;32(10):1176-1185. doi. 10.1007/s00259-005-1830-z [DOI] [PubMed] [Google Scholar]
- 26.Iranzo A, Valldeoriola F, Lomeña F, et al. . Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797-805. doi. 10.1016/s1474-4422(11)70152-1 [DOI] [PubMed] [Google Scholar]
- 27.Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287(13):1653-1661. doi. 10.1001/jama.287.13.1653 [DOI] [PubMed] [Google Scholar]
- 28.Pirker W, Holler I, Gerschlager W, Asenbaum S, Zettinig G, Brücke T. Measuring the rate of progression of Parkinson's disease over a 5-year period with beta-CIT SPECT. Mov Disord. 2003;18(11):1266-1272. doi. 10.1002/mds.10531 [DOI] [PubMed] [Google Scholar]
- 29.Pirker W, Djamshidian S, Asenbaum S, et al. Progression of dopaminergic degeneration in Parkinson's disease and atypical Parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord. 2002;17(1):45-53. doi. 10.1002/mds.1265 [DOI] [PubMed] [Google Scholar]
- 30.Booij J, Habraken JB, Bergmans P, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson's disease. J Nucl Med. 1998;39(11):1879-1884. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available on request to any qualified investigator.