Abstract
Background:
Apolipoprotein C-III (apoC-III) is an important regulator of triglyceride metabolism and was associated with cardiovascular risk in several cohorts. ApoC-III is present in four major proteoforms, a native peptide (C-III0a), and glycosylated proteoforms with zero (C-III0b), one (C-III1, most abundant) or two (C-III2) sialic acids, which may differentially modify lipoprotein metabolism. We studied the relationships of these proteoforms with plasma lipids and cardiovascular risk.
Methods:
ApoC-III proteoforms were measured by mass spectrometry immunoassay in baseline plasma samples of 5,791 participants of Multiethnic Study of Atherosclerosis, an observational community-based cohort. Standard plasma lipids were collected for up to 16 years and cardiovascular events (myocardial infarction, resuscitated cardiac arrest or stroke) were adjudicated for up to 17 years.
Results:
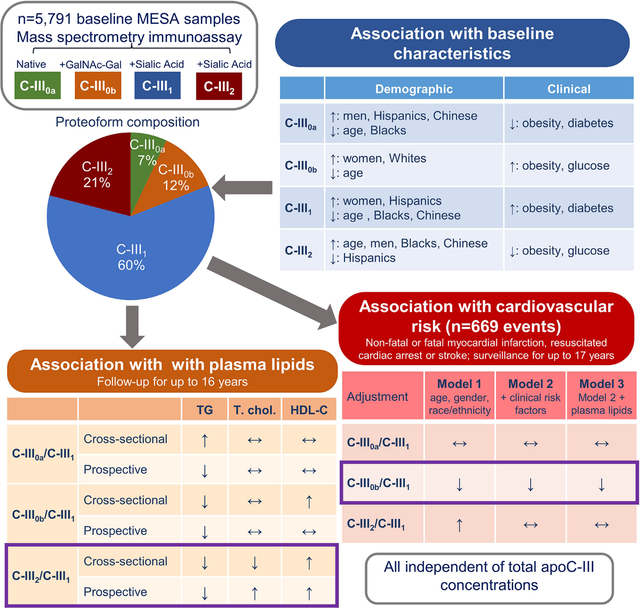
ApoC-III proteoform composition differed by age, gender, race/ethnicity, BMI and fasting glucose. Notably, C-III1 was lower in older participants, men and Blacks and Chinese (versus Whites), and higher in obesity and diabetes. In contrast, C-III2 was higher in older participants, men, Blacks and Chinese, and lower in Hispanics and obesity. Higher C-III2 to C-III1 ratio (C-III2/III1) was associated with lower triglycerides and higher HDL in cross-sectional and longitudinal models, independently of clinical and demographic risk factors and total apoC-III. The associations of C-III0a/III1 and C-III0b/III1 with plasma lipids were weaker and varied through cross-sectional and longitudinal analyses. Total apoC-III and C-III2/III1 were positively associated with CVD risk (n=669 events, Hazard Ratios: 1.14 [95%CI: 1.04–1.25] and 1.21 [1.11–1.31], respectively), however the associations were attenuated after adjustment for clinical and demographic characteristics (1.07 [0.98–1.16]; 1.07 [0.97–1.17]). In contrast, C-III0b/III1 was inversely associated with CVD risk even after full adjustment including plasma lipids (0.86 [0.79–0.93]).
Conclusions:
Our data indicate differences in clinical and demographic relationships of apoC-III proteoforms, and highlight the importance of apoC-III proteoform composition in predicting future lipid patterns and CVD risk.
Graphical Abstract:
Introduction
Apolipoprotein C-III (ApoC-III) is a major apolipoprotein attached to triglyceride-rich lipoproteins (TRLs), remnant cholesterol particles and is also present in LDL and HDL.1 ApoC-III is a key regulator of lipid metabolism, which increases TRLs levels by inhibiting triglyceride clearance and stimulating VLDL production.2–6 ApoC-III may also directly modulate endothelial function and was shown to promote monocyte adhesion to endothelial cells.7,8 Consistent with these metabolic and vascular actions, several studies have demonstrated a strong association of higher total apoC-III levels in plasma or various lipoprotein particles with increased triglycerides, dyslipidemia, inflammation, atherosclerosis, and cardiovascular disease (CVD).9–16 Conversely, loss of function mutations in APOC3 are associated with decreases in plasma triglyceride levels, coronary atherosclerosis and CVD risk.17–19 Inhibition of apoC-III production by antisense oligonucleotides reduced plasma triglycerides and improved dyslipidemia.20,21
In circulation, apoC-III appears in four major proteoforms, a native peptide (C-III0a), and O-glycosylated (on Threonine 74) proteoforms containing zero (C-III0b), one (C-III1) or two (C-III2) sialic acids.22,23 In preclinical studies, apoC-III2 showed greater affinity to VLDL, but less potent inhibition of triglyceride clearance than apoC-III1.24,25 ApoC-III complexes with higher relative C-III2 content were less potent inhibitors of LPL-mediated lipolysis and hepatocyte VLDL uptake.26 In endothelial cells, sialylation was shown necessary for the proinflammatory effect of apoC-III.27 In humans, plasma concentrations and production rates of sialylated apoC-IIIs showed stronger association with plasma triglycerides than non-sialylated proteoforms.27–29 Higher plasma triglycerides were associated with a greater proportion of C-III1 and lower percentages of C-III2 in adolescents with insulin resistance and in adults with prediabetes and type 2 diabetes.26,30
To extend these initial cross-sectional findings of apoC-III proteoform associations with dyslipidemia into a more general population and to assess the role of apoC-III proteoform composition in longitudinal changes in lipids and prediction of CVD risk, we utilized samples and data from the Multi-Ethnic Study of Atherosclerosis (MESA). Specifically, we (a) identified key demographic and clinical characteristics affecting apoC-III proteoforms composition; (b) determined the cross-sectional and longitudinal associations of apoC-III proteoform composition with plasma lipids; and (c) tested the association between apoC-III proteoform composition and incident CVD.
Methods
Study design and population
Data used in this study were obtained from the Multi-Ethnic Study of Atherosclerosis (MESA) (https://www.mesa-nhlbi.org) in accordance with their published data access policies, including an approved written proposal. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The MESA study is a multicenter longitudinal study to examine factors associated with subclinical CVD and the progression from subclinical to clinical CVD in individuals aged 45 to 84 years, of non-Hispanic White, African American, Hispanic and Chinese American race/ethnicity, and without known CVD at the enrollment.31 Institutional review boards at each of the six MESA study sites (Columbia University, New York, NY; Johns Hopkins University, Baltimore, MD; Northwestern University Chicago, IL; University of California at Los Angeles, Los Angeles, CA; University of Minnesota, St. Paul, MN; and Wake Forest University, Winston-Salem, NC) approved the study protocol and informed consent was obtained from all study participants. The present study which used existing MESA data and plasma samples was approved by the Phoenix VA Health Care System Institutional Review Board. MESA clinical exams occurred in 2000 to 2002 (Exam 1), 2002 to 2004 (Exam 2), 2004 to 2006 (Exam 3), 2005 to 2007 (Exam 4), 2010 to 2012 (Exam 5) and 2016 to 2018 (Exam 6). For MESA, and the current study, Exam 1 data was considered baseline. Demographic information, medical history and physical measures were obtained through standardized protocols as described previously.31 For the present analysis, obesity was defined as BMI ≥ 30 kg/m2 in White, Hispanic and African Americans, and as BMI ≥ 27.5 kg/m2 in Chinese Americans. Classification of diabetes was based on a fasting glucose > 6.99 mmol/l or use of hypoglycemic medications. Hypertension was defined as having systolic blood pressure ≥ 140 mmHg or using antihypertensive medications. Blood samples were obtained after a 12-hour fast. Blood biomarkers were measured at the MESA central laboratory at the University of Minnesota.
Outcomes
Outcomes for the present study included plasma triglycerides, and total, LDL and HDL cholesterol collected at each exam, and hard CVD, including definite myocardial infarction, resuscitated cardiac arrest and fatal or non-fatal stroke. Details on cardiovascular events surveillance has been previously reported.32 Additional details on the MESA study’s follow-up methods and event adjudication are available on the MESA web site at http://www.mesa-nhlbi.org. The present analysis includes CVD events reported through end of 2017, i.e., for up to 17 years of follow-up.
ApoC-III proteoform composition
ApoC-III proteoform composition was measured in 5,791 available samples from Exam 1 by mass-spectrometry immunoassay (MSIA).26 Prior to running the assays, 3 μL of thawed plasma was diluted with 117 μL of PBS, 0.1% Tween (PBST). Then, 40 μL of this diluted plasma was mixed with 120 μL of PBST, yielding 160 μL of analytical sample that was plated onto a 96-well plate. Samples were run in batches of 96; each batch contained 90 analytical samples and 6 quality control samples (two distinct plasma samples aliquoted in triplicate). ApoC-III protein was captured by 250 aspiration and dispensing cycles (100 μL each) of analytical sample using immunoaffinity columns derivatized with anti-apoC-III antibody (Academy Biomedical Co, Houston, TX). Captured apoC-III was then eluted directly onto a 96-well formatted matrix-assisted laser desorption/ionization (MALDI) target using a 5 μL of MALDI matrix solution (33% aqueous acetonitrile and 0.4% trifluoroacetic acid saturated with sinapinic acid). Bruker Autoflex III MALDI-TOF instrument (Bruker, Billerica, MA) was utilized to acquire linear mass spectra from each sample spot. The mass spectra were first externally calibrated with protein calibration standards and then internally calibrated using the highest intensity apoC-III signals. The spectra were baseline subtracted and smoothed using Flex Analysis software (Bruker Daltonics). Areas under the peaks signals were integrated using Zebra 1.0 software (Intrinsic Bioprobes Inc., Tempe, AZ). Percent abundance of each apoC-III proteoform was obtained by dividing individual proteoform peak areas by the integrated peak area of all proteoforms. Mean coefficients of variation for intraassay and between-assay replicates, respectively, were 6.2% and 6.1% for C-III0a, 8.2% and 8.2% for C-III0b, 1.5% and 1.7% for C-III1, and 3.5% and 3.8% for C-III2. Plasma concentrations of total apoC-III were measured in baseline samples by sandwich ELISA as part of a previous study.33 Further details on MSIA materials and instruments are in Supplemental methods.
Measurement of plasma lipids
In fasting blood samples from all visits, triglycerides were measured using a glycerol-blanked enzymatic method (Trig/GB; Roche Diagnostics, Indianapolis, IN). Plasma HDL cholesterol was measured by the cholesterol oxidase method (Roche Diagnostics) after precipitation of non–HDL-C magnesium/dextran. In those with triglycerides <4.5 mmol/l (400 mg/dl), LDL cholesterol levels were calculated by the Friedewald equation.
Statistical analyses
Statistical analyses were conducted using SAS (v9.4, SAS Institute, Cary, NC) and R (v3.4.1). P-values <0.05 were considered statistically significant. For statistical analyses, apoC-III proteoforms were expressed as the percentage of each individual proteoform when assessed as univariate variables. When examined in the multivariate models, apoC-III proteoform composition was expressed as additive natural log-ratios (ALR) of less abundant proteoforms to the most abundant C-III1 to allow accurate estimation of relationships among variables that sum to a constant value as occurs with compositional data.34,35 Covariates were chosen for their known prior association with apoC-III proteoforms or study outcomes.
Pearson correlations were used to describe the associations between total plasma apoC-III concentration and percentages of individual proteoforms. Multiple linear regression was used to test the relationships of percentages of individual apoC-III proteoforms with clinical and demographic characteristics. Models were run unadjusted and then adjusted in a two-stage approach: (1) the associations with non-modifiable demographic characteristics, including age (defined in 10-year increments), gender and race/ethnicity were examined in multivariable models; (2) the associations with modifiable clinical characteristics, including BMI, obesity, fasting plasma glucose, diabetes, use of lipid-lowering medications and kidney function were tested individually in models adjusted for the above-listed demographic characteristics and their significant interactions. The association of overall apoC-III proteoform composition with clinical and demographic characteristics was tested by multivariate linear regression (MANOVA), using ALRs of apoC-III proteoforms to C-III1 as a multivariate outcome variable. Pillai’s trace value was calculated to estimate the percent of variance in apoC-III proteoform composition explained by differences in the levels of covariates.
The cross-sectional associations between ALRs of apoC-III proteoforms and plasma lipids were tested by multiple linear regression models before and after adjusting for total apoC-III concentrations, age, gender, race/ethnicity, BMI, diabetes status, fasting glucose, lipid-lowering therapy and eGFR. The associations between ALRs of apoC-III proteoforms and longitudinal changes in plasma lipids were tested by mixed linear regression for repeated measures with random intercept and fixed effect of time, before and after adjusting for age, gender, race/ethnicity, and baseline and follow-up BMI, diabetes status, fasting glucose, lipid-lowering therapy, tobacco use and eGFR. In secondary analyses, we also tested the cross-sectional and longitudinal associations of plasma lipids with percentages of individual proteoforms.
To evaluate the association between apoC-III measures and CVD risk, we first estimated Kaplan-Meier curves for the survival time to the first event stratified by lowest and highest quartiles of baseline total apoC-III concentrations and ALRs of apoC-III proteoforms to C-III1. Cox proportional hazard regression was used to assess the association between ALRs of apoC-III proteoforms to C-III1 and incident CVD events. Proportional hazard assumptions were confirmed by inspecting Kaplan-Meier curves and calculating Schoenfeld residuals. These analyses were run adjusted for total apoC-III only (Model 1), and then adjusted for age, gender, race/ethnicity, smoking status, BMI, diabetes, systolic blood pressure, eGFR, and use of antihypertensive and lipid lowering medications (Model 2), and further adjusted for plasma triglycerides and HDL cholesterol levels (Model 3). To determine if the relationship of apoC-III proteoform composition to CVD risk was similar in different age, gender, and race or ethnic groups, we modelled the interaction of these variables with ALRs of apoC-III proteoforms to C-III1. We also tested the associations of CVD risk with percentages of individual proteoforms. All continuous variables in the regression models were natural log transformed and scaled to a mean of zero and standard deviation of one.
Concordance statistics (C-index), category-free net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated to quantify improvement in 10-year CVD risk prediction after adding apoC-III proteoforms measures (based on 10-year follow-up survival estimates) to the Pooled Cohort Equation (PCE) estimator of CVD risk in asymptomatic adults.36 The confidence intervals were computed using 100 bootstrap samples.
Results
The final sample for this analysis included 5,790 participants after exclusion of one sample due to excess oxidation. Participants included nearly equal numbers of men and women and were racially and ethnically diverse, with relatively low rates of diabetes, and, on average, normal kidney function and near optimal plasma lipids (Table 1). Although nearly 50% had a history of hypertension, mean blood pressure levels were on average well within normal ranges. At baseline, 17% of participants were on lipid-lowering medications which was primarily statins (91% of the time).
Table 1:
Clinical and demographic characteristics of the cohort at baseline.
| Variable | n | Mean ± SD or % |
|---|---|---|
|
| ||
| Age (Years) | 5,790 | 63 ± 10 |
| Non-Hispanic Whites | 2,152 | 37% |
| Blacks | 1,670 | 29% |
| Hispanics | 1,264 | 22% |
| Chinese | 704 | 12% |
| Women | 3,017 | 52% |
| Former tobacco use | 2136 | 37% |
| Current tobacco use | 723 | 13% |
| BMI (kg/m2) | 5.790 | 28.3 ± 5.5 |
| Obesity | 1,916 | 33% |
| Hypertension | 2,634 | 46% |
| Antihypertensive medication use | 2,190 | 38% |
| Systolic Blood Pressure (mmHg) | 5,788 | 127 ± 22 |
| Diastolic Blood Pressure (mmHg) | 5,788 | 72 ± 10 |
| Fasting glucose (mmol/l) | 5,780 | 5.44 ± 1.72 |
| Diabetes | 744 | 13% |
| Diabetes medication use | 574 | 10% |
| Triglycerides (mmol/l) | 5,783 | 1.48 ± 0.90 |
| Total cholesterol (mmol/l) | 5,783 | 5.02 ± 0.93 |
| HDL-cholesterol (mmol/l) | 5,780 | 1.32 ± 0.39 |
| LDL-cholesterol (mmol/l) | 5,710 | 3.03 ± 0.83 |
| Lipid-lowering therapy use | 973 | 17% |
| Statins | 891 | 15% |
| Fibrates | 64 | 1.1% |
| eGFR (ml/min/1.73 m2) | 5,780 | 89 ± 21 |
| Total apoC-III (mg/dl) | 5,784 | 9.4 ± 4.1 |
C-III1 was the most abundant proteoform (median, 60% of total peak area), followed by C-III2 (21 %), C-III0b (12 %) and C-III0a (7 %)(Figure S1). Percentages of all proteoforms correlated only modestly to moderately with total apoC-III concentration; inversely for C-III2 (r= −0.30) and positively for other proteoforms (r= 0.07, C-III0a; r= 0.14, C-III0b and r= 0.25, C-III1) (all p<0.0001, Table S1).
The percentages of apoC-III proteoforms were associated with all tested clinical and demographic characteristics in the unadjusted models (Table S2). In adjusted models, we first examined the relationship of apoC-III proteoform composition with non-modifiable demographic characteristics (Table 2). C-III2 was higher in older participants; C-III0b and C-III1 were higher in women; and compared with Whites, C-III2 was higher in Blacks, C-III0a and C-III1 were higher in Hispanics and C-III0a and C-III2 were higher in Chinese. As indicated by Pillai’s trace values, 9% of the variance in the apoC-III proteoform composition in the model was explained by age, 6% by gender and 18% by race/ethnicity (Table 2). The association of apoC-III proteoform composition with both age and race/ethnicity differed by gender (p<0.0001 for interaction, Figure S2). Additionally, among women in the age group of 45 to 54 years with known menopausal status, those who had gone through menopause had significantly lower C-III2 and higher C-III1 (Figure S3).
Table 2.
ApoC-III proteoforms by age, gender and race/ethnicity.
| Variable | N | C-III0a | C-III0b | C-III1 | C-III2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| % peak area Mean ± SD |
% SD change β [95% CI] |
% peak area Mean ± SD |
% SD change β [95% CI] |
% peak area Mean ± SD |
% SD change β [95% CI] |
% peak area Mean ± SD |
% SD change β [95% CI] |
||
|
|
|||||||||
| Age group | Pillai’s Trace = 0.09, p<0.001 | ||||||||
| 45–54 years | 1,560 | 7.6 ± 3.6 | 0 | 13 ± 2.6 | 0 | 60 ± 5.2 | 0 | 20 ± 6.1 | 0 |
| 55–64 years | 1,562 | 7.7 ± 3.7 | 0.3 [−6.5, 7.1] | 12 ± 2.6 | −6.6 [−13, −0.1] | 60 ± 5.2 | 2.3 [−4.3, 8.9] | 20 ± 6.1 | 1.0 [−5.3, 7.5] |
| 65–74 years | 1,785 | 7.4 ± 3.5 | −6.8 [−13, −0.3] | 11 ± 2.6 | −42 [−48, −35] | 59 ± 5.4 | −10 [−17, −4.1] | 22 ± 6.5 | 30 [24, 37] |
| 75–84 years | 883 | 7.2 ± 3.5 | −12 [−19, −3.8] | 10 ± 2.4 | −78 [−86, −71] | 58 ± 5.8 | −25 [−33, −17] | 24 ± 7.1 | 57 [50, 65] |
|
|
|||||||||
| Gender | Pillai’s Trace = 0.06, p<0.001 | ||||||||
| Men | 2,773 | 7.8 ± 3.7 | 0 | 11 ± 2.6 | 0 | 58 ± 5.2 | 0 | 23 ± 6.6 | 0 |
| Women | 3,017 | 7.2 ± 3.4 | −17 [−22, −12] | 12 ± 2.7 | 25 [20, 29] | 60 ± 5.3 | 40 [36, 45] | 20 ± 6.3 | −37 [−41, −32] |
|
|
|||||||||
| Race/ethnicity | Pillai’s Trace = 0.18, p<0.001 | ||||||||
| White | 2,152 | 7.1 ± 3.5 | 0 | 12 ± 2.7 | 0 | 60 ± 5.2 | 0 | 20 ± 6.2 | 0 |
| Black | 1,670 | 6.6 ± 3.0 | −14 [−20, −7.7] | 11 ± 2.6 | −48 [−54, −42] | 58 ± 5.6 | −44 [−50, −38] | 24 ± 6.7 | 65 [59, 71] |
| Hispanic | 1,264 | 8.2 ± 3.6 | 31 [24, 38] | 12 ± 2.6 | −15 [−21, −8.1] | 61 ± 4.7 | 7.9 [1.4, 14] | 19 ± 5.5 | −16 [−22, −9.9] |
| Chinese | 704 | 9.6 ± 4.0 | 64 [56, 73] | 11 ± 2.6 | −42 [−51, −35] | 57 ± 5.0 | −58 [−66, −50] | 22 ± 6.1 | 32 [24, 39] |
Data are means ± SD, and percent difference in apoC-III proteoforms (scaled to 1 SD of natural log-transformed values) by each demographic variable compared with the reference group (indicated as “0”). Multiple linear regression was used to test the association of each individual apoC-III proteoform with age, gender and race/ethnicity (all included in the same multivariable model). For example, C-III2 was higher in Blacks compared to Whites by 65% of 1 SD. Pillai’s Trace values indicate the variance in overall apoC-III proteoform composition (defined as additive log-ratios of apoC-III proteoforms to C-III1) explained by differences in the levels of the demographic variables tested by multivariate analysis of variance (MANOVA). For example, race/ethnicity accounted for 0.18, i.e., 18% of the variance in proteoform composition. All 95% CI not crossing zero value are consistent with p<0.05.
Next, we tested the relationship of apoC-III proteoform composition with clinical variables. After adjustment for age, gender, race/ethnicity and total apoC-III concentrations, apoC-III proteoforms were associated with BMI, obesity, fasting plasma glucose and/or diabetes status, tobacco use, use of lipid lowering therapy and eGFR (Table 3). C-III0a and C-III2 were lower while C-III0b and C-III1 were higher in those with higher BMI and obesity, and in those with higher fasting glucose and/or diabetes. C-III2 was higher and all other proteoforms were lower in those receiving lipid lowering therapy. C-III2 was also higher in current tobacco users and those with worse kidney function.
Table 3:
Relationship of key clinical characteristics with apoC-III proteoform composition.
| Variable | C-III0a (% SD) |
P-value | C-III0b (% SD) |
P-value | C-III1 (% SD) |
P-value | C-III2 (% SD) |
P-value | Pillai’s trace |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| BMI (1 SD) | −10 [−13, −7.7] | <0.001 | 12 [9.4, 15] | <0.001 | 27 [25, 30] | <0.001 | −22 [−24, −19] | <0.001 | 0.10 |
| Obesity | −17 [−22, −11] | <0.001 | 14 [8.7, 20] | <0.001 | 40 [35, 45] | <0.001 | −31 [−36, −26] | <0.001 | 0.05 |
| Fasting glucose (1 SD) | −13 [−16, −11] | <0.001 | 2.7 [0.2, 5.2] | 0.034 | 17 [15, 20] | <0.001 | −8.2 [−11, −5.8] | <0.001 | 0.03 |
| Diabetes | −45 [−52, −37] | <0.001 | 6.1 [−1.2, 13] | 0.10 | 21 [14, 28] | <0.001 | −0.1 [−7.0, 6.9] | 0.96 | 0.02 |
| Lipid lowering therapy | −33 [−40, −26] | <0.001 | −9.4 [−16, −2.9] | 0.004 | −7.0 [−13, −0.5] | 0.034 | 24 [18, 30] | <0.001 | 0.02 |
| Tobacco use | 0.003 | ||||||||
| Former (vs. never) | 2.0 [−3.6, 7.7] | 0.47 | −1.6 [−7.0, 3.8] | 0.56 | 2.8 [−2.6, 8.2] | 0.31 | −1.2 [−6.3, 3.9] | 0.64 | |
| Current (vs. never) | −0.7 [−8.7, 7.4] | 0.87 | −9.2 [−17, −1.5] | 0.020 | −15 [−23, −7.2] | <0.001 | 17 [9.6, 24] | <0.001 | |
| eGFR (1 SD) | 3.2 [0.5, 5.9] | 0.018 | 3.5 [0.9, 6.1] | 0.008 | 9.3 [6.8, 12] | <0.001 | −10 [−13, −7.8] | <0.001 | 0.007 |
Data are β-estimates [95% CI] of percent difference in 1 SD of natural log-transformed of each apoC-III proteoform for each category, or 1 SD of natural log-transformed continuous variables. Multiple linear regression models were adjusted for age, gender and race/ethnicity, interaction terms of age and race/ethnicity with gender, and total apoC-III concentrations. For example, after these adjustments, an increase of 1 SD in BMI was associated with an increase of 27% of 1 SD in relative amounts of C-III1. Pillai’s trace values indicate the variance in overall apoC-III proteoform composition (tested as additive log-ratios to C-III1) explained by differences in the levels of the clinical characteristics, assessed by multivariate analysis of variance (MANOVA).
The number of participants with available lipid measurements at each exam is shown in Table S3. In baseline cross-sectional models including total apoC-III and all ALRs of apoC-III proteoforms to C-III1, plasma triglycerides were positively associated with total apoC-III and negatively associated with C-III2/C-III1 (Figure 1A). In longitudinal models (also including all apoC-III measures), follow-up triglyceride levels (adjusted for baseline levels) were positively associated with total apoC-III and negatively associated with all proteoform ratios, with the largest negative estimate for C-III2/C-III1 (Figure 1B). In cross-sectional models, total cholesterol was positively associated with total apoC-III and negatively associated with C-III2/C-III1 (Figure 1C). In longitudinal models, total cholesterol was positively associated with C-III2/C-III1 (Figure 1D). LDL cholesterol was positively associated with total apoC-III and negatively associated with C-III2/C-III1 in cross-sectional models. In longitudinal models, LDL cholesterol was positively associated with total apoC-III after adjustment, and was negatively associated with C-III0a/C-III1 (Figures 1E and F). In cross-sectional analyses, HDL cholesterol was positively associated with total apoC-III, C-III0b/C-III1 and C-III2/C-III1 (Figure 1G). In longitudinal analyses, HDL cholesterol was negatively associated with total apoC-III, and positively associated with C-III0a/C-III1 and C-III2/C-III1 (Figure 1H). In analyses of individual apoC-III proteoform percentages, higher relative amounts of C-III1 and lower C-III2 were associated with higher triglycerides and lower HDL in both cross-sectional and longitudinal models (Figure S4).
Figure 1.
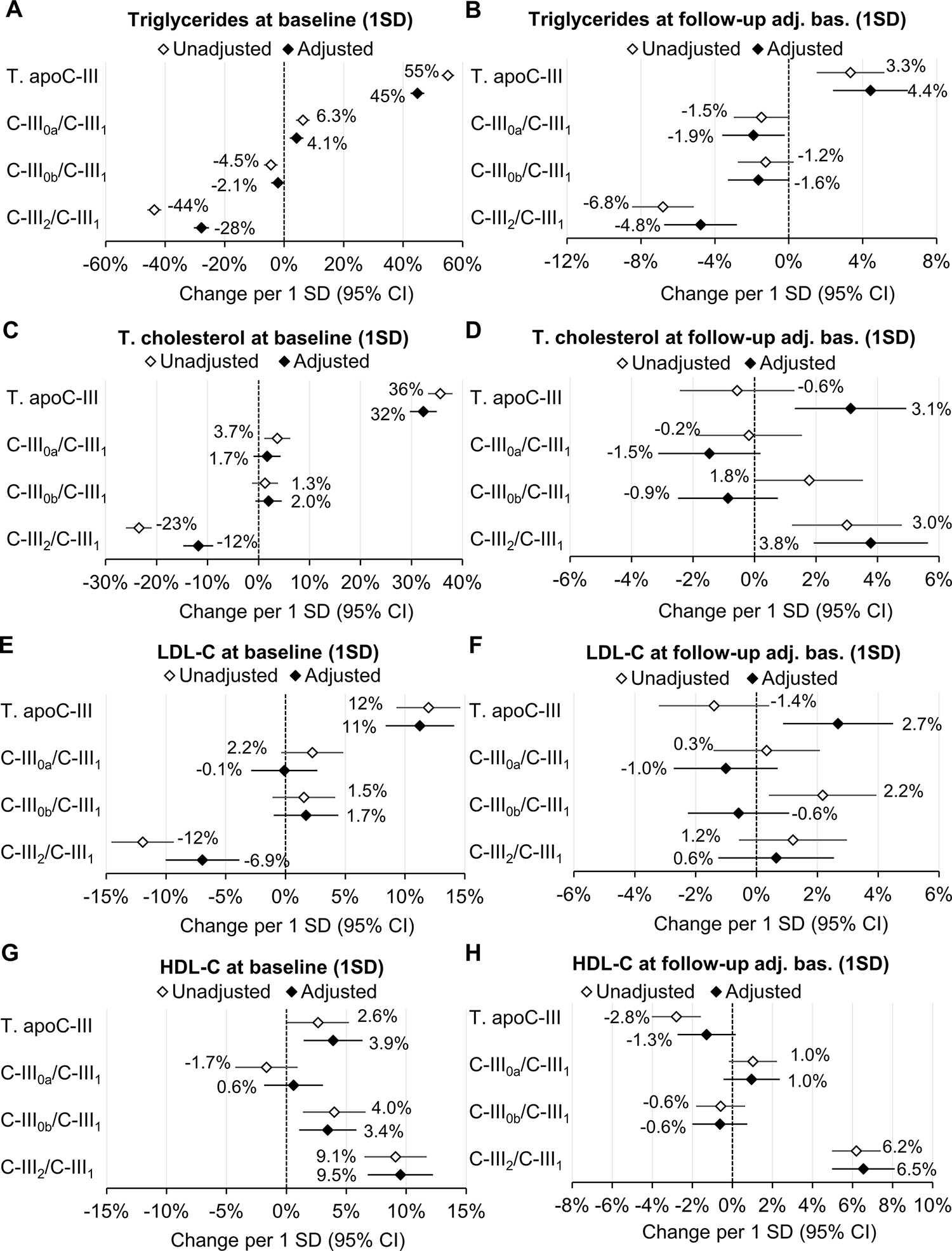
Cross-sectional (baseline; left panels) and longitudinal (follow-up adjusted for baseline and time of follow-up; right panels) relationships of baseline total apoC-III concentrations, and log-ratios of apoC-III proteoforms to C-III1 (all included in the same additive model) with plasma lipids. Multiple linear regression models were run unadjusted (total apoC-III and proteoforms ratios in separate models) and adjusted (total apoC-III and proteoforms ratios in the same model) for baseline age, gender, race/ethnicity, BMI, diabetes status, fasting glucose, tobacco use, lipid-lowering therapy and eGFR. Longitudinal mixed regression models for repeated measures were further adjusted for BMI, diabetes status, fasting glucose, tobacco use, lipid-lowering therapy and eGFR at each follow-up exam. Symbols and labels are β-estimates. All 95% CI not crossing the zero-x-axis value are consistent with p<0.05. All apoC-III and lipid measures were natural log-transformed and scaled to 1 SD, e.g., an increase of 1 SD in C-III2/C-III1 (adjusted model) was associated with reductions of 28% and 4.5% of 1 SD in baseline and follow-up plasma triglycerides, respectively.
We also tested whether the associations of clinical and demographic characteristics with plasma triglycerides and HDL-cholesterol were influenced by adjustment for total apoC-III and apoC-III proteoform ALRs (Table 4). The negative association of age with plasma triglycerides was nullified after adjustment for total apoC-III and became positive after further adjustment for proteoform ALRs. The negative association of Black race with plasma triglycerides was weaker after adjustment for total apoC-III and further attenuated upon adjustment for proteoform ALRs. Higher plasma HDL levels persisted in Black participants and were unchanged after adjustment for total apoC-III, but this HDL elevation was reduced after further adjustment for proteoform ALRs. Adjustment for proteoform ALRs also attenuated the positive association of BMI with plasma triglycerides.
Table 4.
Relationship of clinical and demographic characteristics with plasma triglycerides and HDL cholesterol before (Model 1) and after adjustment for total apoC-III (Model 2) and log-ratios of apoC-III proteoforms to C-III1 (additive model, Model 3).
| Characteristic | Triglycerides (% SD) | HDL-cholesterol (% SD) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
|
| ||||||
| Age (1 SD) | −3.3 [−5.9, −0.8] | −0.7 [−2.9, 1.5] | 2.4 [0.3, 4.6] | 7.4 [4.9, 9.8] | 7.5 [5.0, 9.9] | 7.0 [4.5, 9.5] |
| Women | −4.0 [−8.9, 0.9] | −19 [−23, −14] | −27 [−31, −23] | 80 [75, 84] | 79 [74, 84] | 82 [78, 87] |
| Blacks (vs. Whites) | −53 [−59, −46] | −29 [−35, −24] | −11 [−16, −5.8] | 16 [9.9, 21] | 16 [11, 22] | 11 [4.6, 17] |
| Hispanics (vs. Whites) | 21 [15, 28] | 23 [18, 29] | 21 [16, 27] | −17 [−23, −11] | −17 [−23, −11] | −16 [−23, −10] |
| Chinese (vs. Whites) | 29 [21, 37] | 30 [23, 37] | 35 [28, 42] | −29 [−36, −21] | −29 [−36, −21] | −31 [−39, −23] |
| BMI (1 SD) | 19 [17, 22] | 18 [16, 21] | 12 [10, 15] | −26 [−28, −23] | −26 [−28, −23] | −24 [−26, −21] |
| Fasting glucose (1 SD) | 17 [14, 21] | 12 [8.9, 15] | 8.8 [6.0, 12] | −8.5 [−12, −5.4] | −8.8 [−12, −5.6] | −7.4 [−11, −4.2] |
| Diabetes | −1.5 [−11, 8.3] | −4.1 [−12, 4.2] | 4.3 [−3.6, 12] | −11 [−20, −1.6] | −11 [−20, −1.7] | −14 [−23, −4.6] |
| Lipid lowering therapy | 12 [5.7, 19] | 2.4 [−3.1, 7.9] | 9.7 [4.4, 15] | −1.7 [−7.7, 4.4] | −2.0 [−8.1, 4.1] | −3.9 [−10, 2.2] |
| Former tobacco use | −2.6 [−8.0, 2.7] | −4.2 [−8.7, 0.3] | −4.7 [−8.9, −0.4] | 8.8 [3.8, 14] | 8.8 [3.8, 14] | 8.9 [4.0, 14] |
| Current tobacco use | 28 [20, 36] | 25 [19, 32] | 29 [23, 35] | −21 [−28, −14] | −21 [−28, −14] | −22 [−29, −15] |
| eGFR (1 SD) | −6.4 [−8.9, −3.8] | −0.3 [−2.5, 1.9] | −3.3 [−5.4, −1.2] | 4.0 [1.6, 6.4] | 1.8 [−0.5, 4.1] | 5.3 [2.8, 7.7] |
Data are β-estimates [95% CI] of percent changes of 1 SD of natural log-transformed plasma lipids by clinical category or 1 SD of continuous variables. For example, a 53% reduction (of 1 SD) in plasma triglycerides in Blacks (vs. Whites) was attenuated to a 29% reduction (of 1 SD) in Model 2 and to 11% in Model 3. All 95% CI not crossing zero value are consistent with p<0.05.
A total of 669 participants developed a CVD event out of 5,766 with available surveillance data through the end of 2017 (median time to an event 8.5 years). Kaplan-Meier curves showed significant differences in CVD risk between those with high and low C-III0b/C-III1 and C-III2/C-III1, while there were no significant differences between those with high and low total apoC-III and C-III0a/C-III1 (Figure 2A). In Cox regression models, C-III0b/C-III1 was inversely associated with CVD risk in the minimally adjusted model (containing all proteoform ALRs and total apoC-III) and after adjustments for baseline clinical and demographic characteristics, plasma triglycerides and HDL cholesterol (Figure 2B). Higher C-III2/C-III1 was also associated with higher CVD risk in the minimally adjusted model, however the association was attenuated after further adjustment for clinical characteristics (Figure 2B). These associations of apoC-III proteoforms with CVD were similar between different demographic subgroups, except for a weaker association of C-III0b/C-III1 with CVD in Whites (HR 0.88 [95% CI 0.77–1.001], p=0.002 for interaction) (Table S4). In analyses of individual apoC-III proteoform percentages, higher CVD risk was associated with lower C-III0b and higher C-III2 in all models (Figure S5).
Figure 2. Association between baseline total apoC-III concentrations and log-ratios of apoC-III proteoforms to apoC-III1 (most abundant), and CVD events.
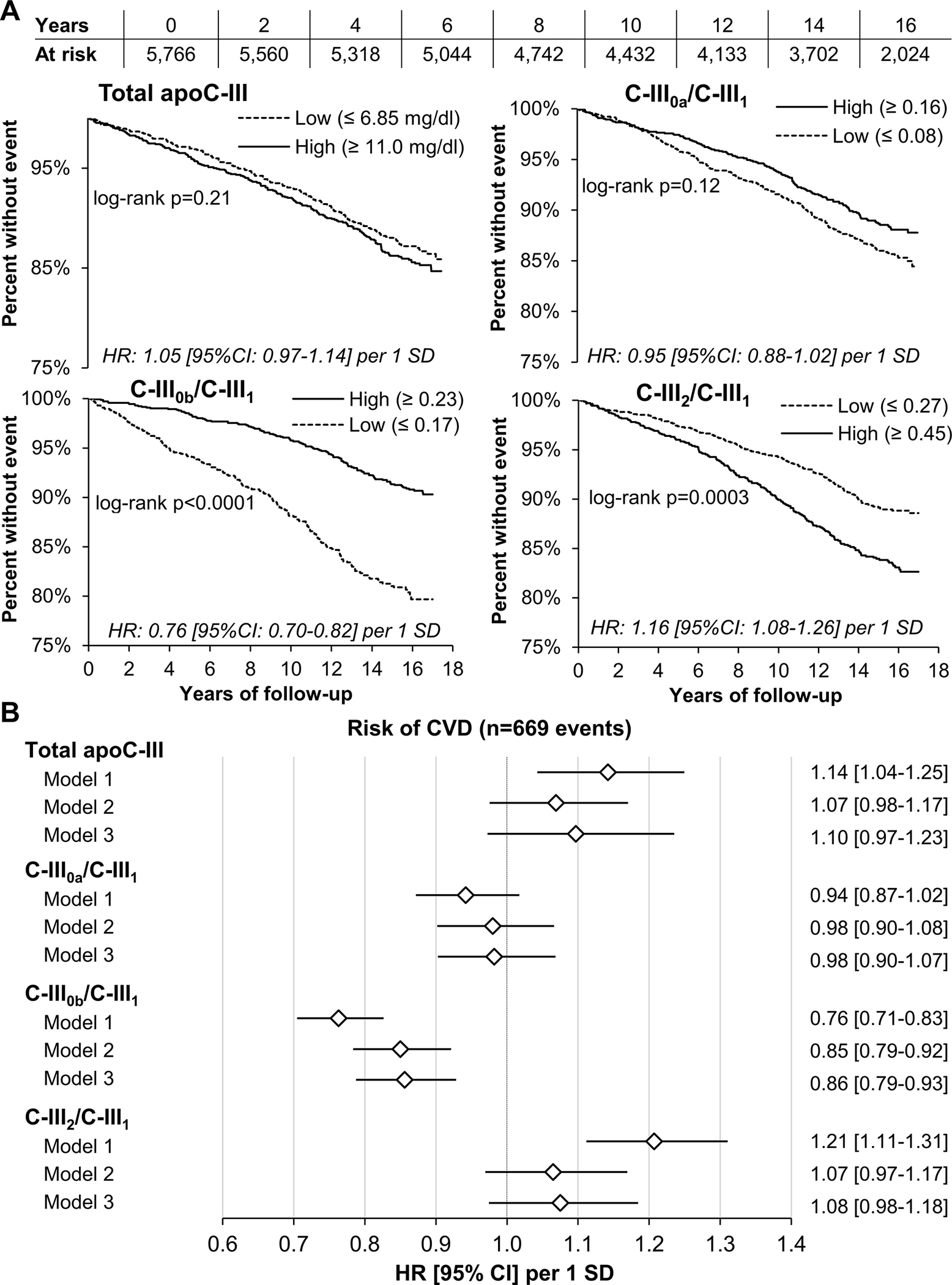
Panel A: Kaplan-Meier curves of top (High) and bottom (Low) quartile of apoC-III measures for entire follow-up period. Unadjusted Cox proportional hazard ratios (HR) and 95% confidence intervals (95% CI) per 1 SD of apoC-III measures are listed in each figure. Panel B: Cox proportional risk models of CVD risk. Model 1 included total apoC-III and apoC-III proteoforms ratios; Model 2 also included baseline age, gender, race/ethnicity, BMI, diabetes, systolic blood pressure, use of antihypertensive and lipid lowering medications, smoking status and eGFR, and Model 3 was further adjusted for plasma triglycerides and HDL cholesterol. All apoC-III measures were natural log-transformed and expressed per 1 SD unit.
The significant inverse association between C-III0b/C-III1 and CVD was also observed for the 10-year CVD risk (Figure S6). Addition of total apoC-III and proteoform ALRs to the 10-year PCE estimator did not change the C-index but improved NRI, i.e., the proportion of individuals correctly upgraded or downgraded in their eventual risk by 24%, and relative IDI, i.e., the difference between slopes of prediction curves by 16% for CVD risk (p<0.0001 for both) (Table S5).
Discussion
The present analyses revealed relationships of apoC-III proteoform composition with multiple demographic and clinical characteristics, including age, gender, race/ethnicity, obesity and fasting glucose status, cross-sectional and longitudinal changes in plasma lipids as well as incident CVD events. Most notably, C-III2 levels were higher in older individuals, in males and in Black and Chinese participants. Higher C-III2 levels were also associated with leaner phenotype and lower fasting glucose levels, more favorable plasma lipid profiles but not with future CVD events. CVD risk was, however, inversely related to baseline C-III0b, a proteoform that showed a relative weak association with typical clinical and demographic determinants of CVD risk, including plasma lipids. Importantly, all of these associations were independent of total plasma apoC-III levels, suggesting posttranslational apoC-III proteoforms may be influenced by clinical characteristics and may have distinct roles in regulation of plasma lipids levels and future CVD risk.
The cross-sectional associations between apoC-III proteoforms and plasma triglycerides shown in this community-based setting are consistent with previous observations in smaller cohorts comprised of individuals with various degrees of impaired glucose regulation.26,30,37 The novel finding of an inverse association between baseline C-III2/C-III1 and longitudinal changes in plasma lipids supports a direct role for the type of apoC-III sialylation in long-term regulation of triglyceride metabolism. The inverse associations of C-III2/C-III1 with plasma triglycerides was paralleled by a positive association between C-III2/C-III1 and HDL cholesterol. Although this could be explained by increased cholesterol transfer from VLDL to HDL due to improved triglyceride clearance with this apoC-III proteoform pattern,38 further studies are needed to test the possibility of direct effects of apoC-III sialylation on HDL metabolism.
ApoC-III proteoform composition was also associated with several other metabolic characteristics. C-III2 and C-III0a were lower while C-III1 and C-III0b were higher in obese participants. Recently, Mendoza et al. demonstrated that three years of dietary weight-loss intervention was associated with significant reductions in plasma concentrations of total apoC-III and all apoC-III proteoforms except for C-III2.39 In that study, C-III2 concentrations trended higher after the intervention, indicating even greater, and presumably significant increases in the relative C-III2 amounts. In addition to their relationships with obesity, C-III1 was higher while C-III2 was lower in participants with higher fasting glucose in our study. In the liver, lipoproteins with higher amounts of C-III2 are cleared primarily by heparan sulfate proteoglycans (HSPGs), whereas lipoproteins containing more C-III1 are preferentially cleared by LDL-receptor (LDL-R) and LDL-R related protein 1 (LRP1).40 The translocation of LRP1 from intracellular vesicles to the plasma membrane depends on intact insulin action.41 Thus, the membrane LRP1 content may be reduced in insulin resistance, potentially leading to retention of C-III1 enriched lipoproteins in circulation. Further supporting the role of insulin resistance in altering apoC-III proteoform composition, treatment with insulin-sensitizing drug pioglitazone increased C-III2 and reduced C-III1 in persons with prediabetes.26 Altogether these data indicate that altered apoC-III proteoform composition may help explain the link between insulin resistance and obesity with high triglycerides and low HDL levels.
The finding of higher C-III2 levels in Black participants is consistent with previous observation from a smaller clinical trial cohort of individuals with prediabetes.26 Significant differences in composition of apoC-III proteoforms between Whites and Hispanics or Chinese provide additional evidence for a broader role of racial and ethnic background in posttranslational apoC-III processing. Moreover, our analyses indicate that higher C-III2 may be a key factor explaining lower triglyceride and higher HDL cholesterol levels in Blacks. Here we show for the first time that apoC-III proteoforms also differ by age and gender and that higher apoC-III2 may in part underly the previously shown decline in triglycerides in elderly.42 We have also observed that premenopausal women had even higher C-III2 and lower C-III1 compared with postmenopausal women, suggesting involvement of sex hormones in controlling apoC-III proteoforms composition.
Our analyses demonstrated a strong inverse association of CVD risk with C-III0b, which did not show a particularly strong relationship with typical CVD risk factors, including plasma lipid levels. In a recent report, however, higher C-III0b when transported in LDL particles was associated with a less proatherogenic phenotype characterized by larger LDL size and a lower lipoprotein-insulin resistance score.43 C-III0b did not show an association with incident CVD in our previous analyses of a subcohort of Veterans Affairs Diabetes Trial (VADT) 26; however, that was a far smaller, predominantly White and much higher CVD risk cohort. Of note, as suggested by the present data, the association between C-III0b and CVD appears weaker in Whites.
Lower C-III0b may indicate reduced glycosylation of its precursor C-III0a and/or increased downstream formation of sialylated apoC-III proteoforms. Greater methylation, i.e., epigenetic modification typically suppressing gene expression, of the N-Acetylgalactosaminyltransferase 2 (GALNT2) gene catalyzing O-glycosylation of C-III0a (which in turn may decrease formation of C-III0b) is associated with increased risk of coronary heart disease.44 Loss of function mutations in GALNT2 are also associated with lower HDL cholesterol levels.45 Nevertheless, the association between C-III0b and CVD risk remained significant even after adjustment for both plasma triglycerides and HDL cholesterol, indicating involvement of more complex facets of lipid metabolism, beyond standard plasma lipid levels, and potential lipid-unrelated mechanisms.
Higher C-III2/C-III1, indicating increased sialylation, was associated with increased CVD risk in the minimally adjusted model. However, the association was attenuated after adjustment for clinical and demographic risk factors. Given the strong positive association of C-III2 with age, male gender and Black race, the apparent CVD risk associated with increased C-III2/C-III1 may be secondary to these demographic factors. However, the positive association between the individual percentage of C-III2 and CVD in the fully adjusted model suggests a direct adverse effect of increased apoC-III sialylation in the cardiovascular system. In preclinical studies, sialic acid triggered myocardial injury and promoted coagulation while sialylation was required for proinflammatory action of apoC-III in endothelial cells.27,46,47 In humans, both CVD or thrombotic events were positively associated with sialic acid levels.48,49
There is increasing awareness of the complex relationships between apolipoproteins, lipid levels and CVD risk. Recent mass spectrometry analysis using samples from several clinical trials demonstrated that levels of several apolipoproteins including apoC-III, apoC-I, apoE and apoB were associated with a 2–3 fold higher risk of coronary heart disease after adjustment for traditional clinical risk factors, including plasma lipids.50 Our data show that apoC-III proteoform composition may account for some of the additional variation in both lipid levels and CVD risk. Moreover, apoC-III proteoform levels differed substantially between gender and racial/ethnic groups and accounted for some of their differences in plasma lipid levels. Thus, measurement of apolipoprotein modifications may further improve personalized CVD risk evaluation.
Besides the prognostic value for future plasma lipids and CVD risk, assessment of apoC-III proteoform composition may have potential therapeutic implications. Inhibition of apoC-III expression with the first-generation antisense oligonucleotide volanesorsen in individuals with hypertriglyceridemia was associated with profound increases in C-III2/C-III1.40 It is possible that this may in part explain the triglyceride-lowering action of volanesorsen. On the other hand, the increase in relative amount of C-III2 may warrant some caution regarding the long-term CVD effects of this type of lipid-lowering therapy.
A major strength of the present study was the measurement of apoC-III proteoform composition in a large demographically diverse and systematically observed longitudinal cohort. The cohort size allowed robust statistical modeling with adjustment for many relevant covariates as well as analyses in several subgroups. For almost all participants we were able to match apoC-III mass spectrometry results with total plasma concentrations measured previously by enzymatic assay.14 Similar to the previous report spanning 11 years of follow-up,13 the association between total apoC-III levels in plasma and incident CVD over 17-year follow period was abolished once adjusted for clinical and demographic covariates. By including apoC-III proteoforms measures with total apoC-III in additive models we were able to demonstrate their independence and added prognostic value to plasma lipids and CVD risk.
Although the cohort size and comprehensive phenotyping permitted robust statistical modeling, the study conclusions are based on association analyses. Many of the novel relationships need to be examined by more direct mechanistic models to confirm causality and to identify underlying pathways. Previous studies have showed that the associations of apoC-III with atherosclerosis depend on the type of lipoproteins carrying apoC-III.14,51 In fact, apoC-III carried on HDL was shown to be a better predictor of both subclinical and clinical coronary atherosclerosis than total apoC-III in plasma.13,14 We were unable to ascertain whether the associations between apoC-III proteoform composition and study outcomes differ by lipoprotein species. However, according to a recent report, the distribution of apoC-III proteoforms is relatively uniform across different lipoprotein species.43 Thus, the measurement of apoC-III proteoforms in whole plasma appears to be a valid overall indicator of their distribution in distinct lipoproteins.
In summary, our results suggest that apoC-III proteoforms may provide additional information beyond that indicated by total apo-CIII measurements. Measuring apoC-III proteoforms may be a useful prognostic tool for future lipid patterns and cardiovascular risk. Greater understanding of the factors regulating their concentrations and composition may not only provide new insights into lipid metabolism and differences in CVD risk, but may in turn lead to development of new therapeutic strategies for dyslipidemia and prevention of CVD.
Supplementary Material
Highlights.
ApoC-III proteoform composition was associated with age, sex and race/ethnicity
Higher relative amount of disialylated apoC-III (C-III2) was associated with more favorable cardiometabolic profile, including lower BMI, lower fasting plasma glucose and triglycerides levels, and higher plasma HDL cholesterol levels
Increased relative amount of glycosylated and non-sialylated apoC-III (C-III0b) was associated with reduced cardiovascular risk
The relationship of apoC-III proteoform composition with clinical and demographic characteristics, and cardiovascular risk was independent of total apoC-III concentrations
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The contents of this article do not represent the views of the Department of Veterans Affairs or The United States Government.
Sources of funding:
This study was supported by the National Heart, Lung, and Blood Institute grant R01- HL-138969. MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Non-standard Abbreviations and Acronyms:
- ALR
additive log-ratio
- apoC-III
apolipoprotein C-III
- CVD
cardiovascular disease
- HR
hazard ratio
- LDL-R
LDL receptor
- LRP1
LDL receptor related protein 1
- MESA
Multi-Ethnic Study of Atherosclerosis
- MSIA
mass spectrometry immunoassay
- TRL
triglyceride-rich lipoproteins
Footnotes
Disclosures: Jeremy Furtado is currently an employee of Biogen.
References
- 1.Sacks FM, Zheng C, Cohn JS. Complexities of plasma apolipoprotein C-III metabolism. J Lipid Res. 2011;52:1067–1070. doi: 10.1194/jlr.E015701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller M Apolipoprotein C-III: The Small Protein With Sizeable Vascular Risk. Arterioscler Thromb Vasc Biol. 2017;37:1013–1014. doi: 10.1161/ATVBAHA.117.309493 [DOI] [PubMed] [Google Scholar]
- 3.Brown WV, Baginsky ML. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972;46:375–382. [DOI] [PubMed] [Google Scholar]
- 4.Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985;26:556–565. [PubMed] [Google Scholar]
- 5.Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest. 1985;75:384–390. doi: 10.1172/JCI111711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, Hussain MM, Parks RJ, Wang Y, Yao Z. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51:150–161. doi: 10.1194/M900346-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–687. doi: 10.1161/CIRCULATIONAHA.106.622514 [DOI] [PubMed] [Google Scholar]
- 8.Kawakami A, Osaka M, Tani M, Azuma H, Sacks FM, Shimokado K, Yoshida M. Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation. 2008;118:731–742. doi: 10.1161/CIRCULATIONAHA.108.784785 [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, Pfeffer MA, Braunwald E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–1892. [DOI] [PubMed] [Google Scholar]
- 10.Klein RL, McHenry MB, Lok KH, Hunter SJ, Le NA, Jenkins AJ, Zheng D, Semler AJ, Brown WV, Lyons TJ, et al. Apolipoprotein C-III protein concentrations and gene polymorphisms in type 1 diabetes: associations with lipoprotein subclasses. Metabolism. 2004;53:1296–1304. doi: 10.1016/j.metabol.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 11.Hodis HN, Mack WJ, Azen SP, Alaupovic P, Pogoda JM, LaBree L, Hemphill LC, Kramsch DM, Blankenhorn DH. Triglyceride- and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation. 1994;90:42–49. doi: 10.1161/01.cir.90.1.42 [DOI] [PubMed] [Google Scholar]
- 12.Pek SLT, Sum CF, Yeoh LY, Lee SBM, Tang WE, Lim SC, Tavintharan S. Association of apolipoprotein-CIII (apoC-III), endothelium-dependent vasodilation and peripheral neuropathy in a multi-ethnic population with type 2 diabetes. Metabolism. 2017;72:75–82. doi: 10.1016/j.metabol.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 13.Jensen MK, Aroner SA, Mukamal KJ, Furtado JD, Post WS, Tsai MY, Tjonneland A, Polak JF, Rimm EB, Overvad K, et al. High-Density Lipoprotein Subspecies Defined by Presence of Apolipoprotein C-III and Incident Coronary Heart Disease in Four Cohorts. Circulation. 2018;137:1364–1373. doi: 10.1161/CIRCULATIONAHA.117.031276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aroner SA, Koch M, Mukamal KJ, Furtado JD, Stein JH, Tattersall MC, McClelland RL, Jensen MK. High-Density Lipoprotein Subspecies Defined by Apolipoprotein C-III and Subclinical Atherosclerosis Measures: MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7:e007824. doi: 10.1161/JAHA.117.007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner T, Shao B, Eckel RH, Heinecke JW, Bornfeldt KE, Snell-Bergeon J. Association of apolipoprotein C3 with insulin resistance and coronary artery calcium in patients with type 1 diabetes. J Clin Lipidol. 2021;15:235–242. doi: 10.1016/j.jacl.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanter JE, Shao B, Kramer F, Barnhart S, Shimizu-Albergine M, Vaisar T, Graham MJ, Crooke RM, Manuel CR, Haeusler RA, et al. Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J Clin Invest. 2019;129:4165–4179. doi: 10.1172/jci127308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027 [DOI] [PubMed] [Google Scholar]
- 18.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo GT, Meigs JB, Cupples LA, Demissie S, Otvos JD, Wilson PWF, Lahoz C, Cucinotta D, Couture P, Mallory T, et al. Association of the Sst-I polymorphism at the APOC3 gene locus with variations in lipid levels, lipoprotein subclass profiles and coronary heart disease risk: the Framingham offspring study. Atherosclerosis. 2001;158:173–181. doi: 10.1016/s0021-9150(01)00409-9 [DOI] [PubMed] [Google Scholar]
- 20.Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, et al. Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. J Am Coll Cardiol. 2017;69:789–800. doi: 10.1016/j.jacc.2016.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, et al. Antisense Inhibition of Apolipoprotein C-III in Patients with Hypertriglyceridemia. N Engl J Med. 2015;373:438–447. doi: 10.1056/NEJMoa1400283 [DOI] [PubMed] [Google Scholar]
- 22.Bondarenko PV, Cockrill SL, Watkins LK, Cruzado ID, Macfarlane RD. Mass spectral study of polymorphism of the apolipoproteins of very low density lipoprotein. Journal of Lipid Research. 1999;40:543–555. [PubMed] [Google Scholar]
- 23.Ito Y, Breslow JL, Chait BT. Apolipoprotein C-III0 lacks carbohydrate residues: use of mass spectrometry to study apolipoprotein structure. Journal of Lipid Research. 1989;30:1781–1787. [PubMed] [Google Scholar]
- 24.Holdsworth G, Stocks J, Dodson P, Galton DJ. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J Clin Invest. 1982;69:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann CJ, Troussard AA, Yen FT, Hannouche N, Najib J, Fruchart JC, Lotteau V, Andre P, Bihain BE. Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride-rich lipoproteins to the lipolysis-stimulated receptor. J Biol Chem. 1997;272:31348–31354. doi: 10.1074/jbc.272.50.31348 [DOI] [PubMed] [Google Scholar]
- 26.Koska J, Yassine H, Trenchevska O, Sinari S, Schwenke DC, Yen FT, Billheimer D, Nelson RW, Nedelkov D, Reaven PD. Disialylated apolipoprotein C-III proteoform is associated with improved lipids in prediabetes and type 2 diabetes. J Lipid Res. 2016;57:894–905. doi: 10.1194/jlr.P064816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiukka A, Stahlman M, Pettersson C, Levin M, Adiels M, Teneberg S, Leinonen ES, Hulten LM, Wiklund O, Oresic M, et al. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 2009;58:2018–2026. doi: 10.2337/db09-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauger JF, Couture P, Bergeron N, Lamarche B. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J Lipid Res. 2006;47:1212–1218. doi: 10.1194/jlr.M500455-JLR200 [DOI] [PubMed] [Google Scholar]
- 29.Kashyap ML, Srivastava LS, Hynd BA, Gartside PS, Perisutti G. Quantitation of human apolipoprotein C-III and its subspecie by radioimmunoassay and analytical isoelectric focusing: abnormal plasma triglyceride-rich lipoprotein apolipoprotein C-III subspecie concentrations in hypertriglyceridemia. J Lipid Res. 1981;22:800–810. [PubMed] [Google Scholar]
- 30.Yassine HN, Trenchevska O, Ramrakhiani A, Parekh A, Koska J, Walker RW, Billheimer D, Reaven PD, Yen FT, Nelson RW, et al. The Association of Human Apolipoprotein C-III Sialylation Proteoforms with Plasma Triglycerides. PLoS One. 2015;10:e0144138. doi: 10.1371/journal.pone.0144138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, acobsJr DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. American Journal of Epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 32.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–245. doi: 10.1161/ATVBAHA.109.197830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aitchison J The statistical analysis of compositional data. Chapman \& Hall, Ltd.; 1986. [Google Scholar]
- 35.Sinari S, Nedelkov D, Reaven P, Billheimer D. The analysis of human serum albumin proteoforms using compositional framework. In: Datta S, Mertens B, eds. Statistical Analysis of Proteomics, Metabolomics, and Lipidomics Data Using Mass Spectrometry. Springer International Publishing; 2016. [Google Scholar]
- 36.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 37.Yassine HN, Trenchevska O, Dong Z, Bashawri Y, Koska J, Reaven PD, Nelson RW, Nedelkov D. The association of plasma cystatin C proteoforms with diabetic chronic kidney disease. Proteome science. 2016;14:7. doi: 10.1186/s12953-016-0096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldoni F, Sinke RJ, Kuivenhoven JA. Mendelian disorders of high-density lipoprotein metabolism. Circ Res. 2014;114:124–142. doi: 10.1161/CIRCRESAHA.113.300634 [DOI] [PubMed] [Google Scholar]
- 39.Mendoza S, Trenchevska O, King SM, Nelson RW, Nedelkov D, Krauss RM, Yassine HN. Changes in low-density lipoprotein size phenotypes associate with changes in apolipoprotein C-III glycoforms after dietary interventions. J Clin Lipidol. 2017;11:224–233 e222. doi: 10.1016/j.jacl.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kegulian NC, Ramms B, Horton S, Trenchevska O, Nedelkov D, Graham MJ, Lee RG, Esko JD, Yassine HN, Gordts P. ApoC-III Glycoforms Are Differentially Cleared by Hepatic TRL (Triglyceride-Rich Lipoprotein) Receptors. Arterioscler Thromb Vasc Biol. 2019;39:2145–2156. doi: 10.1161/ATVBAHA.119.312723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laatsch A, Merkel M, Talmud PJ, Grewal T, Beisiegel U, Heeren J. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2009;204:105–111. doi: 10.1016/j.atherosclerosis.2008.07.046 [DOI] [PubMed] [Google Scholar]
- 42.Ettinger WH, Wahl PW, Kuller LH, Bush TL, Tracy RP, Manolio TA, Borhani NO, Wong ND, O’Leary DH. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation. 1992;86:858–869. doi: 10.1161/01.cir.86.3.858 [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez M, Rehues P, Iranzo V, Mora J, Balsells C, Guardiola M, Ribalta J. Distribution of seven ApoC-III glycoforms in plasma, VLDL, IDL, LDL and HDL of healthy subjects. J Proteomics. 2022;251:104398. doi: 10.1016/j.jprot.2021.104398 [DOI] [PubMed] [Google Scholar]
- 44.Peng P, Wang L, Yang X, Huang X, Ba Y, Chen X, Guo J, Lian J, Zhou J. A preliminary study of the relationship between promoter methylation of the ABCG1, GALNT2 and HMGCR genes and coronary heart disease. PloS one. 2014;9:e102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khetarpal SA, Schjoldager KT, Christoffersen C, Raghavan A, Edmondson AC, Reutter HM, Ahmed B, Ouazzani R, Peloso GM, Vitali C, et al. Loss of Function of GALNT2 Lowers High-Density Lipoproteins in Humans, Nonhuman Primates, and Rodents. Cell Metab. 2016;24:234–245. doi: 10.1016/j.cmet.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okude M, Yamanaka A, Morimoto Y, Akihama S. Sialic acid in fibrinogen: effects of sialic acid on fibrinogen-fibrin conversion by thrombin and properties of asialofibrin clot. Biol Pharm Bull. 1993;16:448–452. doi: 10.1248/bpb.16.448 [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Wei TT, Li Y, Li J, Fan Y, Huang FQ, Cai YY, Ma G, Liu JF, Chen QQ, et al. Functional Metabolomics Characterizes a Key Role for N-Acetylneuraminic Acid in Coronary Artery Diseases. Circulation. 2018;137:1374–1390. doi: 10.1161/CIRCULATIONAHA.117.031139 [DOI] [PubMed] [Google Scholar]
- 48.Gopaul KP, Crook MA. Sialic acid: a novel marker of cardiovascular disease? Clin Biochem. 2006;39:667–681. doi: 10.1016/j.clinbiochem.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 49.Reganon E, Vila V, Martinez-Sales V, Vaya A, Mira Y, Ferrando F, Aznar J. Sialic acid is an inflammation marker associated with a history of deep vein thrombosis. Thromb Res. 2007;119:73–78. doi: 10.1016/j.thromres.2005.12.017 [DOI] [PubMed] [Google Scholar]
- 50.Clarke R, Von Ende A, Schmidt LE, Yin X, Hill M, Hughes AD, Pechlaner R, Willeit J, Kiechl S, Watkins H, et al. Apolipoprotein Proteomics for Residual Lipid-Related Risk in Coronary Heart Disease. Circ Res. 2023;132:452–464. doi: 10.1161/CIRCRESAHA.122.321690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–2072. doi: 10.1161/CIRCULATIONAHA.111.056986 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


