Abstract
Background:
Current breastfeeding guidelines promote initiating breastfeeding ≤1 h after birth to establish long-term breastfeeding. Previous studies dichotomized initiation to ≤1 h versus subsequent hours combined. There are limited data evaluating the effect of initiation in each subsequent hour on breastfeeding duration. Our objective was to evaluate the association between breastfeeding initiated at ≤1 h versus the subsequent 23 hours after birth and outpatient breastfeeding duration.
Methods:
In this retrospective cohort study, we analyzed real-time, discretely documented electronic health record (EHR) breastfeeding data for 3315 infants born at a university center and followed to age ≥12 mo at 27 university primary care clinics. The primary outcome was breastfeeding duration. The exposure variable was hour of breastfeeding initiation within 24 h postnatally. Data were analyzed by univariable and multivariable linear regression separately for infants born by vaginal versus cesarean delivery.
Results:
In adjusted models, initiating breastfeeding during each hour from age >1 to ≤6 h and during ages >6 to ≤24 h was not associated with decreased breastfeeding duration versus initiating breastfeeding at ≤1 h after birth for infants born via vaginal or cesarean delivery.
Conclusions:
Delaying breastfeeding initiation to >1 to ≤24 h after birth is not associated with decreased breastfeeding duration compared with initiating breastfeeding at ≤1 h after birth. Integration of breastfeeding measures into inpatient and outpatient EHR discrete data fields may clarify best practices that support long-term breastfeeding as a public health imperative.
Keywords: electronic health record, lactation, newborn, nursery
Long-term breastfeeding is associated with major health benefits, including decreases in infant morbidity and mortality, maternal cancer risk, and maternal and childhood diabetes risk.1 It is important to study the effects of inpatient breastfeeding practices on breastfeeding duration because hospital events such as giving gift packs of formula, separation of infants from mothers, and not feeding on demand predict early breastfeeding cessation.2–5 The Baby Friendly Hospital Initiative guidelines, which are implemented globally to support breastfeeding in birthing facilities, include requirements about the timing of breastfeeding initiation for a hospital to qualify for Baby Friendly accreditation. These requirements specify that the hospital must help mothers begin to breastfeed within 30 minutes after birth (World Health Organization 1989 version),6 as soon as possible after birth (global 2018 version),5 or within 1 hour after birth (US 2020 version).7
Previous studies to evaluate outcomes associated with breastfeeding initiation typically dichotomize initiation between the first 1 hour versus the remaining hours of the first day, but do not evaluate each of the first several hours after birth separately.2,3,8−11 In countries with high infant mortality, breastfeeding initiation within 1 hour after birth may be associated with fewer infant deaths.5,8,12 In hospital settings with low infant mortality, early skin-to-skin contact between mothers and infants may be associated with higher frequency of breastfeeding initiation and duration,13 but there is limited evidence that initiating breastfeeding within the first hour after birth increases the duration of outpatient breastfeeding more than initiation during each of the subsequent 23 hours after birth.5,11 Studies about US birth hospital practices and long-term duration of breastfeeding typically are based on retrospective recall surveys of mothers that are performed several months after birth.2,3,11 In a study of 1045 mothers, survey responses answered up to 9 months postpartum showed greater odds of quitting breastfeeding at 3−4 weeks (odds ratio, 1.44) when no breastfeeding had been reported in the first hour after birth.2 In a study of 657 Utah mothers who responded up to 42 weeks postpartum, there were no differences in the adjusted prevalence ratio of breastfeeding duration (<2 mo vs ≥2 mo) between mothers who did or did not report breastfeeding in the first hour after birth.3 Studies that dichotomize initiation to within the first hour versus all subsequent hours after birth may not provide sufficient granularity to assess accommodation of infant readiness to feed during fetal to neonatal transition.14
There is increasing interest in using electronic health record (EHR) data to answer clinical research questions,15 and the use of structured EHR forms containing discrete data fields may be associated with improved data quality.16 Structured documentation in the EHR may facilitate improved collection of data about postnatal breastfeeding, which may improve the ability to characterize the relationship between the timing of initial breastfeeding and outpatient breastfeeding duration.17 However, our literature search showed limited information about real-time documentation of inpatient postnatal breastfeeding and its potential relationship to discrete, longitudinally-collected, outpatient breastfeeding duration data.18
The purpose of this study was to assess whether breast-feeding initiated within the first hour after birth is associated with longer duration of outpatient breastfeeding compared with initiation during the subsequent hours after birth.
Methods
Study Population
This was a retrospective cohort study of infants who were born at a single academic hospital and followed up within the same health care system using the same EHR throughout (Epic, Epic Systems Corporation, Verona, Wis). The University of Utah Hospital has a birthing center where 4000−5000 infants are born annually, including 3500 infants at gestational age ≥ 34 weeks who transition to couplet care in the mother’s room or are treated in an intermediate nursery. The University of Utah Health system includes 27 primary care clinics that have the option of using structured templates for well-child visit documentation. This study was exempted for review from the University of Utah Institutional Review Board.
There were 17,359 infants who were born at gestational age ≥34 wk between July 2, 2014 and November 30, 2018 at University of Utah Hospital. After excluding 7087 infants who did not have primary care follow-up in the University of Utah system, we queried the EHRs of the remaining 10,272 infants who were discharged from the nursery and followed up in the system. Infants were included who had inpatient feeding documented in discrete data fields within a structured EHR template, presented for primary care in the University of Utah Health system, had breastfeeding documented at any or all well-child visits by the primary care provider using an outpatient structured EHR template with discrete data fields, and reached age ≥12 mo (Fig. 1). Infants who were discharged from the hospital at <24 h after birth were excluded because the time of breastfeeding initiation was not available for infants initiating after discharge. Data were queried on November 30, 2019. In addition to infants who were breastfed from birth, we included infants who had delayed oral feeding due to resuscitation and stabilization interventions immediately after birth but who initiated breastfeeding later in the hospital stay.
Figure 1.
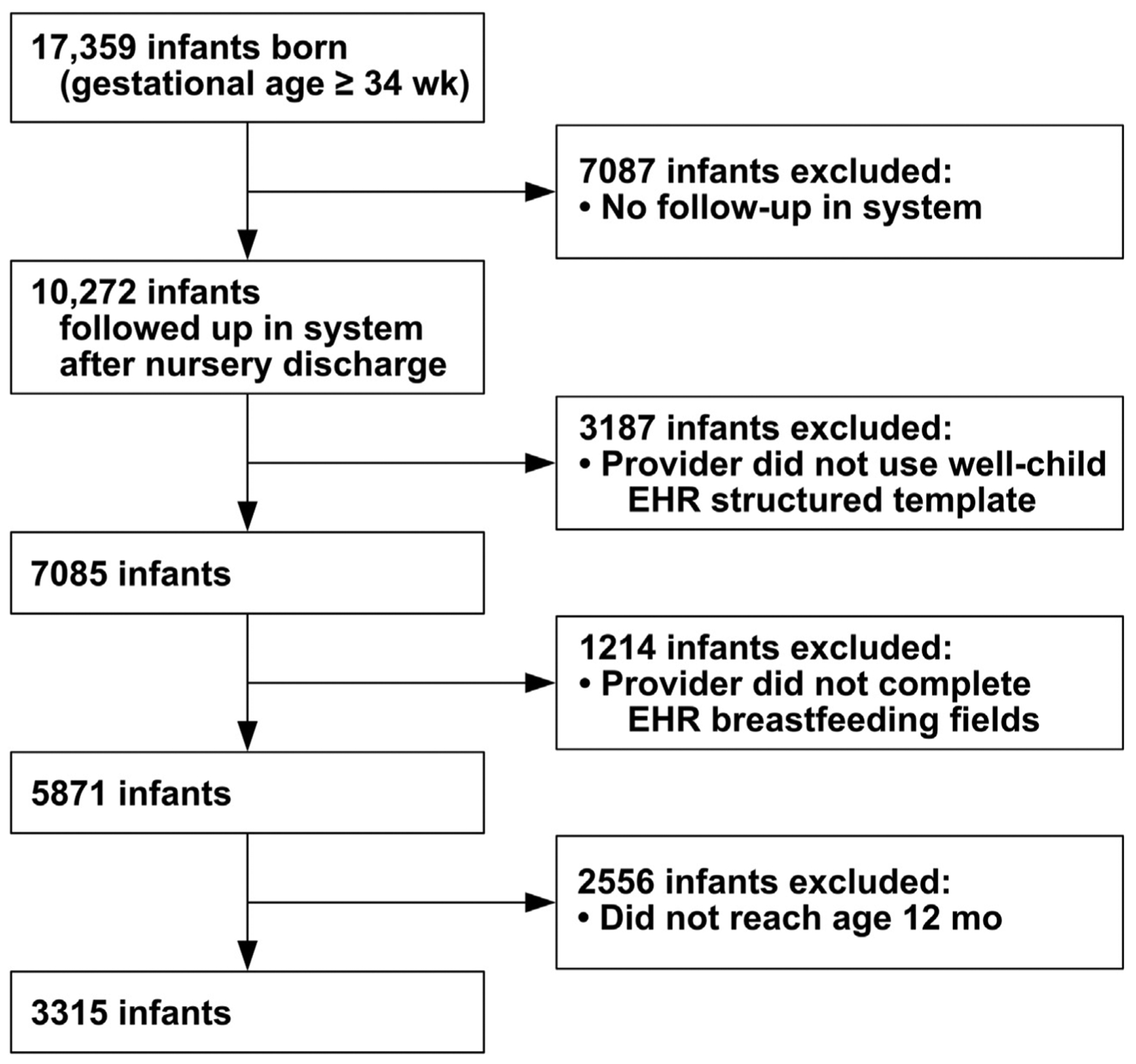
Flow diagram of infants who were born at gestational age ≥34 weeks and followed to age ≥12 months with electronic health record documentation of breastfeeding. EHR, electronic health record.
Exposure Variable
Inpatient nursing documentation of time of breastfeeds was used to generate the exposure variable, which was the hour after birth of breastfeeding initiation during the first 24 hours. For this study, breastfeeding initiation was defined as the earliest of either the first feed at the breast or first expression of milk. The age at initiation of breast-feeding was calculated as the time of first breastfeeding minus birth time.
Inpatient Breastfeeding Documentation
The use of a standardized structured EHR form (flow-sheet) was required for inpatient documentation of feeding. Documentation of the timing of breastfeeding (at breast or expression) was in a discrete data field, and an additional free-text field also was used for documentation of expression. Hospital nurses had at least 12 hours of breastfeeding support training and were required to enter all breastfeeding events into the EHR flowsheet, indicating the time (hour and minute) that breastfeeding began and duration of feeding or expression from each breast (no. of minutes). The nurses documented breastfeeding in real time when they were present during feeding, including an assessment of latch quality. When feeding was not observed, the nurse asked the mother to report the minutes of feeding from each breast when the nurse entered the room to provide care. Infants were put to breast at least every 3 hours, but nurses requested breastfeeding updates with all patient care. There also was a rover nurse at every shift to help support and document breastfeeding.
Outpatient Breastfeeding Documentation and Outcome Measure
The well-child structured EHR template for all ages through 5 years contained the question “Breastfeeding?” followed by 2 answer buttons (yes and no). When the yes button was clicked, the provider was prompted to ask the mother about number of breastfeeds per day and her breastfeeding duration goal. When the no button was clicked, the provider was prompted to fill in a numeric field for “Duration of breastfeeding (months).” Use of the well-child template or breastfeeding fields was voluntary.
The outcome measure was duration, in months, of any outpatient breastfeeding. The duration of outpatient breastfeeding variable was created by using either the numeric field for duration or the last yes response when the duration field was blank.
Control Variables
Control variables, chosen a priori to adjust for infant characteristics known to affect breastfeeding duration, were gestational age at birth,19 documented reasons for giving inpatient formula feeds,14 and receiving inpatient or outpatient phototherapy.19−21 Covariables to adjust for maternal characteristics known to affect breastfeeding included obesity (body mass index >30 kg/m2),22 depression (Edinburgh Postnatal Depression Scale score ≥10),23,24 maternal age,25 parity,25 duration of hospital stay,26 maternal self-identification as white,27 maternal self-identification as Hispanic,27 and Medicaid insurance (as an indicator of economic status).22–28 The Edinburgh Postnatal Depression Scale was offered to mothers for completion at infant well-child visits up to 12 months postpartum, but the infant age upon collection varied between clinics.
Statistical Analysis
Descriptive statistics were used to summarize demographic characteristics of the study sample using numbers (percentage) for categorical variables and means for continuous variables. To assess for association of hour of breast-feeding initiation with breastfeeding duration, we fit univariable linear regression. We adjusted for candidate covariates using least absolute shrinkage and selection operator (LASSO) linear regression29 to select the variables that stayed in the model, mixed-effects linear regression with clinic as a random effect to account for patients being nested within clinics, and multiple imputation by chained equations30 to impute for missing body mass index and depression scale data. We built separate models for infants born by vaginal versus cesarean birth because of the observed delay in breastfeeding initiation with cesarean birth. A truncation approach was used to replace breast-feeding duration longer than 24 mo to equal 24 mo to avoid the potential skewing by extreme scores on breast-feeding duration. The exposure variable was modeled using dummy variables with initiation of breastfeeding at age ≤1 hour as the referent. Breastfeeding initiation at each hour from >1 to ≤6 h after birth was compared separately to the referent, and infants with breastfeeding initiation at ages >6 to ≤24 h were combined into a single group because of small sample sizes due to most mothers initiating breastfeeding earlier. We dichotomized race to white and nonwhite for the analysis because of the small number of mothers in each nonwhite race. We graphed the proportion of infants initiating breastfeeding during each hour in the first 24 h after birth. Statistical analyses were performed using Stata Version 16.1. Two-sided P values less than or equal to .05 were considered statistically significant.
Results
There were 10,272 infants who were born at gestational age ≥34 wk, stayed in the hospital for ≥24 h after birth, and presented for follow-up care after discharge from the nursery within the same health care system. There were 3187 infants (31%) excluded because the providers did not use the well-child structured EHR template, 1214 infants (12%) excluded because the breastfeeding EHR fields were not completed, and 2556 infants (25%) excluded for not having reached age 12 mo. There were 3315 of the 10,272 infants (32%) who were followed to age ≥12 mo and had providers who used the structured well-child visit templates to document outpatient breast-feeding (Fig. 1).
The mean duration of breastfeeding was slightly greater for infants born by vaginal versus cesarean birth (Table 1). Mothers who delivered by cesarean versus vaginal birth were older and more frequently primiparous, obese, and non-Hispanic. Infants born by cesarean versus vaginal birth were less frequently breastfed exclusively during the birth hospitalization (Table 1). The frequency of initiating breastfeeding was greatest ≤1 h after birth for infants born by vaginal birth versus >1 to ≤2 h for infants born by cesarean birth (Fig. 2).
Table 1.
Demographic and Clinical Characteristics of Mothers and Infants Who Had Real-Time Breastfeeding Documentation in the Electronic Health Record*
| Variable | Vaginal Birth | Cesarean Birth | P |
|---|---|---|---|
| No. of infants | 2588 | 727 | |
| Maternal characteristics | |||
| Maternal age (y) | 29 ± 5(14–52) | 31 ± 6(16–49) | <.001 |
| Maternal | |||
| Primiparous | 1237 (48) | 384 (53) | .02 |
| Obesity† | 575 (44) | 193 (60) | <.001 |
| Depression‡ | 89 (5) | 30 (5) | .50 |
| Medicaid insurance | 814(31) | 211 (29) | .20 |
| Maternal race§ | |||
| White | 1789 (69) | 512(70) | .50 |
| Asian | 227 (9) | 64 (9) | .99 |
| Black | 69 (3) | 30 (4) | .05 |
| Native Hawaiian/Pacific Islander | 27(1) | 13(2) | .12 |
| American Indian/Alaska Native | 25(1) | 4(1) | .37 |
| Other | 407(16) | 100(14) | .20 |
| Chose not to disclose‖ | 44 (2) | 4(1) | .02 |
| Maternal ethnicity§ | |||
| Non-Hispanic | 2069 (80) | 613(84) | .01 |
| Hispanic | 519(20) | 114(16) | .01 |
| Duration of hospital stay (h) | 47 ± 30 (24–700) | 81 ± 31 (24–411) | <.001 |
| Birth and neonatal history | |||
| Gestational age (wk) | 39.2 ± 1.3(34–42) | 38.8 ± 1.5(34–42) | <.001 |
| Inpatient antibiotics | 99 (4) | 60 (8) | <.001 |
| Inpatient or outpatient phototherapy | 287(11) | 84 (12) | .70 |
| Feeding during birth hospital stay | |||
| Exclusive breastfeeding | 1793 (69) | 292 (40) | <.001 |
| Any formula feeding | 795 (31) | 435 (60) | <.001 |
| Reasons for giving formula during birth hospital stay | |||
| Requested by mother, no medical indication | 345(13) | 176 (24) | <.001 |
| Medically indicated for infant | 231 (9) | 126(17) | <.001 |
| Medically indicated for mother | 40 (2) | 35 (5) | <.001 |
| No reason documented | 179 (7) | 62 (9) | .20 |
| Time of breastfeeding initiation (h) | |||
| ≤ 1 (reference) | 929 (36) | 65 (9) | <.001 |
| > 1 to ≤ 2 | 716(28) | 254 (35) | <.001 |
| > 2 to ≤ 3 | 309(12) | 83 (11) | .70 |
| > 3 to ≤ 4 | 161 (6) | 65 (9) | .12 |
| > 4 to ≤ 5 | 75 (3) | 40 (6) | .001 |
| > 5 to ≤ 6 | 47 (2) | 27 (4) | .01 |
| > 6 to ≤ 24 | 194 (7) | 116(16) | <.001 |
| No breastfeeding from age 0 to 24 h | 157 (6) | 77 (11) | <.001 |
| Breastfeeding duration (mo) | 9 ± 8 (0–60) | 8 ± 8 (0–60) | <.001 |
N = 3315 infants. Data reported as mean ± SD (range, minimum to maximum) or no. of infants (%).
Maternal obesity defined as body mass index >30 kg/m2. Data available only for 1619 infants (1295 vaginal and 324 cesarean births) because of 1696 missing values.
Maternal depression defined as Edinburgh Postnatal Depression Scale score ≥10. Data available only for 2472 infants (1914 vaginal and 558 cesarean births) because of 843 missing values.
Self-identification by mother.
Includes missing race information.
Figure 2.
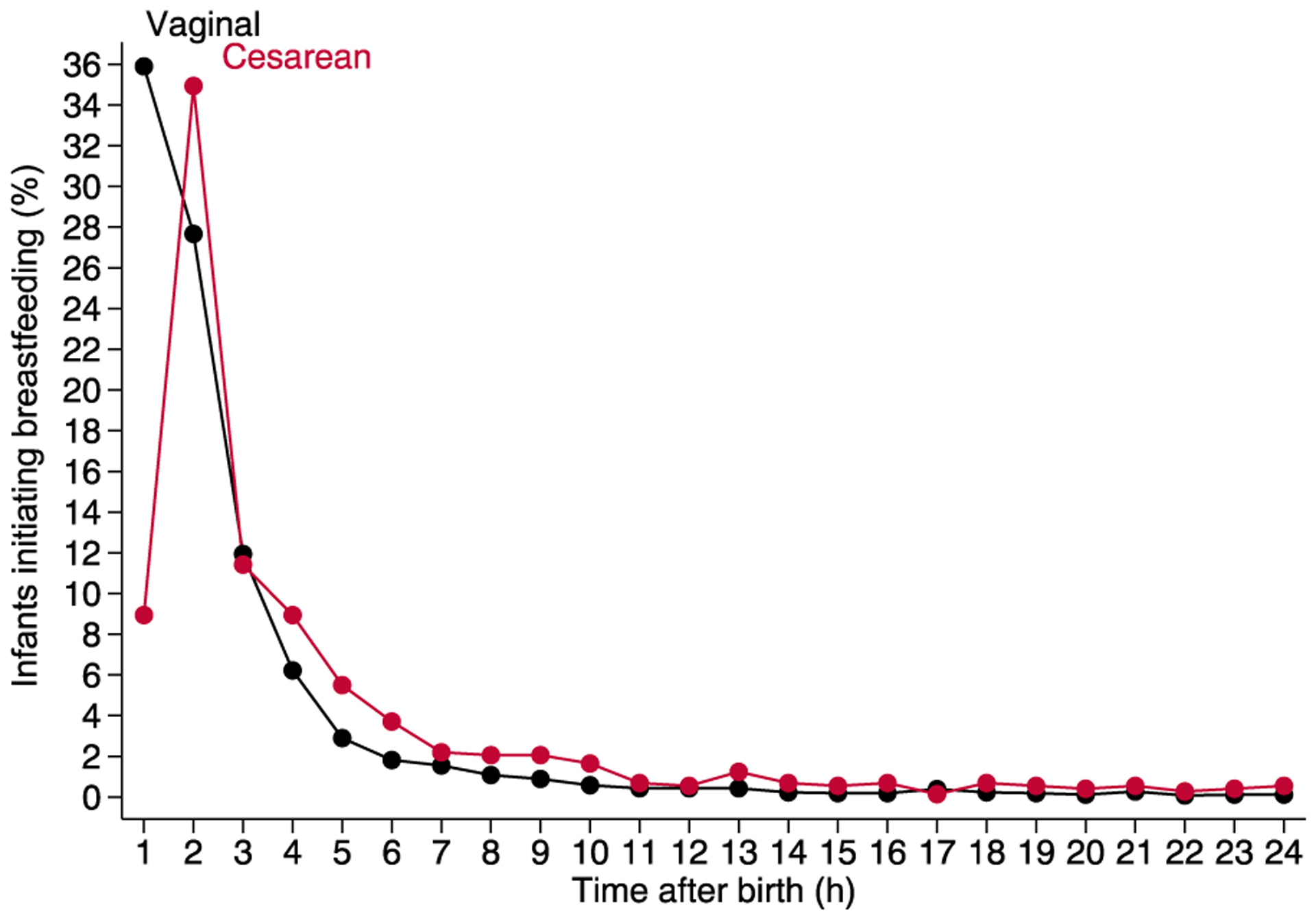
Relation between proportions of infants initiating breast-feeding vs age. Each point represents the percentage of 2588 infants (vaginal birth) or 727 infants (cesarean birth) who initiated breastfeeding at or during the hour before the time noted.
In vaginally born infants, unadjusted linear regression showed no difference in breastfeeding duration for initiation between any hour from >1 to ≤6 h after birth versus ≤1 h after birth, but infants who initiated breastfeeding at ages >6 to ≤24 h had a mean of 1.7 mo shorter breastfeeding duration versus initiation in the first hour (mean, −1.7 mo; 95% CI: − 2.8, −0.67 mo; P < .001) (Table 2). However, the adjusted model showed no difference in breastfeeding duration for initiation from >1 to ≤24 h after birth versus ≤1 h after birth. Vaginally born infants who did not initiate breastfeeding in the first 24 h had lower mean breastfeeding duration (unadjusted mean, −7.5 mo; adjusted mean, −4.5 mo) than infants who initiated in the first hour. Giving formula for any reason was associated with a shorter breastfeeding duration, with maternal medical indication (−4.2 mo) and maternal request for formula without medical indication (−4.0 mo) showing a larger effect than infant medical indication (−2.0 mo; Table 2). Other covariates associated with shorter breastfeeding duration for vaginal births included maternal obesity and depression, and each year of increased maternal age was associated with an additional 0.18 mo of breastfeeding duration.
Table 2.
Linear Regression for Breastfeeding Duration and Time of Breastfeeding Initiation After Vaginal Birth*
| Variable | Vaginal Birth (Unadjusted) | Vaginal Birth (Adjusted) | ||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | |||
| Time of breastfeeding initiation (h) | ||||||
| ≤ 1 (reference) | – | – | – | – | – | – |
| > 1 to ≤ 2 | −0.18 | (−0.85, 0.49) | .60 | −0.12 | (−0.75, 0.51) | .70 |
| > 2 to ≤ 3 | −0.78 | (−1.7, 0.10) | .08 | −0.53 | (−1.4, 0.30) | .21 |
| > 3 to ≤ 4 | −0.28 | (−1.4, 0.86) | .63 | −0.05 | (−1.1, 1.0) | .93 |
| > 4 to ≤ 5 | 1.0 | (−0.62, 2.6) | .23 | 0.87 | (−0.65, 2.4) | .26 |
| > 5 to ≤ 6 | 1.2 | (−0.81, 3.2) | .24 | 1.4 | (−0.53, 3.2) | .16 |
| > 6 to ≤ 24 | −1.7 | (−2.8, −0.67) | <.001 | −0.67 | (−1.7, 0.35) | .20 |
| No breastfeeding from age 0 to 24 h | −7.5 | (−8.6, −6.3) | <.001 | −4.5 | (−5.7, −3.3) | <.001 |
| Reason formula was given | ||||||
| Requested by mother, no medical indication | −4.0 | (−4.8, −3.2) | <.001 | |||
| Medically indicated for infant | −2.0 | (−2.9, −1.0) | <.001 | |||
| Medically indicated for mother | −4.2 | (−6.3, −2.1) | <.001 | |||
| No reason documented | −2.1 | (−3.1, −1.1) | <.001 | |||
| Maternal | ||||||
| Obesity | −0.86 | (−1.6, −0.14) | .02 | |||
| Depression | −1.5 | (−2.9, −0.06) | .04 | |||
| Age (y) | 0.18 | (0.13, 0.23) | <.001 | |||
| Self-identification as Hispanic | −0.56 | (−1.3, 0.16) | .13 | |||
| Gestational age (wk) | 0.17 | (−0.04, 0.37) | .11 | |||
| Medicaid insurance coverage | −0.59 | (−1.2, 0.07) | .08 | |||
| Duration of hospital stay (h) | 0.02 | (0.01, 0.02) | <.001 | |||
N = 2588 infants. Breastfeeding duration > 24 mo truncated to 24 mo. Race was dichotomized into white vs nonwhite because of small numbers of infants in nonwhite categories. White vs nonwhite, inpatient antibiotics, and inpatient or outpatient phototherapy were not included in the model selected by least absolute shrinkage and selection operator (LASSO).
In cesarean born infants, unadjusted linear regression showed that delayed initiation to >2 to ≤3 h was associated with a mean of 2.6 mo lower breastfeeding duration versus ≤1 h after birth (P = .02), and infants who initiated breastfeeding at >6 to ≤24 h after birth had a mean of 3.6 mo shorter breastfeeding duration (P < .001; Table 3). However, the adjusted model showed no difference in breastfeeding duration for initiation from >1 to ≤24 h after birth versus ≤1 h after birth. Cesarean born infants who did not initiate breastfeeding in the first 24 h had lower mean breastfeeding duration (unadjusted mean, −6.8 mo; adjusted mean, −4.2 mo) than infants who initiated in the first hour. Covariates associated with shorter breastfeeding duration for cesarean births included giving formula for maternal medical indication (−3.3 mo), maternal request without medical indication (−3.1 mo), medical indication for the infant (−2.1 mo), and Medicaid insurance coverage (−1.3 mo).
Table 3.
Linear Regression for Breastfeeding Duration and Time of Breastfeeding Initiation After Cesarean Birth*
| Variable | Cesarean Birth (Unadjusted) | Cesarean Birth (Adjusted) | ||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | |||
| Time of breastfeeding initiation (h) | ||||||
| ≤ 1 (reference) | – | – | – | – | – | – |
| > 1 to ≤ 2 | −1.6 | (−3.5, 0.27) | .09 | −1.5 | (−3.3, 0.22) | .09 |
| > 2 to ≤ 3 | −2.6 | (−4.8, −0.35) | .02 | −2.0 | (−4.1, 0.15) | .07 |
| > 3 to ≤ 4 | 0.56 | (−1.8, 2.9) | .64 | 0.88 | (−1.4, 3.1) | .44 |
| > 4 to ≤ 5 | −1.7 | (−4.4, 0.99) | .21 | −0.49 | (−3.0, 2.1) | .71 |
| > 5 to ≤ 6 | −2.7 | (−5.8, 0.38) | .09 | −1.6 | (−4.5, 1.4) | .29 |
| > 6 to ≤ 24 | −3.6 | (−5.7, −1.5) | <.001 | −2.0 | (−4.0, 0.08) | .06 |
| No breastfeeding from age 0 to 24 h | −6.8 | (−9.1, −4.6) | <.001 | −4.2 | (−6.5, −2.0) | <.001 |
| Reason formula was given | ||||||
| Requested by mother, no medical indication | −3.1 | (−4.4, −1.7) | <.001 | |||
| Medically indicated for infant | −2.1 | (−3.4, −0.79) | .002 | |||
| Medically indicated for mother | −3.3 | (−5.7, −0.92) | .007 | |||
| No reason documented | −0.10 | (−1.9, 1.7) | .91 | |||
| Maternal | ||||||
| Obesity | −1.1 | (−2.3, 0.13) | .08 | |||
| Depression | −2.1 | (−4.4, 0.22) | .08 | |||
| Gestational age (wk) | 0.21 | (−0.13,0.55) | .23 | |||
| Medicaid insurance coverage | −1.3 | (−2.4, −0.18) | .02 | |||
N = 727 infants. Breastfeeding duration > 24 mo truncated to 24 mo. Race was dichotomized into white vs nonwhite because of small numbers of infants in nonwhite categories. White vs nonwhite, inpatient antibiotics, inpatient or outpatient phototherapy, maternal age, self-identification as Hispanic, and duration of hospital stay were not included in the model selected by least absolute shrinkage and selection operator (LASSO).
Discussion
In this study to determine the relationship between the timing of the first breastfeeding and the ultimate duration of breastfeeding, we observed that initiation of breastfeeding in the first hour after birth was not associated with increased duration compared with initiation between >1 to ≤24 h after birth in the adjusted models. Not initiating at all in the first 24 h was associated with shorter breastfeeding duration for all infants. The data we collected were from discrete data fields in structured EHR documents that may be more accurate than nondiscrete documentation.16 Real-time breastfeeding documentation in the EHR provided data that enabled analyses of the hour of breastfeeding initiation that were not previously feasible in retrospective studies based on recall2,3,8 or studies that dichotomized initiation to ≤1 h after birth versus all subsequent hours.8
Infants should be encouraged to breastfeed as soon as possible after birth, but approximately 10% of infants require intervention to support transition from fetal to newborn physiology,31 and 32% of US births are cesarean.32 Therefore, some maternal-infant dyads may not be ready to initiate breastfeeding by ≤1 h after birth. Studying the effect of delayed initiation by smaller units of time after birth, instead of combining infants who initiate from >1 to ≤24 h into a single group, may help clarify best practices about the timing of breastfeeding initiation to optimally support newborn care and provide reassurance to parents for whom initiation is delayed.
Discrete documentation of inpatient and outpatient breastfeeding, as in the present study, may enable further evaluation and definition of optimal infant feeding practices. With the beneficial effects of long-term breastfeeding on maternal, child, and community health,33 discrete documentation of outpatient breastfeeding outcomes may potentially be useful as an electronic clinical quality measure,34 which may incentivize institutions to improve the accuracy of EHR feeding documentation.16 In this study, all infants had inpatient feeding data entered because the documentation was required in the nursery EHR. In contrast, 43% of the 10,272 infants who followed up in our system were excluded because providers did not use the voluntary outpatient well-child structured EHR template or breastfeeding EHR fields, potentially causing selection bias (Fig. 1).
Limitations of the present study include limited generalizability due to performance at a single center with support for breastfeeding documentation in the EHR. The present results may be most relevant to dyads who deliver in a hospital with inpatient breastfeeding support and follow up with providers who continue to provide support at outpatient visits. Generalizability also may be limited because of the unavailability of outpatient results for infants who did not follow up in our system. In addition, our population had limited racial and ethnic diversity, factors which may affect breastfeeding initiation or continuation.35,36 As we defined breastfeeding as feeding directly from the breast or expression, the effects of the time of first expression of breast milk versus first direct feeding were not evaluated separately. Expression during the first hour after birth may increase breast milk volume and reduce the time to lactogenesis II, and it may be beneficial to create discrete documentation fields specific for expression that were not available in this study.37 Although we included infants who had delayed oral feeding due to resuscitation and stabilization interventions immediately after birth but who initiated breastfeeding later in the hospital stay, we did not analyze this subgroup separately because the documented information was not queried. In addition, the use of the structured well-child visit template with prompts to ask about breastfeeding may have increased the duration of outpatient breastfeeding by encouraging conversations about maternal breast-feeding goals.38 Furthermore, we did not study exclusivity of breastfeeding, and the present analysis did not evaluate other variables that may be associated with breastfeeding such as smoking and breastfeeding education.39
Conclusions
The present study showed associations between the timing of inpatient breastfeeding initiation in the first 24 h after birth and outpatient breastfeeding duration. Our study does not support the notion that breastfeeding initiation ≤1 h after birth has greater association with long-term outpatient breastfeeding, when compared with each hour between >1 to ≤6 h and the period from >6 to ≤24 h after birth. The results support the proposed change of the Baby Friendly USA guideline to initiate breastfeeding from within 1 hour to as soon as possible after birth.40 Discrete EHR documentation of inpatient and outpatient breastfeeding outcomes enabled evaluations that may not be feasible with recall survey data. Further integration of breastfeeding measures into inpatient and outpatient EHR discrete data fields may clarify best practices that support long-term breastfeeding as a public health imperative.
What’s New.
In this study, real-time electronic health record documentation showed that initiation of breastfeeding ≤1 h after birth was not associated with longer breastfeeding duration vs initiation from >1 to ≤24 h after birth.
Funding
The project was done with no specific funding support.
Abbreviations:
- CI
confidence interval
- EHR
Electronic health record
- LASSO
Least absolute shrinkage and selection operator
- MICE
Multiple imputation by chained equations
Footnotes
Financial Disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have indicated that they have no potential conflicts of interests to disclose.
Reference
- 1.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 2.Pounds L, Shostrom V. Analyzing factors that impact breastfeeding duration in the postpartum period: a secondary analysis of PRAMS data. Breastfeed Med. 2018;13:335–340. 10.1089/bfm.2018.0020. [DOI] [PubMed] [Google Scholar]
- 3.Schliep KC, Denhalter D, Gren LH, et al. Factors in the hospital experience associated with postpartum breastfeeding success. Breastfeed Med. 2019;14:334–341. 10.1089/bfm.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guala A, Boscardini L, Visentin R, et al. Skin-to-skin contact in cesarean birth and duration of breastfeeding: a cohort study. Scient World J. 2017;2017: 1940756. 10.1155/2017/1940756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Implementation Guidance: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: the Revised Baby-friendly Hospital Initiative. Geneva: World Health Organization; 2018. [Google Scholar]
- 6.World Health Organization. Protecting, Promoting and Supporting Breast-feeding: the Special Role of Maternity Services. Geneva: World Health Organization; 1989. [Google Scholar]
- 7.The ten steps to successful breastfeeding. Baby-Friendly USA Web site. Available at: https://www.babyfriendlyusa.org/for-facilities/practice-guidelines/10-steps-and-international-code/. Accessed January 3, 2022. [Google Scholar]
- 8.NEOVITA Study Group. Timing of initiation, patterns of breast-feeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Glob Health. 2016;4:e266–e275. [DOI] [PubMed] [Google Scholar]
- 9.Edmond KM, Zandoh C, Quigley MA, et al. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics. 2006;117: e380–e386. 10.1542/peds.2005-1496. [DOI] [PubMed] [Google Scholar]
- 10.Mullany LC, Katz J, Li YM, et al. Breast-feeding patterns, time to initiation, and mortality risk among newborns in southern Nepal. J Nutr. 2008;138:599–603. 10.1093/jn/138.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boccolini CS, de Carvalho ML, de Oliveira MI, et al. Breastfeeding during the first hour of life and neonatal mortality. J Pediatr (Rio J). 2013;89:131–136. 10.1016/j.jped.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Khan J, Vesel L, Bahl R, et al. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity − a systematic review and meta-analysis. Matern Child Health J. 2015;19:468–479. 10.1007/s10995-014-1526-8. [DOI] [PubMed] [Google Scholar]
- 13.Moore ER, Bergman N, Anderson GC, et al. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11: CD003519. 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton SU, Brodsky D. Fetal physiology and the transition to extra-uterine life. Clin Perinatol. 2016;43:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richesson RL, Marsolo KS, Douthit BJ, et al. Enhancing the use of EHR systems for pragmatic embedded research: lessons from the NIH Health Care Systems Research Collaboratory. J Am Med Inform Assoc. 2021;28:2626–2640. 10.1093/jamia/ocab202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman MR, Colorafi K, Daratha KB. The reliability of electronic health record data used for obstetrical research. Appl Clin Inform. 2018;9:156–162. 10.1055/s-0038-1627475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnipper JL, Linder JA, Palchuk MB, et al. “Smart Forms” in an electronic medical record: documentation-based clinical decision support to improve disease management. J Am Med Inform Assoc. 2008;15:513–523. 10.1197/jamia.M2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Cheng J, Yan S, et al. Early feeding behaviors and breast-feeding outcomes after cesarean section. Breastfeed Med. 2019;14:325–333. 10.1089/bfm.2018.0150. [DOI] [PubMed] [Google Scholar]
- 19.Rayfield S, Oakley L, Quigley MA. Association between breastfeeding support and breastfeeding rates in the UK: a comparison of late preterm and term infants. BMJ Open. 2015;5: e009144. 10.1136/bmjopen-2015-009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vehling L, Chan D, McGavock J, et al. Exclusive breastfeeding in hospital predicts longer breastfeeding duration in Canada: implications for health equity. Birth. 2018;45:440–449. 10.1111/birt.12345. [DOI] [PubMed] [Google Scholar]
- 21.Waite WM, Taylor JA. Phototherapy for the treatment of neonatal jaundice and breastfeeding duration and exclusivity. Breastfeed Med. 2016;11:180–185. [DOI] [PubMed] [Google Scholar]
- 22.Marshall NE, Lau B, Purnell JQ, et al. Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern Child Nutr. 2019;15:e12732. 10.1111/mcn.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith-Nielsen J, Matthey S, Lange T, et al. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393. 10.1186/s12888-018-1965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123:e736–e751. 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- 25.Buckman C, Diaz AL, Tumin D, et al. Parity and the association between maternal sociodemographic characteristics and breastfeeding. Breastfeed Med. 2020;15:443–452. [DOI] [PubMed] [Google Scholar]
- 26.Heck KE, Schoendorf KC, Chávez GF, et al. Does postpartum length of stay affect breastfeeding duration? A population-based study. Birth. 2003;30:153–159. [DOI] [PubMed] [Google Scholar]
- 27.McKinney CO, Hahn-Holbrook J, Chase-Lansdale PL, et al. Racial and ethnic differences in breastfeeding. Pediatrics. 2016;138: e20152388. 10.1542/peds.2015-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornsby PP, Gurka KK, Conaway MR, et al. Reasons for early cessation of breastfeeding among women with low income. Breastfeed Med. 2019;14:375–381. 10.1089/bfm.2018.0206. [DOI] [PubMed] [Google Scholar]
- 29.Tibshirani R Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Series B. 2011;73:273–282. [Google Scholar]
- 30.Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson JR, Sinkin RA. Transition from fetus to newborn. Pediatr Clin North Am. 2015;62:329–343. [DOI] [PubMed] [Google Scholar]
- 32.Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. National Vital Statistics Reports. 2021;70. Centers for Disease Control and Prevention Web site. March 23, 2021. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr70/nvsr70-02-508.pdf. Accessed January 3, 2022. [PubMed] [Google Scholar]
- 33.Walters DD, Phan LT, Mathisen R. The cost of not breastfeeding: global results from a new tool. Health Policy Plan. 2019;34:407–417. 10.1093/heapol/czz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Electronic clinical quality measures basics. Centers for Medicare & Medicaid Services Web site. December 14, 2020. Available at: https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/ClinicalQualityMeasures. Accessed January 3, 2022. [Google Scholar]
- 35.Li R, Perrine CG, Anstey EH, et al. Breastfeeding trends by race/ethnicity among US children born from 2009 to 2015. JAMA Pediatr. 2019;173: e193319. 10.1001/jamapediatrics.2019.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang KV, Li R, Anstey EH, et al. Racial and ethnic disparities in breastfeeding initiation − United States, 2019. MMWR Morb Mortal Wkly Rep. 2021;70:769–774. 10.15585/mmwr.mm7021a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker LA, Sullivan S, Krueger C, et al. Effect of early breast milk expression on milk volume and timing of lactogenesis stage II among mothers of very low birth weight infants: a pilot study. J Perinatol. 2012;32:205–209. 10.1038/jp.2011.78. [DOI] [PubMed] [Google Scholar]
- 38.DiGirolamo A, Thompson N, Martorell R, et al. Intention or experience? Predictors of continued breastfeeding. Health Educ Behav. 2005;32:208–226. 10.1177/1090198104271971. [DOI] [PubMed] [Google Scholar]
- 39.Cohen SS, Alexander DD, Krebs NF, et al. Factors associated with breastfeeding initiation and continuation: a meta-analysis. J Pediatr. 2018;203:190–196.e21. 10.1016/j.jpeds.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Practice guidelines: the guidelines and evaluation criteria. Baby-Friendly USA Web site. Available at: https://www.babyfriendlyusa.org/for-facilities/practice-guidelines/. Accessed January 8, 2022. [Google Scholar]


