Summary
Root-knot nematodes (RKNs) are important nematode pests, causing huge economic losses on vegetable crops worldwide. A decline in the yield of Swiss chard (Beta vulgaris L.) which was associated with RKNs was observed on an organic vegetable farm in the Western Cape Province of South Africa. Nematodes were extracted from galled plant roots and identified using molecular tools. PCR-based Sequence Characterised Amplified Region (SCAR) primers was used to confirm the specie of the RKN associated with the infected plants. Thereafter, a pot assay was conducted to determine the response of artificially infected Swiss chard plants to varying concentrations of bio-synthesized silver nanoparticle Ag-NP (1 μg/mL, 2 μg/mL, and 3 μg/mL) under controlled conditions. The results of the study showed that Swiss chard is highly susceptible to M. javanica with an egg-laying-female index of >5 in all infected plants. Significantly lower values (at P=0.05) in egg masses (EM), juveniles (J2s), and reproduction factor (RF) of nematodes were recorded on plants treated with 3 μg/mL, indicating a potential for nematode control. A negative correlation was also observed in the number of egg masses, J2s, and RF of the nematodes with increasing concentrations of the Ag-NP. This study confirms that Swiss chard is highly susceptible to M. javanica and demonstrates the potential nematicidal property of Ag-NP in controlling the nematode pest of Swiss chard.
Keywords: Nanoparticles, Root-knot nematode, Swiss chard, Vegetables
Introduction
Root-knot nematodes, Meloidogyne species are among the most important nematode pests of horticultural and agricultural crops causing huge economic losses worldwide (Jones et al., 2013). Huge economic losses have been attributed to damage caused by the root-knot nematodes (RKNs), Meloidogyne species on vegetable crops globally (Abad et al., 2008). In Southern Africa, RKNs have been implicated as among the major pests of economic importance in vegetable production (Muimba-Kankolongo, 2018).
Reports on the association and damaging effects of RKNs on Swiss chard (Beta vulgaris L.) have been well documented in countries such as Cyprus; Germany, Spain, Turkey, Nigeria, Iraq, Mexico, Egypt, and South Africa (Philis, 1983; Yang & Eisenback, 1983; Decker, 1989; Millán de Aguirre, 1991; Steyn et al., 2014; Daramola et al., 2015; Mashela et al., 2017; Kareem et al., 2017; Bastidas et al., 2019; Akyazi & Felek, 2020). Significant research has been dedicated and much emphasis has been placed on the prevention and disease monitoring to reduce the spread and severity of RKN infections in agricultural fields, however much is still desired in achieving complete eradication of the pests in infested fields. In many cases, the strict quarantine measures that are put in place to avoid the spread of RKN to uninfected areas constitute additional limitations to vegetable growers in resource-poor economies.
There is a huge compulsion to reduce dependence on pesticides and other environmentally hazardous pest management options used in nematode control. Many scientists and farmers have exploited various potential alternatives to synthetic nematicides. These strategies ranged from the use of organic amendments, plant-based fumigants, biological control agents, and planting resistant crop cultivars, to several cultural practices such as the use of cover crops, crop rotation, fallowing, and solarisation (Duncan, 1991). However, the use of traditional plant protection strategies in controlling parasitic nematodes has been confronted with several limitations and has often proved insufficient to provide complete eradication of nematodes once the infection has been established in the field. Moreover, these approaches are laborious, slow in action, and not as effective in comparison to the chemical-based approach. Thus, there is a continued search for a suitable alternative to nematicides, that could provide nematode control that is similar to pesticides in effectiveness but which is also benign and less hazardous to the environment.
In recent times, the potential of nanotechnology in plant disease management has come to the fore (Ardakani, 2013; Elmer et al., 2018; Khan et al., 2019; Ali et al., 2022; Kumar et al., 2022) and the nematicidal activities of nanoparticles (NPs) against root-knot nematodes have been mentioned in some empirical studies emanating from developing countries such as Egypt, India, and Pakistan (Cromwell et al., 2014; Abdellatif et al., 2016; Hammed et al., 2019; Baronia et al., 2020; Fouda, et al., 2020; Heflish et al., 2021; Ghareeb et al., 2022; El-Ashry et al., 2022). Some of the benefits that have been attributed to biosynthesized NPs include their ability to enhance plant growth, reduce root galling and cause mortality of motile stages of nematodes, thereby constituting a form of biological control. However, the use of biosynthesized Ag-NP in the control of M. javanica in field and pot experiments is yet to be established. The aim of this study, therefore, is to explore the nematicidal potential of bio-synthesized Ag-NP in controlling the root-knot nematode, M. javanica associated with Swiss chard under a controlled growth condition.
Material and Methods
Sample collection and Nematode Extraction
A decline in the yield of Swiss chard was observed on an organic vegetable farm which was located in the Western Cape Province of South Africa located at 34°05′30.7″S 18°51′19.4″E. The plants were purposively selected based on the presentations of aboveground symptoms which included, poor growth, lack of vigour, and discoloured (yellowing) leaves. The root system of the sampled plants was further examined visually for symptoms of root-knot nematode infection and positive samples (with galled roots) were transported to the laboratory for further investigation. Nematodes were extracted from root and soil samples using a modified extraction tray method of Whitehead and Hemming, 1965. The root samples were also dissected to show all the developmental stages. The extracted life stages of the nematodes were examined under the compound microscope (Leica) and identified to the genus using the morphological and morphometric parameters as a guide. Second-stage juveniles (J2s) and adult female nematodes were separated from the root tissues and used for molecular studies.
DNA extraction and PCR assays
Molecular tools were used to confirm species identification of the RKN nematodes that were isolated from the root tissues. DNA was extracted from single J2s adult females, which were placed separately in 30uL of lysis buffer inside 0.5 mL sterile Eppendorf tubes. The nematodes were gently punctured with sterile needles and placed in a −80°C freezer for 15 mins and thereafter incubated at 65°C for 60 min and 95°C for 15 min. The samples were kept at −20°C until use.
PCR assays by species-specific primers were conducted to confirm the identity of the species as described by Adam et al. (2007). The PCR reaction was done in a thermocycler using KAPA2G™ Robust Hotstart ReadyMix (KAPA Biosystems) with the primer combination of a forward primer Fjav (GGTGCGCGATTGAACTGAGC) and a reverse primer Rjav (CAGGCCCTTCAGTGGAACTATAC). The cycling condition was as described by Adam et al. (2007). The amplification products were separated by electrophoresis in 1.5 % agarose gel which was stained with ethidium bromide and visualized under UV light.
Biosynthesis of Ag-NPs and characteristics
The Ag-NPs used in this study was obtained from the Department of Chemistry, University of Johannesburg, South Africa. The Ag-NPs was biosynthesized from water hyacinth plant leaves extract (Oluwafemi et al., 2019) in a reaction comprising 5 mL of water hyacinth water extract mixed with 50 mL aqueous solution of AgNO3 (1 × 10−3 M) at 75°C. The obtained Ag-NP were of average particle size of 15.71nm, TEM micrograph indicated the Ag-NPs to be uniformly distributed and spherical in shape, with an average diameter of 10.46 nm (Oluwafemi et al., 2019).
Nematicidal effect of Ag-NP on the M. javanica eggs, J2, and root galling of Swiss chard
A pot assay was set up to assess the nematicidal potential of Ag-NP on the root galling and reproduction of RKNs on Swiss chard under controlled conditions. Three-week-old Swiss chard seedlings which were planted in steam-sterilized soils inside 15cm diameter plastic pots were inoculated with pure cultures of M. javanica at 3,000 eggs or juvenile/pot. Thereafter, the plants were treated with 30 ml of biosynthesized Ag-NPs (Oluwafemi et al., 2019) which were obtained from the Department of Chemistry, University of Johannesburg. The treatments comprised varying concentrations of Ag-NP at 1 μg/mL, 2 μg/mL, and 3 μg/mL which were applied in drenches around the plants. The treatments were replicated seven times and nematode-inoculated Swiss chard plants without Ag-NP were used as the control. The experiment was arranged in a complete randomized design and the plants were watered daily as required. Treatment with varying concentrations of Ag-NP was repeated at 6 Weeks After Planting (WAP).
The experiment was terminated after eight weeks and the root systems of the plants were examined for symptoms of root-knot nematode infection. The root systems were harvested, carefully washed free of soil, and examined for galling and egg mass production. Root samples were stained with acid fuchsin to facilitate observation and counting of the egg masses under stereoscopic microscopes. The nematode eggs/juveniles were recovered from the infected roots using 1 % NaOCl adapted method of Riekert (1995). The egg-laying-female index was determined for each root system (Hussey & Boerma, 1981), also the reproduction factor (Rf) of the female nematodes was calculated (Rf = Pf/pi) where Pi = initial inoculum level and Pf = newly produced eggs (Windham & Williams, 1987).
Ethical and conflict of interest statements
This article does not contain any studies with human participants or animals. Authors also state no conflict of interest.
Results
Molecular identification of M. javanica
Molecular identification of the individual juveniles and adult females of RKN with the SCAR-PCR assay using the primer combination Fjav/Rjav was positive and consistent for M. javanica. The PCR products readily amplified from the J2 and adult female nematodes at 670 bp-fragment with the Fjav/Rjav primers.
Pathogenicity of M. javanica on infected plants
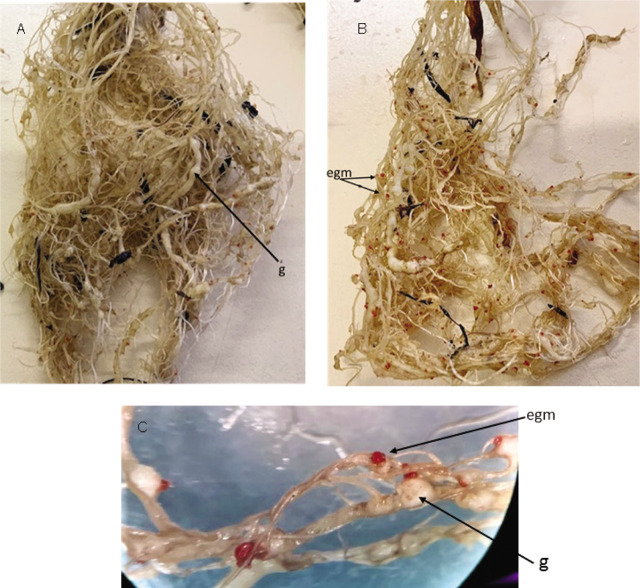
Visual examination of the root system of the Swiss chard plants showed that numerous galls and egg masses were abundant and visible on the infected plants. Examination of the plant roots under the stereomicroscope showed extensive galling with large numbers of egg masses of female nematodes visible on the roots as red spots on the roots (Fig. 1a–c). All developmental stages of the RKN were present in the root samples, however, males were not found in the plant tissues.
Fig. 1.
A – B: Root systems of infected Swiss chard plants showing root galls and egg masses of M. javanica. C: Galled roots with stained egg masses of infected Swiss chard as viewed under a stereomicroscope. egm=egg mass; g=galls.
More galls and egg masses were observed on plants treated with 1 μg/mL of Ag-NP and the control plants that did not receive any Ag-NP treatment (Fig. 1a–c). There was however a lower number of galls and egg masses on plants treated with 2 μg/mL and 3 μg/mL. The infected Swiss chards also showed symptoms of stunted growth with pale-coloured leaves and plants lacking vigour (Fig. 2). Wilting of young leaves was also observed under heat stress (temperature: 35 ± 2°C; day length: 14 hours).
Fig. 2.

RKN infected Swiss chards showing pale coloured leaves.
Effect of Ag-NP on the reproduction of M. javanica on Swiss chard
The result of the pot assay showed that all the inoculated plants were infected with RKNs with visible galls observed on their root systems. An egg-laying-female index of 5 (>100 egg masses) was recorded for most of the infected plants, thus indicating that Swiss chard is highly susceptible to infections by M. javanica. However, egg masses (EM), juveniles (J2), and RF varied with different concentrations of Ag-NP with 3 μg/mL Ag-NP showing higher potential for nematode control by recording significantly lower values of EM, J2, and RF. However, plants treated with 1 μg/mL of Ag-NP recorded significantly higher values for EM, J2, and RF, suggesting lower nematicidal activities (Table 1). A negative correlation was also recorded (Table 2) in the number of egg masses, juveniles, and reproduction potential of the RKNs, with increasing concentrations of the Ag-NP.
Table 1.
Effect of Ag-NP on the number of egg mass, juveniles, and reproduction of Meloidogyne javanica on Swiss chard.
| Treatments (Ag-NP) | Egg masses /root system | Juveniles/root system | Reproduction factor RF (pf/pi) |
|---|---|---|---|
| Control | 254.14a | 29946.14a | 9.98a |
| 1 μg/mL | 244.43ab | 28021.71a | 9.34a |
| 2 μg/mL | 130.86ab | 13661.71ab | 4.55ab |
| 3 μg/mL | 115.14b | 2909.71b | 0.92b |
The mean is significant at the 0.05 level.
Table 2.
Pearson correlations between variables of M. javanica reproduction and Ag-NP treatments.
| Variables | Treatments | Egg masses/root system | Juveniles/root system | Reproduction factor (RF) |
|---|---|---|---|---|
| Treatments | 1 | |||
| Egg masses/root system | −.482** | 1 | ||
| Juveniles/root system | −.604** | .781** | 1 | |
| Reproduction factor | −.606** | .780** | 1.000** | 1 |
Correlation is significant at the 0.01 level (2-tailed).
Discussion
Green leafy vegetables such as Swiss chard are important dietary components because they offer a rich source of essential minerals and vitamins, particularly in the diets of the population in developing countries where access to exotic food varieties and good health care facility is exclusive, and in most cases is beyond the reach of the resource-poor families. Reduction in the yield of vegetables and food crops due to damage by RKN nematodes poses a great threat to Agriculture and food security in Africa (Onkendi et al., 2014). According to Anwar and Mckenry (2010), damage caused by nematodes accounts for about 40 % reductions in the quantity and quality of the yield of harvested vegetables. In the current study, high reproduction of M. javanica and the female-egg-producing index that is greater than 5 suggests that Swiss chard is highly susceptible to infection by the RKN. This result is in tandem with those of Steiner et al. (2014) who first reported the association of M. javanica with Swiss chard in South Africa.
Swiss chard requires a comparatively cool climate within a temperature range of 7°C to 24°C for optimum leave production and yield. In the current study, however, smaller-sized leaves and symptoms of wilting were observed on the vegetable plants. This condition must have been exacerbated by infection with RKN, due to poor water and nutrient uptake as a result of impaired and dys-functional root system caused by the nematodes. Unfortunately, high summer temperatures that were recorded during this study could also favour the multiplication of M. javanica (Agenbag, 2016; Karssen et al., 2013) thus compounding the stress and damage to the plants.
In the current study, the molecular identification of individual juveniles and adult females isolated from the organically grown Swiss chard was positive and consistent for M. javanica. The use of species-specific SCAR primers as indicated in this study provided an effective and inexpensive method for identifying the Meloidogyne spp. The result obtained is supported by previous studies (Zijlstra et al., 2000; Fourie et al., 2001; Adam et al., 2007; Daramola et al., 2015; 2020) where the identification of Meloidogyne species such as M. javanica, M. arenaria, M. incognita, and M. hapla have been effectively done using PCR-based SCAR techniques. Accurate and precise nematode identification is crucial for diagnosing nematode problems and important for developing good and effective nematode management strategies. Nematode identification with molecular tools is now common and fast becoming a routine diagnostic tool in many laboratories in the developed world (Subbotin, 2021) because it offers a quick and more reliable option for disease diagnosis when compared with morphological-based techniques that are usually more tedious, less reliable and require the skill of well-trained personnel.
The present investigation also demonstrates the nematicidal potential of Ag-NP on the Swiss chard plants growing under controlled conditions. The result obtained in this study shows reducing number of egg masses, juveniles, and nematode reproduction in response to low concentrations of Ag-NP. Similar results have been recorded by Khan & Siddiqui (2018) where treatment with ZnO-NP caused a reduction in galling and nematode multiplication of M. incognita infected eggplants. Heflish et al., 2021 had also demonstrated in-vitro, the nematicidal activities of biosynthesized Ag-NP from Acalypha wilkesiana extract against M. incognita and reported reduced nematode activity, mortality, egg hatching, and movement of larvae.
A negative correlation was also observed in the number of egg masses, juveniles, and reproduction potential of the RKNs, with varying concentrations of the Ag-NP. From this study, treatment with a low concentration of Ag-NP (3 μg/Ml) produced nematicidal activities without observed adverse effects on the treated plant. The fact that NPs are required in very minute amounts which are usually non-toxic to plants and their ability to bio-stimulate plant growth (Akhtar, 2022) makes their use more desirable than the conventional chemical nematicides. Nanomaterials have also shown great potential for suppressing nematode on tomato and many vegetable crops (Ardakani, 2013; Abdellatif et al., 2016) while improving crop productivity.
The mode of application of nanoparticles and their effectiveness has also been likened to that of chemical nematicides (Cromwell et al., 2014) with the benefit of lower associated health or environmental effects at recommended concentrations. According to Kumar et al. (2022), NPs can decrease the risk of agrochemical inputs and play a role in plant health monitoring. Although chemical control remains the most effective control option, the attending environmental and health risk make their use undesirable for vegetable growers. Ag-NP when used at low concentrations as suggested in this study can be considered as a potential alternative to chemical nematicides for the management of plant-parasitic nematodes.
Conclusion
This study confirms that Swiss chard is highly susceptible to M. javanica. Identification of the nematode with the PCR-SCAR primers also provided an accurate, rapid, and efficient tool for diagnosing the nematode species. We have also shown in this study that Ag-NP at low concentrations can potentially reduce nematode reproduction on Swiss chard, however further studies will be required to provide more insight into how this can effectively be utilized as a suitable alternative to chemical nematicides.
Acknowledgment
The authors are grateful to Dr. Rinus Knoetze of Agriculture Research Institute (ARC), Nematology Lab, ARC Infruitec-Nietvoorbij, South Africa for assisting with nematode cultures and Prof. Samuel Oluwafemi of the Department of Chemistry, University of Johannesburg, South Africa, for providing the biosynthesized Ag-NP used in the study.
Reference
- Abad P., Gouzy J., Aury M.-J., Castagnone-Sereno P., Danchin E.G., Deleury E., Perfus-Barbeoch L., Anthouard V., Artiguenave F., Blok V.C.. et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Abdellatif K.F., Abdelfattah R.H., El-Ansary M.S.M.. Green Nanoparticles Engineering on Root-knot Nematode Infecting Eggplants and Their Effect on Plant DNA Modification. Iran J Biotechnol. 2016;14(4):250–259. doi: 10.15171/ijb.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam M.A.M., Phillips M.S., Blok V.C.. Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.) Plant Pathol. 2007;56(1):190–197. doi: 10.1111/j.1365-3059.2006.01455.x. [DOI] [Google Scholar]
- Agenbag M. Identification and reproduction potential of South African Meloidogyne species. Department of Environmental Sciences, Potchefstroom Campus of the North-West University; 2016. MSc Dissertation. [Google Scholar]
- Akhtar N., Ilyas N., Meraj T.A., Pour-Aboughadareh A., Sayyed R.Z., Mashwani Z.U.R., Poczai P.. Improvement of Plant Responses by Nanobiofertilizer: A Step towards Sustainable Agriculture. Nanomaterials (Basel) 2022;12(6):965. doi: 10.3390/nano12060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akyazi F., Felek A.F.. Root-knot nematode Meloidogyne arenaria infecting Swiss Chard (Beta vulgaris subsp. cicla) Akademik Ziraat Dergisi. 2020;9(1):43–48. doi: 10.29278/azd.641550. [DOI] [Google Scholar]
- Ali M.A., Ahmed T., Wu W., Hossain A., Hafeez R., Islam Masum M. M., Wang Y., An Q., Sun G., Li B.. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials (Basel) 2020;10(6):1146. doi: 10.3390/nano10061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S.A., Mckenry M.V.. Incidence and reproduction of Meloidogyne incognita on vegetable crop geno-types. Pak J Zool. 2010;42:135–14. [Google Scholar]
- Ardakani A.S.. Toxicity of silver, titanium and silicon nano-particles on the root-knot 184 nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology. 2013;15(6):671–67. [Google Scholar]
- Baronia R., Kumar P., Singh S.P., Walia R.K.. Silver nano-particles as a potential nematicide against Meloidogyne graminicola. J Nematol. 2020;52(1):e2020–02. doi: 10.21307/jofnem-2020-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas M.R., Curiel M.G. M., Fasio A.C., Contreras M.R., Rubio J.S.H., Osuna J.De D.D.. Identification and distribution of Meloidogyne species in Baja California Sur, Mexico. Rev. Mex. Cienc. Agríc. 2019;10(2):337–349. doi: 10.29312/remexca.v10i2.1603. [DOI] [Google Scholar]
- Cromwell W.A., Yang J., Starr J.L., Jo Y.K.. Nematicidal Effects of Silver Nanoparticles on Root-knot Nematode in Bermudagrass. J Nematol. 2014;46(3):261–26. [PMC free article] [PubMed] [Google Scholar]
- Daramola F.Y., Malgas R., Malan A.P.. First record and molecular identification of root-knot nematodes from honeybush tea plants (Cyclopia spp.) in South Africa. Nematology. 2020;22(5):591–593. doi: 10.1163/15685411-bja10023. [DOI] [Google Scholar]
- Daramola F.Y., Popoola J.O., Eni A.O., Sulaiman O.. Characterization of Root-knot Nematodes (Meloidogyne spp.) Associated with Abelmoschus esculentus, Celosia argentea and Corchorus olitorius. Asian J. Biol. Sci. 2015;8:42–50. doi: 10.3923/ajbs.2015.42.50. [DOI] [Google Scholar]
- Decker H. Sveshnicova N.M. Plant Nematodes and Their Control, Phytonematology. E.J. Brill (USA): Pauls Press; 1989. (Ed) New Delhi. Sf 262 den. 540 pp. [Google Scholar]
- Duncan L.W.. Current options for nematode management. Annu Rev Phytopathol. 1991;29(1):469–490. doi: 10.1146/annurev.py.29.090191.002345. [DOI] [PubMed] [Google Scholar]
- El-Ashry R.M., El-Saadony M.T., El-Sobki A., El-Tahan A.M., Al-Otaibi S., El-Shehawi A.M., Saad A.M., Elshaer N.. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J Biol Sci. 2022;29(2):920–932. doi: 10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer W., Ma C., White J.. Nanoparticles for plant disease management. Curr Opin Environ Sci Health. 2018;6:66–70. doi: 10.1016/j.coesh.2018.08.002. [DOI] [Google Scholar]
- Fouda M.M., Abdelsalam N.R., Gohar I.M.A., Hanfy A.E., Othman S.I., Zaitoun A.F., Allam A.A., Morsy O.M., El-Naggar M.. Utilization of High throughput microcrystalline cellulose decorated silver nanoparticles as an eco-nematicide on root-knot nematodes. Colloids Surf B Biointerfaces. 2020;188:110805. doi: 10.1016/j.colsurfb.2020.110805. [DOI] [PubMed] [Google Scholar]
- Fourie H., Mcdonald A.H., Mothata T.S., Ntidi Kn., De Waele D.. Indications of variation in host suitability to root-knot nematode populations in commercial tomato varieties. Afr J Agric Res. 2011;7(15):2344–2352. doi: 10.5897/AJAR11.706. [DOI] [Google Scholar]
- Ghareeb R.Y., Shams El-Din N., Maghraby D., Ibrahim D., Abdel-Megeed A., Abdelsalam N.R.. Nematicidal activity of seaweed-synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Sci Rep. 2022;12(1):3841. doi: 10.1038/s41598-022-06600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammed S.M., Hagag E.S., El-Raouf N.A.. Green production of silver nanoparticles, evaluation of their nematicidal activity against Meloidogyne javanica and their impact on growth of faba bean. Beni-Suef Univ J Basic Appl Sci. 2019;8:9. doi: 10.1186/s43088-019-0010-3. [DOI] [Google Scholar]
- Heflish A.A., Hanfy A.E., Ansari M.J., Dessoky E.S., Attia A.O., Elshaer M.M., Gaber M.K., Kordy A., Doma A.S., Abdelkhalek A., Behiry S.I.. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J. King Saud Univ.-Sci. 2021;33(6):101516. doi: 10.1016/j.jksus.2021.101516. [DOI] [Google Scholar]
- Hussey R.S., Boerma H.R.. A greenhouse screening procedure for root-knot nematode resistance in soybeans. Crop Sci. 1981;21:794–796. doi: 10.2135/cropsci1981.0011183X002100050041x. [DOI] [Google Scholar]
- Jones J.T., Haegeman A., Danchin E.G.J., Gaur H.S., Helder J., Jones M.G.K., Kikuchi T., Manzanilla-López R., Palomares-Rius J.E., Wesemael W.M.L., Perry R.N.. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 2013;14(9):946–961. doi: 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem K.H., Ahmed N.H., Gürkan T., Akbay N.G., Salai S.A.F., Çetintaş R.. Diagnosis of Nematode Populations Found in Chard, Barley and Onion Grown in North of Iraq and South of Turkey. Kahramannaraş Sütçü İmam univ. doğa bilim. derg. 2017;20(1):28–34. [Google Scholar]
- Karssen G., Wesemael W., Moens M. Perry R.N., Moens M. Plant Nematology. 2nd edition. CAB International; Wallingford, UK: 2013. Root-knot Nematodes; pp. 73–108. (Eds) [Google Scholar]
- Khan M., Siddiqui Z.A.. Zinc oxide nanoparticles for the management of Ralstonia solanacearum, Phomopsis vexans and Meloidogyne incognita incited disease complex of eggplant. Indian Phytopathol. 2018;71:355–364. doi: 10.1007/s42360-018-0064-5. [DOI] [Google Scholar]
- Khan M.R., Ahamad F., Rizvi T.F. Ghorbanpour M., Wani S.H. Advances in Phytonanotechnology. Academic Press; 2019. Effect of Nanoparticles on Plant Pathogens; pp. 215–240. (Eds) [DOI] [Google Scholar]
- Kumar A., Choudhary A., Kaur H., Guha S., Mehta S., Husen A.. Potential Applications of Engineered Nanoparticles in Plant Disease Management: A Critical Update. Chemosphere. 2022;295:133798. doi: 10.1016/j.chemosphere.2022.133798. [DOI] [PubMed] [Google Scholar]
- Mashela P.W, De Waele D., Dube Z., Khosa M.C., Pofu K.M., Tefu G., Daneel M.S., Fourie H. Fourie H., Spaull V.W., Jones R.K., Daneel M.S., De Waele D. Nematology in South Africa: a view from the 21st century. Switzerland: Springer; 2017. Alternative nematode management strategies; pp. 151–181. (Eds) [Google Scholar]
- Millán De Aguirre J.R. Estudios de Fitopatología [Phytopathology Studies] Consejería de Agricultura, Badajoz, Spain [Department of Agriculture, Badajoz, Spain]; 1991. Especies del género Meloidogyne presentes en los cultivos de la C. A. Vasca [Species of the genus Meloidogyne present in the crops of the Basque A.C.] pp. 164–167. In: S. E. F. (Ed) (In Spanish) [Google Scholar]
- Muimba-Kankolongo A. Food Crop Production by Smallholder Farmers in Southern Africa. Academic Press; 2018. Climates and Agroecologies; pp. 5–13. [DOI] [Google Scholar]
- Oluwafemi O.S., Anyik J.L., Zikalala N.E.. Biosynthesis of silver nanoparticles from water hyacinth plant leaves extract for colourimetric sensing of heavy metals. Nano-Struct. Nano-Objects. 2019;20:100387. [Google Scholar]
- Onkendi E.M., Kariuki G.M., Marais M., Moleleki L.N.. The threat of root-knot nematodes (Meloidogyne spp.) in Africa: a review. Plant Pathol. 2014;63:727–737. doi: 10.1111/ppa.12202. [DOI] [Google Scholar]
- Philis J.. Occurrence of Meloidogyne spp. and races on the island of Cyprus. Nematol Mediterr. 1983;11:13–1. [Google Scholar]
- Steyn W.P., Daneel M.S., Slabbert M.M.. Host suitability and response of different vegetable genotypes to Meloidogyne incognita race 2 and Meloidogyne javanica in South Africa. Int J Pest Manag. 2014;60:59–66. doi: 10.1080/09670874.2014.900587. [DOI] [Google Scholar]
- Subbotin S. Perry R.N., Hunt D.J., Subbotin S.A. Techniques for Work with Plant and Soil Nematodes. CAB International; 2021. Molecular identification of nematodes using Polymerase Chain Reaction; pp. 218–239. (Eds) [DOI] [Google Scholar]
- Whitehead A.G., Hemming J.R.. A Comparison of some quantitative methods extracting small vermiform nematodes from the soil. Ann Appl Biol. 1965;55:25–38. doi: 10.1111/j.1744-7348.1965.tb07864.x. [DOI] [Google Scholar]
- Windham G.L., Williams W.P.. Host suitability of commercial corn hybrids to Meloidogyne arenaria and Meloidogyne incognita. J Nematol. 1987;19:13–1. [PMC free article] [PubMed] [Google Scholar]
- Yang B., Eisenback J.D.. Meloidogyne enterolobii n.sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpot tree in China. J Nematol. 1983;15:381–39. [PMC free article] [PubMed] [Google Scholar]
- Zijlstra C., Donkers-Venne D.T.H.M., Fargette M.. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology. 2000;2(8):847–853. doi: 10.1163/156854100750112798. [DOI] [Google Scholar]