Summary
A study of the parasite fauna of feral cats in Dubai revealed the presence of two Joyeuxiella species, J. pasqualei (Diamare, 1893) and J. fuhrmanni (Baer, 1924). While the wide distribution of J. pasqualei includes countries of the Middle East, Africa, Asia and Europe, J. fuhrmanni was previously reported from felid hosts from southern Africa and has not been found in other cat parasite surveys in the Middle East, except from Dubai. The availability of historical references, however, raised doubts about the correctness of the allocation of the small Joyeuxiella sp. from Dubai cats to J. fuhrmanni and for this reason, a reexamination of stored material in the parasite collection of the Central Veterinary Research Laboratory in Dubai was carried out. A total of 40 specimens of the small Joyeuxiella sp. with a strobila length between 30 and 60 mm and consisting of 52 to 85 segments obtained from domestic cats and formerly allocated to J. fuhrmanni were studied. In complete specimens, 10 – 13 rows of rostellar hooks were counted. Mature segments were wider than long, round testes were concentrated posterior to coiled vasa deferentia and did not reach the anterior rim of the proglottids. Narrow cirri reached up to 520 μm in length. Gravid segments were longer than wide and egg capsules were restricted to the space between longitudinal excretory vessels. The examination revealed that the morphology of these cestodes matched the main characteristics of J. fuhrmanni. However, the little known cestode, J. gervaisi (Setti, 1895), that had been described from Genetta abyssinica imported from Eritrea 29 years earlier and was declared a species inquirenda met the same main morphological criteria. In this paper, the status of J. gervaisi as a valid species was resurrected and J. fuhrmanni was declared a junior synonym.
Keywords: Joyeuxiella gervaisi, J. fuhrmanni, Family Dipylidiidae, cat, Dubai, United Arab Emirates
Introduction
Joyeuxiella Fuhrmann, 1935 is one of the three genera of the family Dipylidiidae and according to a revision of the genus by Jones (1983) includes three different species J. echinorhynchoides (Sonsino, 1889), J. pasqualei (Diamare, 1893) and J. fuhrmanni (Baer, 1924). Starting from the description of its first species onwards, the current genus Joyeuxiella underwent several changes. Joyeuxiella echinorhynchoides was originally described from a fennec, Vulpes zedra (Zimmermann) in Egypt under the name Taenia echinorhynchoides by Sonsino (1889). Diamare (1893) described the second species (Dipylidium pasqualei) found in a domestic cat in Egypt and provided more details of this species the following year (Diamare, 1894). Setti (1895) described Dipylidium gervaisi from a genet, Genetta abyssinica (Rüppel), from Eritrea. Compared to previously known species of the genus Joyeuxiella, J. echinorhynchoides and J. pasqualei, Setti's description of Dipylidium gervaisi was more comprehensive, detailed and supported by drawings. A further species, J. chyceri (von Ratz, 1897), became a junior synonym of J. pasqualei later on. Among cestodes collected by A. Theiler from African carnivores, Baer (1924, 1926) described another tapeworm, Dipylidium fuhrmanni, from a serval, Leptaiurus serval (Schreber), hunted near Rustenburg in the Transvaal Province (today: North West Province) of South Africa. Lopez-Neyra (1927) transferred D. echinorhynchoides, D. pasqualei, D. gervaisi, D. chyceri and D. fuhrmanni, into the genus Joyeuxia. Since Joyeuxia as genus name was already preoccupied for a sponge (Joyeuxia belli), Fuhrmann (1935) proposed Joyeuxiella as a new genus name.
Joyeuxiella gervaisi fell into oblivion and was declared a species inquirenda by Jones (1983). However, the allegedly lost syntype material collected by Setti (1895) and used for the description of J. gervaisi was found in the zoological museum of the University of Naples.
The presence of J. fuhrmanni in feral cats in Dubai was reported in two papers (Schuster et al., 2009, 2016). Beside other helminths collected at the Central Veterinary Research Laboratory in Dubai, Poon et al. (2017) sequenced the cytochrome oxidase subunit 1 (COI) gene of J. pasqualei and J. fuhrmanni from feral cats in Dubai and registered the sequence in the GenBank Database (KY310707 for J. fuhrmanni and KY310708 for J. pasqualei).
The allocation of small cestodes of the genus Joyeuxiella from cats in Dubai to J. fuhrmanni was based on the revision of the genus Joyeuxiella by Jones (1983) but the eventual availability of the original description of J. gervaisi by Setti (1895) caused doubts about the correctness of the determination and was the reason for this paper.
Materials and Methods
Twenty-five specimens of J. gervaisi initially identified as J. fuhrmanni from the parasitological collection of the Central Veterinary Research Laboratory in Dubai were used. All samples were originally preserved in 70 % alcohol. For the creation of temporary mounts, samples were rehydrated and stained with lactic acid carmine before being dehydrated in rising alcohol concentrations and transferred into clove oil. Temporary preparations were examined under the microscope. For the preparations of permanent mounts, stained and dehydrated cestodes were cleared in clove oil and mounted in DPX medium. Since the structural integrity of rostellar hooks was partly lost through the use of this medium, another 15 cestodes collected from a freshly deceased cat sent to the CVRL for necropsy were fixed in hot 10 % formalin for examination. For comparison and identification, detail pictures of J. pasqualei tapeworms were utilized. Measurements are given in μm unless stated otherwise.
The molecular analysis involved genomic DNA isolation from the individual worms of Joyeuxiella pasqualei (n = 8) and Joyeuxiella gervaisi (n = 3) which were washed thrice with phosphate buffered saline (PBS) to remove residual ethanol and the DNA was extracted following manufacturer's protocol (G-spin total DNA extraction kit, Intron Biotechnology, South Korea). A partial segment of mitochondrial COI gene (400 bp) was amplified using previously described JB3/JB4.5 primer pair (Bowles et al., 1992). The PCR conditions were: initial denaturation: 95 °C for 5 min, denaturation: 95 °C for 1 min, annealing: 45 °C for 1 min, extension: 72 °C for 1 min and final extension: 72 °C for 5 min. The PCR products were sent for bidirectional sequencing using the same primer pair as previously mentioned.
The obtained sequence chromatograms were viewed through FinchTV viewer (Geospiza, Seattle, WA, USA) and checked individually for base-calling errors after ascertaining the peak qualities. Comparison for generated DNA sequences was carried out in NCBI BLAST algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to search for the reference sequences. The sequences were submitted in the GenBank database under following accession numbers: OR081741-OR081751. Additionally, a maximum likelihood tree was generated through MEGA-X software through Kimura-2-parameter substitution model with gamma distributed sites and 1000 bootstrap replicates to understand phylogenetic patterns and species alignment with the reference sequences (Joyeuxiella pasqualei: KY310707 (Poon et al., 2017) and ON981095 (Bezerra-Santos et al., 2022); Joyeuxiella fuhrmanni: KY310708 (Poon et al., 2017)) and an outgroup (Taenia hydatigena NC_012896; Jia et al., 2010).
Ethical Approval and/or Informed Consent
Not applicable. For this study, formal consent is not required.
Results
Redescription of Joyeuxiella gervaisi (Setti, 1895) Fuhrmann, 1935 (Figs. 1 – 4)
Fig. 1.
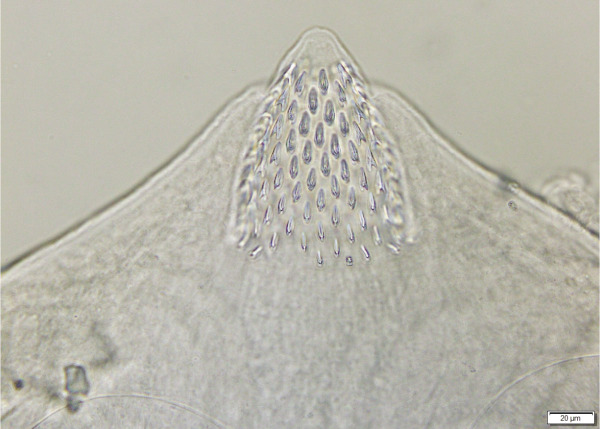
Joyeuxiella gervaisi (Setti, 1895) scolex from domestic cat from Dubai, UAE. The stinkhorn-shaped rostellum consists of a slender and longer stalk and conical shaped armed cap.
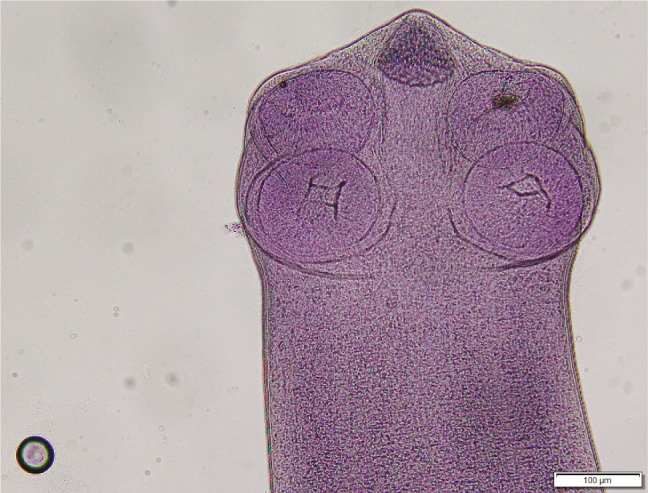
Cyclophyllidea, Dipylidiidae. Strobila 30 to 60 mm long, consisting of 52 to 85 segments. Mature segments with maximum width of 1.2 mm. Scolex (Fig. 1) dorso-ventrally flatted 282 – 436 by 360 – 470; slightly oval, unarmed suckers 115 – 135; rostellum 205 – 225 long and 205 – 225 wide stinkhorn-shaped, divided into lower unarmed cylindrical, followed by conical upper part (Fig. 2), armed with up to 10 – 12 (13) alternating rows of rose-thorn shaped hooks, base of hooks slightly longer than blades, upper rows 10 – 12, rows at the base 6 – 7; neck 388 – 760 × 245 – 425. First proglottids with primordia of reproductive organs (ovaries and vasa deferens) in segments 11 to 25, first signs of testes 2 – 5 segments later. Three to four fully developed rectangle-shaped mature proglottids (Fig. 3), broader (825 – 1230) than long (330 – 690), slightly convex in anterior third at genital pores; genital pores funnel-shaped, 30 – 78 wide and 31 – 69 deep. Gravid segments (Fig. 4) longer than wide, 2100 – 4050 × 840 – 1170, with genital pores in first quarter. Round testes 50 – 70 in diameter, numbering between 28 and 38, positioned anteriorly, medially and posteriorly to female reproductive glands, not reaching anterior part of the segment; vasa deferentia in anterior part of segments, heavily coiled; cirrus sac oval-shaped 150 – 240 × 75 – 120, oblique, with basis directed anteriorly and crossing excretory vessels; unarmed cirrus everted in some segments, 360 – 520 long, diameter 15 – 21. Fan shaped ovaries posterior and median to cirrus pouch, 130 – 250, seminal receptacle fusiform, 75 – 130 × 25 – 60 between ovary lobes; vitelline glands round, oval or kidney-shaped postovarian, 60 – 96. Vagina with diameter comparable to that of cirrus, opening posterior to cirrus sac.; egg capsules in gravid segments 50 – 80 in diameter, located medially to longitudinal excretory vessels; eggs 30 – 42, oncospheres: 28 – 38; embryonal hooks: 15 – 18.
Fig. 2.
Joyeuxiella gervaisi (Setti, 1895) rostellum armed with rose-thorn hooks from domestic cat from Dubai, UAE.
Fig. 3.
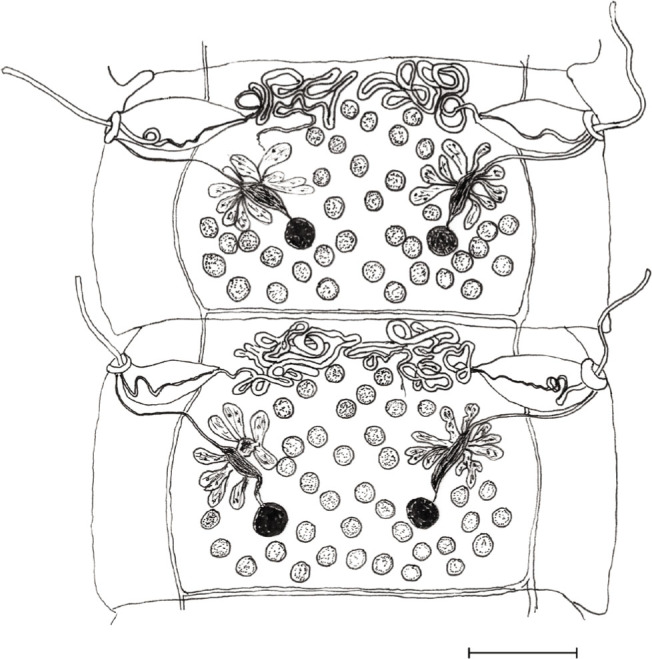
Joyeuxiella gervaisi (Setti, 1895) from domestic cat from Dubai, UAE, mature segment. The anterior end of the segment is occupied by convolutes of vasa deferens (bar: 100 μm).
Fig. 4.
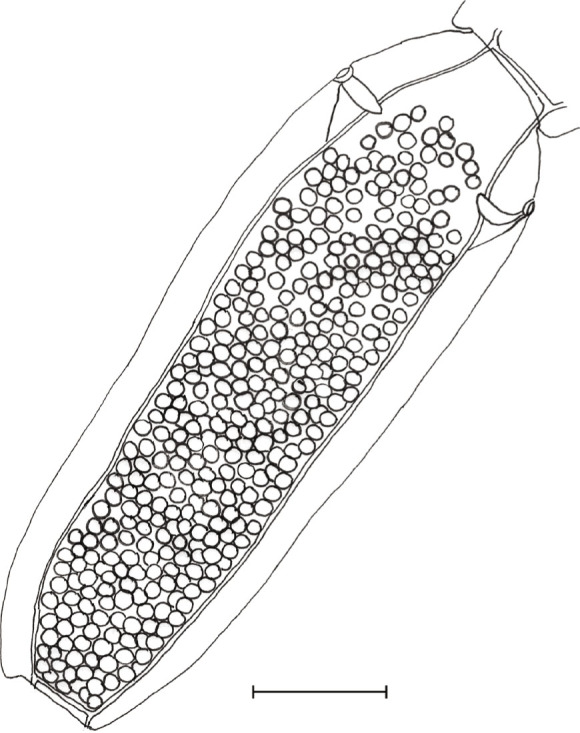
Joyeuxiella gervaisi (Setti, 1895) gravid segment from domestic cat from Dubai, UAE. Egg capsules are located between longitudinal excretory vessels (bar: 500 μm).
Taxonomic summary
Type host: Felis catus Linneus, 1758 (Carnivora: Felidae)
Type locality: Dubai, United Arab Emirates (25°12′42.05″N; 55°17′24.29″E)
Type specimens: MPM coll. No: 21896 (Meguro Parasitological Museum in Tokyo/Japan)
Site in host: small intestine
Prevalence: (see discussion)
Synonymy: F. furmanni (Theiler, 1924) is considered a junior synonym
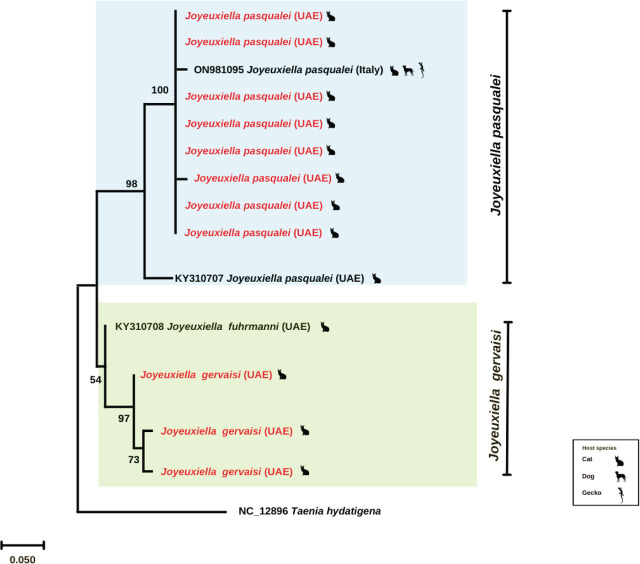
The molecular analysis backed the morphological assessment of cestodes revealing two species namely J. pasqualei and J. gervaisi. The BLAST results yielded 99.11 % homology and 100 % query coverage for the J. pasqualei partial COI sequences with the reference sequence (GenBank accession number: ON981095). For the J. gervaisi sequences, the homology was low as the available sequence of J. fuhrmanni (GenBank accession number: KY310708) is only a stretch of 100 base pairs, however the multiple alignment showed high similarity for the partial COI segment. To further discern the genealogical relationships between the species, a maximum likelihood tree was generated in which COI sequences for both cestode species, J. pasqualei and J. gervaisi, positioned along the respective reference sequences having a strong tree support.
Remarks
Cestodes found by E. Setti in the small intestine of a genet from Eritrea and described as Dipylidium gervaisi were part of the helminthological collection created by Corrado Parona, director of the Natural History Museum of the University of Genova. After Paronas's death in 1922, this collection housing more than 700 different worm species was donated to the Zoological Institute of the University of Naples and was united with the helminthological collections of Monticelli and Stossich to form the ‘Collezione Elmintologica Centrale Italiana’ (Salefi, 1956). A jar labeled ‘Cest 254’ in the aforementioned collection contained the type material (syntype) still classified under the old name of Dipylidium gervasi from Genetta abyssinica from Eritrea. The species name ‘gervasi’ on the label is obviously a typo (Fig. 5, 6).
Fig. 5.

Cylindrical jar ‘Cest 254’ of the zoological museum of University of Naples. This vessel contains the cestodes collected by E. Setti. The host Gazella abyssinica is wrong.
Fig. 6.

Label taken from inside of the jar ‘Cest 254’. Here, the spelling of the host is correct but the species name, Dipylidium gervasi, name is misspelled.
According to Setti (1895), the main features of this small, up to four-centimeter long dipylidiid cestode were the striking long cirri (0.5 mm) and the positioning of testes posterior to the heavily coiled vasa deferentia. Setti's illustrations showed the number of testes to be between 30 – 46 and the positioning of egg capsules in gravid segments to lay between the longitudinal excretory vessels. However, taking into consideration that solely 10 tapeworms and many gravid segments were in Setti's disposition, it is possible that J. gervaisi might have had a longer total length.
As far as molecular analysis is concerned, only a single sequence of J. furhmanni is available in the GenBank (accession no. KY310708; 100 bp) which differs at four nucleotide positions from the currently sequenced specimens (partial COI; 372 bp) of J. gervaisi. However, these sequences shared the same clade with a strong nodal support and that the haplotypic variations exist within the genome manifesting such differences (Fig.7).
Fig. 7.
Maximum-likelihood tree (partial mitochondrial COI) computed through MEGA-X software based on kimura-2 parameter and 1000 bootstrap replicates. Taenia hydatigena was taken as the outgroup. The geographical origin and host from which the sequence was identified are also mentioned. Bootstrap values are given as number on the nodes. The sequences for Joyeuxiella gervaisi and Joyeuxiella pasqualei retrieved in this study are depicted in red.
Discussion
The morphological examination of small Joyeuxiella cestodes found in feral cats in Dubai matched the typical characteristics described for J. gervaisi as well as for J. fuhrmanni. Since J. gervaisi was described 29 years earlier, J. fuhrmanni has to be considered a junior synonym.
Although the verbal characterization of J. gervaisi by Setti (1895) is limited, drawings of the scolex, the rose-thorn-shaped rostellar hooks and mature and gravid proglottids completed the description of the species. The author drew attention to the dichotomous structure of the rostellum consisting of a club-like armed upper part and a bulb-like base. In reality, the whole rostellum resembles a stinkhorn with a slender and longer stalk and a conical shaped armed cap.
Unfortunately, rostellar hooks easily fall off and can only be perceived in freshly isolated cestodes. In such specimens, 10 to 12 rows of hooks can be seen. In a few specimens however, up to 13 alternating rows with a bare outmost apex of the rostellum were observed. In the original description, Setti (1895) counted eight to 12 rows of rostellar hooks.
Mature segments were wider than long and gravid segments resembled a miniature cucumber seed. Within the material observed in this research, in each strobila, only three to four fully developed mature segments were seen. Ovaries, yolk glands and testes were replaced rapidly by the developing uterus in the following proglottids. Baer (1926) acknowledged J. fuhrmanni to show certain affinities with J. gervaisi but to differ in terms of ovarian and yolk gland structure. Additionally, Baer examined the shape of the rostellum which he described as acorn-shaped. However, some details in Baer's description were obscure. The author detailed the ovary as fan-shaped, irregularly lobed and touching the cirrus pouch, but drawings showed a rather compact ovary consisting of a large number of small follicles. Besides, the explicit figure of a pronounced trapezoid-shaped mature segment did not show receptaculi seminis mentioned in the verbal description. Contrary to this, the type material of J. fuhrmanni studied by Witenberg (1932) had lobed ovaries shifted to the centre of the segment with distinct receptaculi seminis. In that material, the majority of testes were situated in the space posterior to the ovaries. Some testes were seen between the coiled vasa deferens at the anterior end of the segment. Witenberg (1932) concluded that the type material of J. fuhrmanni was extremely contracted and thus, did not show a normal organ arrangement. Similar scolex structures of J. fuhrmanni and J. pasqualei led to the synonymization of both species.
Ortlepp (1933) disagreed with this view following the isolation of over a hundred small cestodes (2.0 – 6.5 cm long) from the small intestine of a cat in Pretoria. Examination of the hermaphrodite segments of these tapeworms revealed a similar testes distribution as described by Baer (1926). Ortlepp's material is not available anymore, but the helminthological collection of the Onderstepoort Veterinary Institute stores a glass container with the label: S325: Joyeuxia fuhrmanni; wild cat; small intestine; Kaalplaats (Limpopo Province), Onderstepoort; 7/6/1937. These dipylidiid cestodes were up to 54 mm long and consisted of 78 to 110 segments. Although the collector is not mentioned, it was most probably also R. J. Ortlepp who worked as helminthologist at Onderstepoort Veterinary Institute from 1930 to 1954.
Southwell (1930) mentioned J. gervaisi from fishing cats (Prionailurus viverrinus Bennett) and Asian palm civets (Paradoxurus hermaphroditus Pallas) from a Zoological garden in Calcutta. The seven-line long, not very specific paragraph described these worms as a one to four cm long cestodes with a 150 μm long neck that was not sharply divided from scolex. The small rostellum bore 8 – 12 rows of hooks. No information was given regarding the distribution of testes and egg capsules nor the cirrus length. Another mention of Setti's cestode can be found in a description of morphological abnormalities in D. caninum by Honigberg (1944) but does not provide any important morphological details.
Hudson (1934) reported J. fuhrmanni from a serval in Kenya without any further description and the last reported finding of J. fuhrmanni was from domestic cats in South Africa by Barker et al. (1989) without providing further morphological details.
In Setti's drawings, as well as in our material, mature proglottids were more or less rectangle-shaped, broader than long and slightly convex. Ovaries were shifted towards the centre, leaving a distinct space to the cirrus pouch. The long cirri, a key feature according to Baer (1926), were not noticed in the type material of J. fuhrmanni studied by Witenberg (1932). These structures, which are well represented in Setti's drawings of both mature and gravid proglottids, were perceived in our material as everted delicate long cirri and were noticed in live cestodes as well as in most of the segments during fixation and processing.
Mettrick and Beverley-Burton (1961) gave a description of J. fuhrmanni from a serval from Southern Rhodesia (today: Zimbabwe). This cestode had a length of up to 94 mm and a rostellum covered with 17 to 19 rows of hooks. The tapeworm had fan shaped ovaries and 270 μm long cirri (Table 1). In the same paper, the authors described J. paucitestis Mettrick et Beverley-Burton, 1961 as a new species from a rusty spotted genet (Genetta maculata Grey) hunted at Lake Kariba in the Zambezi Valley of Southern Rhodesia (today: Zimbabwe). Joyeuxiella paucitestis measured only 20 mm in length. Its scolex had a 50 to 80 μm long rostellum bearing 10 rows of small hooks. An obvious differential feature was the elongated shape of mature segments. As in the case of J. fuhrmanni, testes did not occur in front of the vasa deferens. Cirri reached a length of 140 μm.
Table 1.
Metrical data of J. gervaisi and J. fuhrmanni according to various sources. (Sizes are given in μm unless stated otherwise, – : these parameters were not given in the description)
| Author: | Setti (1895) | Baer (1926) | Mettric & Beverly-Burton (1961) | Jones (1983) | This paper | |
| Species: | J. gervaisi | J. fuhrmanni | J. fuhrmanni | J. fuhrmanni | J. gervaisi | |
| Host: | genet | serval | genet | wild felids, domestic cat | domestic cat | |
| (Origin): | (Eritrea) | (South Africa) | (Rhodesia) | (South Africa) | (Dubai) | |
|
| ||||||
| Parameter | ||||||
|
| ||||||
| Strobila | length | 10 – 40 mm | up to 30 mm | up to 94 mm | 10.5 – 80 mm | 30 – 60 mm |
| width | 1 – 1.5 mm | 1.6 mm | 1.6 | 1 mm | 0.9 – 1.2 mm | |
| Proglottids | number | – | 180 – 200 | – | 34 – 165 | 52 – 85 |
| Scolex | width | 250 | 240 | 350 | 132 – 333 | 360 – 470 |
| length | 150 | – | 350 | 141 – 2500 | 282 – 436 | |
| Rostellum | length | 120 | 230 | 140 – 170 | 141 – 268 | 205 – 225 |
| width | – | 100 | 90 | 56 – 137 | 61 – 98 | |
| Hooks | circles | 8 – 12 | 14 – 16 | 17 – 19 | 12 – 16 | 10 – 12 (13) |
| size | 10 | 8 | 12 – 16 | 12 – 17 | 10 – 12 | |
| Suckers | diameter | 100 | 200 | 90×130 − 140 | 94 – 165 | 115 – 135 |
| Mature segment | length | wider than long | trapezoid | 1100 | 114 – 1188 | 330 – 690 |
| width | 700 | 619 – 1667 | 825 – 1230 | |||
| Cirrus sac | length | – | 250 – 300 | 240 – 260 | 141 – 273 | 150 – 240 |
| diameter | – | 20 – 50 | 50 – 70 | 47 – 118 | 75 – 120 | |
| Cirrus | length | 500 | 300 | 270 | – | up to 520 |
| diameter | 15 | 20 | 20 | 14 – 21 | 15 – 21 | |
| Testes | number | 36 – 46 | 40 – 50 | 33 – 42 | 20 – 60 | 28 – 38 |
| diameter | – | 70 | – | 47 – 81 | 50 – 70 | |
| Ovary | diameter | – | – | 120 – 160 | 188 – 282 | 130 – 250 |
| Yolk gland | size | – | – | 40 – 60 | 45 – 165 | 60 – 96 |
| Receptaculum seminis | length | – | – | 100 | 66 – 212 | 75 – 130 |
| diameter | – | – | 50 | 28 – 85 | 25 – 60 | |
| Gravid segment | length | – | 2100 | 4900 | 1400 – 4000 | 2100 – 4050 |
| width | – | 900 | 1600 | 600 – 1800 | 840 – 1170 | |
| Egg capsule | diameter | 50 | 42 – 46 | 54 – 59 | 50 – 71 | 50 – 80 |
Based on examination of cestodes from felids: servals from South Africa and Belgian Congo, African wild cat (F. lybica Forster) and domestic cats from South Africa and Genetta rubriginosa (Grey) from Southern Rhodesia), Jones (1983) gave an extended diagnosis for J. fuhrmanni and resurrected the status of this cestode. According to this, single egg capsules can also be present externally to the longitudinal excretory vessels but the extremely long cirri, one of the key morphological features according to Baer (1924) were not mentioned. Also, Jones (1983) synonymized J. fuhrmanni with J. paucitestes although in the latter, mature segments were clearly longer than wide. Jones (1983) declared J. gervaisi a species inquirenda because of the unavailability of a deposited sample.
Beside J. fuhrmanni, Jones (1983) recognized J. pasqualei and J. echinorhynchoides as the only valid species of the genus Joyeuxiella. Table 1 summarizes morphometrical data on J. gervaisi and J. fuhrmanni according to different authors. Jones (1983) regarded also Dipylidium sp. of Kofend (1917) as synonym of J. fuhrmanni.
In a preliminary communication, Kofend (1917) merely mentioned the finding of a single cestode in a serval from the Sudan. According to Kofend (1917) the worm resembled D. echinorhynchoides. Dipylidium sp. however, differed from D. echinorhynchoides based on the arrangement of the rostellar hooks. A more detailed description was given six years later (Kofend, 1923). According to this, the cestode was 1.5 to 6 cm long with a rostellum armed with 16 to 18 rows of hooks. The description however, did not mention the arrangement, size and number of testes and the length of the cirrus. The uterus in gravid proglottids appeared as a dorsal cavity with branches in all directions and end eggs were also situated externally to longitudinal excretory vessels. A drawing of a mature segment of Kofend's Dipylidium sp. by Witenberg (1932) showed that the testes in this species were present also in the anterior part of the proglottide. For these reasons, the allocation of Dipylidium sp. of Kofend (1917) to J. fuhrmanni remained doubtful. Neither Witenberg (1932) nor Jones (1983) mentioned the enormous cirrus length of J. fuhrmanni. One reason for this might have been that these authors examined stained mounts. During the fixation, staining and embedding processes, these extremely thin structures often break and get lost.
It is interesting that the ‘J. fuhrmanni’ type was so far only detected in Africa and in the United Arab Emirates. In a study of parasites of feral cats in Dubai, both J. pasqalei and J. fuhrmanni were detected with an overall prevalence of 65.8 % (Schuster et al., 2009). A further study revealed that 11 out of 13 cestode infected cats in an untreated control group in an anthelminthic efficacy trial of a new dewormer for cats harbored J. fuhrmanni while 13 out of 13 cats were infected with J. pasqualei (Schuster et al., 2016). J. fuhrmanni was not mentioned in cat surveys carried out in other countries of the Middle East, like Qatar (Abu-Madi et al., 2010), Kuwait (Abdul-Salam & Baker, 1990; El Azazi et al., 2016), Iraq (Al-Rammahi et al., 2014; Al-Rubae et al., 2015) or Iran (Dalimi et al., 2006; Changizi et al., 2007; Esmaeilzadeh et al., 2009; Arabi & Hooshyar 2009; Borij et al., 2011). The mention of J. gervaisi in India by Southwell (1930) needs further confirmation. A more recent publication of cat parasites in Sudan named Joyeuxiella spp. in 60 % of feral cats trapped in Khartoum and figure 2 of this paper pictures mature proglottids with genital pores shifted to the anterior end with heavily coiled vasa deferens (Mohammed et al., 2021).
Key to species of Joyeuxiella Fuhrmann, 1935
- Rostellum cylindrical, hook blades longer than bases, number of alternating rows of rostellar hooks: 20-30…………………… ………………………………………………. J. echinorhynchoides
- - Dichotomous rostellum consisting of a cylindrical base and conical armed top. Hook blades shorter than bases, number of alternating rows of rostellar hooks less than 20……………………………………..2
- Testes in numbers between 40 and 130 present anterior and posterior to vasa deferentia, egg capsules in gravid segments median and lateral to longitudinal excretory vessels, strobila length 200 – 400 mm……………………………………………… ………………………………………………J. pasqualei
- - Testes in numbers between 28 to 60, absent anterior to heavily coiled vasa deferentia, egg capsules median to longitudinal excretory vessels. Strobila length 10 – 94 mm…………… ……………J. gervaisi
Conclusion
Summarizing all these facts, it became clear that J. gervaisi is a valid species that shares the main morphological features with its junior synonym, J. fuhrmanni, e.g. small strobila length, long cirri, heavily coiled vasa deferentia at the anterior end of mature segments and positioning of egg capsules between the longitudinal excretory vessels.
Acknowledgements
The authors are grateful to Dr. Roberto Poggi, Museo Civico di Storia Naturale “Giacomo Doria” in Genova for information about Corrado Parona and Ernesto Setti. For sending images of J. fuhrmanni from museum collections we thank Dr. Isabel Blasco Costa from the Museum d’ Histoire Naturelle Geneva, Switzerland and Dr. Kerstin Juncker from Onderstepoort Veterinary Institute, Onderstepoort, South Africa.
Footnotes
Conflict of Interest
Authors state no conflict of interest.
– Diamare (1892) transferred this species into the genus Dipylidium.
– The French naturalist Paul Gervais was the first person who described cestodes from a genet from southern France (Gervais 1847). Halysis genettae was am 8 cm long cestode with a rostellum armed with multiple thorn like hooks. No doubt that H. genettae belonged to the Dipylidiidae family but the description and illustration were insufficient.
– The article was reviewed by F. Zschocke (1895).
– This paper was a preliminary note that gave the key characteristics of the new species. A more detailed description followed two years later.
– In the description of J. gervaisi, Setti did not mention yolk glands. The pictured hermaphrodite segments had not reached the full maturity since yolk glands could not be distinguished from testes.
– The viewed syntype MHNG PLAT 0060956 of J. fuhrmanni stored in the Natural History Museum of Geneva showed the same trapezoid shaped mature segments.
References
- Abdul-Salam J., Baker K.. Prevalence of intestinal helminths in stray cats in Kuwait. Pakistan Vet J. 1990;10:17–2. [Google Scholar]
- Abu-Madi M.A., Behnke J.M., Prabhaker K.S., Al-Ibrahim R., Lewis J.W.. Intestinal helminths of feral cat population from urban and suburban districts of Qatar. Vet Parasitol. 2010;168:248–292. doi: 10.1016/j.vetpar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Al-Rammahi H.M., Safa M.K., Hammadi A.K.. Prevalence of intestinal helmintes in feral cats in Babylon province/Iraq, urban and rural locations. Mirror Res Vet Sci Anim. 2014;3:44–5. [Google Scholar]
- Al-Rubaie A.-R.L., Mhaisen F.T., Al-Tae A.-R. A.. Survey of some gastrointestinal cestodes and nematodes from stray cats at Baghdad city, Iraq. Am J Biol Life Sci. 2015;3(6):246–253. [Google Scholar]
- Arbabi M., Hooshyar H.. Gastrointestinal parasites of stray cats in Kashan, Iran. Trop Biomed. 2009;26:16–2. [PubMed] [Google Scholar]
- Baer J-G.. Contribution a la faune helminthologique Sud-Africaine. [Contribution to the helminth fauna of South Africa] Ann Parasitol. 1924;2:239–247. (In French) [Google Scholar]
- Baer J.G. Contribution to the helminth fauna of South Africa. Mammalian cestodes. Union of South Africa; 1926. pp. 63–136. Department of Agriculture 11th & 12th Reports of the Director of Veterinary Education and Research. [Google Scholar]
- Baker M.K., Lange L., Vester A., Van Der Plaat S.. A survey of helminths in domestic cats in the Pretoria area of Transvaal. Part 1: The prevalence and comparison of burdens of helminths in adult and juvenile cats. J S Afr Vet Assoc. 1989;60:139–14. [PubMed] [Google Scholar]
- Bezerra-Santos M.A., Mendoza-Roldan J.A., Lia R.P., Annoscia G., Schuster R., Varcasia A., Sgroi G., Modry D., Otranto D.. Description of Joyeuxiella pasqualei (Cestoda: Dipylidiidae) from an Italian domestic dog, with a call for further research on its first intermediate host. Parasitology. 2022;149(13):1769–1774. doi: 10.1017/S0031182022001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borji H., Razmi G., Ahmadi A., Karami H., Yaghfoori S., Abedi V.. A survey on endoparasites and ectoparasites of stray cats from Mashhad (Iran) and association with risk factors. J Paras Dis. 2011;35:202–206. doi: 10.1007/s12639-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J., Blair D., Mcmanus D.P.. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54(2):165–173. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Changizi E., Mobedi I., Salimi-Bejestani M.R., Rezaei-Doust A.. Gastrointestinal helminthic parasites in stray cats (Felis catus) from North of Iran. Iran J Parasitol. 2007;2:25–2. [Google Scholar]
- Dalimi A., Sattari A., Motamedi G.. A study on intestinal helminthes of dogs, foxes and jackels in the western part of Iran. Vet Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Diamare V.. Di un nuovo cestode del gen. Dipylidium Lt. [On a new cestode of the genus Dipylidium Lt] Boll soc nat Napoli. 1892;6:46–48. (In Italian). [Google Scholar]
- Diamare V.. Note su cestodi. [Note on cestodes]. Boll soc nat Napoli. 1893;7:9–13. (In Italian) [Google Scholar]
- Diamare V.. Il genera Dipylidium. [The genus Dipylidium] Atti della Reale Accademia delle Science Fisiche e Matematiche Napoli. 1894;4:1–31. (In Italian) [Google Scholar]
- El-Azazi O.M.E., Abdou N-E., Khalil A., Al-Batel M.K., Henedi A.A-R., Tahrani L.M.A.. Cestodes and nematodes recorded in stray cats in Kuwait. Global Vet. 2016;16:111–118. doi: 10.36478/vr.2014.23.33. [DOI] [Google Scholar]
- Esmaeilzadeh M., Shamsfart M, Kazemi A, Khaqlafi S.A., Altome S.A.. Prevalence of protozoa and gastrointestinal helminthes in stray cats in Zanjan Province, North-West of Iran. Iran J Parasitol. 2009;4:71–7. [Google Scholar]
- Fuhrmann O.. Rectification de nomenclature. [Correction of nomenclature] Ann Parasitol. 1935;13:386. (In French) [Google Scholar]
- Gervais P.. Sur quelques entozoaires tenioides et hydatides. [On entozoon taenoids and hydatides] Memoirs de l’ Academie de Scices et Lettres de Montpellier. Annee. 1847;1947:86–90. (In French) [Google Scholar]
- Honigberg B.. A morphological abnormality in the cestode, Dipylidium caninum. Transact Am Microscop Soc. 1944;63:342–344. [Google Scholar]
- Hudson J.R.. A list of cestodes known to occur in East African mammals, birds and reptiles. J East Afr Uganda Nat Hist Soc. 1934;49 – 50:205–217. [Google Scholar]
- Jia W.Z., Yan H.B., Guo A.J., Zhu X.Q., Wang Y.C., Shi W.G., Chen H.T., Zhan F., Zhang S.H., Fu B.Q., Littlewood D.T.J., Cai X.P.. Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genomics. 2010;11:1–13. doi: 10.1186/1471-2164-11-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.. A revision of the cestode genus Joyeuxiella Fuhrmann, 1935 (Dilepididae: Dipylidiinae. Syst Parasitol. 1983;5:203–213. doi: 10.1007/BF00009352. [DOI] [Google Scholar]
- Kofend L.. Cestoden aus Säugetieren und aus Agama colonorum. [Cestodes of mammals and Agama colonorum]. Vorlaeufige Mitteilung. Anz Kaiserl Akad Wiss, Math-Naturwiss Kl. 1917;54(1–27):229–231. (In German) [Google Scholar]
- Kofend L.. Cestoden aus Säugetieren und aus Agama colonorum. [Cestodes of mammals and Agama colonorum] Denkschr Kaiserl Akad Wissensch, Math-Naturwiss Kl. 1921;98:1–10. (In German) [Google Scholar]
- Lopez-Neyra C.R.. Considerations sur le genre Dipylidium Leuckart. [Considerations about the genus Dipylidium] Bull Soc Pathol Exot. 1927;20:434–449. (In French) [Google Scholar]
- Mettrick D.F., Beverly-Burton M.. Some cyclophyllidean cestodes from carnivores in Southern Rhodesia. Parasitology. 1961;51:355–54. doi: 10.1017/s0031182000070785. [DOI] [PubMed] [Google Scholar]
- Mohammed S.I., Haroun E.M., Jusif M., Mursal W.I., Abdelsalam E.B.. Prevalence and pathology of some internal parasites in stray cats (Felis catus) in Khartoum North Town, Sudan. Am J Res Commun. 2021;9:13–3. [Google Scholar]
- Ortlepp R.J.. Joyeuxia fuhrmanni Baer, 1924, hitherto unrecorded cestode parasite of the domestic cat in South Africa. Onderstepoort J Vet Sci Animal Ind. 1933;1:97–9. [Google Scholar]
- Poon R.W.S., Tam E.W.T., Lau S.K.P., Cheng V.C.C., Yuen K.-Y., Schuster R.K., Woo P.C.Y.. Molecular identification of cestodes and nematodes by cox1 gene real-time PCR and sequencing. Diagn Microbiol Infect Dis. 2017;89:185–190. doi: 10.1016/j.diagmicrobio.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Salefi M.. Nel 150 anniversario della istituzione della Cattedra di zoologia dell’ateneo Napoletano [150 annyversary of the chair of zoology of Naples university]. Ital J Zool. 1956;23:89–98. (In Italian) [Google Scholar]
- Schuster R.K., Thomas K., Sivakumar S., O’donovan D.. The parasite fauna of stray domestic cats (Felis catus) in Dubai, United Arab Emirates. Parasitol Res. 2009;105:125–134. doi: 10.1007/s00436-009-1372-6. [DOI] [PubMed] [Google Scholar]
- Schuster R.K., Mustafa M.B., Baskar J.V., Rosentael J., Chester T., Knaus M.. Efficacy of a topical combination of fipronil, (S)-methoprene, eprinomectin and praziquantel (Broadline®) against naturallyacquired infections with cestodes of the genus Joyeuxiella in cats. Parasitol Res. 2016;115:2679–2684. doi: 10.1016/j.vetpar.2014.02.038. [DOI] [PubMed] [Google Scholar]
- Setti E.. Dipylidium gervaisi n. sp. e qualche considerazione sui limiti specifici nei cestodi. [Dipylidium gervaisi n. sp. and some considerations on limits in specifity of cestodes] Bolletino dei Musei di Zoologia e Anatomia Comparata della R. Universiteta di Genova. 1895;(32):1–8. (In Italian) [Google Scholar]
- Sonsino P.. Noticie elmintologiche. [Helminthological notes]. Atti della Societa Toscana di Scienze Naturali Residente in Piesa. Processi Verbali. 1889;6:191–194. (In Italian) [Google Scholar]
- Southwell T. The fauna of British India, including Ceylon and Burma. Cestoda. Vol. 2. London: Taylor and Francis; 1930. 262 pp. [Google Scholar]
- Witenberg G.. On the cestode subfamily Dipylidiinae Stiles. Z Parasitenk. 1932;4:542–584. [Google Scholar]
- Zschokke F.. Setti, E. Dipylidium gervaisi n. sp. e qualche considerazione sui limiti specifici nei cestodi. In: Atti soc. ligust. Sc. Nat. geogr. Anno VI. 8 pag., 1 Taf. Zool Centralbl. 1895;2:341–34. [Google Scholar]